Abstract
Background
In the United States, anabolic sex steroids are administered to cattle for growth promotion. There is concern regarding the reproductive consequences of this practice for men who eat beef. We investigated whether meat consumption was associated with semen quality parameters and reproductive hormone levels in young men.
Methods
Semen samples were obtained from 189 men aged 18-22 years. Diet was assessed with a previously validated food frequency questionnaire. We used linear regression to analyze the cross-sectional associations of meat intake with semen quality parameters and reproductive hormones, while adjusting for potential confounders.
Results
There was an inverse relation between processed red meat intake and total sperm count. The adjusted relative differences in total sperm counts for men in increasing quartiles of processed meat intake were 0 (ref), −3 (95% confidence interval = −67 to 37), −14 (−82 to 28), and −78 (−202 to −5) million (test for trend, P = 0.01). This association was strongest among men with abstinence time less than 2 days and was driven by a strong inverse relation between processed red meat intake and ejaculate volume (test for trend, P =0.003).
Conclusions
In our population of young men, processed meat intake was associated with lower total sperm count. We cannot distinguish whether this association is due to residual confounding by abstinence time or represents a true biological effect.
In the United States, anabolic sex steroids are administered to cattle for growth promotion 60 to 90 days before slaughter. Estrogen, progesterone, testosterone and three synthetic hormones (zeranol, melengestrol acetate, and trenbolone acetate) are the main hormones used for this purpose. Levels of hormone residues in edible tissues are higher in treated than in non-treated animals,1,2 and there is concern that hormonal residues in edible tissues, particularly those of synthetic hormones, may result in adverse reproductive consequences among beef eaters.3-6 Because of this, the European Union banned this practice in 1989.1,7
Despite the concerns, data on the relation of meat intake to semen quality parameters or reproductive hormone levels is scarce and inconsistent.4,8-10 To further investigate this, we examined whether meat consumption was associated with semen quality parameters and reproductive hormones among young healthy men in the United States. We hypothesized that higher red meat consumption would be associated with lower semen quality parameters. Furthermore, since hormone residue levels differ across edible tissues,2,11 our secondary hypothesis was that meats previously reported to have higher concentration of hormone residues would be more strongly related to semen quality parameters (processed red meats>organ meats>unprocessed red meats>poultry> fish).
Methods
Study population
The Rochester Young Men's Study is a cross-sectional study that enrolled men at the University of Rochester (New York) in 2009 and 2010. Men were recruited into the study through flyers and newspapers as described elsewhere.12 The Rochester study is part of a multi-center international study (U.S., Spain, Finland, and Denmark) aimed at evaluating the association of environmental contaminants (specifically, maternal beef consumption) with semen quality parameters during pregnancy. Men were eligible to participate if they were born in the United States after 31 December 1987, able to read and speak English, and able to contact their mother and ask her to complete a questionnaire. A total of 389 men contacted the study coordinator between spring 2009 and spring 2010. Of these, 305 met all eligibility criteria and 222 men (73%) enrolled in the study. A food frequency questionnaire (FFQ) was introduced in the fall of 2009, after enrollment had started. All men after this point (n=194) completed the FFQ. Among them, 3 had missing data on sperm morphology, and 2 had implausible total caloric intakes (<600 kcals or >15,000 per day), leaving a final sample size of 189 men.
Men underwent a physical examination during which height and weight were measured, the presence of reproductive disorders (e.g. varicocele) documented, and anogenital distance measured. Participants also completed a brief lifestyle and medical history questionnaire at this time. Participants received $75 upon study completion. The study was approved by the University of Rochester Research Subjects Review Board and written informed consent was obtained from all men.
Semen collection and analysis
Men produced semen samples by masturbation into a specimen cup at the clinic on the day of the physical examination. Lubricants were not used for masturbation. The men were asked to abstain from ejaculation for 48 hours prior to the clinic visit and to report the time of their previous ejaculation, but men who failed to follow these instructions were not excluded. Abstinence times >240 hr (n=7) were truncated at 240 hr. Samples were processed within 30 minutes of collection.
Ejaculate volumes were estimated by specimen weight, assuming a semen density of 1.0 g/mL. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific Inc., Horsham, PA, USA). Motility was assessed in accordance with the World Health Organization 1999 criteria13 and classified as both progressive motile (A + B), total motile (A + B + C) or immotile (D). Smears for morphology were made, air-dried, fixed, and shipped to the University Department of Growth and Reproduction at the Rigshospitalet (Copenhagen, Denmark). The slides were Papanicolaou stained and assessed using strict criteria.14 Total sperm count was calculated as concentration × volume and total progressive motile count was defined as concentration × volume × % progressive motility.
Reproductive hormone measurement
Blood samples were drawn from participant's cubital vein and centrifuged; the serum was separated, stored, and frozen at −80°C. Serum samples were then shipped to Copenhagen, Denmark on dry ice and stored at −20°C until hormone analysis was performed at Rigshospitalet. The methods have been described previously.15 Briefly, hormone assessments were done simultaneously to reduce intralaboratory variations. Serum levels of follicle-stimulating hormone (FSH), luteinizing hormone (LH), and sex hormone-binding globulin (SHBG) were determined using time-resolved immunofluorometric assays (DELFIA; PerkinElmer, Skovlund, Denmark). Intra- and interassay variations were < 5% in each of the three assays. Serum testosterone (T) levels were determined using a time-resolved fluoroimmunoassay (DELFIA; PerkinElmer) with intra- and interassay variation < 8%. Estradiol (E2) was measured by radioimmunoassay (Pantex, Santa Monica, CA, USA) with an intraassay variation of < 8% and an interassay variation of < 13%. Inhibin B levels were determined by a specific two-sided enzyme immunometric assay (Oxford Bio-Innovation Ltd, Bicester, UK) with intra- and interassay variation of 13% and 18%, respectively. Free testosterone (FT) was calculated using the equation of Vermeulen et al.16 assuming a fixed albumin of 43.8 g/L.
Dietary assessment
Diet was assessed using a previously validated 131-item FFQ.16,17 Men reported how often, on average, they consumed specified amounts of food, beverages, and supplements during the previous year. Nutrient intakes were estimated using the nutrient database from the U.S. Department of Agriculture (USDA),18 with additional information from manufacturers when necessary. In a validation study, the de-attenuated correlation coefficient between meat intake assessed with the FFQ and the 1-year average of prospectively collected diet records ranged from 0.56 for chicken and turkey to 0.83 for processed red meats.17 Unprocessed red meat intake was defined as the sum of beef, pork, and ham consumed as sandwiches, mixed dishes, or main dishes. Processed red meat intake was defined as the sum of hamburger, hot dog, bacon, and other processed red meats (e.g. salami). Organ meat intake was defined as the sum of liver from beef, calf, pork, chicken, and turkey. Poultry intake was defined as chicken or turkey cooked with or without skin, as main dish, sandwich, or frozen dinner. Two data-derived dietary patterns previously described in this population, the “Prudent Pattern” and the “Western Pattern”,19 were calculated as summary measures of global food choices.
Statistical analysis
We first summarized participant characteristics and compared them across quartiles of meat intake, using the Kruskal-Wallis test for continuous measures and an extended Fisher's Exact test for categorical variables. We used linear regression models to assess the association of meat intake (in categories) with semen quality parameters by comparing semen parameter levels in men with higher intake levels to those in the lowest quartile of intake (reference) while adjusting for potential confounders. Robust estimators of the variance were used in the computation of 95% confidence intervals (CIs). Total sperm count and sperm concentration were log-transformed to more closely approximate a normal distribution and to be consistent with previous literature. Results for these parameters were back-transformed to allow presentation of results on the original scale. Population marginal means20 were utilized to present marginal population averages adjusted for the covariates in the model. Tests for linear trend were performed using the median values of meat intake in each category as a continuous variable and semen parameters as the response variable. Departures from linearity were evaluated by introducing quadratic and cubic terms to the models and comparing these to models where meat intake was modeled as a linear term using a likelihood ratio test.
We considered as potential confounders baseline characteristics that were associated with meat intake and semen parameters, as well as factors previously reported to predict semen parameters. Based on these criteria, all models were adjusted for age (continuous), body mass index (BMI) (continuous), abstinence time (<2, 2-5, ≥5 days), smoking status (yes or no), hours of moderate to vigorous physical activity per week (continuous), hours of TV-watching per week (continuous), race (black, all other), recruitment period (2009, 2010), and caloric intake (continuous). In addition, sperm motility models were adjusted for time from semen collection to start of semen analysis (continuous). We further adjusted for overall dietary patterns (continuous) to determine whether any observed association was specific to a particular meat type or was explained by overall food choices. Additional models also included adjustment for animal fat and animal protein intakes (continuous) to examine whether these nutrients were responsible for any observed associations. The same set of covariates was used for adjustment of semen quality parameters and reproductive hormone levels with three exceptions: (1) hormones were not adjusted for abstinence time or time from semen collection to start of semen analysis; (2) hormones were adjusted for time of blood sampling (continuous), to take into consideration circadian variation in blood levels of some hormones; and (3) hormones were adjusted for alcohol intake (continuous), as some studies have found lower testosterone levels among men with high alcohol intake.21 We assessed effect modification of dietary associations with semen parameters by BMI (<25 kg/m2 and ≥25 kg/m2) and smoking status (current and never/former smokers) using cross-product terms. We also used cross-product terms to test for heterogeneity across strata of abstinence time. We analyzed the data using SAS (version 9.2; SAS Institute Inc., Cary, NC, USA), and two-sided p-values ≤ 0.05 were considered statistically significant.
Results
Participants were primarily white (83%), with a median age of 19.6 (interquartile range [IQR] = 18.9 to 20.5) years. Their median time spent on moderate to vigorous activity was 8 h/wk (5 to 14). Forty-one percent were overweight or obese (BMI≥25 kg/m2). The median sperm concentration was 53.0 × 106/ml (IQR = 20.5 to 95.5×106/ml); the percent progressively motile sperm was 60.5% (49.5% to 69.5%); and percent morphologically normal sperm was 8.5% (5.0% to 12.0%). Median total meat intake was 2.3 servings/day (IQR = 1.6 to 3.2 servings/day). Processed red meats were the most commonly consumed meat product, accounting for 40% of total meat intake, followed by poultry (31%), unprocessed red meats (17%), fish (11%) and organ meats (1%).
Total meat intake was positively related to moderate-to-vigorous physical activity, TV-watching, and smoking (Table 1). There was also an inverse relation between total meat intake and abstinence time; the median difference in abstinence time between the top and bottom quartile of total meat intake was 21 hours. Higher meat intake was associated with higher intake of total energy, saturated fat, mono-unsaturated fat, animal fat, total protein, and animal protein, as well as higher summary scores reflecting the Prudent and Western dietary patterns.
Table 1.
Participants’ characteristicsa according to quartiles of total meat intake.
| Quartiles of total meat intake |
||||
|---|---|---|---|---|
| Q1 (lowest) | Q2 | Q3 | Q4 (highest) | |
| Intake (servings/day): | 0 – 0.42 | 0.44 – 0.85 | 0.87 – 1.44 | 1.45 – 5.26 |
| No.: | (n = 48) | (n = 47) | (n = 48) | (n = 47) |
| Background characteristics | ||||
| Age (years) | 19.9 (18.9 to 20.8) | 19.4 (18.8 to 20.6) | 19.5 (19.0 to 20.3) | 19.6 (18.9 to 20.4) |
| Race/ethnicity; No. (%) | ||||
| White, not Hispanic | 35 (78) | 43 (88) | 43 (90) | 35 (75) |
| Black, not Hispanic | 4 (9) | 3 (6) | 1 (2) | 4 (8) |
| Hispanic or Latino | 3 (7) | 2 (4) | 0 (0) | 2 (4) |
| Asian | 2 (4) | 1 (2) | 1 (2) | 3 (6) |
| Other | 1 (2) | 0 (0) | 3 (6) | 3 (6) |
| Body Mass Index, kg/m2 | 24.5 (22.5 to 25.9) | 23.9 (22.6 to 26.0) | 24.9 (23.6 to 28.7) | 24.6 (22.5 to 27.5) |
| Moderate to vigorous physical activity (hours/week) | 8.0 (4.0 to 10.5) | 7.0 (4.0 to 15.0) | 10.0 (5.0 to 12.0) | 10.0 (7.0 to 17.0) |
| TV watching (hours/week) | 10.0 (4.0 to 14.0) | 14.0 (0.0 to 20.0) | 14.0 (4.0 to 17.0) | 14.0 (10.0 to 25.0) |
| Current smoker; No. (%) | 5 (11) | 17 (35) | 13 (27) | 8 (17) |
| Abstinence time (hours) | 84.6 (61.8 to 124.0) | 73.7 (54.7 to 110.9) | 64.7 (50.7 to 93.8) | 63.3 (50.7 to 86.6) |
| Self-reported reproductive history | ||||
| Undescended testes at birth; No. (%) | 2 (4) | 2 (4) | 0 (0) | 1 (2) |
| Varicocele; No. (%) | 3 (6) | 1 (2) | 0 (0) | 1 (2) |
| Hydrocele; No. (%) | 0 (0) | 1 (2) | 0 (0) | 2 (4) |
| Inguinal hernia repair; No. (%)b | 5 (11) | 1 (2) | 3 (6) | 1 (2) |
| History of genital disease; No. (%)c | 2 (4) | 2 (4) | 3 (6) | 3 (6) |
| Use of hormones; No. (%)d | 3 (6) | 3 (6) | 10 (21) | 3 (6) |
| Physical examination findings | ||||
| Testes low in scrotum; No. (%) | 45 (96) | 46 (98) | 46 (96) | 37 (79) |
| Varicocele; No. (%) | 7 (15) | 7 (15) | 4 (8) | 4 (9) |
| Hydrocele; No. (%) | 0 (0) | 1 (2) | 1 (2) | 2 (4) |
| Surgical scars; No. (%)e | 3 (6) | 2 (4) | 1 (2) | 2 (4) |
| Diet | ||||
| Total energy intake (kcal/day) | 2086.2 (1615.1 to 2566.8) | 2815.3 (2199.0 to 3210.3) | 3022.6 (2367.5 to 3590.8) | 3840.7 (3340.5, to 4943.0) |
| Caffeine intake (mg/day) | 51.0 (17.3 to 108.7) | 53.8 (14.2 to 126.6) | 66.2 (27.4 to 118.4) | 81.2 (39.1 to 133.4) |
| Alcohol intake (g/day) | 10.8 (2.7 to 31.7) | 10.4 (3.4 to 31.5) | 16.1 (7.6 to 27.7) | 11.2 (4.2 to 21.6) |
| Saturated fat (% energy) | 9.6 (8.1 to 10.9) | 10.4 (8.9 to 11.7) | 10.5 (9.3 to 12.2) | 10.6 (9.7 to 12.0) |
| Mono unsaturated fat (% energy) | 10.7 (9.5 to 12.5) | 11.1 (9.9 to 12.3) | 11.7 (10.6 to 13.0) | 11.8 (10.7 to 13.3) |
| Polyunsaturated fat (% energy) | 5.4 (4.9 to 6.4) | 5.2 (4.4 to 5.9) | 5.5 (4.9 to 5.8) | 5.5 (4.9 to 6.0) |
| Trans fat (% energy) | 1.2 (1.0 to 1.4) | 1.2 (0.9 to 1.4) | 1.3 (1.1 to 1.5) | 1.2 (1.1 to 1.6) |
| Animal fat (% energy) | 12.1 (10.3 to 14.7) | 15.1 (11.9 to 17.8) | 16.1 (13.1 to 19.3) | 16.5 (14.5 to 20.0) |
| Protein intake (% energy) | 14.1 (12.6 to 15.4) | 16.0 (14.5 to 17.9) | 16.7 (15.0 to 18.6) | 17.6 (16.1 to 20.1) |
| Animal protein intake (% energy) | 8.4 (7.2 to 9.7) | 10.7 (14.5 to 12.9) | 11.3 (10.1 to 13.1) | 12.1 (10.8 to 14.8) |
| Prudent pattern scoref | −0.52 (−0.84 to −0.05) | −0.29 (−0.63 to 0.26) | −0.19 (−0.58 to 0.12) | 0.32 (−0.37 to 1.42) |
| Western pattern scoreg | −0.68 (−1.09 to −0.28) | −0.37 (−0.82 to 0.14) | 0.14 (−0.30 to 0.68) | 0.71 (0.08 to 1.52) |
Median (IQR) unless otherwise specified
Inguinal hernia repair n=188
Self-report of any of the following: infection of epididymis, testicle, prostate, urinary tract infection, gonorrhea, genital warts or herpes, chlamydia, or other diseases of the penis, testicles, urinary tract or scrotum
Self-report of any of the following: dehydroepiandrosterone (DHEA), creatinine, or other muscle-building compounds
From hernia repair, appendectomy, orchidopexy, or other lower abdomen/inguinal procedures
Characterized by high intakes of fish, chicken, fruit, cruciferous vegetables, tomatoes, leafy green vegetables, legumes and whole grains
Characterized by high intakes of red and processed meat, butter, high fat dairy, refined grains, pizza, snacks, high energy drinks, mayonnaise and sweets
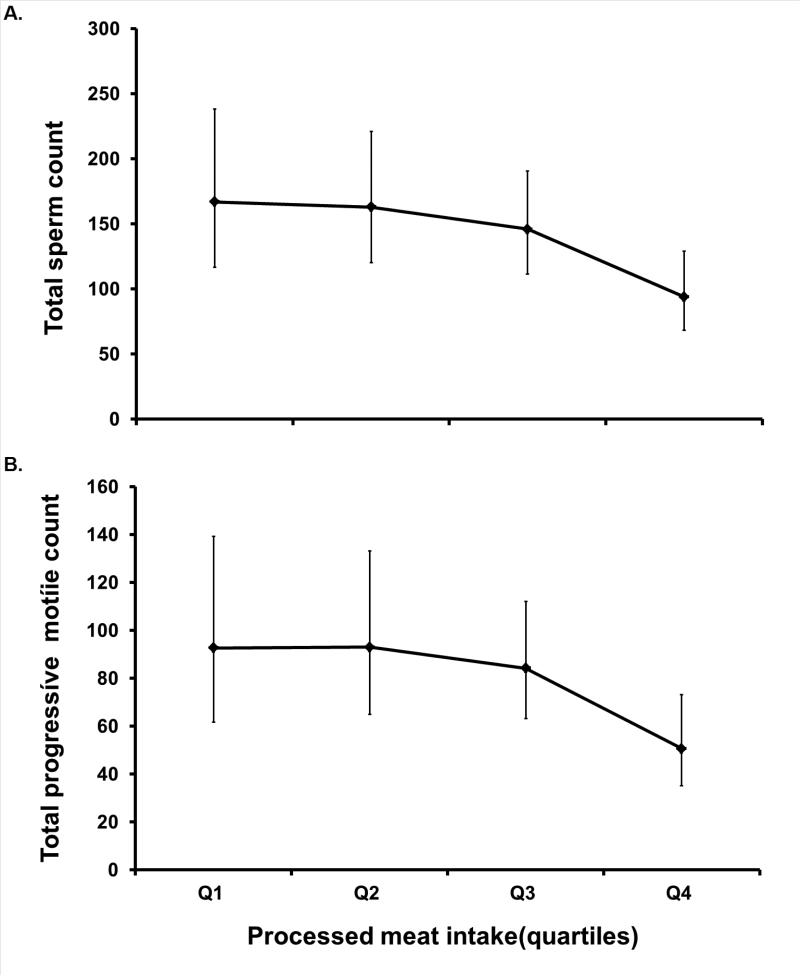
Intake of processed red meats was strongly associated with ejaculate volume in crude analyses. Compared with men in the lowest quartile of processed red meat intake, the adjusted differences in ejaculate volume for men in the second, third and fourth quartiles of intake were, −0.1 (95% CI = −0.8 to 0.6), −0.6 (−1.3 to 0.1), and −1.1 (−1.8 to −0.4) mL (test for trend, P <0.001). This association between processed red meat intake and reduced ejaculate volume persisted in multivariate adjusted models (Table 2). Processed red meat intake was also associated with lower total sperm count and lower total progressive motile count (Figure). Processed red meat intake was unrelated to sperm concentration, progressive motility or morphology (Table 2). The addition of quadratic and cubic terms for processed meat intake to a model with a linear term only did not suggest departures from a linear association for total sperm count, total progressive motile count, or ejaculate volume.
Table 2.
Adjusteda semen parameters according to intake of various meat types
| Meat intake (servings/day); range | No. | Sperm concentration (millions/mL) Mean (95% CI) | Progressive motilityb (% motile) Mean (95% CI) | Sperm morphology (% normal) Mean (95% CI) | Ejaculate volume (mL) Mean (95% CI) |
|---|---|---|---|---|---|
| Total meat intake | |||||
| Q1 (0 - 1.60) | 48 | 42.4 (31.3 to 57.4) | 56.7 (51.7 to 61.7) | 9.0 (7.6 to 10.5) | 3.7 (3.3 to 4.2) |
| Q2 (l.64 - 2.31) | 47 | 45.5 (34.8 to 59.6) | 60.3 (56.9 to 63.6) | 8.7 (7.4 to 10.0) | 3.6 (3.2 to 4.0) |
| Q3 (2.32 - 3.19) | 48 | 48.2 (37.3 to 62.3) | 60.7 (57.0 to 64.4) | 8.6 (7.3 to 9.9) | 3.6 (3.2 to 4.0) |
| Q4 (3.23 - 9.32) | 47 | 44.4 (32.7 to 60.2) | 55.8 (50.8 to 60.8) | 8.2 (6.6 to 9.7) | 2.9 (2.5 to 3.4) |
| Test for trend | P = 0.85 | P = 0.70 | P = 0.48 | P = 0.05 | |
| Processed red meatc intake | |||||
| Q1 (0 - 0.42) | 46 | 46.5 (34.7 to 62.4) | 59.4 (54.7 to 64.1) | 8.3 (7.0 to 9.6) | 3.9 (3.4 to 4.4) |
| Q2 (0.44 - 0.85) | 47 | 47.0 (36.3 to 61.0) | 60.2 (56.4 to 64.1) | 9.2 (7.7 to 10.6) | 3.8 (3.4 to 4.3) |
| Q3 (0.87 - 1.44) | 49 | 48.7 (37.8 to 62.8) | 58.6 (55.2 to 61.9) | 8.7 (7.4 to 10.0) | 3.3 (2.9 to 3.7) |
| Q4 (1.45 - 5.26) | 47 | 38.7 (28.3 to 53.0) | 55.3 (50.6 to 60.1) | 8.3 (6.9 to 9.7) | 2.8 (2.4 to 3.2) |
| Test for trend | P = 0.38 | P = 0.17 | P = 0.69 | P = 0.003 | |
| Unprocessed red meatd intake | |||||
| Q1 (0 - 0.16) | 53 | 42.7 (32.6 to 56.0) | 55.8 (51.3 to 60.4) | 8.3 (7.1 to 9.6) | 3.4 (3.0 to 3.7) |
| Q2 (0.22 - 0.30) | 42 | 46.1 (35.1 to 60.7) | 60.2 (56.8 to 63.6) | 7.8 (6.5 to 9.0) | 3.9 (3.4 to 4.4) |
| Q3 (0.36 - 0.65) | 51 | 39.9 (30.5 to 52.2) | 57.1 (53.6 to 60.6) | 8.6 (7.3 to 10.0) | 3.3 (2.9 to 3.6) |
| Q4 (0.71 - 2.23) | 43 | 54.5 (42.5 to 69.9) | 61.2 (57.2 to 65.3) | 9.8 (8.4 to 11.3) | 3.4 (2.9 to 3.8) |
| Test for trend | P = 0.15 | P = 0.12 | P = 0.06 | P = 0.65 | |
| Organ meate intake | |||||
| None (0) | 158 | 41.4 (35.8 to 47.8) | 57.1 (55.0 to 59.2) | 8.4 (7.7 to 9.1) | 3.3 (3.1 to 3.5) |
| Any (0.01 - 0.28) | 31 | 70.0 (53.9 to 90.9) | 65.1 (61.2 to 69.0) | 9.7 (8.1 to 11.4) | 4.1 (3.6 to 4.6) |
| Poultryf intake | |||||
| Q1 (0 - 0.28) | 43 | 40.4 (29.8 to 54.6) | 57.0 (53.0 to 61.0) | 8.8 (7.2 to 10.3) | 3.3 (2.9 to 3.7) |
| Q2 (0.30 - 0.59) | 47 | 45.1 (33.7 to 60.4) | 59.1 (55.4 to 62.9) | 9.2 (8.0, 10.4) | 3.7 (3.3 to 4.1) |
| Q3 (0.65 - 1.02) | 51 | 48.1 (39.5 to 58.6) | 60.4 (56.4 to 64.4) | 8.1 (6.9, 9.4) | 3.6 (3.2 to 4.0) |
| Q4 (1.08 - 4.50) | 48 | 46.5 (34.9 to 61.9) | 56.8 (52.7 to 60.8) | 8.5 (7.3, 9.7) | 3.2 (2.7 to 3.7) |
| Test for trend | P = 0.60 | P = 0.71 | P = 0.62 | P = 0.41 | |
| Total fish intake | |||||
| Q1 (0) | 38 | 43.4 (31.8 to 59.2) | 60.3 (56.0 to 64.7) | 9.2 (7.8 to 10.5) | 3.3 (2.3 to 3.7) |
| Q2 (0.08 - 0.16) | 56 | 42.4 (33.0 to 54.6) | 55.6 (51.9 to 59.3) | 8.2 (7.1 to 9.3) | 3.5 (3.2 to 3.9) |
| Q3 (0.22 - 0.38) | 47 | 46.4 (36.1 to 59.6) | 60.5 (57.4 to 63.6) | 9.8 (8.4 to 11.1) | 3.6 (3.1 to 4.0) |
| Q4 (0.40 - 2.25) | 48 | 48.6 (37.1 to 63.8) | 58.0 (53.6 to 62.5) | 7.6 (6.2 to 9.1) | 3.4 (2.9 to 3.9) |
| Test for trend | P = 0.47 | P = 0.89 | P = 0.38 | P = 0.83 |
Adjusted for age, abstinence time, race, smoking status, BMI, recruitment period, moderate-to-intense exercise, TV-watching, dietary patterns, and total calorie intake
Additionally adjusted for time from current ejaculation to start of semen analysis
includes hamburgers, hot dogs, bacon, and other processed meats (e.g. salami, bologna, etc.)
includes beef, pork, and ham consumed as sandwich, mixed dish, or main dish
includes beef, calf, pork, chicken, and turkey liver
includes chicken or turkey cooked with or without skin, as main dish, sandwich, or frozen dinner
Figure. Processed meat intake quartiles in relation to (A) total sperm count (millions) and (B) total progressive motile count (million motile). Meat intake quartiles: Q1 = 0 - 0.42mL; Q2 = 0.44 – 0.85 mL; Q3 = 0.87 – 1.44mL; Q4 = 1.45 – 5.25mL.
Models are adjusted for age, abstinence time, race, smoking status, BMI, recruitment period, moderate-to-intense exercise, TV-watching, dietary patterns, and total calorie intake. Tests for trend were conducted across quartiles using a variable with the median processed meat intake in each quartile as a continuous variable in the linear regression models. for total sperm count, P =0.01; for total progressive motile count, P = 0.02.
Since meat intake was inversely related to abstinence time, and abstinence time was positively related to ejaculate volume, sperm concentration, total sperm count, and total progressive motile count, we performed additional analyses to examine the possibility that the relations of processed red meat intake with total count and total progressive motile count were due to residual confounding by abstinence time. Results were nearly identical regardless of how abstinence time was modeled (eTable 1). To further examine the possibility of residual confounding, we examined the relation of processed red meats with semen parameters within strata of abstinence time (<2, 2-5, ≥5 days). The strongest relation of processed red meat intake with total sperm count was among men with abstinence time < 2 days (eTable 2). When men with abstinence time <2 days were excluded, the adjusted relative differences in total sperm counts for men in increasing quartiles of processed meat intake were 0 (ref), 13 (95% CI = −45 to 48), 3 (−59 to 40), and −17 (−108 to 34) million (test for trend, P = 0.34). The adjusted differences in ejaculate volume for men in increasing quartiles of processed red meat intake were 0 (ref), 0.01 (−0.7 to 0.7), −0.5 (−1.2 to 0.2), and −0.6 (−1.4 to 0.2) mL after this exclusion (test for trend, P = 0.05).
Organ meat intake was related to higher total sperm count, higher sperm concentration and greater sperm motility (Table 3). Compared with non-consumers, men who reported consuming organ meats had 53% (34% to 66%) higher total sperm count, 41% (20% to 56%) higher sperm concentration, and 8 (4 to 12) percentage units higher progressive motility after adjusting for potential confounders. We examined whether nutrients concentrated in organ meats explained these associations. Further adjustment for intakes of animal protein, animal fat, cholesterol, copper, manganese, iron or vitamin B12, alone or in combination, did not affect the association of organ meat intake with semen parameters (data not shown). Intakes of poultry or fish were not related to any of the semen quality parameters examined (Table 2).
Table 3.
Adjusteda mean values (95% CIs) of hormones according to intake of various meat types
| Meat intake (servings/day); range | No. | LH (IU/L) Mean (95% CI) | FSH (IU/L) Mean (95% CI) | E2 (pmol/L) Mean (95% CI) | Free Testosterone (pmol/L) Mean (95% CI) | Total testosterone (nmol/L) Mean (95% CI) | Inhibin B (pg/mL) Mean (95% CI) | SHBG (nmol/L) Mean (95% CI) |
|---|---|---|---|---|---|---|---|---|
| Total meat | ||||||||
| Q1 (0 - 1.60) | 48 | 4.0 (3.5 to 4.5) | 2.5 (2.1 to 2.9) | 92.5 (83.8 to 101.2) | 492.7 (444.7 to 540.7) | 21.5 (19.2 to 23.9) | 203.4 (183.8 to 223.1) | 31.6 (28.1 to 35.1) |
| Q2 (1.64 - 2.31) | 47 | 3.4 (3.0 to 3.8) | 2.6 (2.2 to 3.0) | 91.7 (84.9 to 98.4) | 434.9 (398.1 to 471.8) | 19.6 (17.5 to 21.7) | 185.4 (170.4 to 200.4) | 32.4 (28.3 to 36.5) |
| Q3 (2.32 - 3.19) | 48 | 3.5 (3.1 to 3.9) | 2.3 (2.0 to 2.6) | 85.4 (78.7 to 92.1) | 477.6 (425.4 to 529.7) | 19.8 (17.9 to 21.7) | 210.9 (194.0 to 227.8) | 29.1 (26.5 to 31.6) |
| Q4 (3.23 - 9.32) | 47 | 3.9 (3.4, to 4.4) | 2.9 (2.5 to 3.3) | 95.6 (86.8 to 104.3) | 502.2 (454.1 to 550.3) | 20.6 (18.6 to 22.6) | 179.8 (162.7 to 196.9) | 28.4 (25.2 to 31.7) |
| Test for trend | P = 0.98 | P = 0.35 | P = 0.74 | P = 0.50 | P = 0.73 | P = 0.22 | P = 0.14 | |
| Processed red meatb | ||||||||
| Q1 (0 - 0.42) | 46 | 3.8 (3.3 to 4.2) | 2.5 (2.1 to 2.9) | 92.1 (83.6 to 10.6) | 482.2 (435.8 to 528.7) | 21.5 (18.9 to 24.2) | 202.2 (183.0 to 221.3) | 32.8 (27.8 to 37.8) |
| Q2 (0.44 - 0.85) | 47 | 3.5 (3.1 to 4.0) | 2.3 (2.0 to 2.7) | 88.3 (81.1 to 95.5) | 488.0 (435.5 to 540.5) | 20.5 (18.3 to 22.8) | 200.0 (181.2 to 218.9) | 30.1 (27.3 to 33.0) |
| Q3 (0.87 - 1.44) | 49 | 3.7 (3.3 to 4.1) | 2.7 (2.4 to 3.2) | 92.4 (85.8 to 99.0) | 476.7 (439.7 to 513.7) | 20.2 (18.7 to 21.7) | 194.3 (178.4 to 210.1) | 29.9 (27.1 to 32.7) |
| Q4 (1.45 - 5.26) | 47 | 3.8 (3.4 to 4.2) | 2.6 (2.3 to 3.0) | 92.2 (84.5 to 99.9) | 460.6 (418.6 to 502.6) | 19.3 (17.4 to 21.2) | 183.6 (165.5 to 201.6) | 28.8 (25.1 to 32.4) |
| Test for trend | P = 0.60 | P = 0.28 | P = 0.72 | P = 0.45 | P = 0.28 | P = 0.16 | P = 0.32 | |
| Unprocessed red meatc | ||||||||
| Q1 (0 - 0.16) | 53 | 3.7 (3.3 to 4.1) | 2.3 (2.0 to 2.7) | 87.6 (81.1 to 94.1) | 453.6 (416.4 to 490.8) | 19.7 (17.9 to 21.5) | 196.9 (182.2 to 211.6) | 30.5 (27.5 to 33.5) |
| Q2 (0.22 - 0.30) | 42 | 3.6 (3.2 to 4.0) | 2.6 (2.2 to 3.0) | 93.3 (85.6 to 101.0) | 476.0 (432.4 to 519.6) | 21.5 (18.9 to 24.1) | 200.3 (183.7 to 216.9) | 33.1 (28.4 to 37.9) |
| Q3 (0.36 - 0.65) | 51 | 3.9 (3.5 to 4.3) | 2.6 (2.3 to 3.0) | 93.5 (85.4 to 101.6) | 483.6 (433.0 to 534.1) | 20.6 (18.5 to 22.6) | 186.1 (167.3 to 204.9) | 30.7 (27 to 833.7) |
| Q4 (0.71 - 2.23) | 43 | 3.6 (3.2 to 4.0) | 2.7 (2.3 to 3.1) | 91.1 (83.1 to 99.1) | 498.4 (450.9 to 545.8) | 20.0 (18.2 to 21.7) | 197.8 (178.9 to 216.8) | 27.1 (24.1 to 30.1) |
| Test for trend | P = 0.70 | P = 0.30 | P = 0.77 | P = 0.21 | P = 0.83 | P = 0.91 | P = 0.05 | |
| Organ meatd | ||||||||
| None (0) | 158 | 3.7 (3.5 to 3.9) | 2.5 (2.4 to 2.8) | 91.9 (88.0 to 95.9) | 478.0 (452.8 to 503.2) | 20.5 (19.4 to 21.7) | 194.8 (185.3 to 204.2) | 30.7 (28.8 to 32.6) |
| Any (0.01 - 0.28) | 31 | 3.8 (3.2 to 4.3) | 2.5 (2.1 to 3.0) | 87.8 (80.5 to 95.1) | 470.9 (438.3 to 503.6) | 19.7 (18.2 to 21.1) | 196.0 (176.3 to 215.6) | 28.7 (25.4 to 32.1) |
Adjusted for age, hour of blood sampling, race, smoking status, BMI, recruitment period, moderate-to-intense exercise, TV watching, dietary patterns, alcohol, and total calorie intake
includes hamburgers, hot dogs, bacon, and other processed meats (e.g. salami, bologna, etc.)
includes beef, pork, and ham consumed as sandwich, mixed dish, or main dish
includes beef, calf, pork, chicken, and turkey liver
LH= luteinizing hormone, FSH= follicle stimulating hormone, SHBG= sex hormone binding globulin
To gain further insights into how meat consumption might influence male reproductive function, we also investigated the relation between meat intake and reproductive hormone levels (Table 3). Unprocessed red meat intake was inversely related to SHBG. No other associations with reproductive hormone levels were observed.
Discussion
We investigated the association of meat intake with semen quality parameters and reproductive hormone levels among young men. We had hypothesized that meat consumption would be associated with poor semen quality parameters and that this relation would be stronger for meats where higher levels of hormone residues have been previously documented. In support of our hypothesis, we found that processed red meat intake was inversely related to total sperm count. However, in contradiction to our hypothesis, we also found that organ meat intake was associated with higher total sperm count, sperm concentration, and motility. Our results should be interpreted with caution as they may represent chance findings or, in the case of total sperm count, residual confounding by abstinence time. Additional analyses aimed at addressing residual confounding could not rule out this possibility as an explanation for our findings. More importantly, the inverse relation with total count (sperm concentration × ejaculate volume) was driven by a strong inverse relation with volume rather than with sperm concentration. While we cannot rule out that this association represents a true biological effect, further research is needed to clarify this issue.
The literature on the relationship between meat intake and semen quality is scarce. Swan et al. 4 observed that high maternal beef consumption during pregnancy was associated with lower sperm concentration among their sons 30 years later. Compared with men whose mothers reported no beef intake during pregnancy, men whose mothers consumed more than 7 beef meals per week had 24% lower sperm concentration.4 Eslamian and colleagues 10 reported that the odds of asthenozoospermia were 2.03 (1.7 to 2.4) higher among men in the 3rd tertile of processed red meat intake as compared with those in the first tertile of intake, but red meat intake was not associated with the odds of asthenozoospermia. Similarly, Mendiola et al. 9 found that intake of processed red meats was approximately 31% higher among oligoasthenoteratospermic men than among controls, but did not find any difference in unprocessed red meat intake between these groups. Vujkovic et al. 8 found that intake of meat products was unrelated to semen quality parameters. It should be pointed out that the studies by Mendiola and Vujkovic were conducted within the European Union after the ban on steroid hormones, suggesting that the similar findings in Mendiola's study and the current results are probably not due to hormonal residues but rather may be a consequence of other factors such as saturated fat intake, which has been related to lower sperm counts in US and European studies.8,9 Thus, whether meat intake adversely affects semen quality parameters remains an open question.
We observed a strong positive relation between organ meat intake with various semen parameters when we had hypothesized the opposite. These associations were explained neither by intakes of micronutrients such as zinc and vitamin B12, which are highly concentrated in these foods and may have a role in spermatogenesis, nor by animal fat or protein intake. The only previous report on this relation found no association between organ meat intake and the odds of oligoasthenoteratospermia.9 Since these foods were very rarely consumed in this population and had an extremely narrow intake range among the small number of consumers, it is possible that the relation of organ meat consumption with semen quality parameters is either a chance finding or due to unmeasured confounding. Further evaluation of this relation is warranted.
The cross-sectional nature of this study does not allow the determination of causality of the observed associations. However, we adjusted for several determinants of semen quality parameters such as BMI and abstinence time. In addition, the observed associations were independent of overall food choices as summarized by data-derived dietary patterns. Moreover, because participants were young men with no knowledge of their fertility or their semen quality parameters when answering the questionnaire, they were blinded to the outcome measures of this study. This is a major strength of this study, since this feature can all-but-eliminate reverse causation – a common concern of cross-sectional studies in general and of semen quality parameters studies conducted among fertility patients. Second, while the homogeneity of the study population can enhance its internal validity, these findings may not generalize to subfertile men. These results may also not be generalizable to less active men since participants were considerably more physically active than men in the general population.22 Another limitation is that, while our hypothesis was related to previously described hormone residue levels in meats and meat products, we did not measure residual hormone levels in edible meat tissues. Lastly, since semen parameters are less-than-perfect predictors of fertility23 it is not possible to predict how our findings might translate into fertility, particularly when the mean values across quartiles of meat intake were above the WHO reference values of abnormal semen quality parameters.13 Strengths of the study include the size of the study relative to the existing literature, the wide range of meat intake observed in this population (which allowed us to make more extreme comparisons than in the existing literature), and the use of a previously validated diet questionnaire that assessed intake within the relevant window for spermatogenesis.16
In summary, among a group of 189 young men, we found that processed red meat intake was inversely related to total sperm count and total progressive motile count. However, it is not clear whether these associations represent a true biological effect or residual confounding by abstinence time. We also found that organ meat intake was positively related to total sperm count, sperm concentration, and progressive motility, but these may also represent chance findings. Given the paucity of literature on this topic, this question should be further evaluated.
Supplementary Material
Acknowledgements
We thank the men for their participation in RYMS.
Sources of financial support: European Union Seventh Framework Program (Environment), “Developmental Effects of Environment on Reproductive Health” (DEER) grant 212844. Grant P30 DK046200 and Ruth L. Kirschstein National Research Service Award T32 DK007703-16 from the National Institutes of Health.
Footnotes
The authors have no conflicts of interest to declare.
References
- 1.Andersson A, Skakkebaek N. Exposure to exogenous estrogens in food: possible impact on human development and health. European Journal of Endocrinology. 1999;140(6):477–485. doi: 10.1530/eje.0.1400477. [DOI] [PubMed] [Google Scholar]
- 2.Daxenberger A, Ibarreta D, Meyer HHD. Possible health impact of animal oestrogens in food. Human Reproduction Update. 2001;7(3):340–355. doi: 10.1093/humupd/7.3.340. [DOI] [PubMed] [Google Scholar]
- 3.Willingham EJ. Environmental Review: Trenbolone and Other Cattle Growth Promoters: Need for a New Risk-Assessment Framework. Environmental Practice. 2006;8(01):58–65. [Google Scholar]
- 4.Swan SH, Liu F, Overstreet JW, Brazil C, Skakkebaek NE. Semen quality of fertile US males in relation to their mothers' beef consumption during pregnancy. Hum Reprod. 2007;22(6):1497–1502. doi: 10.1093/humrep/dem068. [DOI] [PubMed] [Google Scholar]
- 5.Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010;365(1546):1697–1712. doi: 10.1098/rstb.2009.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauer ER, Daxenberger A, Petri T, Sauerwein H, Meyer HH. Characterisation of the affinity of different anabolics and synthetic hormones to the human androgen receptor, human sex hormone binding globulin and to the bovine progestin receptor. APMIS. 2000;108(12):838–46. doi: 10.1111/j.1600-0463.2000.tb00007.x. [DOI] [PubMed] [Google Scholar]
- 7.European Commission . Directorate General XXIV Cpachp. 1999. Opinion of the scientific commitee on veterinary measures relating to public health. Assessment of potential risks to human health from hormone residues in bovine meat and meat products. [Google Scholar]
- 8.Vujkovic M, de Vries JH, Dohle GR, Bonsel GJ, Lindemans J, Macklon NS, van der Spek PJ, Steegers EA, Steegers-Theunissen RP. Associations between dietary patterns and semen quality in men undergoing IVF/ICSI treatment. Hum Reprod. 2009;24(6):1304–12. doi: 10.1093/humrep/dep024. [DOI] [PubMed] [Google Scholar]
- 9.Mendiola J, Torres-Cantero AM, Moreno-Grau JM, Ten J, Roca M, Moreno-Grau S, Bernabeu R. Food intake and its relationship with semen quality: a case-control study. Fertil Steril. 2009;91(3):812–8. doi: 10.1016/j.fertnstert.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Eslamian G, Amirjannati N, Rashidkhani B, Sadeghi MR, Hekmatdoost A. Intake of food groups and idiopathic asthenozoospermia: a case-control study. Hum Reprod. 2012;27(11):3328–36. doi: 10.1093/humrep/des311. [DOI] [PubMed] [Google Scholar]
- 11.Henricks DM, Gray SL, Owenby JJ, Lackey BR. Residues from anabolic preparations after good veterinary practice. APMIS. 2001;109(4):273–283. doi: 10.1034/j.1600-0463.2001.d01-120.x. [DOI] [PubMed] [Google Scholar]
- 12.Mendiola J, Stahlhut RW, Jørgensen N, Liu F, Swan SH. Shorter Anogenital Distance Predicts Poorer Semen Quality in Young Men in Rochester, New York. Environ Health Perspect. 2011;119(7) doi: 10.1289/ehp.1103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th ed. World Health Organization; Geneva: 1999. [Google Scholar]
- 14.Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5(5):586–92. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- 15.Asklund C, Jørgensen N, Skakkebæk NE, Jensen TK. Increased frequency of reproductive health problems among fathers of boys with hypospadias. Human Reproduction. 2007;22(10):2639–2646. doi: 10.1093/humrep/dem217. [DOI] [PubMed] [Google Scholar]
- 16.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and Validity of an Expanded Self-Administered Semiquantitative Food Frequency Questionnaire among Male Health Professionals. American Journal of Epidemiology. 1992;135(10):1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 17.Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93(7):790–6. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- 18.Gebhardt SE, Lemar LE, Haytowitz DB, Pehrsson PR, Nickle MS, Showell BA, Thomas RG, Exler J, Holden JM. USDA national nutrient database for standard reference, release 21. 2008 [Google Scholar]
- 19.Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27(10):2899–907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34(4):216–221. [Google Scholar]
- 21.La Vignera S, Condorelli RA, Balercia G, Vicari E, Calogero AE. Does alcohol have any effect on male reproductive function? A review of literature. Asian J Androl. 2013;15(2):221–5. doi: 10.1038/aja.2012.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Macera CA, Ham SA, Yore MM, Jones DA, Ainsworth BE, Kimsey CD, Kohl HW., 3rd Prevalence of physical activity in the United States: Behavioral Risk Factor Surveillance System, 2001. Prev Chronic Dis. 2005;2(2):A17. [PMC free article] [PubMed] [Google Scholar]
- 23.Jedrzejczak P, Taszarek-Hauke G, Hauke J, Pawelczyk L, Duleba AJ. Prediction of spontaneous conception based on semen parameters. International Journal of Andrology. 2008;31(5):499–507. doi: 10.1111/j.1365-2605.2007.00799.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.