Abstract
Thyroid hormone (TH) modulates serum cholesterol by acting on TH receptor β1 (TRβ1) in liver to regulate metabolic gene sets. In rodents, one important TH regulated step involves induction of Cyp7a1, an enzyme in the cytochrome P450 family, which enhances cholesterol to bile acid conversion and plays a crucial role in regulation of serum cholesterol levels. Current models suggest, however, that Cyp7a1 has lost the capacity to respond to THs in humans. We were prompted to re-examine TH effects on cholesterol metabolic genes in human liver cells by a recent study of a synthetic TH mimetic which showed that serum cholesterol reductions were accompanied by increases in a marker for bile acid synthesis in humans. Here, we show that TH effects upon cholesterol metabolic genes are almost identical in mouse liver, mouse and human liver primary cells and human hepatocyte cell lines. Moreover, Cyp7a1 is a direct TR target gene that responds to physiologic TR levels through a set of distinct response elements in its promoter. These findings suggest that THs regulate cholesterol to bile acid conversion in similar ways in humans and rodent experimental models and that manipulation of hormone signaling pathways could provide a strategy to enhance Cyp7a1 activity in human patients.
Keywords: TH, TH receptor, Cholesterol, Bile acids, Cyp7a1
1. Introduction
Hepatic bile acid synthesis is a finely coordinated metabolic pathway that is integral to dietary lipid absorption and serum cholesterol regulation but regulation of this process has exhibited divergence throughout mammalian evolution (Ellis et al., 1998; Völzke et al., 2005; Moore et al., 2002). Cholesterol 7α hydroxylase (Cyp7a1) catalyzes the rate limiting step of the classical bile acid synthesis pathway and activity of this enzyme displays inverse correlation with plasma low density lipoprotein (LDL) cholesterol levels in rodents and humans (Li et al., 2011). Phenotypes of Cyp7a1 transgenic and knockout mice and humans with Cyp7a1 mutations confirm that reductions in Cyp7a1 activity lead to elevated serum LDL cholesterol and trigger onset of atherosclerosis. Whereas Cyp7a1 expression is positively regulated by the nuclear hormone receptor (NR) liver X receptor α (LXRα) in mouse liver, human Cyp7a1 does not exhibit similar regulation because the human promoter lacks LXRα binding sites (Menke et al., 2002). It is important to understand evolutionary divergence of regulation of cholesterol metabolic genes in order to fully comprehend whether results of tests of potential lipid-lowering therapeutics in rodent models can be applied to humans.
Thyroid hormones (THs, predominantly triiodothyronine, T3) also act through cognate NRs (TRs α and β) to regulate serum cholesterol (Baxter and Webb, 2009), and current models suggest that THs differentially regulate Cyp7a1 in rodents and human. T3 similarly induces some genes that play important regulatory roles in cholesterol metabolism in both species, such as liver LDL receptor (Bakker et al., 1998). While T3 strongly induces Cyp7a1 expression in rodents (Gullberg et al., 2002; Johansson et al., 2005; Shin et al., 2006; Hashimoto et al., 2006; Kamiya et al., 2003; Ness et al., 1994, 1998; Hylemon et al., 1992), several lines of evidence suggest that the human Cyp7a1 gene could respond differently. First, assessment of cholic acid (CA) levels in humans have indicated that CA secretion rates into the intestine, CA synthesis and pool size are all decreased in hyperthyroid patients and restored by normalization of TH levels (Sauter et al., 1997). Second, initial measurements of a plasma marker of cholesterol to bile acid conversion (7α-hydroxy-4-cholesten-3-one, ‘C4’;) (Gälman et al., 2003; Honda et al., 2007) are consistent with unchanged Cyp7a1 activity in hyperthyroid and hypothyroid patients. Third, TH reduces bile acid production by primary liver cell cultures (Ellis et al., 1998; Völzke et al., 2005). Fourth, THs are reported to suppress human Cyp7a1 promoter activity (Wang et al., 1996) and, while mapping of the human Cyp7a1 promoter revealed two sites that bind to TRα1, these elements confer repression by unliganded TRs but not T3 response upon the Cyp7a1 dependent reporters (Drover and Agellon, 2004). Finally, introduction of the human Cyp7a1 gene into transgenic mouse models and subsequent induction of hypo- and hyperthyroid states indicated that T3 suppressed the human Cyp7a1 gene in male mice (Drover and Agellon, 2004). These findings suggest that THs do not regulate the human Cyp7a1 gene in the same way as mouse Cyp7a1.
Recent results from a human trial with a selective thyromimetic have led us to question that notion that the human Cyp7a1 gene has lost the ability to respond to TH (Berkenstam et al., 2008). Thyromimetics are synthetic TH-like compounds designed to: (i) preferentially bind to TRβ, the major isoform of which (TRβ1) plays major roles in liver to reduce serum cholesterol, versus TRα1, which mediates deleterious effects of hyperthyroidism on heart, muscle and bone, and (ii) to accumulate selectively in liver, the major site of regulation of cholesterol metabolism (Meruvu et al., 2013). Administration of one of these compounds, KB2115/Eprotirome [3-[[3,5-dibromo-4-[4-hydroxy-3-(1-methylethyl)-phenoxy]-phenyl]-amino]-3-oxopropanoic acid)], to moderately overweight and hypercholesterolemic human subjects revealed that KB2115 was well tolerated and elicited striking (≈40%) reductions in serum LDL cholesterol during 14 day trials (Berkenstam et al., 2008). Of note, however, is that these effects were accompanied by dose-dependent elevation of serum C4, without evidence for increased markers of cholesterol synthesis (Gälman et al., 2008), implying that KB2115 enhances cholesterol to bile acid conversion in human subjects.
Direct assessment of hepatic gene regulation in human patients presents logistical and ethical quandaries. Additionally, we found that human liver cell lines and primary cultures express vanishingly low levels of TR proteins (Yuan et al., 2012), meaning that it is also difficult to assess TH regulatory mechanisms in these systems. We therefore turned to an exogenous TR expression strategy to compare TH-dependent regulation of genes involved in cholesterol and bile acid metabolism in panels of mouse and human liver cells to understand evolutionary differences in gene regulation. Our results indicate that TH regulates key genes that regulate important steps in cholesterol reabsorption and bile acid conversion in an almost identical manner in mouse and human cells and that the human Cyp7a1 gene is strongly induced by T3 in cells derived from both species. We confirm that such effects are observed at physiologic TR levels and that the Cyp7A1 promoter region is decorated with functional TH response elements (TREs). We discuss the significance of this finding in terms of novel strategies to reduce elevated serum cholesterol levels.
2. Materials and methods
2.1. Materials
Reagents were acquired from the following sources: oligonucleotides from Integrated DNA Technologies (Coralville, IA), TRβ antibody from Pierce Biotechnologies (Rockford, IL), Cyp7a1 antibody from Abcam (Cambridge, England), tubulin antibody from Pierce (Rockford, IL), T3 from Sigma–Aldrich (St. Louis, MO), 125I-labelled T3 from Perkin–Elmer (Boston, MA), HepG2 cells from the American Type Culture Collection (Manassas, VA), HepaRG cells and human and mouse primary hepatocytes from Life Technologies (Carlsbad, CA), Fugene HD from Roche Applied Science (Penzberg, Germany).
2.2. Plasmids
Luciferase reporter constructs were generated by mutagenesis of pGL4.13 [MinP] (Promega, Madison, WI), using primers containing TREs in Table S1. Open reading frames of TRα1 and TRβ1 were PCR-amplified using the primers indicated in Table S1 and inserted into pENTR D-Topo, which were subsequently used to generate adenovirus-containing genomes with the Adeasy 1.0 system using manufacturer's standard protocols (available, www.invitrogen.com).
2.3. Adenoviruses
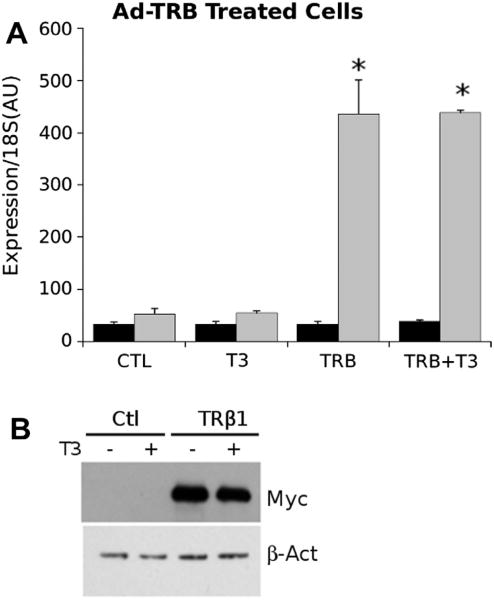
Adenoviruses were generated using HEK293 cells through transfection of cloned adenovirus genomes, using standard protocols (available, www.invitrogen.com). Virus titer was measured using the Viracheck adenovirus quantification system and expression of genes of interest evaluated by realtime PCR (Fig. 1).
Fig. 1.
Adenovirus expression of TRb. (A) Detection of TRβ1 transcripts after adenovirus infection of HepG2 cells. Panel represents qPCR analysis performed on extracts of control and adenovirus TRb infected cells ± T3. Grey panels represent TRβ1 and black panels represent TRα1. Results of T-test indicated by * (P < 0.05). (B) Western blot of SDS–polyacrylamide gels used to separate extracts of control adenovirus and adenovirus TRβ1 infected cells. Blots used antibodies against myc epitope tag and β-actin control to ensure equal loading.
2.4. Cell culture and transfection
HepG2 cells and TR expressing derivatives were cultured in DMEM, with Penicillin and Streptomycin and subcultured every 2–3 days. Prior to transfection assays, cells were supplemented with resin-stripped FBS to remove T3 and transfected with constructs indicated and luciferase reporter activity was normalized by cotransfection of pRL which contains the open reading frame of renilla luciferase. TRβ1-BioChIP cells were created by transfection of vectors bearing TRβ1 and BiRA and selection with corresponding antibiotics as described elsewhere (Ayers et al., 2014). TRα1-BioChIP cells were created in a similar manner.
2.5. Mouse liver extracts
Mouse liver samples were harvested in T-PER tissue lysis buffer with added protease inhibitor cocktail (Pierce Bioreagents) from C57 mice maintained on standard chow diet, sacrificed after 8 h fast by CO2 asphyxiation. Hyperthyroidism or hypothyroidism were induced as described (Yuan et al., 2012). All procedures were conducted with full approval of the TMHRI Institutional Animal Care and Use Committee.
2.6. RNA expression analysis
RNA was extracted from cells using RNEasy columns, according to manufacturer's instructions (Qiagen, Valencia, CA). Realtime PCR was used to analyze gene expression levels using the primer sets (Table S2) and standard SYBR Green reagents (Roche) on a Roche 480 instrument.
2.7. Western blots
Protein expression was assessed by immunoblotting with indicated antibodies; mouse liver samples were homogenized with Glass Bead Tubes (Fisher) T-PER protein extraction reagent with protease inhibitor cocktail (Thermo-Fisher). Proteins were transferred using the iBlot automated blotting system (Life Technologies) and immunoblotting with indicated antibodies assessed with standard ECL reagents (Fisher).
2.8. Mass spectrometry
Analysis of serum soluble metabolites was conducted by the TMHRI Proteomics Core Facility, using previously published methodologies for 7αC4 (Gälman et al., 2003). Briefly, cell culture media samples were mixed with deuterium-labeled C4 (Santa Cruz Biotechnology), precipitated with ammonium sulfate and acetonitrile and resuspended in methanol. Levels of 7αC4 were analyzed by liquid chromatography electrospray ionization-tandem mass spectrometry on an Acuity UPLC system and XevoTQ mass spectrometer operated in MRM mode using positive ion electrospray conditions.
2.9. T3 binding assays
T3 binding was measured in cell or tissue extracts by standard methods. Briefly, cellular protein was incubated with 125I-labelled T3 for 1 h. Protein-bound T3 was subsequently separated by column centrifugation with Centri-Spin 10 columns (Princeton Separations, Adelphia, NJ) and protein-bound T3 quantified on a Capintec Caprac scintillation quantification system (Ramsey, NY). Results indicate the average of 3 independent assays.
2.10. Cell extract preparation and gel shift
Cells were lysed in a binding buffer containing 25 mM HEPES, 50 mM KCl, 1 mM dithiothreitol, 10 M ZnSO4, 0.1% Nonidet P-40, 5% glycerol. TREs were synthesized as single-stranded oligonucleotides with a 5′ amine moiety on their forward strand and were annealed by standard protocols and labeled with 2×-excess of IRDye800-NHS-esther (Li-Cor Biosciences, Lincoln, NE) and purified by silica column. Complexes were formed in binding buffer containing 1 μg of poly(dI-dC) (Sigma) and resolved on a 5% non-denaturing gel and visualized on a Li-Core Odyssey Imaging System.
2.11. Chromatin immunoprecipitation (ChIP)
ChIP was performed using standard protocols. Cells were plated in DMEM containing resin-stripped FBS, treated with 100 nM T3 and cross-linked with 1% formaldehyde and chromatin fragmented with a Bioruptor sonicator (Diagenode, Denville, New Jersey). Binding and washing steps were performed and resulting samples analyzed by QPCR with indicated primers (Table S2). Samples were used in ChIP analyses were analyzed in triplicate.
2.12. TRE-search
Putative TREs were identified in DNA sequences, obtained from UCSC Genome Browser (available: http://genome.ucsc.edu) and processed by progressive base scoring with a position-weighted matrix, compiled from previously identified response elements, using a Perl script. TRE sequences were then verified using described methods.
3. Results
3.1. Evaluation of adenovirus TR expression strategy
To test the ability of our adenovirus-TR vectors to produce functional myc-tagged TRs, we performed trial infections of human HepG2 liver cells and assessed TR mRNA and protein. We selected TRβ1, to express the predominant form of TR expressed in liver. We observed large increases in TRβ1 mRNA in adenovirus-TRβ (Ad-TRβ1) infected samples, but not cells infected with control virus, and there were no changes in TRα mRNA in these conditions (Fig. 1A). Infection was also accompanied by increases in TRβ1 protein, evidenced by appearance of epitope tag at the appropriate molecular weight in Western blot (Fig. 1B). We varied adenovirus titer to select minimum multiplicity of infection required for detectable TR expression and used these conditions for subsequent experiments (not shown).
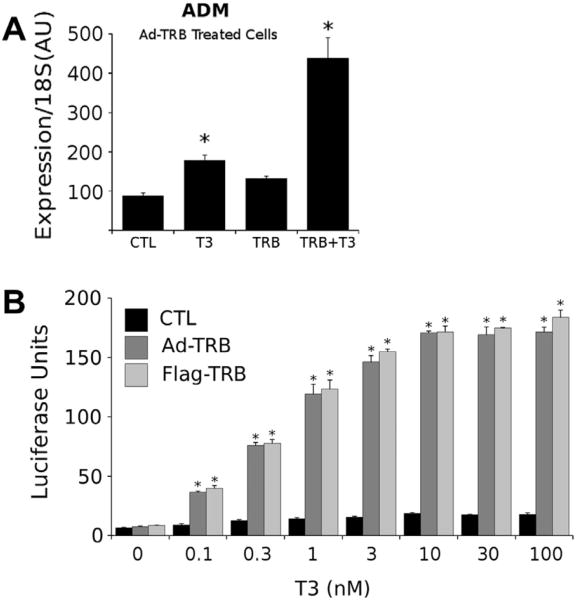
Adenovirus expressed TRs were functional. We assessed expression of a classical TR target gene (adrenomedullin, adm) in infected versus uninfected cells (Ayers et al., 2014). We detected modest T3 induction of adm mRNA(≈ 1.8-fold) in the absence of exogenous TRs, likely a consequence of low levels of TRβ1 expression in this cell line (Yuan et al., 2012), and T3 induction was boosted to approximately 4-fold in the presence of Ad-TRβ1 (Fig. 2A). Likewise, we detected substantial T3 dose dependent induction of a standard DR-4 TRE-driven luciferase reporter gene in Ad-TRβ1 infected cells, versus no significant T3 induction in parental HepG2 cells (Fig. 2B). EC50 values for induction were around 0.3 nM, typical for T3 induction and similar to results obtained with HepG2 cells that stably express transfected Flag-TRβ1 (Ayers et al., 2014; Lin et al., 2013). We conclude that adenovirus expression produces functional TRs in a human liver cell line.
Fig. 2.
Adenovirus expressed TRβ1 is functional. (A) qPCR analysis of adrenomedullin transcripts in control and adenovirus-TRβ1 infected HepG2 cells treated ± T3. Note enhancement of T3 induction of the endogenous adm gene in the presence of adenovirus TRβ versus induction obtained with endogenous TRβ. (B) Comparative transfection assays performed with a DR-4 responsive luciferase reporter in control (black) and adenovirus TRβ1 infected (dark grey) HepG2 cells and in HepG2 cells that stably express TRβ1 and treated with increasing levels of T3. Note lack of enhancement of luciferase activity with T3 in cells that do not express endogenous TRβ1 and that dose response is similar with Ad-TRβ1 infected HepG2 cells and HepG2-TRβ1 cells. Results of T-test indicated by * (P < 0.05).
3.2. Similar effects of T3 on Cyp7a1 expression in mouse and human liver cells
To compare effects of exogenous TRβ1 expression in mouse and human liver cells, we infected mouse (MHEP) and human (HHEP) liver primary cultures with Ad-TRβ1. We utilized these primary cells in order to best mimic effects of exogenous TRβ1 expression in hepatocytes, instead of transformed cell lines. We also employed a human liver cell line, which is reported to display a gene expression pattern that closely resembles native human hepatocytes (HepaRG) (Jennen et al., 2010). We then used high throughput qRT-PCR to examine patterns of T3-dependent gene expression in mock infected and infected cells and to compare changes in gene expression with that of endogenous genes in livers of hypothyroid mice treated with vehicle or T3 for 24 h (Fig. 3A).
Fig. 3.
Similar T3 regulation of cholesterol metabolic genes in mouse liver, mouse and human liver primaries and a human hepatocyte cell line. (A) Heat map depicting high throughput qPCR analysis of expression levels of indicated genes in livers of hypothyroid mice treated ± T3, mouse and human primary hepatocyte cultures and HepaRG cells infected with control adenovirus or Ad-TRβ1 and treated ± T3. (B) Independent single point qPCR validation of changes in mouse and human Cyp7a1 expression levels ± adenovirus TRβ1 expression and T3.
Interestingly, patterns of T3 response observed in human primary liver culture and HepaRG in the presence of adeno-TRβ1 were highly similar to that of native mouse liver and adeno-TRβ1 infected mouse primary hepatocytes (Fig. 3A). As expected (Yuan et al., 2012), liver primary cultures of both species and the HepaRG cells expressed diminished levels of TRβ1 mRNA relative to native liver and Ad-TRβ1 infection restored TRβ1 mRNA and permitted T3-dependent induction of the control gene adm. In these conditions, T3 induced genes involved in cholesterol uptake (LDLR), secretion (ABCG5, ABCG8) and serum transport (APOA1, 5) in native mouse liver and all other cell types. One difference between mouse and human cells is that T3 induced farnesoid X receptor (FXR) expression in human cells, similar to effects noted in HepG2 cells in the past (Yuan et al., 2012). Additionally, exogenous TRβ1 expression led to modest suppression of T3 response of the ABCA1 (cholesterol transporter) gene (see Discussion). Most strikingly, however, we observed similar strong T3 induction of Cyp7a1 mRNA in mouse liver and Ad-TRβ infected MHEP and both human cell types. No T3 induction of Cyp7a1 was observed in the absence of exogenous TRs in any human or mouse cultured cells. We were readily able to verify 4-fold T3 induction of mouse and human Cyp7a1 cells in independent single point qRT-PCR experiments and confirm that these effects were dependent upon exogenous TR expression (Fig. 3B). Thus, TRβ1 expression restores T3 response at a tested subset of cholesterol metabolic genes in mouse and human cells and, in particular, permits T3 induction of both mouse and human Cyp7a1.
To determine whether T3 effects upon human Cyp7a1 involve direct transcriptional regulation, we examined effects of the protein synthesis inhibitor cycloheximide (CHX). We treated human HepG2 transformed hepatocyte cells that stably express TRβ1 (Lin et al., 2013) with T3 for 6 h, including a pretreatment ± CHX for 30 min (Fig. 4A). We observed approximately 2.5-fold T3 response in this cell culture system and found that T3 induction was completely unaffected by CHX.
Fig. 4.
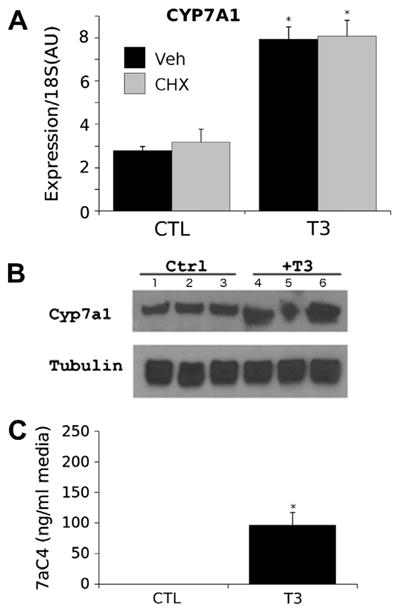
T3 induction of Cyp7a11 in HepG2 cells. (A) Results of qPCR analysis of Cyp7a1 transcripts in HepG2 cells that express TRβ1. Cells treated ± T3 and the protein synthesis inhibitor cycloheximide. Results represent mean of 3 replicates ± SEM, P < 0.05 indicated by * (Student's T-test). (B) Western blot of SDS-polyacrylamide gels used to separate extracts of HepG2-TRβ1 cells treated ± T3. Antibodies used were anti-Cyp7a1 and anti-tubulin loading control. Individual lanes represent different biological replicates in triplicate. (C) C4 levels detected in culture media for HepG2-TRβ1 cells treated ± T3 using approaches described in Section 2. Results represent mean of 3 replicates ± SEM, P < 0.05 indicated by * (Student's T-test).
We also determined T3 effects upon Cyp7a1 protein expression and activity in the cultured HepG2 cells. Western blots of HepG2 cell extracts revealed T3-dependent increases in Cyp7a1 protein levels relative to a control tubulin gene (Fig. 4B). Further, assays of cell culture medium by a mass spectroscopic approach (Section 2) revealed striking increases in levels of C4 in the cell culture medium; C4 is the enzymatic product of Cyp7a1 that is used as a marker for cholesterol to bile acid conversion rates in serum (Gälman et al., 2003). Thus, we conclude that the human Cyp7a1 gene is a TRβ1 target gene that is induced by T3 in cell culture similar to the mouse gene in cultured cells and native mouse liver.
3.3. Cyp7a1 induction in the presence of low levels of TRs
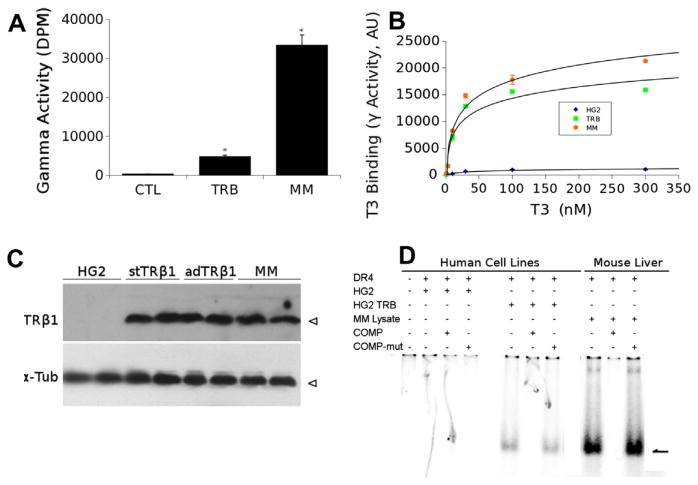
To investigate the possibility that TRβ1 overexpression is required for induction of the human Cyp7a1 gene in cultured cells, we assessed TRβ1 protein levels expressed in representative ad-TRβ1 expressing HepG2 cells and compared this to mouse liver samples. Ligand binding assays revealed that TRβ expressing HepG2 cells (TRβ) displayed around 15% of total ligand binding activity seen in mouse liver (MM, Fig. 5A). We also confirmed that adenovirus expressed TRβ1 and mouse liver extract (5× dilution) displayed similar T3 binding curves (Fig. 5B). Western analysis confirmed similar levels of TRβ1 in HepG2 cells that stably express TRβ1 (stTRβ1), HepG2 cells infected with TRβ1 expressing adenovirus (adTRβ1) and 5× dilution of mouse liver extract (MM) (Fig. 5C). Further, gel shift analysis performed with extracts of parental HepG2 cells (HG2) and HepG2 cells infected with ad-TRβ1 (HG2 TRb) revealed specific complex formation with a TRE DR-4 element only in adTRβ1 infected cell extracts and that amounts of shifted complex were much lower than obtained with equivalent amounts of mouse liver extracts (Fig. 5D). We conclude that T3-dependent Cyp7a1 induction is obtained with TRβ1 levels that are relatively low compared to TR levels in native mouse liver.
Fig. 5.
TRβ1 is expressed at low levels in HepG2 cells relative to mouse liver. (A) Results of single point T3 binding analysis performed on extracts of HepG2 cells (control, ctl) or HepG2 cells expressing ad-TRβ1 (TRβ) compared to native mouse liver extracts (MM). Results represent mean of 3 replicates ± SEM, P < 0.05 indicated by * (Student's T-test). (B) Saturation T3 binding analysis performed with HepG2 cells (HG2), HepG2 infected with Ad-TRβ1 expression vector (HG2-TRβ) and mouse liver extracts (5× dilution; MM). Results of each data point represent mean of 3 replicates ± SEM, P < 0.05 indicated by * (Student's T-test). (C) Western analysis of HepG2 cells (HG2), TRβ1 expressing HepG2 cells (stable and adenovirus expression stTRβ1 and adTRβ1) versus mouse liver (5× dilution, MM). Antibodies detect TRβ1 or tubulin as a loading control. Individual lanes represent duplicate biological replicates. (D) Results of gel shift analysis performed with extracts on native HepG2 (HG2), HepG2 infected with ad-TRβ1 (HG2TRβ) and mouse liver (MM). Triplet sets of lanes represent shifting with native DR-4, with unlabeled competitor DR-4 oligonucleotide or mutant oligonucleotide.
3.4. TR binding to the human Cyp7a1 promoter in cultured cells
Next, we assessed TR binding events near the human Cyp7a1 gene (Fig. 6). We utilized a ChIP strategy with tiled primer sets to measure TR binding to several different regions (R1–6) of the Cyp7A1 promoter in HepG2 cells that stably express TRα1 or TRβ1 (Ayers et al., 2014) relative to IgG controls. We observed significant enrichment for both TRα and TRβ at R5 and a second peak at R3 (Fig. 6).
Fig. 6.
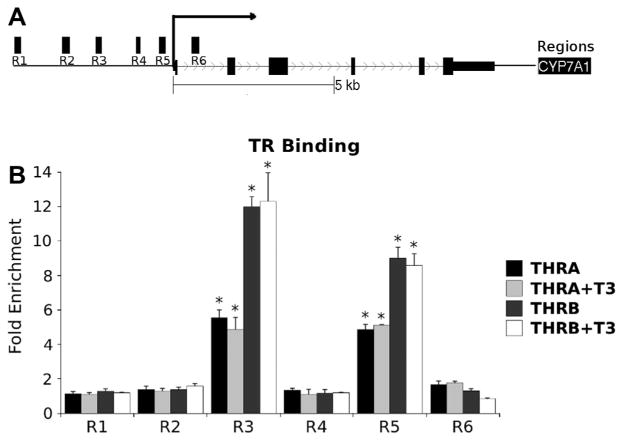
TRs bind to the Cyp7a1 proximal promoter. Upper panel is a schematic of the Cyp7a1 gene locus with positions of regions that were probed with ChIP primers. The lower panel depicts ChIP assays performed upon extracts of cells that express tagged TRα1 or TRβ1, as described in Section 2. Bars represent enrichment over IgG control, which was set to a value of 1. Note that TR binding only exceeds background in the R3 and R5 regions. Results of T-test indicated by * (P < 0.05).
To investigate whether TR binding peaks within the CYP7A1 promoter harbor functional TREs, we employed a standard position-weighted matrix-based comparison to previously characterized TREs to detect TRE-like sequences within both TRβ peaks (Table 1). This method revealed two previously identified TRE-like sequences within R5 that were reported to bind TRα1, but did not confer T3 response upon the Cyp7a1 promoter (defined sites, D) (Drover and Agellon, 2004). In addition, we identified a novel additional TRE-like sequence (novel site 1, N1) within the R3 region. We cloned these elements into a standard luciferase reporter gene. Both defined (D) elements within the R5 region also conferred T3 responses on the luciferase reporter that were almost as robust as a standard DR-4 element and the native Cyp7a1 promoter. In these cases, fold induction was similar in the presence of TRα1 and TRβ1. The novel TRE like sequence (N1) within the R3 region also conferred striking T3 responses on the luciferase reporter that were as large as obtained with a classic DR-4 element (Fig. 7). Interestingly, N1 also displayed modest TRβ1 selectivity. T3 responses from this novel element were abolished by mutation of a putative TRE half-site (Table 1). We conclude that the human Cyp7a1 promoter contains several TR binding sites that harbor functional TREs.
Table 1.
TRE-like sequences within TR binding peaks in the Cyp7a1 proximal promoter.
| Name | Sequence | Position |
|---|---|---|
| DI | TACCTG TGGACT T AGTTCA AGGCCAGTT | chr8: 59, 412, 844–59, 412, 871 |
| DII | TAGC TGTTGTCCC C AGGTCC GAAT | chr8: 59, 412, 950–59, 412, 973 |
| N1 | GAGATGA TTTAGG GTAT CTGGCA GAAGAA | chr8: 59, 415, 249–59, 415, 276 |
Fig. 7.
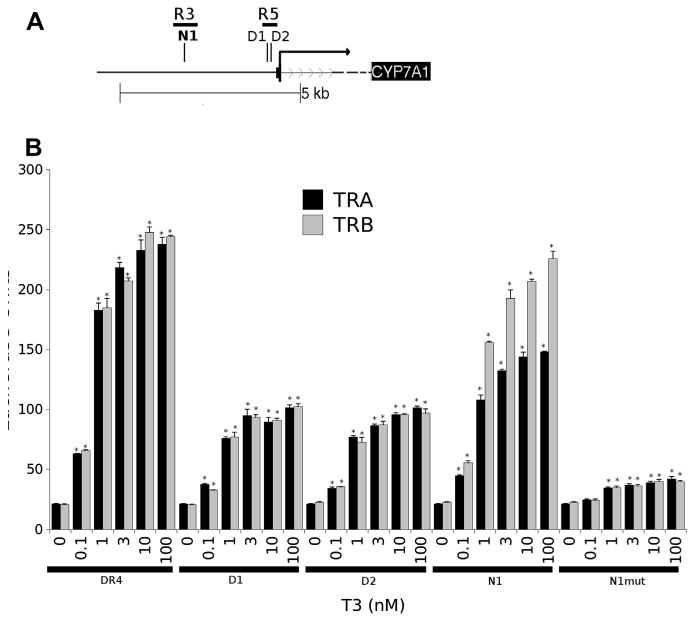
Analysis of function of TREs in TR binding peaks. Upper panel represents approximate positions of defined TREs in TR binding peaks. Lower panel depicts results of luciferase assays performed with reporter genes drive by a standard DR-4 element, the Cyp7a1 promoter or individual predicted TREs in HepG2 cells that stably express TRα or TRβ. Note specific dose-dependent enhancement of luciferase activity with T3. Results of T-test indicated by * (P < 0.05).
4. Discussion
Whereas mouse and rat Cyp7a1 is a defined TR target gene and T3-dependent induction of Cyp7a1 in mouse liver enhances rates of cholesterol to bile acid conversion, Cyp7a1 has not generally been considered to be a TR target gene in humans. The present study was initiated in response to observations made in a human study designed to measure effects of the experimental thyromimetic KB2115/Eprotirome on LDL cholesterol in humans (Berkenstam et al., 2008; Ladenson et al., 2010). As expected, two week KB2115 treatments led to striking reductions serum total and LDL cholesterol in patients. Surprisingly, these effects were accompanied by transient dose-dependent elevation of serum C4, a marker for hepatic bile acid synthesis. This usually indicates that Cyp7a1 activity is elevated and this observation prompted us to re-examine TR and T3 regulation of human Cyp7a1 in cultured cells in vitro.
Several lines of evidence convince us that human Cyp7a1 is a bona fide T3 target gene in cultured human cells. We assessed effects of adenovirus TRβ1 expression on several key cholesterol metabolic genes in human liver primary cells and a human liver cell line (HepaRG) that displays a gene expression profile that resembles native hepatocytes. T3 strongly induced human Cyp7a1 in both of these hepatocyte cell types and, in general, effects of T3 treatment on selected genes were very similar in human and mouse primary cultures and native mouse liver. We also observed T3 induction of Cyp7a1 transcripts in transformed human HepG2 liver cells that stably express TRβ1 and these effects were direct, as assessed by cycloheximide treatment, and mirrored by changes in Cyp7a1 protein levels and enzymatic activity. Finally, we detected two strong TRβ binding peaks within the Cyp7a1 proximal promoter in HepG2 using a tiled ChIP approach and verified that these peaks contain functional TREs. While we recognize that our studies have not assessed TH-regulation of Cyp7a1 in human subjects, we nevertheless therefore suggest that there is no intrinsic block to TH induction of the human Cyp7a1 gene locus and that such regulation could potentially also be observed in native liver.
We do not think that T3-dependent induction of Cyp7a1 is an artifact of TRβ1 overexpression. We turned to stable transfection and adenovirus expression strategies to elevate endogenous TR levels in human cells because cultured liver cell lines and primary hepatocytes only express vanishingly low amounts of functional TRs (Yuan et al., 2012 and data not shown). We remained concerned about the possibility that some gene expression changes were related to overexpression of TRs and would not be obtained with physiologic TR expression levels. We therefore verified that exogenous TRβ1 expression levels are maintained within a range that is below that seen in native liver and that exogenous TRs display normal function.
In general, we and others have found that exogenous TRs regulate a physiologically sensible gene set in apparently normal manner (Lin et al., 2013; Chan and Privalsky, 2009). In this study, we observed that adenovirus TR expression restores T3 response at genes that regulate steps in cholesterol metabolism and that gene expression profiles mostly resemble mouse liver. Of 12 genes tested, we found two exceptions. First, T3 induces ABCA1 in primary hepatocytes and this effect is suppressed by adenovirus expressed TRs. We have not followed this observation, but we previously observed similar effects with angiopoietin-like factor 4 (angptl4), which is well induced by low levels of endogenous TRβ1 in HepG2 cells and silenced by actions of exogenously expressed unliganded TRs (Lin et al., 2013). We suspect that similar silencing effects by exogenous unliganded TRs are at play for ABCA1. Second, we uncovered one instance in which T3 responses are seen at a human gene, for the bile acid activated nuclear receptor FXR, but not the equivalent mouse gene. We noted that FXR is a T3 target in other human cell lines (Yuan et al., 2012), raising the possibility that this gene has acquired T3 responsiveness through evolution. In general, however, our data are consistent with the idea that emergence of T3 response seen in the presence of exogenous TRs recapitulates normal T3 responses and that Cyp7a1 is a true TR target gene in cultured human cells.
It is important to consider possible reasons that other investigators did not observe evidence for T3 induction of Cyp7a1 in a variety of experimental systems. For studies in cell lines, several factors may be at play. As mentioned, human primary liver cultures and liver cell lines only express limited levels of TRs, meaning that physiologically appropriate TH responses may have been reduced or lost in these conditions. Moreover, human liver primary cultures tend to produce much more bile acids than those of rodents, implying differences in basal regulatory mechanisms of Cyp7a1 (Ellis et al., 1998). We believe that the fact that we readily detect T3 activation from two TRE elements that were previously shown to bind TRs, but not respond to T3, is probably related to differences in transfection conditions and/or TR expression levels that emphasize T3 response here versus suppressive actions of unliganded TRs in previous studies. In this regard, early studies of TR action at the Cyp7a1 promoter also used TRα1, and Cyp7a1 activation displays a modest TRβ1 preference, suggesting that conditions for detection of T3 activation were not optimal.
It is harder to explain why humanized Cyp7a1 transgenic mice did not display T3 response; functional TREs described here were present in the genomic locus that was transferred to mice. Perhaps T3 response was masked by genomic context of the transgene. Alternatively, it is known that there is complex interplay between factors that regulate Cyp7a1 (Hashimoto et al., 2006) and unspecified factors in the mouse liver cellular milieu could have suppressed T3 response. It will be important to understand how TH regulates Cyp7a1 expression in different conditions of diet and other factors.
As mentioned in Section 1, several human studies failed to detect alterations in C4 levels in response to changes in TH status and this was considered further evidence that human Cyp7a1 was not a TR target gene. Thus, it was surprising that the thyromimetic KB2115 altered C4 levels in human patients (Sauter et al., 1997), and hard to reconcile this observation with previous literature. While we cannot explain this discrepancy, we note that effects of KB2115 upon C4 levels appear transient (Berkenstam et al., 2008) and we suspect that these transient alterations may have been missed in situations in which there are long-term changes in TH levels.
Finally, if our findings using human cell culture models can be applied to human patients and TH actions in native liver, then they may have implications for development of strategies to reduce serum cholesterol levels. As mentioned, Cyp7a1 expression levels and activity strongly influence serum cholesterol levels and are related to atherosclerosis risk (Li et al., 2011; Menke et al., 2002; Baxter and Webb, 2009). We and others have previously shown that thyromimetic and T3 dependent reductions in serum LDL cholesterol can be obtained in mice that lack functional LDL-receptors and such effects are strongly dependent on Cyp7a1 induction (Lin et al., 2012; Goldberg et al., 2012). While we were initially skeptical that similar effects would be observed in humans that totally lack functional LDL receptors (familial hypercholesterolemia, FH) or in humans with heterozygous forms of FH that do not respond to statins because of the previous lack of evidence for TH induction of Cyp7a1, data presented here suggests that human Cyp7a1 could be indeed be induced by T3 or thyromimetics. Thus, it seems likely that safe thyromimetics could induce cholesterol-lowering effects in human subjects with FH, as seen in LDLR knockout mice, and that thyromimetics may offer one possible treatment avenue for such patients. In general, our findings indicate that it is important to understand regulatory influences that target the human Cyp7a1 gene in order to develop improved strategies to obtain significant cholesterol reductions in both normal humans and patients with homo- or heterozygous FH.
5. Conclusions
The human Cyp7a1 gene, hitherto thought to have lost the capacity to respond to THs during evolution, is a bona fide T3 inducible target gene. Cyp7a1 is directly regulated by TRs, which bind to several verified TREs in the proximal promoter. This finding opens up possibilities to manipulate Cyp7a1 expression in order to reduce serum cholesterol levels.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health Grant RC4 DK090849 (PW), a grant from the American Thyroid Association (SDA) and CONACYT, México (JALL).
Abbreviations
- TRA1
TH Receptor Alpha 1 (NR1A1)
- TRB1
TH Receptor Beta 1 (NR1A2)
- TCPOBOP
1,4-Bis-[2-(3,5-dichloropyridyloxy)]benzene
- KB2115
3-[[3, 5-dibromo-4-[4-hydroxy-3-(1-methylethyl)phenoxy]phenyl]amino-3-oxo-propanoic acid, a TRB-selective thyromimetic
- T3
3′,3,5-triiodothyronine
- VLDLR
very low density lipoprotein receptor
- LDLR
low-density lipoprotein receptor
- LDL
low-density lipoprotein
- LDLC
low-density lipoprotein
- HDL
high-density lipoprotein
- SR-B1 (SCARB1)
scavenger receptor B1
Footnotes
Appendix A. Supplementary material: Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.mce.2014.02.003.
Contributor Information
Paul Webb, Email: PWebb@tmhs.org.
Stephen D. Ayers, Email: SDAyers@tmhs.org.
References
- Ayers S, Switnicki M, Angajala A, Lammel J, Webb P. Genome-wide binding patterns of TH receptor beta. PLoS One. 2014;9(2):e81186. doi: 10.1371/journal.pone.0081186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker O, Hudig F, Meijssen S, Wiersinga WM. Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem Biophys Res Commun. 1998;249(2):517–521. doi: 10.1006/bbrc.1998.9174. [DOI] [PubMed] [Google Scholar]
- Baxter JD, Webb P. TH mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev. 2009;8(4):308–320. doi: 10.1038/nrd2830. [DOI] [PubMed] [Google Scholar]
- Berkenstam A, Kristensen J, Mellstrom K, Carlsson B, Malm J, Rehnmark S, et al. The TH mimetic compound KB2115 lowers plasma LDL cholesterol and stimulates bile acid synthesis without cardiac effects in humans. Proc Natl Acad Sci USA. 2008;105(2):663–667. doi: 10.1073/pnas.0705286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan IH, Privalsky ML. Isoform-specific transcriptional activity of overlapping target genes that respond to TH receptors alpha1 and beta1. Mol Endocrinol Baltim Md. 2009;23(11):1758–1775. doi: 10.1210/me.2009-0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drover VAB, Agellon LB. Regulation of the human cholesterol 7alpha-hydroxylase gene (CYP7A1) by TH in transgenic mice. Endocrinology. 2004;145(2):574–581. doi: 10.1210/en.2003-0993. [DOI] [PubMed] [Google Scholar]
- Ellis E, Goodwin B, Abrahamsson A, Liddle C, Mode A, Rudling M, et al. Bile acid synthesis in primary cultures of rat and human hepatocytes. Hepatol Baltim Md. 1998;27(2):615–620. doi: 10.1002/hep.510270241. [DOI] [PubMed] [Google Scholar]
- Gälman C, Arvidsson I, Angelin B, Rudling M. Monitoring hepatic cholesterol 7alpha-hydroxylase activity by assay of the stable bile acid intermediate 7alpha-hydroxy-4-cholesten-3-one in peripheral blood. J Lipid Res. 2003;44(4):859–866. doi: 10.1194/jlr.D200043-JLR200. [DOI] [PubMed] [Google Scholar]
- Gälman C, Bonde Y, Matasconi M, Angelin B, Rudling M. Dramatically increased intestinal absorption of cholesterol following hypophysectomy is normalized by TH. Gastroenterology. 2008;134(4):1127–1136. doi: 10.1053/j.gastro.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Goldberg IJ, Huang LS, Huggins LA, Yu S, Nagareddy PR, Scanlan TS, et al. TH reduces cholesterol via a non-LDL receptor-mediated pathway. Endocrinology. 2012;153(11):5143–5149. doi: 10.1210/en.2012-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullberg H, Rudling M, Saltó C, Forrest D, Angelin B, Vennström B. Requirement for TH receptor beta in T3 regulation of cholesterol metabolism in mice. Mol Endocrinol Baltim Md. 2002;16(8):1767–1777. doi: 10.1210/me.2002-0009. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Cohen RN, Yamada M, Markan KR, Monden T, Satoh T, et al. Cross-talk between TH receptor and liver X receptor regulatory pathways is revealed in a TH resistance mouse model. J Biol Chem. 2006;281(1):295–302. doi: 10.1074/jbc.M507877200. [DOI] [PubMed] [Google Scholar]
- Honda A, Yamashita K, Numazawa M, Ikegami T, Doy M, Matsuzaki Y, et al. Highly sensitive quantification of 7alpha-hydroxy-4-cholesten-3-one in human serum by LC–ESI–MS/MS. J Lipid Res. 2007;48(2):458–464. doi: 10.1194/jlr.D600032-JLR200. [DOI] [PubMed] [Google Scholar]
- Hylemon PB, Gurley EC, Stravitz RT, Litz JS, Pandak WM, Chiang JY, et al. Hormonal regulation of cholesterol 7 alpha-hydroxylase mRNA levels and transcriptional activity in primary rat hepatocyte cultures. J Biol Chem. 1992;267(24):16866–16871. [PubMed] [Google Scholar]
- Jennen D, Magkoufopoulou C, Ketelslegers H, van Herwijnen M, Kleinjans J, et al. Comparison of HepG2 and HepaRG by whole-genome gene expression analysis for the purpose of chemical hazard identification. Toxicol Sci Off J Soc Toxicol. 2010;115(1):66–79. doi: 10.1093/toxsci/kfq026. [DOI] [PubMed] [Google Scholar]
- Johansson L, Rudling M, Scanlan TS, Lundåsen T, Webb P, Baxter J, et al. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc Natl Acad Sci USA. 2005;102(29):10297–10302. doi: 10.1073/pnas.0504379102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Zhang XY, Ying H, Kato Y, Willingham MC, Xu J, et al. Modulation by steroid receptor coactivator-1 of target-tissue responsiveness in resistance to TH. Endocrinology. 2003;144(9):4144–4153. doi: 10.1210/en.2003-0239. [DOI] [PubMed] [Google Scholar]
- Ladenson PW, Kristensen JD, Ridgway EC, Olsson AG, Carlsson B, et al. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N Engl J Med. 2010;362(10):906–916. doi: 10.1056/NEJMoa0905633. [DOI] [PubMed] [Google Scholar]
- Li T, Matozel M, Boehme S, Kong B, Nilsson LM, Guo G, et al. Overexpression of cholesterol 7α-hydroxylase promotes hepatic bile acid synthesis and secretion and maintains cholesterol homeostasis. Hepatol Baltim Md. 2011;53(3):996–1006. doi: 10.1002/hep.24107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JZ, Martagón AJ, Hsueh WA, Baxter JD, Gustafsson JÅ, Webb P, et al. TH receptor agonists reduce serum cholesterol independent of the LDL receptor. Endocrinology. 2012;153(12):6136–6144. doi: 10.1210/en.2011-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JZ, Sieglaff DH, Yuan C, Su J, Arumanayagam AS, Firouzbakht S, et al. Gene specific actions of TH receptor subtypes. PloS One. 2013;8(1):e52407. doi: 10.1371/journal.pone.0052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke JG, Macnaul KL, Hayes NS, Baffic J, Chao YS, Elbrecht A, et al. A novel liver X receptor agonist establishes species differences in the regulation of cholesterol 7alpha-hydroxylase (CYP7a) Endocrinology. 2002;143(7):2548–2558. doi: 10.1210/endo.143.7.8907. [DOI] [PubMed] [Google Scholar]
- Meruvu S, Ayers S, Winnier G, Webb P. TH analogues – where do we stand in 2013? Thyroid Off J Am Thyroid Assoc. 2013 doi: 10.1089/thy.2012.0458. [DOI] [PubMed] [Google Scholar]
- Moore JT, Goodwin B, Willson TM, Kliewer SA. Nuclear receptor regulation of genes involved in bile acid metabolism. Crit Rev Eukaryot Gene Expr. 2002;12(2):119–135. doi: 10.1615/critreveukaryotgeneexpr.v12.i2.30. [DOI] [PubMed] [Google Scholar]
- Ness GC, Pendelton LC, Zhao Z. TH rapidly increases cholesterol 7 alpha-hydroxylase mRNA levels in hypophysectomized rats. Biochim Biophys Acta. 1994;1214(3):229–233. doi: 10.1016/0005-2760(94)90068-x. [DOI] [PubMed] [Google Scholar]
- Ness GC, Lopez D, Chambers CM, Newsome WP, Cornelius P, Long CA, et al. Effects of L-triiodothyronine and the thyromimetic L-94901 on serum lipoprotein levels and hepatic low-density lipoprotein receptor, 3-hydroxy-3-methylglutaryl coenzyme A reductase, and apo A-I gene expression. Biochem Pharmacol. 1998;56(1):121–129. doi: 10.1016/s0006-2952(98)00119-1. [DOI] [PubMed] [Google Scholar]
- Sauter G, Weiss M, Hoermann R. Cholesterol 7 alpha-hydroxylase activity in hypothyroidism and hyperthyroidism in humans. Horm Metab Res Horm Stoffwechselforschung Horm Métab. 1997;29(4):176–179. doi: 10.1055/s-2007-979016. [DOI] [PubMed] [Google Scholar]
- Shin DJ, Plateroti M, Samarut J, Osborne TF. Two uniquely arranged TH response elements in the far upstream 5′ flanking region confer direct TH regulation to the murine cholesterol 7alpha hydroxylase gene. Nucl Acids Res. 2006;34(14):3853–3861. doi: 10.1093/nar/gkl506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Völzke H, Robinson DM, John U. Association between thyroid function and gallstone disease. World J Gastroenterol WJG. 2005;11(35):5530–5534. doi: 10.3748/wjg.v11.i35.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DP, Stroup D, Marrapodi M, Crestani M, Galli G, Chiang JY. Transcriptional regulation of the human cholesterol 7 alpha-hydroxylase gene (CYP7A) in HepG2 cells. J Lipid Res. 1996;37(9):1831–1841. [PubMed] [Google Scholar]
- Yuan C, Lin JZH, Sieglaff DH, Ayers SD, Denoto-Reynolds F, Baxter JD, et al. Identical gene regulation patterns of T3 and selective TH receptor modulator GC-1. Endocrinology. 2012;153(1):501–511. doi: 10.1210/en.2011-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.