Abstract
Mucus presents a formidable barrier to nanoparticle drug-delivery systems, but adding a coating of polymer molecules helps them sneak through the net.
Mucus, the amorphous, sticky substance that often invokes unpleasant images, may not be a favourite topic at the dinner table, but we all rely on it for a healthy life. The mucus barrier is essential in preventing viruses and bacteria from entering our tissues; but this function also poses a perennial problem for drug delivery (Fig. 1). Ying-Ying Wang and colleagues have now demonstrated a method that could help drug-loaded nanoparticles to slip through the mucus barrier1. Their coating of a non-degradable polymer opens opportunities for particles to be used in localized drug-and gene-delivery in mucus-coated areas. This could include treatment of diseases such as cancer and infections in the respiratory and female-reproductive tracts.
Figure 1.
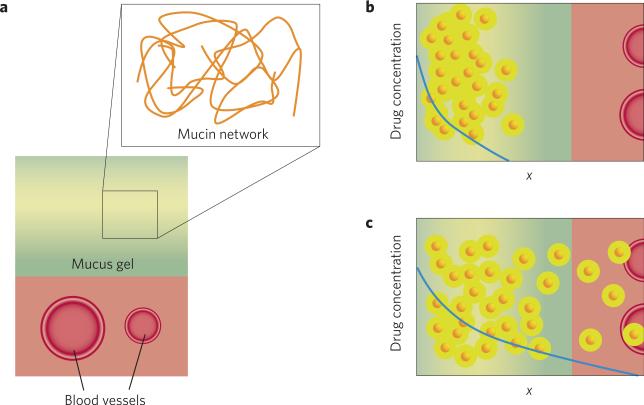
Mucus structure and its role in drug delivery. a, The value of the mucus gel can be appreciated at both the macroscopic and microscopic scales. At the large scale, the mucus gel is viscous enough to trap pathogens, but thin enough to flow along the mucosal surface so that it can be continuously removed, often by shedding. At the smaller scale, the gel is a highly entangled network of long proteins called mucins. The physical characteristics of this porous network, such as pore size, depend on both concentration and state of entanglement of mucins. Furthermore, the mucin fibres can bind with high affinity to some molecules, preventing harmful invaders from reaching the body by immobilizing them onto the scaffold of the mucus gel. The pathogen-laden mucus is then shed from the body and replaced by fresh mucus secreted from the cells beneath. b,c, In the context of drug delivery, drug molecules or carriers that do not diffuse well in mucus gel (b) are less effective at producing a therapeutic effect (c). A drug carrier with fast diffusion in mucus can penetrate this barrier to deliver drugs to cell layers beneath, or access the systemic circulation via blood vessels.
Mucus provides an essential barrier for humans that protects vulnerable surfaces in the lung, intestinal, eye and reproductive tissues from invasion by bacteria, viruses, allergens and irritants. Mucus is home to a wide range of microorganisms that exist in symbiosis with our body. It also acts as a lubricant, making possible everyday functions that we often take for granted, such as blinking and passing food through the digestive tract.
It was long thought that the mucus barrier prevents uptake of large molecules by hindering their ability to diffuse. We now know that most proteins diffuse easily through mucus — at least through human cervicovaginal mucus, which is the most easily collected and studied — because the mesh size of mucin fibres within mucus (~1 μm) is much larger than most proteins2.
Particles the size of viruses have more difficulty in penetrating mucus for two reasons: first, they are too large to easily penetrate through the mucin mesh and, second, they often have adhesive interactions with the mucin fibres3. These properties make mucus a good barrier for viruses, but are frustrating for bioengineers, who would like to produce drug- or gene-loaded particles capable of penetrating mucus to treat disease in underlying cells, or even deeper within the body.
The work of Wang and co-workers provides some clues that may help in the engineering of particles that can penetrate through mucus1. The investigators used poly(ethylene glycol) (PEG), an inert, non-degradable polymer, to coat the surface of negatively charged 220-nm-diameter polystyrene particles that usually diffuse poorly in mucus. The idea of using PEG to modify drugs, proteins and particles is not new: PEG has been conjugated to various drugs and particulate drug carriers to increase their circulation time after intravenous injection4,5; and to enhance the penetration of drugs through brain tissue6. It was not clear, however, that this same concept would work for enhancing the penetration of particles through mucus. In fact, some previous studies have shown that PEG is mucoadhesive, sticking to mucus strands in a way that would inhibit penetration7.
To examine the effect of PEG surface attachment on particle diffusion, Wang and colleagues used a microscopy technique called multiple particle tracking, which can be used to study the movement of a relatively small number of particles in solution. The path travelled by each particle over time is recorded and the mean displacement of particles is fitted to a mathematical model. This allows the investigators to estimate the particle diffusion coefficient — a larger coefficient denotes faster diffusion and, hence, more rapid penetration of particles through the mucus gel. To examine the effect of the addition of PEG to particles on their diffusion rate in cervicovaginal mucus, the authors produced particles coated at high density with short-length PEG molecules. This resulted in the particles’ negative surface charge being neutralized, enabling them to diffuse readily in mucus gel at a rate just ten times slower than in water, an unhindered medium. In contrast, a high-density coating of longer PEG molecules reduced the diffusion ratio by three orders of magnitude. The authors attribute this effect to the longer PEG molecules entangling with mucin fibres in the gel network. Additionally, when the density of PEG coverage on the particle surface was lowered by 40%, the negative charge of the particle surface was less effectively screened, which caused a 700-fold decrease in particle transport rate, due to charge-facilitated binding to mucin fibres.
These results suggest that two factors are responsible for improving particle transport in mucus: a net neutral surface to avoid binding, and a small radius to avoid entanglement with the mucin network (Fig. 2). In fact, this approach mimics the properties of virus particles that are known to infect mucosal tissues efficiently.
Figure 2.
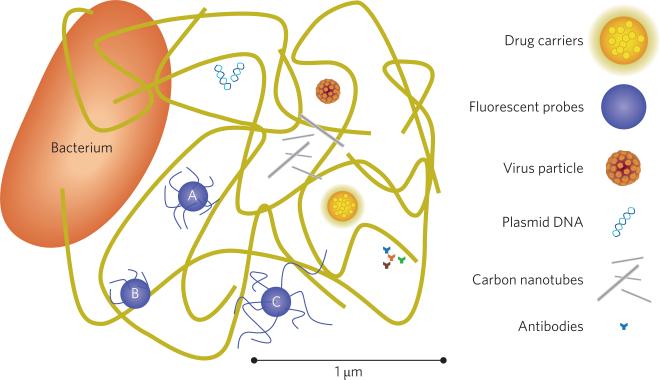
A scaled representation of the mucus gel depicted as a complex, porous network, and some relevant foreign constituents. Large bacteria, which are several micrometres in width and length, do not migrate through the mucus gel but may infect tissues where the mucus surface is disrupted or thin. Antibodies (~10 nm) diffuse readily, as do plasmid DNA; however, these small and potentially therapeutic molecules may be degraded by acidic environments or enzymes in the mucus. Long and narrow carbon nanotubes, and other particulate matter (pollutants) may be present, but are typically sequestered due to entanglement and binding to mucin fibres. Some virus particles, typically <200 nm, can migrate quickly through the mucus gel by reducing their surface interaction with mucin fibres. Fluorescent polystyrene particles that are surface-modified by PEG can mimic this behaviour (A), but still adhere if the particle surface is too sparsely coated (B) or entangle if coated with PEG polymers that are too long (C)1. PEG coatings have also been shown to enhance diffusion of polymer particles that are endowed with the potential to carry drugs through the mucus gel8.
It is important to note that the imaging technique used by the authors involves some manipulation of the mucus gel to introduce the particles. These manipulations may have some impact on particle diffusion, although those effects are likely to be small compared with rather large changes that were measured. More importantly, the nanoparticles studied by Wang et al. are not easily adapted for drug-or gene-delivery purposes. Fortunately, a similar study that supports the findings of Wang et al. has just appeared. This paper shows that the addition of PEG enhances the penetration of biodegradable drug-delivery particles synthesized from poly(lactide-co-glycolide), a Food and Drug Administration approved material8.
We are slowly beginning to gain an appreciation for the biophysical properties of mucus. Engineering a drug-delivery vehicle that can penetrate the mucus barrier offers great promise for increasing efficacy of treatments against diseases of the mucosal surfaces such as cystic fibrosis, and lung and cervical cancers, as well as more potent vaccines for influenza, herpes, human papilloma virus and HIV: important steps towards a healthier life for all.
This approach mimics the properties of virus particles that are known to infect mucosal tissues efficiently.
Biography
Nature versus Naturoid
 PhILIP BALL Can there be metameric devices in the same way that there are metameric colours? The latter are colours that look identical to the eye but have different spectra. Might we make devices that, although made up of different components, perform identically?
PhILIP BALL Can there be metameric devices in the same way that there are metameric colours? The latter are colours that look identical to the eye but have different spectra. Might we make devices that, although made up of different components, perform identically?
Of course we can, you might say. A vacuum tube performs the same function as a semiconductor diode. Clocks can be driven by springs or batteries. But the answer may depend on how much similarity you want. Semiconductor diodes will survive a fall on a hard floor. Battery-operated clocks don't need winding. And what about something considerably more ambitious, such as an artificial heart?
These thoughts are prompted by a recent article by methodologist Massimo Negrotti of the University of Urbino in Italy (Design Issues 24, 26–36; 2008). Negrotti has for several years pondered the concept of what, in science and engineering, is commonly called biomimesis, aiming to develop a general framework for what this entails and what its limitations might be. His vision is informed less by the usual engineering concerns, evident in materials science, of learning from nature and imitating its clever solutions to design problems. Rather, Negrotti wants to develop something akin to a philosophy of the artificial, analogous to (but different from) that expounded by Herbert Simon in his 1969 book The Sciences of the Artificial (MIT Press, Massachusetts).
To this end, Negrotti has coined the term ‘naturoid’ to describe “all devices that are designed with natural objects in mind, by means of materials and building procedures that differ from those that nature adopts.” A naturoid could be a robot, but also a synthetic-polymer-based enzyme, an artificial-intelligence program or even a simulant of a natural odour. This concept was explored in Negrotti's 2002 book Naturoids: On the Nature of the Artificial (World Scientific, New Jersey).
Can one say anything useful about such a broad category? That might remain a matter of taste. But Negrotti's systematic analysis of the issues has the virtue of stripping away some of the illusions and myths attached to attempts to ‘copy nature’.
It won't surprise anyone that these attempts will always fall short of perfect mimicry; indeed, such replication is often explicitly not intended, with biomimetic materials generally imitating just one function of a biological material or structure, such as adhesion or toughness. Negrotti calls this the ‘essential performance’, which itself also implies a selected ‘observation level’ — we might make a comparison solely at the level of bulk mechanical behaviour, irrespective of, say, microstructure or chemical composition.
This inevitably means that the mimicry breaks down at some other observational level, just as colour metamerism can fail depending on the observing conditions (daylight or artificial illumination, say, or different viewing angles).
This reasoning leads Negrotti to conclude that there is no reason to suppose the capacities of naturoids can ever converge on those of the natural models. In particular, the idea that robots and computers will become ever more humanoid in features and function, forecast by some prophets of AI, has no scientific foundation.
References
- 1.Wang YY, et al. Angew. Chem. Int. Ed. 2008;47:9726–9729. doi: 10.1002/anie.200803526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saltzman WM, et al. Biophys J. 1994;66:508–515. doi: 10.1016/s0006-3495(94)80802-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olmsted SS, et al. Biophys J. 2001;81:1930–1937. doi: 10.1016/S0006-3495(01)75844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romberg B, Hennink WE, Storm G. Pharm. Res. 2008;25:55–71. doi: 10.1007/s11095-007-9348-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosqueira VC, et al. Pharm. Res. 2001;18:1411–1419. doi: 10.1023/a:1012248721523. [DOI] [PubMed] [Google Scholar]
- 6.Fleming AB, Haverstick K, Saltzman WM. Bioconjug. Chem. 2004;15:1364–1375. doi: 10.1021/bc034180o. [DOI] [PubMed] [Google Scholar]
- 7.Huang Y, et al. J. Control Release. 2000;65:63–71. doi: 10.1016/s0168-3659(99)00233-3. [DOI] [PubMed] [Google Scholar]
- 8.Cu Y, Saltzman WM. Mol. Pharmaceutics. 2008 doi: 10.1021/mp8001254. doi:10.1021/mp8001254. [DOI] [PMC free article] [PubMed] [Google Scholar]