Abstract
Data demonstrating the superiority of nilotinib over imatinib in the frontline treatment of chronic myeloid leukemia (CML) and ongoing studies with dasatinib and bosutinib are rapidly changing the treatment landscape for CML. In this review, the authors discuss currently available therapies for CML, focusing on mechanisms of resistance to imatinib and treatment strategies to overcome resistance. Relevant articles were identified through searches of PubMed and abstracts from international hematology/oncology congresses. Additional information sources were identified from the bibliographies of these references and from the authors’ own libraries and expertise. In vitro 50% inhibitory concentration (IC50) data alone are not sufficient to guide the choice of a tyrosine kinase inhibitor (TKI) in the presence of a mutant breakpoint cluster region-v-abl Abelson murine leukemia viral oncogene homolog (BCR-ABL) clone, because there is a lack of data regarding how well such IC50 values correlate with clinical response. A small subset of BCR-ABL mutant clones have been associated with impaired responses to second-generation TKIs (tyrosine to histidine mutation at codon 253 [Y253H], glutamic acid to lysine or valine mutation at codon 255 [E255K/V], and phenylalanine to cysteine or valine mutation at codon 359 [F359C/V] for nilotinib; valine to leucine mutation at codon 299 [V299L] and F317L for dasatinib); neither nilotinib nor dasatinib is active against the threonine to isoleucine mutation at codon 315 (T315I). For each second-generation TKI, the detection of 1 of a small subset of mutations at the time of resistance may be helpful in the selection of second-line therapy. For the majority of patients, comorbidities and drug safety profiles should be the basis for choosing a second-line agent. Clinical trial data from an evaluation of the response of specific mutant BCR-ABL clones to TKIs is needed to establish the role of mutation testing in the management of CML.
Keywords: imatinib, nilotinib, dasatinib, BCR-ABL, mutation, chronic myeloid leukemia
The 2001 approval in the United States of imatinib (Glivec/Gleevec; formerly STI571; Novartis Pharmaceuticals, East Hanover, NJ), a tyrosine kinase inhibitor (TKI) that targets the breakpoint cluster region-v-abl Abelson murine leukemia viral oncogene homolog (BCR-ABL), started a new paradigm for the treatment of newly diagnosed Philadelphia chromosome (Ph)-positive, chronic phase (CP) chronic myeloid leukemia (CML). In the International Randomized Study of Interferon and STI571 (IRIS) trial, imatinib demonstrated superiority to combined interferon-α plus cytarabine and produced significantly higher rates of hematologic response and cytogenetic response (CyR), better tolerability, and a lower likelihood of progression to accelerated phase (AP) or blast crisis (BC).1 Long-term follow-up of the IRIS trial has demonstrated durable responses, prolonged survival, and a low risk of progression among imatinib-treated patients.2
Strategies for Overcoming Resistance/Intolerance to Imatinib
Although high response rates are observed in patients who receive imatinib treatment, some patients (approximately 33%)2 are refractory to therapy. Patients are classified with 2 types of resistance: primary and secondary. Primary resistance is defined as a lack of response and is subdivided further into primary hematologic resistance and primary cytogenetic resistance.3,4 Hematologic resistance occurs in 2% to 4% of patients,5 whereas cytogenetic resistance is more common and occurs in 15% to 25% of patients.5 Mutations in BCR-ABL rarely are responsible for primary resistance.6 Elevated transcript levels of prostaglandin-endoperoxide synthase 1/cyclooxgenase 1 (PTGS1/COX1), which encodes an enzyme that metabolizes imatinib, may be associated with primary resistance. Thus, PTGS1/COX1 may serve as a biomarker to identify patients with primary resistance to imatinib.7 Secondary resistance is defined by the achievement and then subsequent loss of a hematologic response or CyR.
Multiple factors may contribute to imatinib resistance, including altered intracellular drug availability caused by drug influx and efflux transporters. Overexpression of the adenosine triphosphate (ATP)-binding cassette (ABC), subfamily B, member 1 (ABCB1) gene, which encodes the P-glycoprotein drug efflux pump, has been observed in imatinib-resistant cell lines,8,9 and the addition of PSC833 (valspodar), a P-glycoprotein inhibitor, can increase the sensitivity of resistant patient-derived CML cells to imatinib.8 In addition, a study of 33 patients who were receiving imatinib demonstrated that those who did not achieve at least major cytogenetic remission and those who experienced disease progression had P-glycoprotein overexpression.10 Imatinib also is a substrate for the drug-efflux transporter ABC subfamily G, member 2 (ABCG2),11,12 but it is not a substrate for the multidrug-resistance protein 1 (MRP-1).9 Low activity of the drug-intake protein human organic cation transporter 1 (hOCT-1) has been associated with suboptimal cytogenetic and molecular responses to imatinib.13–15 Unlike imatinib, the efficacy of nilotinib is not affected by P-glycoprotein or hOCT-1 expression,16,17 although nilotinib is a high-affinity substrate for ABCG2.11,17 Overexpression of P-glycoprotein and ABCG2 can confer resistance to dasatinib,17 but dasatinib is not a substrate for hOCT-1.18 Bosutinib is not a substrate of P-glycoprotein or ABCG2.17 At higher concentrations, imatinib, nilotinib, dasatinib, and bosutinib all can inhibit both P-glycoprotein and ABCG2 in vitro.11,17
Multiple strategies to overcome failure on standard-dose (400 mg daily) imatinib are under investigation. These strategies include the dose escalation of imatinib, the switch to a second-generation TKI, or, for patients with secondary resistance because of the threonine to isoleucine mutation at codon 315 (T315I), allogeneic stem cell transplantation in eligible patients or a clinical trial. It has been demonstrated that imatinib dose escalation to 600 mg or 800 mg daily is effective and safe in patients who have mutations with high sensitivity to imatinib.19–22 Dasatinib and nilotinib currently are approved for the treatment of patients with CML who have developed resistance or intolerance to imatinib and other previous therapies.23,24
Dasatinib (Sprycel; Bristol-Myers Squibb, Princeton, NJ) is an orally bioavailable, multikinase inhibitor25 that currently is approved for the treatment of imatinib-resistant or imatinib-intolerant CML in all phases and for the treatment of Ph-positive acute lymphoblastic leukemia (ALL).24 The efficacy of dasatinib in imatinib-resistant and imatinib-intolerant disease has been evaluated in several trials. In the SRC/ABL Tyrosine Kinase Inhibition Activity Research Trials of Dasatinib in Chronic Phase Patients (START-C), dasatinib induced responses in most patients (N = 387) and had acceptable tolerability, although grade 3/4 neutropenia and thrombocytopenia were experienced in 49% and 48% of patients, respectively.26 The most common (>20% patients) nonhematologic adverse events (AEs) were diarrhea, headache, fatigue, dyspnea, pleural effusion, rash, and nausea.
In a subsequent phase 3 dasatinib dose-optimization study, efficacy was similar between patients who received 100 mg once daily and those who received 70 mg twice daily, and lower AE rates were observed in the patients who received 100 mg dasatinib once daily.27 After a minimum follow-up of 2 years, the progression-free survival (PFS) rate was 81%, and the overall survival (OS) rate was 90% for patients who received 100 mg dasatinib once daily.27 This dose is now approved for patients with CML-CP.24 In patients with CML in AP or BC, comparable efficacy and better tolerability were observed with dasatinib doses of 140 mg once daily compared with 70 mg twice daily.28 Consequently, 140 mg once daily is now the approved dose of dasatinib for patients with advanced phase disease.24
Preliminary results recently have become available from the phase 3 randomized Dasatinib Versus Imatinib Study in Treatment-Naive CML Patients (the DASISION trial), which is comparing 100 mg once daily dasatinib with 400 mg once daily imatinib.29 In that trial, dasatinib had superior complete CyR (CCyR) rates and major molecular response (MMR) rates compared with imatinib. The rate of confirmed CCyR by 12 months, which was the primary endpoint of the trial, was 77% in the dasatinib arm versus 66% in the imatinib arm (P = .007). Progression rates were similar between the 2 arms. More deaths occurred on the dasatinib arm (10 deaths vs 6 deaths, respectively), and the estimated OS rate at 12 months was 97% for dasatinib-treated patients and 99% and imatinib-treated patients. Rates of discontinuation were similar for the dasatinib and imatinib arms, and dasatinib generally was well tolerated. These results are supported by an independent phase 2 trial with dasatinib in newly diagnosed patients that currently is ongoing at The University of Texas M. D. Anderson Cancer Center (MDACC).30
Nilotinib (Tasigna; Novartis Pharmaceuticals) is a rationally designed BCR-ABL inhibitor that currently is approved for the treatment of imatinib-resistant/intolerant patients who have CML-CP and CML-AP at a dose of 400 mg twice daily.23 In clinical trials with up to 24 months of follow-up, nilotinib has demonstrated efficacy and safety in patients with CML-CP and CML-AP.31–33 At 24 months of follow-up, nilotinib induced responses in most of the CML-CP patients, including a major CyR (MCyR) in 59% of patients, a CCyR in 44% of patients, and an MMR in 28% of patients.31 The median PFS has not been reached. The estimated OS rate at 24 months was 87%.31 In general, nilotinib was well tolerated in the phase 2 registration study and in the expanded access study (Expanding Nilotinib Access in Clinical Trials [ENACT]); the most common grade 3/4 AEs associated with therapy were thrombocytopenia (20%–33% of patients), neutropenia (13%–31% of patients), elevated bilirubin (7% of patients), and elevated serum lipase (5%–15% of patients).31,34 Nilotinib has minimal cross-intolerance with imatinib.35
Nilotinib also has demonstrated promise as frontline therapy in patients with CML-CP.36–39 In the Evaluating Nilotinib Efficacy and Safety in Clinical Trials of Newly Diagnosed Philadelphia Chromosome-Positive CML Patients (the ENESTnd study) study, nilotinib (300 mg or 400 mg twice daily) was compared with imatinib (400 mg daily) in 846 patients with newly diagnosed CML-CP. At 12 months of follow-up, the patients who received nilotinib had superior rates of MMR, CCyR, and undetectable BCR-ABL transcript levels (≤0.0032% International Scale [IS]; 4.5-log reduction).36 The MMR rate at 12 months, which was the primary endpoint of the study, was 44% for nilotinib 300 mg twice daily, 43% for nilotinib 400 mg twice daily, and 22% for imatinib (P < .0001 for imatinib vs both nilotinib doses).36
It is noteworthy that patients who received nilotinib had a significant improvement in the time to progression to AP/BC (P = .01 and P = .004 for nilotinib 300 mg twice daily and 400 mg twice daily vs imatinib, respectively) at a time early in therapy when the risk of progression on imatinib is greatest.36 These superior response rates and progression advantage were maintained at a median follow-up >18 months, with progression to AP/BC observed in 0.7%, 0.4%, and 4.2% of patients on nilotinib 300 mg twice daily, 400 mg twice daily, and imatinib, respectively, and with OS rates of 98.5%, 99.3%, and 96.9%, respectively.40,41
Low rates of grade 3/4 AEs were observed during the trial. The most common AEs were neutropenia (12% of patients who were receiving 300 mg nilotinib twice daily, 10% of patients who were receiving 400 mg nilotinib twice daily, and 20% of patients who were receiving imatinib) and thrombocytopenia (10% of patients who were receiving 300 mg nilotinib twice daily, 12% of patients who were receiving 400 mg nilotinib twice daily, and 9% of patients who were receiving imatinib).36,40,41 The results from the ENESTnd trial confirm the findings of independent phase 2 studies of frontline nilotinib treatment in patients with newly diagnosed CML that was conducted by the All-Ireland Cooperative Oncology Research Group,39 MDACC,38 and the Italian Group for Adult Hematologic Diseases.37 Nilotinib is now approved by the US Food and Drug Administration for the frontline treatment of CML.
Mutations in BCR-ABL
Mutations in the BCR-ABL kinase domain account for 50% to 90% of the imatinib resistance observed in patients.19,42,43 Structurally, the ABL kinase domain is made up of a highly conserved P-loop, catalytic domain, and activation loop. A mutation can affect imatinib binding in 2 ways: direct inhibition by altering an amino acid involved in binding the drug to the kinase (eg, the T315I mutation, the phenylalanine to leucine mutation at codon 317 [F317L], and the phenylalanine to cysteine or valine mutation at codon 359 [F359C/V])42,44 or indirect inhibition by altering BCR-ABL protein conformation (eg, the glycine to glutamic acid mutation at codon 250 [G250E], the glutamine to histidine mutation at codon 252 [Q252H], the tyrosine to histidine mutation at codon 253 [Y253H], and the glutamic acid to lysine or valine mutation at codon 255 [E255K/V]).42 The T315I mutation is identified in 4% to 15% of imatinib-resistant patients45 and commonly is called the gatekeeper mutation, because its structural effect confers resistance to imatinib and to the second-generation TKIs nilotinib and dasatinib.46 The T315I mutation forms a hydrogen bond with imatinib through the insertion of a bulky isoleucine, which is thought to disrupt hydrogen bonding and, thus, sterically hinder imatinib binding to BCR-ABL.47,48 In addition, the T315I mutation is associated with an increased affinity for ATP that may contribute to resistance.49,50
Mutational Status and Response to Therapy
In vitro mutation data: imatinib
The presence of a BCR-ABL mutation does not necessarily lead to clinical resistance; in fact, the extent of resistance depends on the sensitivity of the specific mutation to imatinib.51 In addition, potency observed in vitro does not necessarily translate to an in vivo response, in part because in vitro assays do not directly predict in vivo plasma or target cell concentrations of compounds.52
In vivo mutation data: imatinib
Data are conflicting about the prognostic significance of BCR-ABL mutations in patients. In several retrospective analyses of patients with CML-CP who developed resistance, mutations in the P-loop (excluding residue 244) and in T315I were associated with worse PFS and OS.20,53–55 In contrast, a study by Jabbour et al56 at MDACC indicated that P-loop mutations had no effect on OS. The treatment that those patients received after imatinib failure may explain the conflicting findings. For example, the study by Branford et al53 was conducted before the approval of dasatinib and nilotinib, whereas patients in the MDACC study56 received a second-generation TKI after imatinib failure.
Baseline mutation screening for patients with newly diagnosed CML has demonstrated no benefit for predicting response57 and should not be used routinely. In a study that used highly sensitive DNA sequencing techniques, among patients who received imatinib, no correlation was observed between baseline mutation status and response (PFS or OS).57 Additional work has confirmed that the identification of mutations before therapy does not predict insensitivity to imatinib.58
In vitro mutation data: Second-generation and third-generation tyrosine kinase inhibitors
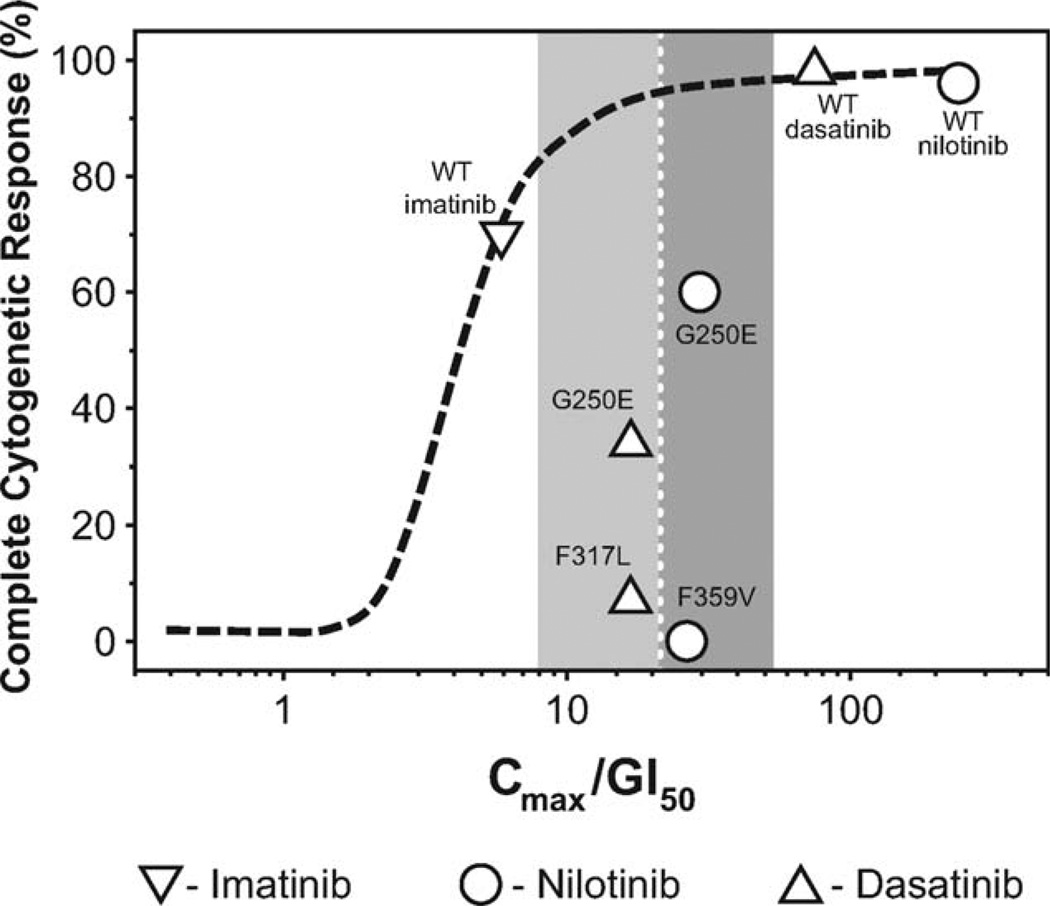
In the case of imatinib resistance, the utility of using in vitro mutation data to select a second-generation TKI is a topic of much debate. Recently, the activities of imatinib, bosutinib, nilotinib, and dasatinib against 18 BCR-ABL mutations spanning the protein were assessed in vitro by Redaelli et al.59 The 8 most commonly mutated amino acids (T315I; Y253F/H; the glutamic acid to aspartic acid, lysine, arginine, or valine mutation at codon 255 [E255D/K/R/V]; the methionine to threonine mutation at codon 351 [M351T]; the glycine to alanine or glutamic acid mutation at codon 250 [G250A/E]; F359C/L/V; the histidine to proline or arginine mutation at codon 296 [H396P/R]; and M244V), which are identified in 85% of patients with mutations, were included in the analysis.60 The mutations were stratified according to IC50 values as sensitive, moderately resistant, resistant, or highly resistant.59 The authors of that report concluded that the data offered physicians a tool for selecting a patient-tailored TKI therapy.59 Laneuville et al42 offered a contrasting view of the use of in vitro data to predict in vivo response, arguing that the in vitro potency data do not take into account other relevant factors, such as whether drug exposure is adequate to block BCR-ABL in vivo. They also noted that the IC50 table published by Redaelli et al49 did not allow a side-by-side comparison of data, because the columns for each inhibitor were normalized to the data within that column. Furthermore, when the ratio of the in vivo maximum drug concentration to 50% growth inhibition (Cmax/GI50) of in vitro CML cell colonies values were plotted for each mutation and compared with published CyR rates for patients who had these mutations, a poor correlation was observed between in vitro predictions of TKI efficacy toward particular BCR-ABL mutant clones and clinical data (Fig. 1). However, clinical data regarding the efficacy of TKIs against most BCR-ABL mutant clones are limited. In addition, proposals to use these IC50 values for the selection of second-generation therapy have not been prospectively evaluated. Furthermore, IC50 values differ among publications. Caution should be applied when making clinical decisions based on the detection of BCR-ABL mutations in patients with CML.
Figure 1.
This chart compares the predicted Cmax/GI50 values (ie, ratio of the in vivo maximum drug concentration to 50% growth inhibition of in vitro chronic myeloid leukemia cell colonies) from in vitro data and complete cytogenetic responses (CCyRs) in clinical trials.52 Previously published CCyR rates for patients with specific mutations were plotted against adjusted values to assess the correlation between in vitro GI50 data (adjusted for in vivo plasma levels) and observed clinical responses in patients. The shaded areas indicate that, despite similar Cmax/GI50 values, clinical responses differed between mutations, suggesting that the 50% concentration values have limited use in predicting clinical resistance. WT indicates wild type; G250E, glycine to glutamic acid mutation at codon 250; F317L, phenylalanine to leucine mutation at codon 317; F359V, phenylalanine to valine mutation at codon 359. (Reprinted with permission: VC American Society of Clinical Oncology 2010. Laneuville P, Di Lea C, Yin O, Woodman R, Mestan J, Manley P. Comparative in vitro cellular data alone are insufficient to predict clinical responses and guide the choice of BCR-ABL inhibitor for treating imatinib-resistant chronic myeloid leukemia. J Clin Oncol. 2010; 28:e169–e171.)
Mutation Status and Response to Therapy: In Vivo Mutation Data With Second-Generation Tyrosine Kinase Inhibitors
In-depth analyses of the effect of mutations on responses to both dasatinib and nilotinib have been performed using data from the clinical trials of these drugs. Of 1043 patients who received treatment with second-line dasatinib in phase 2/3 trials, 39% had a pre-existing BCR-ABL mutation, including 48% of 805 patients who had either imatinib resistance or a suboptimal response.61 Despite this high prevalence of mutations at baseline, patients with imatinib resistance and patients who had a suboptimal response exhibited similar responses to dasatinib regardless of the presence or absence of baseline mutations (MCyR rate: 55% vs 58%, respectively; CCyR rate: 40% vs 41%, respectively). Rates of PFS and OS also were comparable between patients with and without baseline mutations. To further analyze responses according to mutation status, patients were stratified according to the in vitro IC50 value of their mutation(s). Patients who had the T315I mutation were excluded from the analyses. Specific mutations with an IC50 >3 nm were associated with lower rates of CCyR, including F317L (1 of 14 patients; 7%), Q252H (1 of 6 patients; 17%), V299L (0 of 1 patient; 0%), and L384M (0 of 2 patients; 0%). However, several patients (n = 75) who had a variety of mutations with IC50 values >3 nM had favorable responses to dasatinib.61 In a separate study, F317L mutations were detected in 12 of 99 patients who had mutations after imatinib failure.62 Three of those 12 patients were treated with dasatinib, and none achieved a CCyR. Indeed, the best response was a partial hematologic response in 1 patient and a complete hematologic response in 2 patients (who were lost to follow-up after 10 months and 12 months). Furthermore, F317L was detected in 8 of 16 patients who had new mutations after dasatinib failure.62 Two patients were rescued with a higher dose of imatinib, and the remaining 6 patients responded to nilotinib.
To examine the impact of mutations on responses to nilotinib at 400 mg twice daily, patients with available mutation data who had received second-line nilotinib in the phase 2 registration study (n = 281) were stratified into the following 3 groups: patients with no mutations, patients with sensitive mutations, and patients with less sensitive mutations (ie, with IC50 values >150 nM; Y253H, E255V/K, and F359V/C). Patients with T315I mutations were excluded. Like rates of response to dasatinib treatment, the response rates with nilotinib did not differ significantly between patients with or without mutations at baseline.63,64 In patients with CML-CP, an MCyR, a CCyR, and an MMR were achieved by 52 of 87 patients (60%), 35 of 87 patients (40%), and 22 of 76 patients (29%), respectively, who did not have baseline mutations compared with 49 of 100 patients (49%), 32 of 100 patients (32%), and 19 of 87 patients (22%), respectively, who had baseline mutations (P>.05 for all comparisons) and evaluable samples. However, patients who had low-sensitivity mutations achieved lower CyR rates (1 of 8 patients [13%], 3 of 7 patients [43%], and 1 of 11 patients [9%] with Y253H, E255V/K, and F359V/C mutations, respectively, achieved an MCyR; none achieved a CCyR) and lower molecular response rates (0 of 7 patients [0%], 1 of 7 patients [14%], and 0 of 10 patients [0%], respectively).
In a study of imatinib-resistant and imatinib-intolerant patients who received either nilotinib or dasatinib as second-line or third-line therapy, relapse after an initial response with a new mutation was more common in patients who had mutations at baseline (23 of 51 patients; 45%) than in patients who had wild-type BCR-ABL at baseline (8 of 44 patients; 18%).65 Forty-three patients did not respond or relapsed when they received treatment with a second-line TKI, including 26 patients who received third-line therapy. After a median of 4 months of treatment with third-line therapy, 13 of 20 patients who had mutations at baseline relapsed with a newly acquired mutation after an initial response, whereas 1 of 6 patients without mutations at baseline relapsed with a newly acquired mutation. All patients who had baseline mutations and lost responses to second-line or third-line treatments (36 of 36 patients) had newly acquired mutations. A relapse after an initial response was associated with new mutations in 45 of 54 patients (83%). With the exception of T315I, the mutations that conferred resistance to nilotinib or dasatinib were nonoverlapping. The mutations that conferred resistance specifically to dasatinib were T315A, V299L, and F317L/I/C/V and those that conferred resistance specifically to nilotinib were Y253H, E255K/V, L273M, and F359V. In a separate study of imatinib-intolerant or imatinib-resistant patients who received dasatinib and nilotinib as second-line and third-line therapies, 29 of 112 patients (26%) acquired new mutations, including 24 patients who had evidence of drug resistance.66 Soverini et al65 observed that the mutations that conferred resistance to nilotinib and dasatinib did not overlap with the exception of T315I.66 Mutations at residues 299 and 317 conferred resistance to dasatinib, whereas mutations at residues 253, 255, 311, and 359 conferred resistance to nilotinib.66
It is important to note that factors other than mutations affect the response to TKI therapy. Studies have indicated that a proportion of patients who progress do not have mutations.19,58 In addition, other studies have demonstrated that patients who have a CCyR can develop BCR-ABL mutations and maintain their CCyR.67,68
Future Chronic Myeloid Leukemia Therapies
Although the T315I mutation is identified in only a small proportion of patients with CML-CP, it confers resistance to all 3 currently approved TKIs and is associated with a poor prognosis.19,69 However, prognosis is associated with disease stage at the time of detection, and patients with this mutation who have an indolent disease course have been reported.45 New therapies are in development for patients with the T315I mutation and for other patients who fail second-line therapies. Therapies currently in development target not only the BCR-ABL pathway but also other kinases and the modulation of cellular processes, such as apoptosis.
The most clinically mature of these new agents is bosutinib (SKI606)—an SRC/ABL inhibitor that currently is in clinical trials and has demonstrated efficacy in patients with CML-CP who failed imatinib and were not treated with second-generation TKIs (Table 1).70 However, bosutinib is not active clinically against T315I mutations. A head-to-head, phase 3, randomized trial of bosutinib versus imatinib in patients with newly diagnosed CML-CP is ongoing. Preliminary results are expected in late 2010. Another promising third-line agent is omacetaxine mepesuccinate (OMAPRO; ChemGenex Pharmaceuticals Ltd., Menlo Park, Calif), a subcutaneously bioavailable form of homoharringtonine. Omacetaxine works independently of BCR-ABL, and it blocks protein synthesis and induces apoptosis. On the basis of encouraging efficacy and safety results from a phase 2 trial of omacetaxine in imatinib-resistant patients in all phases of CML with the T315I mutation,71 omacetaxine currently is under review by the US Food and Drug Administration for patients with T315 mutations (Table 1).
Table 1.
Tyrosine Kinase and Aurora Kinase Inhibitors Currently In Clinical Trials
| Compound | Mechanism of Action |
Clinical Trials |
Data |
|---|---|---|---|
| Tyrosine kinase inhibitor | |||
| Bosutinib (SKI606; Wyeth, New York, NY) | ABL/SCR | Phase 2 trial | Imatinib-resistant-intolerant patients (N=295) had CHR, MCyR, and CCyR rates of 91%, 64%, and 50%, respectively, and favorable safety and tolerability profiles (Cortes 201070); binds both active and intermediate BCR-ABL conformations (Puttini 200691) |
| AP24534 (ARIAD Pharmaceuticals, Cambridge, Mass)a | Multikinase, including BCR-ABL | Phase 1 trial | Patients with Ph+ leukemia (N=44) treated with AP24534; 83% of evaluable patients with CML-CP achieved a CHR, 48% achieved an MCyR, 33% achieved a CCyR (Talpaz 201074); pancreatitis and increased lipase/amylase were DLTs (Cortes 200973) |
| Aurora kinase inhibitor | |||
| XL-228 (Exelixis, South San Francisco, Calif)a | Multikinase, including ABL, aurora kinase A, SFT, and IFGR1 | Phase 1 dose-escalation trial | Patients (N=35) who failed multiple therapies (or with T315I) received weekly doses ranging from 0.45 to 10.8 mg/kg or 3.6 mg/kg twice weekly; 9 patients had T315I, and 5 patients had F317L; therapy was well tolerated up to 7.2 mg/kg/d; hyperglycemia and syncope were DLTs at 10.8 mg/kg/d; common grade 2 toxicities included hyperglycemia, fatigue, nausea, vomiting, and bradycardia; 7 of 35 patients (20%) responded (1 returned to CP, 2 had a minor CyR, 2 had an MCyR, and 2 had a CCyR); of the patients with T315I, 1 had a minor CyR, 1 had an MCyR, and 1 had a CCyR (Cortes 200875) |
| Apoptosis inhibitor | |||
| Omacetaxin mepesuccinate (OMAPRO; Chem-Genex Pharmaceuticals Ltd., Menlo Park, Calif)a | Inhibition of protein synthesis | Multicenter phase 2/3 trial | In 81 patients with CML who had T315I, the CHR, MCyR, and CCyR rates were 86%, 27%, and 18%, respectively, in patients with CML-CP; omacetaxine generally was well tolerated, with mainly hematologi toxicities (grade 3/4 thrombocytopenia [58%], anemia [36%], and neutropenia [33%]) (Cortes-Franco 200971) |
ABL indicates the Abelson leukemia virus tyrosine kinase; SRC, tyrosine kinase encoded by the Rous sarcoma virus; CHR, complete hematologic response; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; BCR, breakpoint cluster region; Ph+, Philadelphia chromosome positive; CML, chronic myeloid leukemia; CP, chronic phase; DLT, dose-limiting toxicity; SFT, simulator of Fe transport; IFGR1, insulin-like growth factor receptor 1; T315I, threonine to isoleucine mutation at codon 315; F317L, phenylalanine to leucine mutation at codon 317; CyR, cytogenetic response.
Active against T315I.
Other new kinase therapies include AP24534, a multikinase inhibitor with in vitro and in vivo activity against wild-type BCR-ABL, and the T315I clone.72 Once-daily oral dosing has proven to be effective in mouse models,72 and a phase 1 trial in patients with Ph-positive leukemia is ongoing (Table 1).73,74 Another new class of agents being tested for the treatment of CML is aurora kinase inhibitors (eg, XL-22875). Aurora kinases regulate cell division and are overexpressed in leukemic cells.76 Aurora kinase inhibitors also inhibit BCR-ABL, but they remain active against the T315I mutation, because binding with these agents does not occur as deeply in the hydrophobic pocket as it does with other TKIs. Thus, they are not subject to steric hindrance by isoleucine.76
DCC-2036 belongs to a novel class of agents known as switch pocket inhibitors, which bind to kinases in the regions that regulate the switch between active and inactive conformation. Therefore, DCC-2036 binding is not affected by the T315I mutation. The switch pocket structure varies between kinases; thus, inhibitors are kinase specific. In vitro, DCC-2036 inhibited T3151, Y253F, and M351T with an IC50 <25 nM. Levels of phosphorylated ABL decreased in mice who were treated with 100 mg/kg DCC-2036, and daily dosing improved OS.77 DCC-2036 currently is in phase 1 clinical trials for patients with resistant or intolerant CML or ALL.
The Role of Monitoring and Mutation Screening in Imatinib Therapy
The European LeukemiaNet (ELN) and the National Comprehensive Cancer Network (NCCN) provide guidance concerning the monitoring of patients with CML.3,4 The criteria for defining optimal response, suboptimal response, and failure to respond are outlined by the ELN.3 Both the NCCN and ELN recommend mutation analysis in instances of inadequate response and suggest that mutation screening may assist the in choice of a second-generation TKI.3,4 However, neither the NCCN nor ELN provides clear guidance on altering or selecting therapy based on mutational analysis, and neither discusses the significance of particular mutations (with the exception of T315I).
A recent report from the Agency for Healthcare Research and Quality (AHRQ) on the assessment of BCR-ABL mutations and their utility in predicting response also should be considered. Thirty-one publications involving BCR-ABL testing (20 with dasatinib, 7 with imatinib, 3 with nilotinib, and 1 with various TKIs) were reviewed.78 The report concluded that, with the exception of T315I, the presence of mutations did not predict clinical outcomes with TKI therapy. Thus, the value of mutation testing in patient management remains uncertain, and prospective data from international registries are needed.
Current Treatment Algorithm for Patients With Newly Diagnosed Chronic Myeloid Leukemia in Chronic Phase
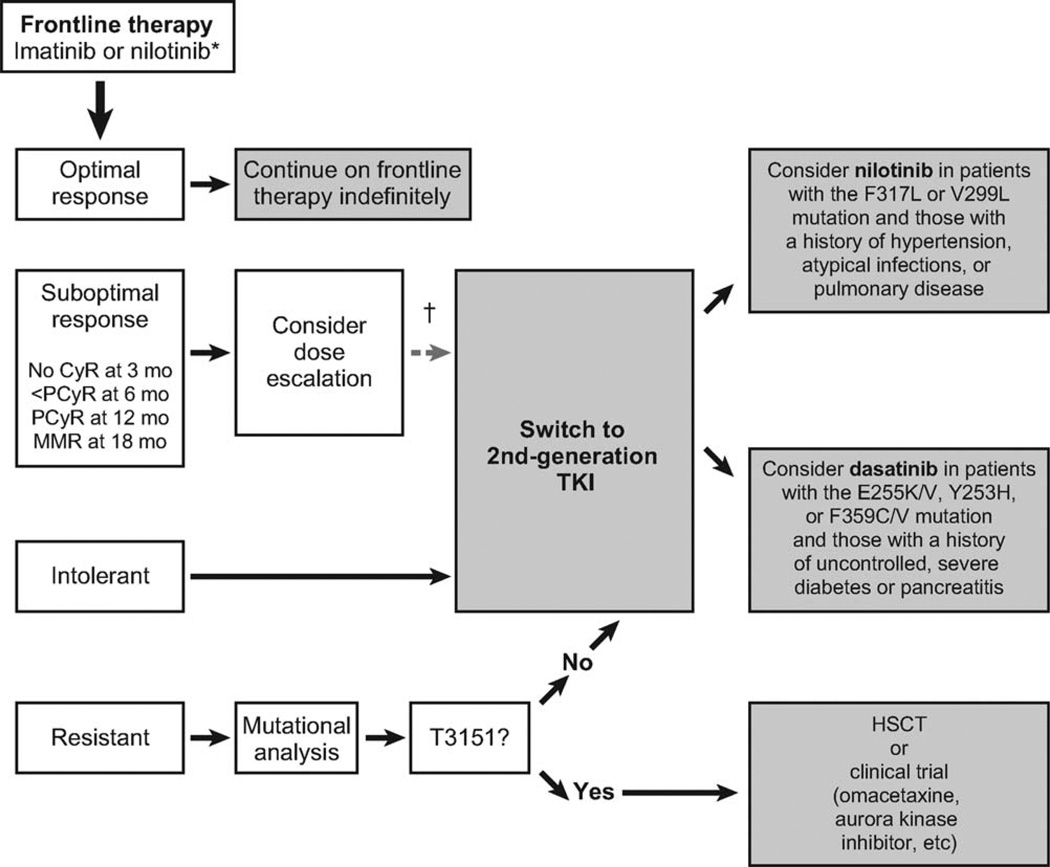
Imatinib 400 mg daily is the recommended frontline treatment for patients with CML (Fig. 2). The efficacy of nilotinib and dasatinib was superior to that of imatinib in 2 independent phase 3 randomized trials.29,36 These drugs currently are under review by health authorities for the frontline indication. Bosutinib is also being evaluated in randomized trials for patients with newly diagnosed CML, and the results are expected in 2010. The results from these studies may affect the current treatment paradigm for patients with newly diagnosed CML. Nilotinib, dasatinib, or bosutinib may become frontline treatments of choice, and patients may continue indefinitely if they achieve an optimal response, may switch to an alternative TKI if they are intolerant or resistant (except for those with the T315I mutation), or may consider hematopoietic stem cell transplantation (HSCT) or clinical trials if it is determined that they have the T315I mutation.
Figure 2.
This chart illustrates the suggested treatment algorithm for patients with newly diagnosed chronic myeloid leukemia (CML) in chronic phase. An asterisk indicates that imatinib and nilotinib are the only approved tyrosine kinase inhibitors (TKIs) for the frontline treatment of CML. Nilotinib recently was approved by the US Food and Drug Administration after it demonstrated superior response rates and a significant improvement in the time to progression compared with imatinib36; those improvements persisted at a median follow-up >18 months in an international, phase 3, randomized trial.40,41 Dasatinib also demonstrated superior response rates compared with imatinib in a phase 3 randomized trial and is under review for regulatory approval in several countries.29 Bosutinib is being evaluated in a randomized trial of patients with newly diagnosed CML, and those results are expected in 2010. The dagger indicates that randomized clinical trials currently are underway to examine the value of an early switch to second-generation TKIs in patients who have a suboptimal response to imatinib. CyR indicates cytogenetic response; PCyR, partial cytogenetic response; MMR, major molecular response; F317L, phenylalanine to leucine mutation at codon 317; V299L, valine to leucine mutation at codon 299; E255K/V, glutamic acid to lysine or valine mutation at codon 255; Y253H, tyrosine to histidine mutation at codon 253; F359C/V, phenylalanine to cysteine or valine mutation at codon 359; HSCT, hematopoietic stem cell transplantation.
It has not been demonstrated that the presence of baseline mutations can predict the outcome of treatment with imatinib; thus, baseline mutation testing is not necessary. In the event of treatment failure on imatinib, an alternative approach is required. Dose escalation of imatinib can be considered, but it is not likely to be effective in patients who never achieved a hematologic or cytogenetic response with imatinib or in those with known imatinib-resistant mutations. A change to a second-generation therapy may be a better option for most patients. In vitro and in vivo data have demonstrated that both dasatinib and nilotinib have a small and distinct set of mutants that confer decreased sensitivity: Y253H, E255K/V, and F359C/V for nilotinib63 and V299L and F317L for dasatinib61 If it is known that patients have 1 of these mutations, then they should be considered for treatment with the agent that retains activity against their particular mutation.
Most patients, however, do not have these mutations.79 For this reason, the AHRQ concluded that the presence of BCR-ABL mutations does not predict the response to TKI therapy.78 Although it is clear that a few mutations, in fact, are associated with inferior responses to TKIs, treatment for most patients should be guided by previous medical history and by the safety profiles of the drugs. Severe, uncontrolled diabetes and past pancreatitis are considered risk factors for nilotinib use because of the occurrence of grade 3/4 elevated lipase levels (18%), elevated bilirubin levels (7%), and hyperglycemia (12%) in some patients who receive second-line nilotinib,31 although most biochemical laboratory abnormalities associated with nilotinib were transient, asymptomatic, and not clinically relevant. Caution should be exercised before prescribing dasatinib for patients with hypertension, asthma, pneumonia, gastrointestinal bleeding, chronic obstructive pulmonary disease, chest wall injury, congestive heart failure, autoimmune disorders, and concomitant aspirin use because of the risk of pleural and pericardial effusion,80,81 bleeding,81–83 and infection84 associated with this TKI. Patients with the T315I mutation are insensitive to both nilotinib and dasatinib in vitro,85 and a clinical trial or HSCT can be considered for such patients.3,4,86
The impact of mutations on clinical response is under investigation. The treatment algorithm should be modified accordingly as new data become available. Recent reports indicate that baseline characteristics at the time second-generation therapy is initiated may predict response. The ultimate goal is the development of a scoring system to predict patient prognosis based on multiple factors, which will allow a more accurate prediction of the response to therapy. These scoring systems include 1 or more of the following: Eastern Cooperative Oncology Group performance score, previous cytogenetic response to imatinib, baseline mutations with low sensitivity to a particular TKI, hemoglobin concentration, basophilia, Sokal score, and recurrent neutropenia.87–89 Such scoring systems may guide future treatment algorithms by predicting the response to particular TKIs.
In conclusion, given the incomplete data and the presence of other mechanisms of TKI resistance, in vitro IC50 data alone are not sufficient to guide the choice of TKI in the presence of a mutant BCR-ABL clone. For example, whereas in vitro data indicate that dasatinib is more potent that nilotinib,90 it has been demonstrated in vivo that nilotinib delivers higher concentrations to CML cells.52 Clinically, both second-line therapies are effective against most mutations that cause resistance to imatinib, but each remains relatively ineffective against certain mutations. In vivo data indicate that, for each second-generation TKI, the presence of a subset of mutations at the time resistance is detected may be helpful in the selection of the appropriate second-line therapy. Fewer BCR-ABL mutations are associated with decreased sensitivity to nilotinib and dasatinib than to imatinib, possibly because of the improved binding properties of these drugs to the BCR-ABL target. In patients who have the T315I mutation, HSCT or participation in a clinical trial with 1 of the newer agents that have demonstrated activity against this mutant can be considered. We are only beginning to understand the utility of mutation testing in monitoring the response to TKIs or in guiding treatment choices. Clinical trial data from an evaluation of the response of specific clones to TKIs will be essential to further refine the role of mutation testing in patient therapy. Thus, as our understanding of predictors of response to imatinib and second-generation TKIs increases, the development of a scoring system to predict patient response may be possible. Patient comorbidities and TKI safety profiles always must be considered when choosing a TKI therapy. Furthermore, the current treatment algorithm for CML is likely to be revised as more data become available about second-generation TKIs in the frontline setting.
Acknowledgments
FUNDING STATEMENT:
This article funded by NIH-Grant number-P30 CA016672.
We thank Stacey Rose, PhD (Articulate Science) and Erinn Goldman, PhD (Articulate Science) for medical editorial assistance with this article.
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. Dr. Jabbour acted as a consultant for and received honoraria from Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb. Dr. Branford received honoraria from Bristol-Myers Squibb and Novartis Pharmaceuticals Corporation. Dr. Saglio acted as a consultant for and received honoraria from Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb, and received research funding from Novartis Pharmaceuticals Corporation. Dr. Cortes received research funding from Novartis Pharmaceuticals Corporation, Wyeth, and Bristol-Myers Squibb. Dr. Kantarjian acted as a consultant for Novartis Pharmaceuticals Corporation, and received research funding from Novartis Pharmaceuticals Corporation and Bristol-Myers Squibb.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Jones has no conflicts of interest to declare.
REFERENCES
- 1.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 2.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23:1054–1061. doi: 10.1038/leu.2009.38. [DOI] [PubMed] [Google Scholar]
- 3.Baccarani M, Cortes J, Pane F, et al. Chronic myeloid leukemia: an update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol. 2009;27:6041–6051. doi: 10.1200/JCO.2009.25.0779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Chronic Myelogenous Leukemia. Version 2. Jenkintown, PA: NCCN; 2010. NCCN: Clinical Practice Guidelines in Oncology. [DOI] [PubMed] [Google Scholar]
- 5.Shah NP. Medical management of CML. Hematology Am Soc Hematol Educ Program. 2007:371–375. doi: 10.1182/asheducation-2007.1.371. [DOI] [PubMed] [Google Scholar]
- 6.Deininger MW. Optimizing therapy of chronic myeloid leukemia. Exp Hematol. 2007;35:144–154. doi: 10.1016/j.exphem.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 7.Zhang WW, Cortes JE, Yao H, et al. Predictors of primary imatinib resistance in chronic myelogenous leukemia are distinct from those in secondary imatinib resistance. J Clin Oncol. 2009;27:3642–3649. doi: 10.1200/JCO.2008.19.4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahon FX, Belloc F, Lagarde V, et al. MDR1 gene overexpression confers resistance to imatinib mesylate in leukemia cell line models. Blood. 2003;101:2368–2373. doi: 10.1182/blood.V101.6.2368. [DOI] [PubMed] [Google Scholar]
- 9.Mukai M, Che XF, Furukawa T, et al. Reversal of the resistance to STI571 in human chronic myelogenous leukemia K562 cells. Cancer Sci. 2003;94:557–563. doi: 10.1111/j.1349-7006.2003.tb01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galimberti S, Cervetti G, Guerrini F, et al. Quantitative molecular monitoring of BCR-ABL and MDR1 transcripts in patients with chronic myeloid leukemia during imatinib treatment. Cancer Genet Cytogenet. 2005;162:57–62. doi: 10.1016/j.cancergencyto.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 11.Dohse M, Scharenberg C, Shukla S, et al. Comparison of ATP-binding cassette transporter interactions with the tyrosine kinase inhibitors imatinib, nilotinib, and dasatinib. Drug Metab Dispos. 2010;38:1371–1380. doi: 10.1124/dmd.109.031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burger H, van Tol H, Boersma AW, et al. Imatinib mesylate (STI571) is a substrate for the breast cancer resistance protein (BCRP)/ABCG2 drug pump. Blood. 2004;104:2940–2942. doi: 10.1182/blood-2004-04-1398. [DOI] [PubMed] [Google Scholar]
- 13.White DL, Dang P, Engler J, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010;28:2761–2767. doi: 10.1200/JCO.2009.26.5819. [DOI] [PubMed] [Google Scholar]
- 14.White DL, Saunders VA, Dang P, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110:4064–4072. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- 15.Crossman LC, Druker BJ, Deininger MW, Pirmohamed M, Wang L, Clark RE. hOCT-1 and resistance to imatinib. Blood. 2005;106:1133–1134. doi: 10.1182/blood-2005-02-0694. author reply 1134. [DOI] [PubMed] [Google Scholar]
- 16.Davies A, Jordanides NE, Giannoudis A, et al. Nilotinib concentration in cell lines and primary CD34(+) chronic myeloid leukemia cells is not mediated by active uptake or efflux by major drug transporters. Leukemia. 2009;23:1999–2006. doi: 10.1038/leu.2009.166. [DOI] [PubMed] [Google Scholar]
- 17.Hegedus C, Ozvegy-Laczka C, Apati A, et al. Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. Br J Pharmacol. 2009;158:1153–1164. doi: 10.1111/j.1476-5381.2009.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hiwase DK, Saunders V, Hewett D, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14:3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 19.Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. [DOI] [PubMed] [Google Scholar]
- 20.Nicolini FE, Corm S, Le QH, et al. Mutation status and clinical outcome of 89 imatinib mesylate-resistant chronic myelogenous leukemia patients: a retrospective analysis from the French Intergroup of CML (fi(phi)-LMC Group) Leukemia. 2006;20:1061–1066. doi: 10.1038/sj.leu.2404236. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian HM, Larson RA, Guilhot F, et al. Efficacy of imatinib dose escalation in patients with chronic myeloid leukemia in chronic phase. Cancer. 2009;115:551–560. doi: 10.1002/cncr.24066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jabbour E, Kantarjian HM, Jones D, et al. Imatinib mesylate dose escalation is associated with durable responses in patients with chronic myeloid leukemia after cytogenetic failure on standard-dose imatinib therapy. Blood. 2009;113:2154–2160. doi: 10.1182/blood-2008-04-154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tasigna (Nilotinib) [package insert] East Hanover, NJ: Novartis Pharmaceuticals Corporation; 2010. [Google Scholar]
- 24.Sprycel (Dasatinib) [package insert] Princeton, NJ: Bristol-Myers Squibb Company; 2009. [Google Scholar]
- 25.Apperley JF. Part II: management of resistance to imatinib in chronic myeloid leukemia. Lancet Oncol. 2007;8:1116–1128. doi: 10.1016/S1470-2045(07)70379-0. [DOI] [PubMed] [Google Scholar]
- 26.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 27.Shah NP, Kim DW, Kantarjian H, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Hematologica. 2010;95:232–240. doi: 10.3324/haematol.2009.011452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kantarjian H, Cortes J, Kim DW, et al. Phase 3 study of dasatinib 140 mg once daily versus 70 mg twice daily in patients with chronic myeloid leukemia in accelerated phase resistant or intolerant to imatinib: 15-month median follow-up. Blood. 2009;113:6322–6329. doi: 10.1182/blood-2008-11-186817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kantarjian H, Shah NP, Hochhaus A, et al. Dasatinib versus imatinib in newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2010;362:2260–2270. doi: 10.1056/NEJMoa1002315. [DOI] [PubMed] [Google Scholar]
- 30.Cortes J, Borthakur G, O’Brien S, et al. Efficacy of dasatinib in patients (pts) with previously untreated chronic myelogenous leukemia (CML) in early chronic phase (CML-CP) [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114:143. Abstract 338. [Google Scholar]
- 31.Kantarjian HM, Giles FJ, Bhalla KN, et al. Update on imatinib-resistant chronic myeloid leukemia patients in chronic phase (CML-CP) on nilotinib therapy at 24 months: clinical response, safety, and long-term outcomes [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114:464. Abstract 1129. [Google Scholar]
- 32.le Coutre P, Hochhaus A, Apperley J, et al. Nilotinib in imatinib-resistant or -intolerant patients with chronic myelogenous leukemia in accelerated phase (CML-AP): update of a phase 2 study [abstract] Hematologica. 2008;93:47. Abstract 0118. [Google Scholar]
- 33.Nicolini F, Alimena G, Al-Ali HK, et al. Final safety analysis of 1793 CML patients the from ENACT (Expanding Nilotinib Access in Clinical Trials) study in adult patients with imatinib-resistant or -intolerant chronic myeloid leukemia (CML) [abstract] Hematologica. 2009;94:255.256. Abstract 0630. [Google Scholar]
- 34.le Coutre PD, Ceglarek B, Turkina A, et al. Patterns and management of selected adverse events of adult patients with imatinib-resistant or -intolerant chronic myeloid leukemia (CML) from the ENACT (Expanding Nilotinib Access in Clinical Trials) study [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114:457–458. Abstract 0115. [Google Scholar]
- 35.Jabbour E, Kantarjian HM, Baccarani M, et al. Minimal cross-intolerance between nilotinib and imatinib in patients with imatinib-intolerant chronic myeloid leukemia in chronic phase (CML-CP) or accelerated phase (CML-AP) [abstract] Blood (ASH Annual Meeting Abstracts) 2008;112:1103. Abstract 3215. [Google Scholar]
- 36.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362:2251–2259. doi: 10.1056/NEJMoa0912614. [DOI] [PubMed] [Google Scholar]
- 37.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114:4933–4938. doi: 10.1182/blood-2009-07-232595. [DOI] [PubMed] [Google Scholar]
- 38.Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28:392–397. doi: 10.1200/JCO.2009.25.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Dwyer M, Swords R, Giles F, et al. Nilotinib 300 mg twice daily is effective and well tolerated as first line treatment of Ph-positive chronic myeloid leukemia in chronic phase: updated results of the ICORG 0802 phase 2 study [abstract] Hematologica. 2010;95:340. Abstract 0812. [Google Scholar]
- 40.Larson RA, le Coutre PD, Reiffers J, et al. Comparison of nilotinib and imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): ENESTnd beyond one year [abstract] J Clin Oncol. 2010;28(15S):487s. Abstract 6501. [Google Scholar]
- 41.Hochhaus A, Lobo C, Pasquini R, et al. Continued superiority of nilotinib vs imatinib in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): ENESTnd beyond 1 year [abstract] Hematologica. 2010;95:459. Abstract 1113. [Google Scholar]
- 42.Shah NP, Nicoll JM, Nagar B, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 43.Ernst T, Erben P, Muller MC, et al. Dynamics of BCR-ABL mutated clones before hematologic or cytogenetic resistance to imatinib. Hematologica. 2008;93:186–192. doi: 10.3324/haematol.11993. [DOI] [PubMed] [Google Scholar]
- 44.Azam M, Latek RR, Daley GQ. Mechanisms of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 45.Jabbour E, Kantarjian H, Jones D, et al. Characteristics and outcomes of patients with chronic myeloid leukemia and T315I mutation following failure of imatinib mesylate therapy. Blood. 2008;112:53–55. doi: 10.1182/blood-2007-11-123950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mauro MJ. Defining and managing imatinib resistance. Hematology Am Soc Hematol Educ Program. 2006:219–225. doi: 10.1182/asheducation-2006.1.219. [DOI] [PubMed] [Google Scholar]
- 47.Deininger M. Resistance to imatinib: mechanisms and management. J Natl Compr Canc Netw. 2005;3:757–768. doi: 10.6004/jnccn.2005.0045. [DOI] [PubMed] [Google Scholar]
- 48.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 49.Griswold IJ, MacPartlin M, Bumm T, et al. Kinase domain mutants of Bcr-Abl exhibit altered transformation potency, kinase activity, and substrate utilization, irrespective of sensitivity to imatinib. Mol Cell Biol. 2006;26:6082–6093. doi: 10.1128/MCB.02202-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Corbin AS, Buchdunger E, Pascal F, Druker BJ. Analysis of the structural basis of specificity of inhibition of the abl kinase by STI571. J Biol Chem. 2002;277:32214–32219. doi: 10.1074/jbc.M111525200. [DOI] [PubMed] [Google Scholar]
- 51.Corbin AS, Louisiana Rosee P, Stoffregen EP, Druker BJ, Deininger MW. Several bcr-abl kinase domain mutants associated with imatinib mesylate resistance remain sensitive to imatinib. Blood. 2003;101:4611–4614. doi: 10.1182/blood-2002-12-3659. [DOI] [PubMed] [Google Scholar]
- 52.Laneuville P, Di Lea C, Yin O, Woodman R, Mestan J, Manley P. Comparative in vitro cellular data alone are insufficient to predict clinical responses and guide the choice of BCR-ABL inhibitor for treating imatinib-resistant chronic myeloid leukemia [serial online] J Clin Oncol. 2010;28:e169–e171. doi: 10.1200/JCO.2009.26.4945. [DOI] [PubMed] [Google Scholar]
- 53.Branford S, Rudzki Z, Walsh S, et al. Detection of BCR-ABL mutations in patients with CML treated with imatinib is virtually always accompanied by clinical resistance, and mutations in the ATP phosphate-binding loop (P-loop) are associated with a poor prognosis. Blood. 2003;102:276–283. doi: 10.1182/blood-2002-09-2896. [DOI] [PubMed] [Google Scholar]
- 54.Soverini S, Martinelli G, Rosti G, et al. ABL mutations in late chronic phase chronic myeloid leukemia patients with up-front cytogenetic resistance to imatinib are associated with a greater likelihood of progression to blast crisis and shorter survival: a study by the GIMEMA Working Party on Chronic Myeloid Leukemia. J Clin Oncol. 2005;23:4100–4109. doi: 10.1200/JCO.2005.05.531. [DOI] [PubMed] [Google Scholar]
- 55.Khorashad JS, de Lavallade H, Apperley JF, et al. Finding of kinase domain mutations in patients with chronic phase chronic myeloid leukemia responding to imatinib may identify those at high risk of disease progression. J Clin Oncol. 2008;26:4806–4813. doi: 10.1200/JCO.2008.16.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jabbour E, Kantarjian H, Jones D, et al. Frequency and clinical significance of BCR-ABL mutations in patients with chronic myeloid leukemia treated with imatinib mesylate. Leukemia. 2006;20:1767–1773. doi: 10.1038/sj.leu.2404318. [DOI] [PubMed] [Google Scholar]
- 57.Willis SG, Lange T, Demehri S, et al. High-sensitivity detection of BCR-ABL kinase domain mutations in imatinib-naive patients: correlation with clonal cytogenetic evolution but not response to therapy. Blood. 2005;106:2128–2137. doi: 10.1182/blood-2005-03-1036. [DOI] [PubMed] [Google Scholar]
- 58.Khorashad JS, Anand M, Marin D, et al. The presence of a BCR-ABL mutant allele in CML does not always explain clinical resistance to imatinib. Leukemia. 2006;20:658–663. doi: 10.1038/sj.leu.2404137. [DOI] [PubMed] [Google Scholar]
- 59.Redaelli S, Piazza R, Rostagno R, et al. Activity of bosutinib, dasatinib, and nilotinib against 18 imatinib-resistant BCR-ABL mutants. J Clin Oncol. 2009;27:469–471. doi: 10.1200/JCO.2008.19.8853. [DOI] [PubMed] [Google Scholar]
- 60.Apperley JF. Part I: mechanisms of resistance to imatinib in chronic myeloid leukemia. Lancet Oncol. 2007;8:1018–1029. doi: 10.1016/S1470-2045(07)70342-X. [DOI] [PubMed] [Google Scholar]
- 61.Muller MC, Cortes JE, Kim DW, et al. Dasatinib treatment of chronic-phase chronic myeloid leukemia: analysis of responses according to pre-existing BCR-ABL mutations. Blood. 2009;114:4944–4953. doi: 10.1182/blood-2009-04-214221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jabbour E, Kantarjian HM, Jones D, et al. Characteristics and outcome of patients with F317L BCR-ABL kinase domain mutation after therapy with tyrosine kinase inhibitors. Blood. 2008;112:4839–4842. doi: 10.1182/blood-2008-04-149948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hughes T, Saglio G, Branford S, et al. Impact of baseline BCR-ABL mutations on response to nilotinib in patients with chronic myeloid leukemia in chronic phase. J Clin Oncol. 2009;27:4204–4210. doi: 10.1200/JCO.2009.21.8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radich JP, Martinelli G, Hochhaus A, et al. Response and outcomes to nilotinib at 24 months in imatinib-resistant chronic myeloid leukemia patients in chronic phase (CML-CP) and accelerated phase (CML-AP) with and without BCR-ABL mutations [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114:464–465. Abstract 1130. [Google Scholar]
- 65.Soverini S, Gnani A, Colarossi S, et al. Philadelphia-positive patients who already harbor imatinib-resistant bcr-abl kinase domain mutations have a higher likelihood of developing additional mutations associated with resistance to second- or third-line tyrosine kinase inhibitors. Blood. 2009;114:2168–2171. doi: 10.1182/blood-2009-01-197186. [DOI] [PubMed] [Google Scholar]
- 66.Cortes J, Jabbour E, Kantarjian H, et al. Dynamics of BCR-ABL kinase domain mutations in chronic myeloid leukemia after sequential treatment with multiple tyrosine kinase inhibitors. Blood. 2007;110:4005–4011. doi: 10.1182/blood-2007-03-080838. [DOI] [PubMed] [Google Scholar]
- 67.Chu S, Xu H, Shah NP, et al. Detection of BCR-ABL kinase mutations in CD34+ cells from chronic myelogenous leukemia patients in complete cytogenetic remission on imatinib mesylate treatment. Blood. 2005;105:2093–2098. doi: 10.1182/blood-2004-03-1114. [DOI] [PubMed] [Google Scholar]
- 68.Sherbenou DW, Wong MJ, Humayun A, et al. Mutations of the BCR-ABL-kinase domain occur in a minority of patients with stable complete cytogenetic response to imatinib. Leukemia. 2007;21:489–493. doi: 10.1038/sj.leu.2404554. [DOI] [PubMed] [Google Scholar]
- 69.Nicolini FE, Hayette S, Corm S, et al. Clinical outcome of 27 imatinib mesylate-resistant chronic myelogenous leukemia patients harboring a T315I BCR-ABL mutation. Hematologica. 2007;92:1238–1241. doi: 10.3324/haematol.11369. [DOI] [PubMed] [Google Scholar]
- 70.Cortes JE, Kantarjian H, Brummendorf T, et al. Safety and efficacy of bosutinib (SKI-606) in patients (pts) with chronic phase (CP) chronic myeloid leukemia (CML) following resistance or intolerance to imatinib (IM) [abstract] J Clin Oncol. 2010;28(15S):487s. Abstract 6502. [Google Scholar]
- 71.Cortes-Franco J, Khoury HJ, Nicolini FE, et al. Safety and efficacy of subcutaenous-administered omacetaxine mepesuccinate in imatinib-resistant chronic myeloid leukemia (CML) patients who harbor the bcr-abl T315I mutation—results of an ongoing multicenter phase 2/3 study [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114:267. Abstract 644. [Google Scholar]
- 72.O’Hare T, Shakespeare WC, Zhu X, et al. AP24534, a pan-BCR-ABL inhibitor for chronic myeloid leukemia, potently inhibits the T315I mutant and overcomes mutation-based resistance. Cancer Cell. 2009;16:401–412. doi: 10.1016/j.ccr.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cortes J, Talpaz M, Denininger M, et al. A phase 1 trial of oral AP24534 in patients with refractory chronic myeloid leukemia and other hematologic malignancies: first results of safety and clinical activity against T315I and resistant mutations [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114:267. Abstract 643. [Google Scholar]
- 74.Talpaz M, Cortes JE, Deininger MW, et al. Phase I trial of AP24534 in patients with refractory chronic myeloid leukemia (CML) and hematologic malignancies [abstract] J Clin Oncol. 2010;28(15S):489s. Abstract 6511. [Google Scholar]
- 75.Cortes JE, Paquette R, Talpaz M, et al. Preliminary clinical activity in a phase 1 trial of the BCR-ABL/IGF-1R/aurora kinase inhibitor XL228 in patients with Ph+ leukemias with either failure to multiple TKI therapies or with T315I mutation [abstract] Blood (ASH Annual Meeting Abstracts) 2008;112:1109. Abstract 3232. [Google Scholar]
- 76.Gontarewicz A, Balabanov S, Keller G, et al. Simultaneous targeting of aurora kinases and bcr-abl kinase by the small molecule inhibitor PHA-739358 is effective against imatinib-resistant BCR-ABL mutations including T315I. Blood. 2008;111:4355–4364. doi: 10.1182/blood-2007-09-113175. [DOI] [PubMed] [Google Scholar]
- 77.Van Etten RA, Chan WW, Zaleskas VM, et al. DCC-2036: a novel switch pocket inhibitor of ABL tyrosine kinases with therapeutic efficacy against BCR-ABL T315I in vitro and in a CML mouse model [abstract] Blood (ASH Annual Meeting Abstracts) 2007;110:142a. Abstract 463. [Google Scholar]
- 78.Agency for Healthcare Research and Quality (AHRQ) Imatinib for Chronic Myeloid Leukemia (CML) Rockville, MD: AHRQ; 2009. [Accessed October 26, 2010]. Technology Assessment: Report on the Relative Efficacy of Oral Cancer Therapy for Medicare Beneficiaries Versus Currently Covered Therapy. Part 3. http://www.ahrq.gov/clinic/ta/cml/cml.pdf. [PubMed] [Google Scholar]
- 79.Branford S, Melo JV, Hughes TP. Selecting optimal second-line tyrosine kinase inhibitor therapy for chronic myeloid leukemia patients after imatinib failure: does the BCR-ABL mutation status really matter? Blood. 2009;114:5426–5435. doi: 10.1182/blood-2009-08-215939. [DOI] [PubMed] [Google Scholar]
- 80.Lipton JH, Sriharsha L, Bogomilsky S, et al. Pleural effusions in patients treated with dasatinib: results from 2 institutions, risk factors and management [abstract] J Clin Oncol. 2007;25(18S) Abstract 17503. [Google Scholar]
- 81.Quintas-Cardama A, Kantarjian HM, O’Brien S, et al. Association of pleural effusion and bleeding in patients with chronic myelogenous leukemia receiving dasatinib. Blood. 2008;112:735. Abstract 2112. [Google Scholar]
- 82.Quintas-Cardama A, Kantarjian H, Ravandi F, et al. Bleeding diathesis in patients with chronic myelogenous leukemia receiving dasatinib therapy. Cancer. 2009;115:2482–2490. doi: 10.1002/cncr.24257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Quintas-Cardama A, Han X, Kantarjian H, Cortes J. Dasatinib-induced platelet dysfunction [abstract] Blood (ASH Annual Meeting Abstracts) 2007;110:864a. Abstract 2941. [Google Scholar]
- 84.Sillaber C, Herrmann H, Bennett K, et al. Immunosuppression and atypical infections in CML patients treated with dasatinib at 140 mg daily. Eur J Clin Invest. 2009;39:1098–1109. doi: 10.1111/j.1365-2362.2009.02206.x. [DOI] [PubMed] [Google Scholar]
- 85.Bradeen HA, Eide CA, O’Hare T, et al. Comparison of imatinib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. Blood. 2006;108:2332–2338. doi: 10.1182/blood-2006-02-004580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jabbour E, Hochhaus A, Cortes J, Louisiana Rosee P, Kantarjian HM. Choosing the best treatment strategy for chronic myeloid leukemia patients resistant to imatinib: weighing the efficacy and safety of individual drugs with BCR-ABL mutations and patient history. Leukemia. 2010;24:6–12. doi: 10.1038/leu.2009.193. [DOI] [PubMed] [Google Scholar]
- 87.Kantarjian HM, Jabbour E, Giles FJ, et al. Prognostic factors for progression-free survival in patients with imatinib-resistant or -intolerant chronic myeloid leukemia in chronic phase (CML-CP) treated with nilotinib based on 24 month data [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114:1278–1279. Abstract 3298. [Google Scholar]
- 88.Milojkovic D, Nicholson E, Apperley JF, et al. Early prediction of success or failure of treatment with second-generation tyrosine kinase inhibitors in patients with chronic myeloid leukemia. Hematologica. 2010;95:224–231. doi: 10.3324/haematol.2009.012781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jabbour E, Kantarjian H, O’Brien S, et al. Predictive factors for response and outcome in patients (pts) treated with second generation tyrosine kinase inhibitors (2-TKI) for chronic myeloid leukemia in chronic phase (CML-CP) post imatinib failure [abstract] Blood (ASH Annual Meeting Abstracts) 2009;114:210–211. Abstract 509. [Google Scholar]
- 90.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of bcr-abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 91.Puttini M, Coluccia AM, Boschelli F, et al. In vitro and in vivo activity of SKI-606, a novel src-abl inhibitor, against imatinib-resistant bcr-abl+ neoplastic cells. Cancer Res. 2006;66:11314–11322. doi: 10.1158/0008-5472.CAN-06-1199. [DOI] [PubMed] [Google Scholar]