Abstract
Context
An association between an adjuvanted (AS03) A/H1N1 pandemic vaccine and narcolepsy has been reported in Europe.
Objective
To assess narcolepsy risk following administration of a similar vaccine in Quebec.
Design
Retrospective population-based study.
Setting
Neurologists and lung specialists in the province were invited to report narcolepsy cases to a single reference centre.
Population
Patients were interviewed by two sleep experts and standard diagnostic tests were performed. Immunization status was verified in the provincial pandemic influenza vaccination registry.
Main Outcome Measures
Confirmed narcolepsy with or without cataplexy with onset of excessive daytime sleepiness between January 1st, 2009, and December 31st, 2010. Relative risks (RRs) were calculated using a Poisson model in a cohort analysis, by a self-controlled case series (SCCS) and a case-control method.
Results
A total of 24 cases were included and overall incidence rate was 1.5 per million person-years. A cluster of 7 cases was observed among vaccinated persons in the winter 2009–2010. In the primary cohort analysis, 16-week post-vaccination RR was 4.32 (95% CI: 1.50–11.12). RR was 2.07 (0.70–6.17) in the SCCS, and 1.48 (0.37–7.03) using the case-control method. Estimates were lower when observation was restricted to the period of pandemic influenza circulation, and tended to be higher in persons <20 years old and for cataplexy cases.
Conclusions
Results are compatible with an excess risk of approximately one case per million vaccine doses, mainly in persons less than 20 years of age. However, a confounding effect of the influenza infection cannot be ruled out.
Introduction
Narcolepsy is characterized by the occurrence of excessive daytime sleepiness (EDS), which is usually the most disabling feature of the disease [1]–[3]. EDS is exacerbated when the patient is physically inactive like reading, watching television or driving a car. The other major manifestation of narcolepsy is cataplexy, consisting of sudden drops of muscle tone triggered by emotions such as laughter, anger or surprise. The onset of the disease is usually during adolescence and young adulthood. The manifestations of the disease can be alleviated by medical treatment and their consequences mitigated through adaptation but there is no cure. Narcolepsy is caused by a selective loss of hypocretin-producing cells in the hypothalamus, presumably the consequence of an autoimmune disorder, with a genetic predisposition [4]–[5]. Environmental factors that could trigger the occurrence of narcolepsy include head trauma, stressful life events or infections [6]–[8].
In 2010–2011, an association between the administration of an inactivated adjuvanted (AS03) A/H1N1 pandemic vaccine produced in Dresden, Germany, (Pandemrix, GlaxoSmithKline Biologicals, Wavre, Belgium) and narcolepsy was reported mostly in children in some European countries [9]–[13]. These cases had common features: severe sleepiness and presence of cataplexy, abrupt onset and rapid evolution, presence of the HLA DBQ1*0602 genetic marker, very low CSF hypocretin-1 levels, and positive Multiple Sleep Latency Test (MSLT) test [14]. At the request of the European Medicine Agency, the vaccine manufacturer funded an independent study in the province of Quebec, Canada, where a similar pandemic vaccine produced in Quebec City, Canada, had been used (Arepanrix, GlaxoSmithKline Biologicals, Wavre, Belgium) and where population-based epidemiologic studies had been conducted to assess the risk of other adverse outcomes [15]–[16]. Such study is important as different adjuvants belonging to the same class are likely to be used for other pandemic influenza vaccines in the future.
Methods
Study population and case definition
The study population included all persons 6 months of age or more residing in the province of Quebec (Census data = 7,817,449 as of July 1st, 2009). At the end of the mass immunization campaign, 57% of the target population (≥6 months of age) had been immunized, the vast majority (96%) with one dose of the AS03 vaccine [15]. The primary outcome of interest was narcolepsy with or without cataplexy with onset of EDS during the period of January 1st, 2009 to December 31, 2010.
Identification of cases
In Quebec, patients with complex sleep disorders are usually seen by neurologists or lung specialists in private clinics or hospital outpatient services. When a diagnosis of narcolepsy is suspected, patients are referred to sleep laboratories situated in tertiary care hospitals for special investigations and treatment. The main reference centre is situated at the ‘Hôpital du Sacré-Coeur de Montréal’ (HSCM). Between June 2011 and June 2012, all adult and pediatric neurologists and lung specialists in the province were contacted by letter through their association and invited to report any confirmed or suspected narcolepsy case with onset during the study period. Directors of sleep laboratories, key neurologists and lung specialists were also contacted individually and invited to participate. Records of all patients who consulted for EDS at HSCM were also reviewed. A first screening was performed using available medical records. All patients meeting inclusion criteria (high suspicion of narcolepsy and probable date of onset within study period) were formally invited to participate and to be examined at HSCM (if not already done) for in-depth interview and standard diagnostic tests (details are provided in Table S1). Afterwards, information on immunization status was extracted from the provincial pandemic influenza immunization registry which was established at the time of the mass immunization campaign in 2009. For each person immunized, it contains precise information on the date of vaccine administration and the type of vaccine used. Recruitment extended up to June 30, 2012. The research protocol and the consent procedures were approved by the “Comité d’éthique de la recherche de l’Hôpital du Sacré-Coeur de Montréal”. All participants provided a written consent. The consent of a parent and assent of the patient were requested for those less than 18 years of age.
Classification of cases
All suspected cases were confirmed and classified by two experienced sleep specialists (JM and AD) using all the information available. At this stage, the sleep specialists were blind as to the immunization status of the patient. The criteria proposed by the Brighton Collaboration Narcolepsy Working Group were used to classify narcolepsy cases according to 3 levels of certainty, based on clinical signs and symptoms, hypocretin-1 CSF concentration, and results of daytime MSLT [16]. In cases of discordant opinions, a consensus process was initiated between the two experts.
Determination of the date of disease onset
For each confirmed case, the determination of the most probable date of disease onset (first occurrence of EDS and/or cataplexy symptoms) was established on the basis of the extensive questionnaire completed at the sleep clinic from the interview of the patient, and for children, from an additional interview with a parent. Most importantly, existing medical files were also requested (with patients’ authorization) and reviewed for a date of onset of first symptoms reported at the first medical visit for excessive somnolence and/or cataplexy. For most patients (n = 15), the date of onset of symptoms was established at the first clinical visit. For these patients, the mean delay between the date of onset and the date of the medical visit was 2.9 months. These dates were also corroborated by the detailed interview at the sleep center. For the rest of patients (n = 9), there was no report of a date of symptoms onset at the time of the first medical visit. In these cases, the assessment of the date of onset was determined through the medical interview at study intake. However, these 9 patients had several first medical visits between onset of symptoms and final assessment date which served as indicators of onset date. The final date could be a precise day or a given (usually short) time period (the median day of the period being used in statistical analyses). Confirmed narcolepsy cases with a date of onset clearly outside the 2009–2010 study period were excluded.
Thereafter, each case was classified according to exposure to the vaccine: (1) not vaccinated, (2) vaccinated after disease onset, (3) vaccinated before disease onset and within a time (risk) period compatible with the development of the disease, (4) vaccinated before disease onset but outside the risk period. The third category represents those exposed and the other categories are considered as not exposed. In the primary analysis, the post-vaccination risk period was defined as 16 weeks (112 days), not including the day of vaccine administration, based on series of cases reported in Canada and in Europe [11], [14]. Sensitivity analyses were also performed using 8 weeks (56 days), 24 weeks (168 days) and one year (365 days) risk periods.
Statistical analyses
The primary statistical analyses were performed using Brighton level 1 cases (narcolepsy with cataplexy and hypocretin deficiency), level 2 cases (narcolepsy with cataplexy and specific MSLT abnormalities) and level 3 cases (narcolepsy without cataplexy but with specific MSLT abnormalities). Characteristics of narcolepsy cases were described using absolute numbers, range, median, means and proportions. The Fisher exact test was used to compare proportions and the Wilcoxon rank test for comparing medians. Statistical analyses were performed using SAS 9.3 software (SAS Institute, Cary, NC). Statistical significance was established for p value<0.05 (two-sided tests).
First, the narcolepsy incidence rate in the exposed population was calculated using post-vaccination risk periods as defined above (16 weeks in the primary analysis). Incidence rates in the non-exposed population were calculated by combining the experience of persons not vaccinated and the experience of those vaccinated and observed up to the date of vaccine administration and from the end of the post-vaccination risk period to the end of the study period (with no buffer) in the base analysis [17]. Age- and gender-adjusted rate ratios (RRs) and 95% confidence intervals (95% CI) were estimated using a Poisson model. Attributable risks (per million vaccine doses) were calculated as the number of narcolepsy cases per million vaccine doses observed during a specific risk period and multiplied by (RR−1)/RR.
In order to control for seasonality and circulation of the pandemic virus in 2009, other analyses were performed, curtailing the observation to the period May 1st, 2009 (beginning of the first pandemic wave) to March 31st, 2010 (6 months after the start of the second pandemic wave), and to the period between October 4th, 2009 (beginning of the second pandemic wave) and March 31st, 2010 (6 months after the start of the second pandemic wave). A final analysis was performed, comparing narcolepsy rates among vaccinated persons only (post-vaccination period v.s. pre-vaccination period), among non-vaccinated persons only (post-vaccination period v.s. pre-vaccination period); among vaccinated and non-vaccinated persons during the pre-vaccination period, and among vaccinated and non-vaccinated persons during the pre-vaccination period.
The self-controlled case series (SCCS) method, as described by Whitaker and colleagues [18] was also used for estimating RRs. In this method, the analysis was restricted to narcolepsy patients who had been vaccinated. In the base model, the observation period extended from 6 months (183 days) prior vaccine administration to 6 months after the date of vaccine administration. The risk period was defined as 16 weeks (112 days) following vaccine administration. To take into account the uncertainty in the definition of the risk period, a buffer period (17–24 weeks post-vaccination) was used. Other pre- and post-vaccination observation periods were used as the reference in sensitivity analyses, including or excluding a buffer period (which was then included in the control period).
Finally, a case-control approach was used [19], which is a variant of the ‘case-coverage’ method’ used in the UK study [11]. In this secondary analysis, only cases with onset after the beginning of the mass immunization campaign (October 24, 2009) were included. Using population and immunization registry data, each narcolepsy case was matched to a large number of controls: all persons of the same gender and of the same single-year age-group in the target population. The exposure was vaccinated or not before the day of disease onset of the case. Odds ratios (ORs), which are approximations of rate ratios, and 95% CI were calculated by conditional logistic regression.
Results
Identification of narcolepsy cases
A retrospective review of medical charts of all patients who consulted for somnolence at the sleep clinic of the HSCM between January 1st, 2009 and July 21, 2011 was performed. Of these, 588 cases were excluded because they received another diagnosis (e.g. diagnosis of narcolepsy ruled out clinically or based on PSG results) or because onset of first symptoms was prior to January 1st, 2009 or later than December 31st, 2010. This was determined either by date of referral for somnolence, by the fact that the subject already had a prior visit for the same problem (follow-up visit) or a clear indication by the referring physician that onset of somnolence was outside the target period. Three patients were lost to follow-up and another refused to participate. Out of 33 patients who were retained for a final assessment including standard PSG and MSLT (performed at HSMC in 30 cases), 9 were further excluded as they did not meet Brighton diagnostic criteria. Thus, 24 confirmed narcolepsy cases were retained for statistical analyses (3.8% of the total investigated). Sixteen of these patients had been vaccinated and a precise date of vaccine administration was available for all of them. Details on study patients are presented in Table S2.
Exposure assessment
For 21 out of 24 patients, the date of onset of first symptoms of narcolepsy could be unambiguously assessed (less than one-month apart between the two sleep specialists). The 15th day of the month was arbitrarily selected when the exact date was missing. For the three other patients, establishing a precise date of disease onset was more problematic. One patient reported somnolence as early as September 2008 but this was mild and symptoms became more severe in August 2009 with the onset of cataplexy. This patient was excluded from the primary analyses and included in sensitivity analyses on cataplexy. This patient was vaccinated in November 2009 and was considered as not exposed in all scenarios. For a second patient not vaccinated, EDS occurred in the period November–December 2009. For a third patient, EDS occurred in the period October–November 2009 (probably in early November) and the date of vaccination was November 15, 2009. Arbitrarily, disease onset was assigned on November 1st, 2009 and the case was considered as not exposed in the primary analysis. A sensitivity analysis was performed considering this case as exposed (vaccination before disease onset). The date of vaccination relative to disease onset (before/after) was thus assessed in a non-equivocal way for 15 out of 16 vaccinated patients.
Characteristics of narcolepsy cases
Sixteen out of 24 patients had developed cataplexy (Table 1). This may change in the future as cataplexy symptoms may appear late in the course of the disease. Five patients were classified as Brighton level 1, 11 as level 2, and 8 as level 3. The mean age of the total sample was 24 years, ranging from 6 to 55 years. Although the difference was not statistically significant, exposed cases tended to be younger than non-exposed cases. The male proportion was 50% in the total sample. The proportion of cases positive for the HLA DQB1*0602 marker was 79% (19/24) and this was similar between exposed and non-exposed cases. Interestingly, the HLA marker was present in all of the 16 cases with cataplexy but only in 3 of the 8 cases without cataplexy. The mean interval between the onset of first symptoms and occurrence of cataplexy was 53 days and tended to be shorter for exposed (24 days) than for non-exposed cases (72 days). The average time interval between the onset of first symptoms and the first medical visit was 149 days, shorter for exposed (66 days) than for non-exposed cases (191 days).
Table 1. Distribution of 24 narcolepsy cases according to exposure status and patients’ characteristics.
| Patient’s characteristics Mean ± SD/median/range | All cases (n = 24) | Not vaccinated or vaccinated after disease onset (n = 16) | Vaccinated before disease onset (n = 8) | p value* |
| Age (years) | 24.0±14.3 | 26.1±13.1 | 19.9±16.6 | 0.20 |
| 18.1 | 28.2 | 13.7 | ||
| 6–55 | 8–55 | 6–50 | ||
| Age group | ||||
| <20 years of age | 13 (54%) | 7 (44%) | 6 (75%) | 0.21 |
| ≥20 years of age | 11 (46%) | 9 (56%) | 2 (25%) | |
| Male proportion | 12 (50%) | 10 (62.5%) | 2 (25%) | 0.19 |
| Cases with cataplexy | 16 (67%) | 10 (63%) | 6 (75%) | 0.67 |
| Cases with hypnagogic hallucinations | 14/23 (61%) | 9 (56%) | 5/7 (71%) | 0.66 |
| Cases with sleep paralysis | 10 (42%) | 7 (44%) | 3 (38%) | 1.00 |
| Cases with all signs and symptoms | 5 (21%) | 3 (19%) | 2 (25%) | 1.00 |
| Diagnostic certainty | ||||
| Brighton level 1 | 4 (17%) | 2 (12.5%) | 2 (25%) | 0.73 |
| Brighton level 2 | 12 (50%) | 8 (50%) | 4 (50%) | |
| Brighton level 3 | 8 (33%) | 6 (38%) | 2 (25%) | |
| HLA DQB1*0602 positive | 19/24 (79%) | 12/16 (75%) | 7/8 (88%) | 0.63 |
| Interval between onset first sleep | 53.3±93.1 | 72.3±116.9 | 24.8±27.5 | 1.00 |
| symptoms and onset of cataplexy (days) | 21 | 0 | 23 | |
| 0–330 | 0–330 | 0–91 | ||
| Interval between disease onset and first | 149.1±187.8 | 190.9±218.9 | 65.5±35.7 | 0.42 |
| medical visit (days) | 70.5 | 77.5 | 52.5 | |
| 3–799 | 3–799 | 32–124 | ||
| Interval between disease onset and | 427.8±244.52 | 482.1±239.3 | 319.3±231.4 | 0.15 |
| confirmatory laboratory investigations | 414 | 422 | 246 | |
| (days) | 79–903 | 170–903 | 79–756 |
*Fisher exact test for proportions and Wilcoxon rank test for medians.
Distribution of cases over time
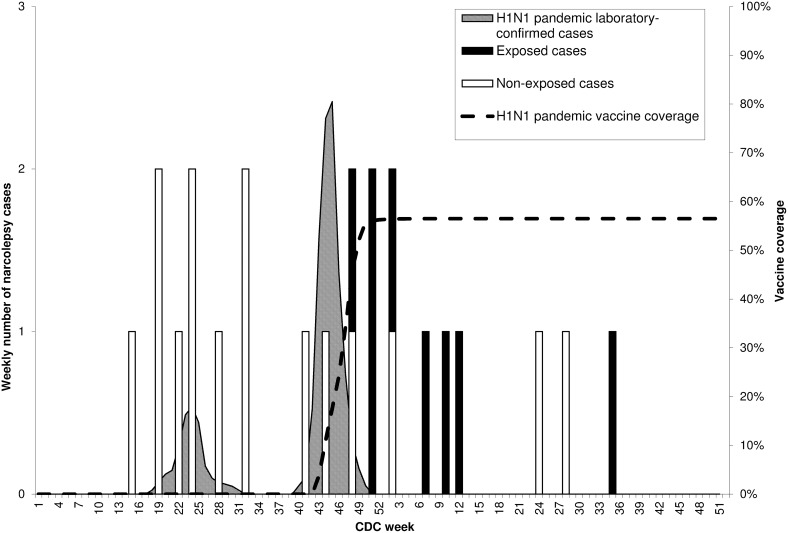
No case was reported with disease onset before week 15, 2009 or after week 35, 2010 (Figure 1). No seasonal pattern (12-month cycle) was observed, but cases occurred in clusters. A first cluster of narcolepsy cases was observed in the spring-summer of 2009, at the time of the first A/H1N1 influenza pandemic wave. A second cluster was observed in the winter 2009–2010, after the second pandemic wave and the mass immunization campaign. In the second cluster, 7 of the 11 cases were patients who developed symptoms shortly after vaccine administration. These seven cases, considered as exposed, included mainly persons less than 20 years of age (5/7), for whom the onset of narcolepsy was abrupt and cataplexy rapidly developed (6/7).
Figure 1. Distribution of dates of onset of 23 narcolepsy cases according to CDC week, in province of the Quebec, 2009–2010.
Risk estimates using the cohort method
The average incidence rate in the study population including 23 narcolepsy cases with onset in 2009–2010 was 1.5 per million person-years (p-y), 2.0 in 2009 and 1.0 in 2010.
In the base cohort analysis, narcolepsy incidence in vaccinated persons during the 16-week period following vaccine administration was 0.52/100,000 p-y and the rate was 0.11/100,000 p-y in the group not exposed, for an age- and gender-adjusted RR of 4.58 (95% CI: 1.59 to 11.77). The absolute attributable risk estimate was close to 1 case per million vaccine doses (Table 2). When the post-vaccination risk period was extended to 365 days post-vaccination or to the end of the study period (December 31st, 2010), risk estimates were lower and no longer significant. On the contrary, when the post-vaccination risk period was shortened (8 weeks), RRs were of the same magnitude than in the base model and statistically significant. When the observation period was restricted to the period of circulation of the pandemic virus (1st and 2nd wave or 2nd wave only), RRs were comprised between 2 and 4 and were not significant.
Table 2. Risk of narcolepsy ± narcolepsy associated with A/H1N1 (2009) vaccination using the cohort method according to observation period and post-vaccination risk period, in Quebec, 2009–2010.
| Observation period | Risk period from dateof vaccination to: | No cases | Incidence rate | Age- and gender-adjusted risk ratio | Attributable cases/million doses(95% confidence intervals) | |||||||
| E+ | E− | Total | E+ | E− | Total | RR | ICinf | ICsup | P-value | |||
| January 1st 2009, toDecember 31, 2010 | End study periodDecember 31st, 2010 | 8 | 15 | 23 | 0.164 | 0.253 | 0.148 | 1.06 | 39.00 | 2.68 | 1.000 | 0.25 [−2.83 to 1.13] |
| One year (365 days)post-vaccination | 8 | 15 | 23 | 0.181 | 0.464 | 0.148 | 1.24 | 0.45 | 3.13 | 0.650 | 0.46 [−2.21 to 1.23] | |
| 24 weeks (168 days)post-vaccination | 7 | 16 | 23 | 0.344 | 1.039 | 0.148 | 2.73 | 0.95 | 7.04 | 0.063 | 1.04 [−0.08 to 1.36] | |
| 16 weeks (112 days)post-vaccination* | 7 | 16 | 23 | 0.516 | 1.237 | 0.148 | 4.32 | 1.50 | 11.12 | 0.007 | 1.24 [0.53 to 1.44] | |
| 8 weeks (56 days)post-vaccination | 4 | 19 | 23 | 0.590 | 0.709 | 0.148 | 4.36 | 1.08 | 13.15 | 0.039 | 0.71 [0.07 to 0.84] | |
| May 1st 2009, toMarch 31 2010 | End study periodDecember 31st, 2010 | 7 | 12 | 19 | 0.448 | 0.822 | 0.266 | 1.91 | 0.63 | 5.28 | 0.272 | 0.82 [−0.93 to 1.28] |
| One year (365 days)post-vaccination | 7 | 12 | 19 | 0.448 | 0.822 | 0.266 | 1.91 | 0.63 | 5.28 | 0.272 | 0.82 [−0.93 to 1.28] | |
| 24 weeks (168 days)post-vaccination | 7 | 12 | 19 | 0.448 | 0.822 | 0.266 | 1.91 | 0.63 | 5.28 | 0.272 | 0.82 [−0.93 to 1.28] | |
| 16 weeks (112 days)post-vaccination | 7 | 12 | 19 | 0.516 | 0.947 | 0.266 | 2.33 | 0.77 | 6.43 | 0.138 | 0.95 [−0.47 to 1.34] | |
| 8 weeks (56 days)post-vaccination | 4 | 15 | 19 | 0.590 | 0.549 | 0.266 | 2.39 | 0.58 | 7.52 | 0.233 | 0.55 [−0.66 to 0.78] | |
| October 4 2009, toMarch 31 2010 | End study periodDecember 31st, 2010 | 7 | 3 | 10 | 0.448 | 0.133 | 0.262 | 2.73 | 0.62 | 16.45 | 0.234 | 1.11 [−0.97 to 1.49] |
| One year (365 days)post-vaccination | 7 | 3 | 10 | 0.448 | 0.133 | 0.262 | 2.73 | 0.62 | 16.45 | 0.234 | 1.11 [−0.97 to 1.49] | |
| 24 weeks (168 days)post-vaccination | 7 | 3 | 10 | 0.448 | 0.133 | 0.262 | 2.73 | 0.62 | 16.45 | 0.234 | 1.11 [−0.97 to 1.49] | |
| 16 weeks (112 days)post-vaccination | 7 | 3 | 10 | 0.516 | 0.122 | 0.262 | 3.63 | 0.82 | 21.82 | 0.098 | 1.21 [−0.35 to 1.51] | |
| 8 weeks (56 days)post-vaccination | 4 | 6 | 10 | 0.590 | 0.191 | 0.262 | 2.73 | 0.57 | 11.55 | 0.229 | 0.61 [−0.68 to 0.83] | |
+: Cases with onset after vaccination during risk period; E−: Cases not vaccinated or with onset before vaccination or after end of risk period.
*Primary analysis defined a priori.
Risk estimates were higher in persons less than 20 years of age (5 cases exposed v.s. 7 cases not exposed in base analysis; RR = 6.39; 95% CI: 1.60 to 23.38), than in those 20 years of age or older (2 cases exposed v.s. 9 cases not exposed in base analysis; RR = 2.44; 95% CI: 0.26 to 11.80) (details in Tables S3−S4).
When the date of first medical visit was used to define disease onset instead of that of EDS occurrence, the total number of cases retained in the base-case analysis was 20 (3 patients had their first visit in 2011), including 3 exposed and 17 considered as not exposed, for a RR of 1.73 (95% CI: 0.32 TO 5.99) (details of sensitivity analyses are in Tables S5−S9).
Risk estimates using the self-controlled case-series method
In the base analysis including a post-vaccination buffer period, RR during the 16-week post-vaccination period was 2.07 (95% CI: 0.70 to 6.17). RR was 2.96 (95% CI: 0.71 to 12.39) among persons less than 20 years of age and was 1.18 (95% CI: 0.20 to 7.09) in those older. RRs tended to be higher when the analysis was restricted to Brighton levels 1–2 cases and when the buffer period was excluded (Table 3).
Table 3. Risk of narcolepsy ± narcolepsy associated with A/H1N1 (2009) vaccination using the ‘self-controlled case-series method’ according to age and diagnostic classification, in the province of Quebec, 2009–2010.
| Observation period | Post-vaccinationrisk period | Post-vaccinationbuffer period | Brighton level | No of casesin analysis | Relative risk | 95% confidenceinterval | Attributable risk per million doses (95% confidence intervals) |
| All ages | |||||||
| 6 months (183 days) beforevaccination to 6 monthsafter vaccination | 16 weeks (112 days) | Weeks 17–24(days 113–168) | 1–3 | 13 | 2.07 | 0.70 to 6.17 | 0.82 [−068 to 1.32] |
| 1–2 | 9 | 3.55 | 0.89 to 14.21 | 0.97 [−0.17 to 1.26] | |||
| No buffer | 1–3 | 13 | 2.66 | 0.89 to 7.90 | 0.99 [−0.19 to 1.38] | ||
| 1–2 | 9 | 4.55 | 1.14 to 18.21 | 1.06 [0.17 to 1.28] | |||
| Persons 6 months to <20 years of age | |||||||
| 6 months (183 days) beforevaccination to 6 monthsafter vaccination | 16 weeks (112 days) | Weeks 17–24(days 113–168) | 1–3 | 8 | 2.96 | 0.71 to 12.39 | 2.98 [−1.84 to 4.14] |
| 1–2 | 5 | 7.10 | 0.79 to 63.59 | 3.09 [−0.96 to 3.54] | |||
| No buffer | 1–3 | 8 | 3.79 | 0.90 to 15.89 | 3.31 [−0.50 to 4.22] | ||
| 1–2 | 5 | 9.11 | 1.02 to 81.48 | 3.21 [0.07 to 3.56] | |||
| Persons ≥20 years of age | |||||||
| 6 months (183 days) beforevaccination to 6 monthsafter vaccination | 16 weeks (112 days) | Weeks 17–24(days 113–168) | 1–3 | 5 | 1.18 | 1.19 to 7.09 | 0.09 [−2.58 to 0.52] |
| 1–2 | 4 | 1.78 | 0.25 to 12.61 | 0.26 [−1.81 to 0.56] | |||
| No buffer | 1–3 | 5 | 1.52 | 0.25 to 9.08 | 0.21 [−1.81 to 0.54] | ||
| 1–2 | 4 | 2.27 | 0.32 to 16.16 | 0.34 [−1.28 to 0.57] | |||
Using the whole observation period (January 1st, 2009 to December 31st, 2010) and the 16-week post-vaccination reference period as in the base cohort analysis with no buffer, the all-age RR for Brighton levels 1–3 was 4.82 (95% CI: 1.75 to 13.31).
Risk estimates using the case-control method
This analysis was restricted to 13 narcolepsy cases with onset on or later than October 24, 2009, the first day of the mass immunization campaign. There were 8 cases with disease onset after vaccine administration and 5 unvaccinated cases, for an OR of 1.48 (95% CI: 0.37 to 7.03). OR was 3.21 (95% CI: 0.37 to 90.37) among persons less than 20 years of age (6 exposed v.s. 2 not exposed) and was 0.73 (95% CI: 0.06 to 6.70) among adults (2 exposed v.s. 3 not exposed).
Discussion
The incidence rate of narcolepsy recorded in Quebec, in 2009–2010, (1.5 per million-p-y) was very low compared with the rate (13.7 per million p-y) reported in a previous study in Olmsted County, Minnesota, in 1960–1989 [20]. In Europe, the incidence rates reported in 6 countries in 2000–2010, ranged from a minimum of 1.9 to a maximum of 14.2 per million p-y [21]. In our study, very strict diagnostic criteria were used to define narcolepsy cases and a majority of patients with suspected diagnosis were referred to a single sleep laboratory serving as a reference centre for the province. At the end of a very intense recruitment process, only 3.8% of the total cases investigated were retained. In a retrospective survey in the United-Kingdom, the median delay between the onset of first symptoms and the confirmation of diagnosis was 10.5 years, but this was much shorter in recent years and for cases with cataplexy [22]. In Quebec, the accessibility of primary care services is not great for patients with mild conditions but this should not be the case for young persons with severe symptoms affecting their performance at school or at work. The incidence of narcolepsy in a given population may be influenced by genetic factors [1], [2]. This is not a likely explanation for the low incidence rate reported in our study. Actually, the prevalence of the HLA-DR2 allele, a close marker to the HLA DQB1*0602 predisposing trait [23] has been estimated to 22% in the Quebec population [24], similar to the 21% reported in Caucasian Americans [25], [26].
In our study, the date of onset of EDS was selected to define exposure for all main statistical analyses. The interval between disease onset and first medical visit exceeded two months for most of our patients (median 71 days) and this interval was shorter in exposed cases. Using the date of first medical visit instead of the date of first symptoms introduced a misclassification bias and diluted any effect of vaccination. The problem was even worse using the date of confirmation of diagnosis. The date of first symptoms may be less precise than other points of reference and some recall bias cannot be excluded. In Quebec, however, media attention to the possible association between the pandemic vaccine and narcolepsy was minimal up to the end of 2012, as shown in a survey we performed recently (data not shown).
There was no obvious seasonal pattern in the incidence of narcolepsy in Quebec during the two-year study period. A first cluster of 9 cases was observed in April−August 2009, concomitantly with the first influenza pandemic wave which occurred in May−June 2009. The circulation of seasonal influenza viruses during the winters 2008–2009 and 2009–2010 was unusually low as compared with previous seasons [27]. The second influenza pandemic wave was observed in October−November 2009, with the peak incidence three weeks before the peak of the mass immunization campaign, generating an association which is difficult to disentangle. A temporal association between seasonal and pandemic influenza has been reported in China [28], [29], but not in a less rigorous study in South-Korea [30]. In the second cluster observed in Quebec in the period of October 2009 to March 2010, 7 of the 11 cases were patients who developed symptoms less than 16 weeks after pandemic vaccine administration. These seven cases rapidly developed narcolepsy, with the presence of cataplexy (6/7). Persons less than 20 years of age were over-represented in this sample (5/7). In our study, no information was retrospectively collected on influenza-like symptoms and medical visits for acute infection, and this is a limitation. In a context of wait times in vaccination clinics during the mass campaign, it is reasonable to assume that people who developed an influenza–like illness did not rush to get a vaccine as soon as they recovered. Also, people who received one dose of adjuvanted pandemic vaccine were rapidly protected [31], [32]. It is thus very unlikely that the rate of pandemic influenza infection would have been much higher in the vaccinated portion than in the unvaccinated portion of the population. Also, the 2009–2010 seasonal influenza vaccination campaign in Quebec was postponed and started late in January 2010 with low uptake [15] and this cannot explain the second cluster. In a recent study in Finland [33], no serologic evidence of a contribution of the A/H1N1 (2009) virus infection in the epidemic of childhood narcolepsy could be found. In this study, antibody reactivity to a NS1 polymorphism specific of wild type A/H1N1 (2009) but not present in the vaccine was used to test this hypothesis.
A priori, a cohort approach was selected for the base-case analysis as in a previous study on Guillain-Barré Syndrome [17], and the choice of a 16-week post-vaccination risk window, based on series of cases reported in Canada and in Ireland [11], [14]. This is the most natural and statistically powerful approach as risk estimates are based on all persons in the study population observed during the whole study period. Two other methods were used: the SCCS method as described by Whitaker and colleagues [18], which allows for the control of all permanent characteristics of patients, and a traditional matched case-control analysis nested in a cohort, which allows for the control of any seasonal or other cyclic effect [19]. An excess post-vaccination risk was measured in almost all analyses although its magnitude and statistical significance were influenced both by the choice of the risk window and of the reference period.
Our results showing an increased narcolepsy risk in vaccinated individuals less than 20 years of age are congruent with those of studies on a similar AS03 adjuvanted pandemic vaccine in Europe although the magnitude of the reported excess risk was much higher in Norway (90 per million) [34], Finland (63 per million) [9], in Sweden (36 per million) [35], in Ireland (53 per million) [11], and in England (18 per million) [13]. The origin of this difference is not known. Differences in the production process and composition of the two vaccines are a possible hypothesis [36]–[38]. Underascertainment of narcolepsy cases in our study could also bias to some extend attributable risk estimates.
Conclusions
We can cautiously conclude that our results are consistent with a risk of narcolepsy of small magnitude (approximately one case per million dose) following administration of the adjuvanted (AS03) A/H1N1 pandemic vaccine manufactured in Quebec, and that this risk occurred preferentially in people under 20 years of age with a short latency (≤16 weeks between vaccine administration and disease onset) and with the presence of cataplexy. However, given the small numbers, the impossibility to completely exclude a confounding effect of A/H1N1 (2009) virus infection and/or a reporting bias, our results are to be considered as weak evidence of a causal relationship. We can, however, reasonably exclude the existence of an excess risk of much higher magnitude as reported in some European countries in which Pandemrix had been used. Further studies comparing influenza and autoimmune responses to Pandemrix versus Arepanrix will be needed to shed light on these differences.
Supporting Information
Standard diagnostic tests performed at HSCM Sleep Disorders Center.
(DOCX)
List of 24 narcolepsy cases included in final analysis, province of Quebec, 2009–2010.
(DOCX)
Risk of narcolepsy associated with A/H1N1 (2009) vaccination using the cohort method according to observation period and post-vaccination risk period in persons less than 20 years of age.
(DOCX)
Risk of narcolepsy associated with A/H1N1 (2009) vaccination using the cohort method according to observation period and post-vaccination risk period in persons ≥20 years of age.
(DOCX)
Sensitivity analysis in cohort approach: Risk of narcolepsy associated with A/H1N1 vaccination using date of onset of narcolepsy, according to observation period and post-vaccination risk period, and including one case (#009) with date of onset of sleep disorders in 2008 but onset of cataplexy in 2009 which is now considered as the date of disease onset.
(DOCX)
Sensitivity analysis in cohort approach: Risk of narcolepsy associated with A/H1N1 vaccination using date of onset of narcolepsy, according to observation period and post-vaccination risk period, and including one case (#020) with uncertain date of onset which is now considered as exposed.
(DOCX)
Sensitivity analysis in cohort approach: Risk of narcolepsy associated with A/H1N1 vaccination using date of onset of cataplexy, according to observation period and post-vaccination risk period, and excluding a case (#011) with uncertain date of onset of cataplexy.
(DOCX)
Sensitivity analysis in cohort approach: Risk of narcolepsy associated with A/H1N1 vaccination using date of first medical visit, according to observation period and post-vaccination risk period.
(DOCX)
Risk of narcolepsy using the cohort method according to vaccination status and risk periods defined around the date of vaccination (for non-vaccinated persons a pseudo date was assigned: the median date of vaccination among vaccinated persons of same age and gender).
(DOCX)
Acknowledgments
The authors thank all the health professionals in Quebec who participated in the data collection. Catherine Cohet from GlaxoSmithKline Biologicals had an important liaison role in the conduct of the study. Nicole Boulianne from the Quebec National Public Health Institute (‘Institut national de santé publique du Québec’) provided assistance to obtain information from the pandemic influenza immunization registry.
Funding Statement
This study was funded by GlaxoSmithKline Biologicals. The sponsor had a role in the design of the study and provided comments on the first draft of the manuscript. It has, however, no role in the conduct of the study, collection, management, analysis of data, and preparation and approval of the final version of the manuscript.
References
- 1. Dauvilliers Y, Arnulf I, Mignot E (2007) Narcolepsy with cataplexy. Lancet 369(9560): 499–511. [DOI] [PubMed] [Google Scholar]
- 2. Burgess CR, Scammell TE (2012) Narcolepsy: neural mechanisms of sleepiness and cataplexy. J Neurosci 32(36): 12305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Longstreth WT Jr, Koepsell TD, Ton TG, Hendrickson AF, van Belle G (2007) The epidemiology of narcolepsy. Sleep 30(1): 13–26. [DOI] [PubMed] [Google Scholar]
- 4. Mignot E, Tafti M, Dement WC, Grumet FC (1995) Narcolepsy and immunity. Adv Neuroimmunol 5(1): 23–37. [DOI] [PubMed] [Google Scholar]
- 5. Singh AK, Mahlios J, Mignot E (2013) Genetic association, seasonal infections and autoimmune basis of narcolepsy. J Autoimmun 43: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Orellana C, Villemin E, Tafti M, Carlander B, Besset A, et al. (1994) Life events in the year preceding the onset of narcolepsy. Sleep 17 Suppl8: S50–3. [DOI] [PubMed] [Google Scholar]
- 7. Lankford DA, Wellman JJ, O’Hara C (1994) Posttraumatic narcolepsy in mild to moderate closed head injury. Sleep 17 Suppl8: S25–8. [DOI] [PubMed] [Google Scholar]
- 8. Picchioni D, Hope CR, Harsh JR (2007) A case-control study of the environmental risk factors for narcolepsy. Neuroepidemiology 29(3–4): 185–192. [DOI] [PubMed] [Google Scholar]
- 9. Nohynek H, Jokinen J, Partinen M, Vaarala O, Kirjavainen T, et al. (2012) AS03 adjuvanted AH1N1 vaccine associated with an abrupt increase in the incidence of childhood narcolepsy in Finland. PLoS One 7(3): e33536 10.1371/journal.pone.0033536. Accessed 2014 Sept 1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Partinen M, Saarenpaa-Heikkila O, Ilveskoski I, Hublin C, Linna M, et al. (2012) Increased incidence and clinical picture of childhood narcolepsy following the 2009 H1N1 pandemic vaccination campaign in Finland. PLoS One 7(3): e33723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Narcolepsy Study Steering Committee (2012) Investigation of an increase in the incidence of narcolepsy in children and adolescents in 2009 and 2010. Dublin, Ireland: Department of Health and Children. Available: http://www.dohc.ie/publications/pdf/Final_Report_of_National_Narcolepsy_Study_Steering_Committee.pdf?direct=1. Accessed 2014 Sept 1. [Google Scholar]
- 12.ECDC Technical Report (2012) Narcolepsy in association with pandemic influenza vaccination. Stockholm: European Centre for Disease Prevention and Control. Available: http://www.ecdc.europa.eu/en/publications/Publications/Vaesco%20in%20brief%20final.pdf. Accessed 2014 Sept 1.
- 13. Miller E, Andrews N, Stellitano L, Stowe J, Winstone AM, et al. (2013) Risk of narcolepsy in children and young people receiving AS03 adjuvanted pandemic A/H1N1 2009 influenza vaccine: retrospective analysis. BMJ 346: f794 10.1136/bmj.f794 [DOI] [PubMed] [Google Scholar]
- 14. Dauvilliers Y, Montplaisir J, Cochen V, Desautels A, Einen M, et al. (2010) Post-H1N1 narcolepsy-cataplexy. Sleep 33(11): 1428–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ministère de la Santé et des Services sociaux du Québec (MSSS) (2011) Bilan de la direction générale de la santé publique de la vaccination contre la grippe pandémique A(H1N1) 2009. Du 22 octobre au 18 décembre 2009. Québec: Direction de la protection de la santé publique, Ministère de la Santé et des Services sociaux du Québec, 2011. Available: http://publications.msss.gouv.qc.ca/acrobat/f/documentation/2011/11-220-02.pdf. Accessed 2014 Sept 1.
- 16. Poli F, Overeem S, Lammers GJ, Plazzi G, Lecendreux M, et al. (2013) Narcolepsy as an adverse event following immunization: Case definition and guidelines for data collection, analysis and presentation. Vaccine 31(6): 994–1007. [DOI] [PubMed] [Google Scholar]
- 17. De Wals P, Deceuninck G, Toth E, Boulianne N, Brunet D, et al. (2012) Risk of Guillain-Barré Syndrome following H1N1 influenza vaccination in Quebec. JAMA 308(2): 175–81. [DOI] [PubMed] [Google Scholar]
- 18. Whitaker HJ, Farrington CP, Spiessens B, Musonda P (2006) Tutorial in biostatistics: The self-controlled case series method. Stat Med 25(10): 1768–97. [DOI] [PubMed] [Google Scholar]
- 19. Comstock GW (1994) Evaluating vaccination effectiveness and vaccine efficacy by means of case-control studies. Epidemiol Rev 16(1): 77–89. [DOI] [PubMed] [Google Scholar]
- 20. Silber MH, Krahn LE, Olson EJ, Pankratz VS (2002) The epidemiology of narcolepsy in Olmsted County, Minnesota: a population-based study. Sleep 25(2): 197–202. [DOI] [PubMed] [Google Scholar]
- 21. Wijnans L, Lecomte C, de Vries C, Weibel D, Sammon C, et al. (2013) The incidence of narcolepsy in Europe: before, during, and after the influenza A(H1N1) pdm09 pandemic and vaccination campaigns. Vaccine 31(8): 1246–54. [DOI] [PubMed] [Google Scholar]
- 22. Morrish E, King MA, Smith IE, Shneerson JM (2004) Factors associated with a delay in the diagnosis of narcolepsy. Sleep Medicine 5(1): 37–41. [DOI] [PubMed] [Google Scholar]
- 23. Mignot E, Lin X, Kalil J, George C, Singh S, et al. (1992) DQB1-0602 (DQw1) is not present in most nonDR2 Caucasian narcoleptics. Sleep 15(5): 415–22. [DOI] [PubMed] [Google Scholar]
- 24. Poirier G, Montplaisir J, Décary F, Momège D, Lebrun A (1986) HLA antigens in narcolepsy and idiopathic central nervous system hypersomnolence. Sleep 9(1 Pt 2): 153–8. [DOI] [PubMed] [Google Scholar]
- 25. Mignot E, Hayduk R, Black J, Grumet FC, Guilleminault C (1997) HLA DQB1*0602 is associated with cataplexy in 509 narcoleptic patients. Sleep 20(11): 1012–20. [PubMed] [Google Scholar]
- 26. Mignot E, Kimura A, Lattermann A, Lin X, Yasunaga S, et al. (1997) Extensive HLA class II studies in 58 non-DRB1*15 (DR2) narcoleptic patients with cataplexy. Tissue Antigens 49(4): 329–41. [DOI] [PubMed] [Google Scholar]
- 27.Institut national de santé publique du Québec (INSPQ) (2010) Bilan épidémiologique de la pandémie d’influenza A(H1N1). Province de Québec–2009. Québec: Direction des risques biologiques et de la santé au travail, Institut national de santé publique du Québec, 2010. Available: http://www.inspq.qc.ca/pdf/publications/1212_BilanAH1N12009.pdf. Accessed 2014 Sept 1.
- 28. Han F, Lin L, Li J, Dong XS, Mignot E (2013) Decreased incidence of childhood narcolepsy 2 years after the 2009 H1N1 winter flu pandemic. Ann Neurol 73(4): 560 10.1002/ana.23799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han F, Lin L, Warby SC, Faraco J, Li J, et al. Narcolepsy onset is seasonal and increased following the 2009 H1N1 pandemic in China. Ann Neurol 70(3): 410–7. [DOI] [PubMed] [Google Scholar]
- 30. Choe YJ, Bae GR, Lee DH (2012) No association between influenza A (H1N1) pdm09 vaccination and narcolepsy in South Korea: An ecological study. Vaccine 30(52): 7439–42. [DOI] [PubMed] [Google Scholar]
- 31. Skowronski DM, Janjua NZ, De Serres G, Hottes TS, Dickinson JA, et al. (2011) Effectiveness of AS03 adjuvanted pandemic H1N1 vaccine: case-control evaluation based on sentinel surveillance system in Canada, autumn 2009. BMJ 342: c7297 10.1136/bmj.c7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Buynder PG, Dhaliwal JK, Van Buynder JL, Couturier C, Minville-Leblanc M, et al. (2010) Protective effect of single-dose adjuvanted pandemic influenza vaccine in children. Influenza Other Respir Viruses 4(4): 171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Melèn K, Partinen M, Tynell J, Sillanpää M, Himanen SL, et al. (2013) No serological evidence of influenza A H1N1pdm09 virus infection as a contributing factor in childhood narcolepsy after Pandemrix vaccination campaign in Finland. PLoS ONE 8(8): e68402 10.1371/journal.pone.0068402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heier MS, Gautvik KM, Wannag E, Bronder KH, Midtlyng E, et al. (2013) Incidence of narcolepsy in Norwegian children and adolescents after vaccination against H1N1 influenza A. Sleep Medicine. 14: 867–71. [DOI] [PubMed] [Google Scholar]
- 35.Medical Product Agency (2011) Occurrence of narcolepsy with cataplexy among children and adolescents in relation to the H1N1 pandemic and Pandemrix vaccinations - Results of a case inventory study by the MPA in Sweden during 2009–2010. Stockholm, Sweden: Läkemedelsverket. Available: http://www.lakemedelsverket.se/upload/nyheter/2011/Fallinventeringsrapport_pandermrix_110630.pdf. Accessed 2014 Sept 1.
- 36.European Medicine Agency (2013) Pandemrix: EPAR-Product Information Annex I: Summary of product characteristics. London (UK): European Medicine Agency. Available: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000832/WC500038121.pdf. Accessed 2014 Sept 1.
- 37.GlaxoSmithKline Inc (2009) Product Information Leaflet Arepanrix H1N1 AS03-Adjuvanted H1N1 Pandemic Influenza Vaccine - Version 4 approved 20 April 2010. Ottawa: Health Canada, 2010. Available: http://www.hc-sc.gc.ca/dhp-mps/alt_formats/pdf/prodpharma/legislation/interimorders-arretesurgence/prodinfo-vaccin-eng.pdf. Accessed 2014 Sept 1. [Google Scholar]
- 38. Ahmed SS, Schur PH, MacDonald NE, Steinman L (2014) Narcolepsy 2009 A(H1N1) pandemic influenza, and pandemic influenza vaccinations: What is known and unknown about the neurological disorder, the role of autoimmunity, and vaccine adjuvants. J Autoimmunity 50: 1–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Standard diagnostic tests performed at HSCM Sleep Disorders Center.
(DOCX)
List of 24 narcolepsy cases included in final analysis, province of Quebec, 2009–2010.
(DOCX)
Risk of narcolepsy associated with A/H1N1 (2009) vaccination using the cohort method according to observation period and post-vaccination risk period in persons less than 20 years of age.
(DOCX)
Risk of narcolepsy associated with A/H1N1 (2009) vaccination using the cohort method according to observation period and post-vaccination risk period in persons ≥20 years of age.
(DOCX)
Sensitivity analysis in cohort approach: Risk of narcolepsy associated with A/H1N1 vaccination using date of onset of narcolepsy, according to observation period and post-vaccination risk period, and including one case (#009) with date of onset of sleep disorders in 2008 but onset of cataplexy in 2009 which is now considered as the date of disease onset.
(DOCX)
Sensitivity analysis in cohort approach: Risk of narcolepsy associated with A/H1N1 vaccination using date of onset of narcolepsy, according to observation period and post-vaccination risk period, and including one case (#020) with uncertain date of onset which is now considered as exposed.
(DOCX)
Sensitivity analysis in cohort approach: Risk of narcolepsy associated with A/H1N1 vaccination using date of onset of cataplexy, according to observation period and post-vaccination risk period, and excluding a case (#011) with uncertain date of onset of cataplexy.
(DOCX)
Sensitivity analysis in cohort approach: Risk of narcolepsy associated with A/H1N1 vaccination using date of first medical visit, according to observation period and post-vaccination risk period.
(DOCX)
Risk of narcolepsy using the cohort method according to vaccination status and risk periods defined around the date of vaccination (for non-vaccinated persons a pseudo date was assigned: the median date of vaccination among vaccinated persons of same age and gender).
(DOCX)