Abstract
Objective
To determine whether women experience greater knee pain severity than men at equivalent levels of radiographic knee osteoarthritis (OA).
Design and Methods
A cross-sectional analysis of 2712 individuals (60% women) without knee replacement or a recent steroid injection. Sex differences in pain severity at each KL grade were assessed by knee using VAS scale and WOMAC with and without adjustment for age, analgesic use, BMI, clinic site, comorbid conditions, depression score, education, race, and widespread pain (WSP) using generalized estimating equations. Effect sizes (Cohen’s d) were also calculated. Analyses were repeated in those with and without patellofemoral OA (PFOA).
Results
Women reported higher VAS pain at all KL grades in unadjusted analyses (d=0.21–0.31, p<0.0001–0.0038) and in analyses adjusted for all covariates except WSP (d=0.16–0.22, p<0.0001–0.0472). Pain severity differences further decreased with adjustment for WSP (d=0.10–0.18) and were significant for KL grade ≤2 (p=0.0015) and 2 (p=0.0200). Presence compared with absence of WSP was associated with significantly greater knee pain at all KL grades (d=0.32–0.52, p<0.0001–0.0008). In knees with PFOA, VAS pain severity sex differences were greater at each KL grade (d=0.45–0.62, p=0.0006–0.0030) and remained significant for all KL grades in adjusted analyses (d=0.31–0.57, p=0.0013–0.0361). Results using WOMAC were similar.
Conclusions
Women reported greater knee pain than men regardless of KL grade, though effect sizes were generally small. These differences increased in the presence of PFOA. The strong contribution of WSP to sex differences in knee pain suggests that central sensitivity plays a role in these differences.
Keywords: sex differences, knee pain, knee osteoarthritis
Introduction
Women are at greater risk for developing knee osteoarthritis (OA)1, 2 compared with men, particularly those over 50 years of age2. Women with OA have also been found to have greater levels of knee pain and lower function3–6. However, a greater prevalence of radiographic knee OA in women7, 8 could account for sex differences in knee pain and function3, 4. Few studies have examined the degree to which the symptoms of knee OA differ between men and women after accounting for the degree of radiographic severity.
In addition to OA, several chronic musculoskeletal pain conditions are overrepresented in women such as temporomandibular disorders, headaches, and fibromyalgia9, all of which are generally thought to involve central sensitization. Indeed, the term, “central sensitivity syndromes”10 has been coined to represent a number of these frequently co-morbid chronic pain conditions that may share altered central pain processing11. The predominance of women with central sensitivity syndrome diseases might suggest that women are at greater risk for enhanced pain related to central sensitization than men.
Experimental pain studies of sex differences in pain sensitivity in healthy subjects, however, have produced somewhat inconclusive results. A meta-analysis found generally greater pain sensitivity in women than men for pain threshold and tolerance to a variety of noxious stimuli, with mean effect sizes ranging from d = 0.09 to 0.8212. In contrast, a recent review of the literature concluded little additional evidence is available to support clear sex differences in acute pain sensitivity9. Others have suggested women may be more susceptible to centrally-mediated pain than men, since they show greater temporal summation to heat pain13 and greater referred pain in response to intramuscular experimental pain14, and less conditioned pain modulation15. However, clinical studies of knee OA have been lacking in their explicit examination of sex differences in pain sensitivity, likely in part due to the inherently confounding issue of disease severity.
Thus, the primary purpose of this study was to examine whether women exhibit greater pain than men despite similar levels of radiographic knee OA, using a large epidemiological study of adults with or at risk for knee OA. Our secondary aim was to define the role of widespread pain in men and women, as an indication of the presence of heightened “central sensitivity.”
Methods
Study Sample
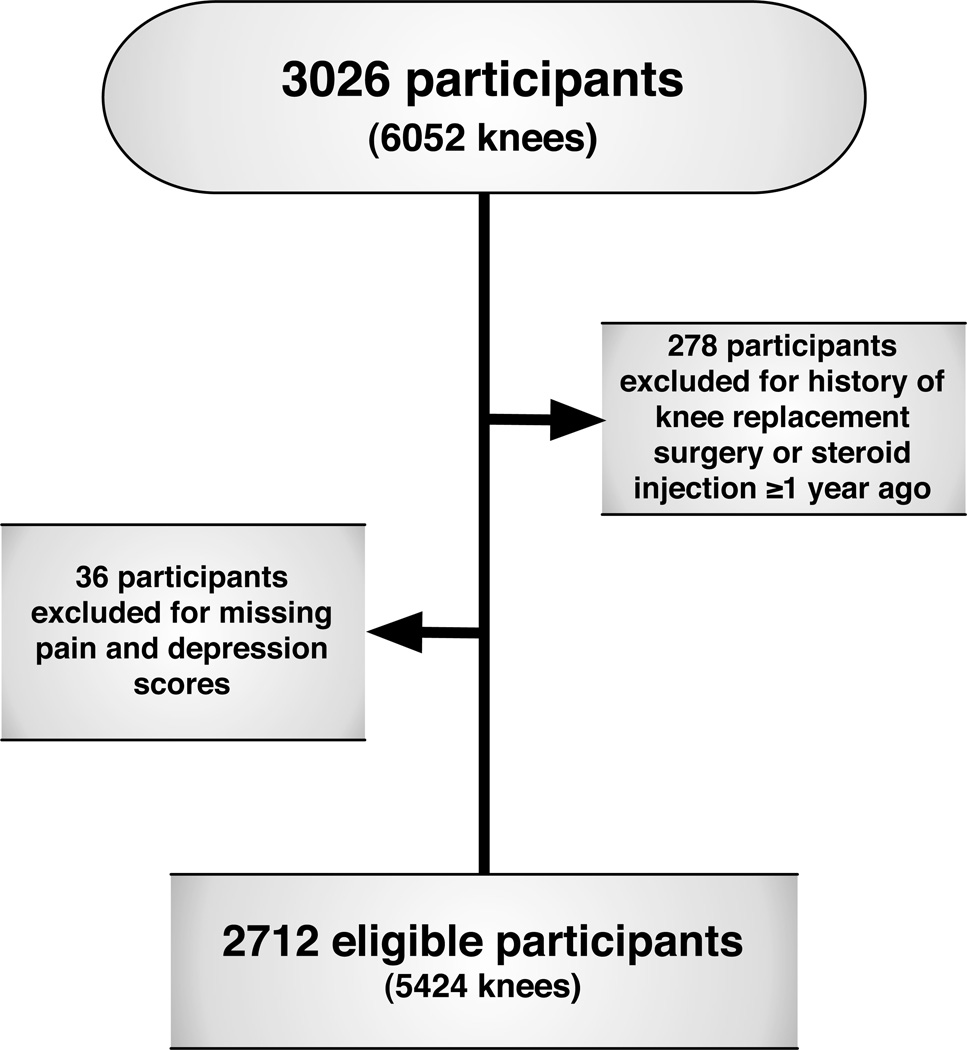
For this cross-sectional analysis, data from the baseline examination of the Multicenter Osteoarthritis Study (MOST) were utilized. MOST is an observational study that enrolled 3026 community-dwelling adults aged 50–79 years with knee OA or known risk factors for knee OA including age, female sex, overweight, and history of knee symptoms, knee injury and/or surgery. Participants were from Iowa City, IA, and Birmingham, AL or the surrounding communities. The study design and participant eligibility has been described previously16. Participants who had a steroid injection in the past year or with a history of total knee replacement were excluded from this study (Figure 1). The MOST study was approved by the institutional review boards at the University of Iowa; University of Alabama, Birmingham; University of California, San Francisco; and Boston University Medical Center. All participants provided written, informed consent.
Figure 1.
Participant Inclusion Diagram
Assessments
Analgesic Use
Participants provided information on analgesic medication (salicylate, non-steroidal anti-inflammatory drug (NSAID), opioid and “other” analgesic that included acetaminophen and other analgesics and antipyretics) use through an interviewer-administered questionnaire at the clinic visit. Participants were asked whether medications were used on an intermittent or regular basis. Analyses controlled for regular use of analgesic medications.
Anthropometric Measures
Body mass index (BMI, kg/m2) was calculated from weight in kilograms divided by the square of the height in meters (stadiometer, Holtain, Wales, UK), as measured by trained and certified staff at the clinic visit.
Comorbid Conditions
Participants completed the Charlson Comorbidity Index, a validated classification system of comorbid conditions17. Responses were categorized as none, one, and two or more comorbid conditions.
Depressive Symptoms Score
The Center for Epidemiologic Studies Depression Scale (CES-D)18 was utilized as an indicator of depressive symptoms. The instrument includes 20 items which query participants’ feelings over the past seven days. A score of 16 or greater has been used as an indicator of depression.19
Education
Participants provided information on the highest grade or year of school completed. Responses were categorized into one of three categories: less than high school education, completion of high school and at least some college.
Knee radiographs
Weight-bearing, fixed-flexion posteroanterior and lateral radiographs of the knees were obtained at baseline according to the MOST radiograph protocol as previously described20. Each participant’s radiographs were scored by two independent readers (an experienced academically-based musculoskeletal radiologist and a rheumatologist experienced in study reading) according to Kellgren-Lawrence scale at the tibiofemoral joint. Participants who attended both the baseline MOST visit and a follow-up visit had their baseline radiographs evaluated for the presence of patellofemoral (PF) OA. Their PFOA status was indicated as present if there was an osteophyte grade ≥ 2 or if there was an osteophyte grade ≥ 1 plus presence of PF JSN ≥ 2 or sclerosis ≥ 2 or cysts ≥ 221.
Pain
We used two assessments to characterize knee-specific pain. Average knee pain over the past 30 days was assessed on a 0–100 mm visual analog scale (VAS), with anchors of 0 indicating ‘no pain’ and 100 indicating ‘pain as bad as it could be’ for each knee. Pain during activities (i.e., walking, standing, stairs) was assessed using a subset of questions from the Western Ontario and McMaster Universities Arthritis Index (WOMAC pain) for each knee. This subscale comprises five items with responses that range from no (0) to extreme (4) pain with a possible total score of 20. Higher scores on the WOMAC indicate greater pain. WOMAC scores were also rescaled to a range of 0 to 100% for analyses. Differences in pain severity between men and women were evaluated according to effect sizes as well as whether differences were clinically important (minimal clinically important differences, MCID). The MCID utilized in this study was a difference of ≥6% of the maximal score22, 23. This corresponded to 6 points for VAS and WOMAC pain.
Widespread Pain
Widespread pain was ascertained using a homunculus. It was defined using American College of Rheumatology 1990 classification criteria24. Widespread pain was considered present if participants reported pain in all five regions of the body including axial pain, pain both above and below the waist and pain on both the right and left sides of the body.
Statistical Methods
Participant characteristics were summarized with frequencies and means for both the eligible study cohort and those excluded. Comparisons between men and women as well as between the eligible and excluded participants for each covariate were assessed using t-tests for continuous and chi-square tests for categorical variables.
Histograms of knee pain severity and results of normality tests (Shapiro-Wilk, Kolmogorov-Smirnov) revealed knee pain severity did not follow a normal distribution. The knee was the basic unit of analysis with each participant providing two knees. Knee-specific pain assessments were compared between men and women using generalized estimating equations to control for the covariance between knees in the same subject and stratified by the radiographic severity of each knee: KL grade <2, 2, 3, and 4. This method may also be used on data that are clustered and do not follow a normal distribution. Differences in knee pain severity were compared between men and women with the following models: Model 1) unadjusted estimate; Model 2) adjusted for age, body mass index (BMI, kg/m2), co-morbidities (none, one, two or more), CESD score, clinic site, educational level, frequent use of pain medications, and race; Model 3) Model 2 further adjusted for the presence of widespread pain. All analyses were adjusted for KL grade of the contralateral knee. We also examined whether PFOA status had an impact on sex differences in pain severity in sub-analyses. These analyses were conducted separately because the severity of PFOA was not graded. PFOA was defined as present or absent. All except for 376 knees from eligible participants met criteria for evaluation of PFOA status. The same analyses (Model 1, Model 2 and Model 3) were repeated in knees with and without PFOA separately. Adjusted and unadjusted least square (LS) means, 95% confidence intervals and (SE) for all analyses and standardized effect sizes (Cohen’s d, positive = greater pain in women) were computed. Effect sizes were defined as small (d=0.2), medium (d=0.5) or large (d=0.8)25.
Least square means are defined as the linear combination of the estimated effects from a linear model. Analyses were completed using the statistical software SAS Version 9.3 (SAS Institute Inc., Cary, NC) and significance was set at an alpha level of 0.05 throughout.
Results
A total of 2712 subjects (5424 knees) were included in the current study (Figure 1). The proportion of men and women did not significantly differ between those excluded due to knee replacement surgery, steroid injection in the past year or missing pain or covariate data (n=314) and those included. However, the excluded cohort tended to be slightly older (mean±SD: 65.3±7.9 vs. 62.2±8.1 years, p<0.0001), have a slightly higher BMI (32.5±6.6 vs. 30.5±5.9 kg/m2, p<0.0001), more comorbid conditions (20.1% vs. 12.6% with ≥2 comorbid conditions, p<0.0001), had a greater percentage with widespread pain (WSP, 55.1% vs. 49.9%, p<0.0001), and less education (35.0% vs. 45.1% with ≥ high school education, p=0.0006) than those included.
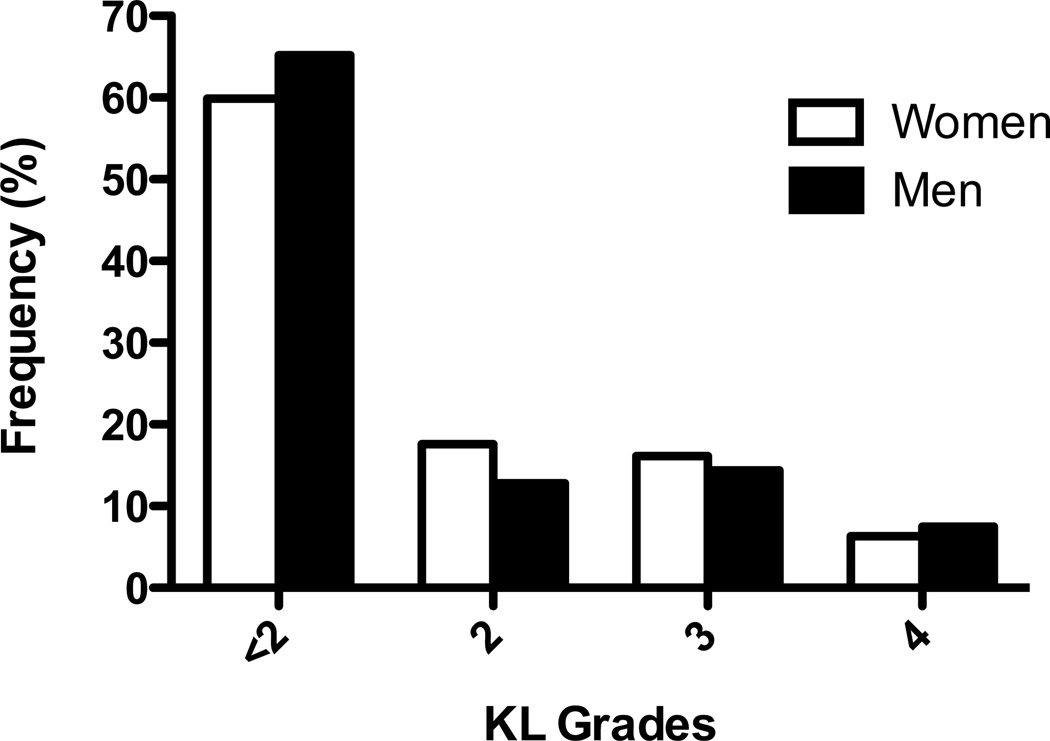
Within the included study cohort, more women than men reported depressive symptoms, WSP, and had bilateral radiographic knee OA and patellofemoral OA (Table 1). Analgesic medication use differed between men and women with a greater percentage of men reporting frequent salicylate analgesic use and a greater percentage of women used NSAIDs (Table 1). The distribution of men and women with knees at each KL grade was significantly different (p < 0.0001) with a higher proportion of women’s knees with radiographic knee OA (40.0% KL ≥ 2) than men (34.8% KL ≥ 2), particularly with KL grade = 2, (Figure 2, 17.6% vs 12.8%). There were 376 out of the eligible 5424 knees (6.9% of eligible knees) missing information about PFOA status. Participants not missing PFOA data were similar in characteristics to those included in the primary analyses. For example, there were no significant differences in age, BMI, percent with bilateral knee OA, percent with at least two comorbid conditions, percent with a CES-D score indicating depressive symptoms, percent with a level of education greater than high school, percent with frequent medication use, percent with widespread pain or percent women.
Table 1.
Characteristics of Women Compared with Men (Mean±SD or % as indicated).
| Characteristic | Women (n = 1618) |
Men (n = 1094) |
p-value |
|---|---|---|---|
| Age at baseline (years) | 62.3±7.9 | 62.0±8.3 | 0.48 |
| BMI (kg/m2) | 30.6±6.3 | 30.4±5.1 | 0.27 |
| Race (% Caucasian) | 83.7% | 85.3% | 0.26 |
| Education (% > high school) | 41.2% | 50.9% | <0.0001 |
| Site (% University of Iowa) | 51.5% | 49.8% | 0.38 |
| Widespread pain % | 56.2% | 40.4% | <0.0001 |
| CES-D Score ≥16 (%) | 13.5% | 7.9% | <0.0001 |
| Comorbidities (% ≥1 reported) | 12.2% | 13.2% | 0.39 |
| Bilateral knee OA (%) | 29.2% | 22.3% | <0.0001 |
| Patellofemoral OA (%) | 22.2% | 18.0% | 0.0089 |
| Salicylate Use (%) | 34.5% | 46.7% | <0.0001 |
| NSAID Use (%) | 25.0% | 17.3% | <0.0001 |
| Opioid Use (%) | 3.5% | 2.7% | 0.29 |
| Other Analgesic Use (%) | 8.8% | 4.9% | 0.0001 |
BMI: Body Mass Index
BMI: Body Mass Index
OA: Osteoarthritis
NSAID: Non-steroidal anti-inflammatory drug
Figure 2.
Frequency of Knees by KL Grade in Women and Men
For those missing PFOA information compared with those not missing PFOA information, age and percent women did not significantly differ, BMI was slightly greater (mean±SD: 31.4±6.2 vs. 30.5±5.8, p=0.0380), percent with widespread pain was greater (58.8% vs. 55.1%, p=0.0110) and percent with more than a high school education was lower (36.4% vs. 45.7%, p=0.0447).
VAS Pain
Women consistently reported greater or equal knee pain compared with men at each KL grade as shown in Table 2. In unadjusted analyses (Model 1), pain was greater in women than men at each KL grade, but only clinically significant for KL grades ≥3. The associated effect sizes for differences were small and ranged from 0.2 (KL grades ≤2) to 0.3 (KL grades ≥3). After adjusting for covariates (Model 2), sex differences in VAS pain remained significantly higher in women than in men for all KL grades. In addition, adjustment for WSP (Model 3) reduced the differences in pain levels between men and women such that differences only remained significant for KL grades ≤2. At all KL grades, presence of WSP (Model 3) was associated with greater pain severity with mean VAS pain scores 7.5–10.7 higher than in those without WSP (p<0.0001 for KL grades <2–3 and p=0.0008 for KL grade 4). There was a significant interaction between sex and presence of WSP (3.0±1.4, 95%CI=0.3–5.6, p=0.0288) such that pain severity was significantly greater in women with WSP compared with men with WSP.
Table 2.
Estimated Differences (LSmean±SE, 95% Confidence Interval, p-value) in Pain Levels in knees Between Women and Men by KL Grade.
| KL Grade | Unadjusted* | Adjusted** | Adjusted*** | |||
|---|---|---|---|---|---|---|
| VAS Pain (0–100) | ||||||
| <2 n=3365 |
4.2±0.7 (2.8–5.7) |
<0.0001 | 3.3±0.7 (1.9–4.6) |
<0.0001 | 2.1±0.7 (0.8–3.5) |
0.0015 |
| 2 n=852 |
5.3±1.6 (2.1–8.5) |
0.0015 | 4.6±1.5 (1.6–7.6) |
0.0036 | 3.5±1.5 (0.6–6.4) |
0.0200 |
| 3 n=838 |
6.4±1.8 (2.9–10.0) |
0.0004 | 4.2±1.7 (0.8–7.5) |
0.0151 | 3.2±1.7 (−0.1–6.6) |
0.0566 |
| 4 n=369 |
7.6±2.6 (2.5–12.7) |
0.0038 | 5.0±2.5 (0.1–9.9) |
0.0472 | 4.4±2.5 (−0.5–9.3) |
0.0792 |
| WOMAC Pain (0–100%) | ||||||
| <2 n=3365 |
3.6±0.6 (2.3–4.9) |
<0.0001 | 2.6±0.6 (1.4–3.7) |
<0.0001 | 1.5±0.6 (0.3–2.6) |
0.0115 |
| 2 n=852 |
4.8±1.4 (2.1–7.5) |
0.0007 | 4.2±1.3 (1.6–6.7) |
0.0016 | 3.1±1.2 (0.7–5.6) |
0.0128 |
| 3 n=838 |
6.0±1.5 (3.0–9.1) |
0.0001 | 3.9±1.4 (1.1–6.7) |
0.0059 | 2.8±1.4 (0.1–5.6) |
0.0436 |
| 4 n=369 |
3.9±2.1 (−0.1–8.0) |
0.0605 | 1.3±1.9 (−2.5–5.1) |
0.4932 | 0.8±2.0 (−3.0–4.7) |
0.6699 |
BMI: Body Mass Index
KL: Kellgren-Lawrence
LSMean: Least Squares Means
SE: Standard Error
VAS: Visual Analog Scale
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Adjusted for contralateral knee KL grade
Adjusted for age, BMI, depression score, education, clinic site, race, comorbid conditions and analgesic medication use
Further adjusted for presence of widespread pain
WOMAC Pain
Analyses repeated using WOMAC pain were similar to those using VAS pain (Table 2). However, in unadjusted (Model 1) and adjusted analyses (model 2), differences in pain severity were not statistically or clinically significant for KL grade 4.
Subanalyses in Knees with PFOA Data
In knees with PFOA, women reported pain of greater severity for all KL grades in unadjusted analyses (Model 1) for VAS pain and for all KL grades <4 for WOMAC pain. Adjusted models (Models 2 and 3) slightly reduced the estimated differences though they remained statistically significant for all KL grades for VAS pain (Table 3) and all KL grades <4 for WOMAC pain (Table 4). In most cases, statistically significant differences also met or exceeded clinically important differences. Effect sizes for unadjusted analyses of PFOA were much higher than for the analyses of TFOA data. For VAS pain, effect sizes were moderate (KL grades <2 and 4=0.5 and KL grade 2=0.6) or small (KL grade 3=0.4). For WOMAC pain, effect sizes were also moderate (KL grades 2 and 3=0.5) or small (KL grade<2=0.4, KL grade 4=0.3).
Table 3.
Estimated Differences (LSmean±SE, 95% Confidence Interval, p-value) in VAS Pain Levels (0–100) Between Women and Men by KL Grade in Knees with PFOA and without PFOA.
| KL Grade | Unadjusted* | Adjusted** | Adjusted*** | |||
|---|---|---|---|---|---|---|
| + PFOA | ||||||
| <2 n=160 |
9.2±2.9 (3.6–14.8) |
0.0025 | 8.9±3.0 (3.1–14.7) |
0.0048 | 7.1±2.9 (1.5–12.7) |
0.0184 |
| 2 n=248 |
12.2±3.1 (6.1–18.4) |
0.0006 | 15.1±3.0 (9.2–21.1) |
<0.0001 | 11.5±3.2 (5.2–17.8) |
0.0013 |
| 3 n=238 |
10.8±3.3 (4.4–17.3) |
0.0018 | 8.3±3.1 (2.2–14.4) |
0.0085 | 7.0±3.1 (1.0–13.0) |
0.0251 |
| 4 n=179 |
11.4±3.7 (4.2–18.6) |
0.0030 | 8.9±3.6 (1.9–16.0) |
0.0165 | 8.0±3.7 (0.7–15.3) |
0.0361 |
| − PFOA | ||||||
| <2 n=3205 |
3.9±0.7 (2.5–5.4) |
<0.0001 | 3.0±0.7 (1.6–4.3) |
<0.0001 | 1.9±0.7 (0.5–3.2) |
0.0062 |
| 2 n=604 |
3.0±1.9 (−0.6–6.7) |
0.1072 | 1.9±1.7 (−1.5–5.3) |
0.2868 | 1.5±1.7 (−1.8–4.8) |
0.3725 |
| 3 n=600 |
5.2±2.1 (1.1–9.3) |
0.0144 | 2.5±2.0 (−1.4–6.4) |
0.2021 | 1.8±2.0 (−2.1–5.7) |
0.3621 |
| 4 n=190 |
5.3±3.5 (−1.6–12.2) |
0.1383 | 2.4±3.2 (−4.0–8.6) |
0.4727 | 1.8±3.1 (−4.3–7.9) |
0.5619 |
BMI: Body Mass Index
SE: Standard Error
KL: Kellgren-Lawrence
PFOA: patellofemoral osteoarthritis
VAS: Visual Analog Scale
Adjusted for contralateral knee KL grade and PFOA status
Adjusted for age, BMI, depression score, education, clinic site, race, comorbid conditions and analgesic medication use
Further adjusted for presence of widespread pain
Table 4.
Estimated Differences (LSmean±SE, 95% Confidence Interval, p-value) in WOMAC Pain Levels (0–100%) Between Women and Men by KL Grade in Knees with PFOA and without PFOA.
| KL Grade | Unadjusted* | Adjusted** | Adjusted*** | |||
|---|---|---|---|---|---|---|
| + PFOA | ||||||
| <2 n=160 |
6.2±2.6 (1.2–11.2) |
0.0185 | 5.3±2.5 (0.4–10.1) |
0.0408 | 3.9±2.5 (−0.9–8.7) |
0.1282 |
| 2 n=248 |
9.3±2.7 (4.1–14.6) |
0.0019 | 12.3±2.5 (7.5–17.2) |
<0.0001 | 8.9±2.6 (3.8–14.0) |
0.0019 |
| 3 n=238 |
9.8±2.9 (4.2–15.4) |
0.0013 | 7.6±2.8 (2.2–13.0) |
0.0075 | 6.3±2.8 (0.9–11.7) |
0.0269 |
| 4 n=179 |
5.2±2.7 (−0.01–10.4) |
0.0537 | 3.5±2.5 (−1.5–8.4) |
0.1808 | 2.8±2.7 (−2.4–8.0) |
0.3026 |
| − PFOA | ||||||
| <2 n=3205 |
3.5±0.7 (2.2–4.7) |
<0.0001 | 2.4±0.6 (1.2–3.6) |
<0.0001 | 1.4±0.6 (0.2–2.5) |
0.0214 |
| 2 n=604 |
3.1±1.6 (0.05–6.2) |
0.0484 | 1.9±1.5 (−1.0–4.8) |
0.2086 | 1.5±1.4 (−1.3–4.3) |
0.2868 |
| 3 n=600 |
4.9±1.8 (1.4–8.4) |
0.0064 | 2.4±1.6 (−0.7–5.6) |
0.1384 | 1.4±1.6 (−1.7–4.5) |
0.3681 |
| 4 n=190 |
3.2±2.8 (−2.4–8.7) |
0.2716 | 0.5±2.6 (−4.6–5.6) |
0.8460 | 0.1±2.5 (−4.9–5.0) |
0.9836 |
BMI: Body Mass Index
KL: Kellgren-Lawrence
SE: Standard Error
PFOA: patellofemoral osteoarthritis
WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index
Adjusted for contralateral knee KL grade and PFOA status
Adjusted for age, BMI, depression score, education, clinic site, race, comorbid conditions and analgesic medication use
Further adjusted for presence of widespread pain
In knees without PFOA, however, statistically significant differences were only present in knees with a KL grade <2, KL grade=2 (WOMAC only) or Kl grade=3 in unadjusted analyses (Model 1). In adjusted analyses (Models 2 and 3), differences remained statistically significant for KL grade <2 (i.e., no evidence of radiographic TFOA or PFOA). Sex differences in VAS pain scores for knees without PFOA are shown in Table 3 by KL grade while differences in WOMAC pain are shown in Table 4. In all cases, where statistically significant, differences among knees without PFOA were small and did not meet the cut-offs for clinical significance. In addition, all effect sizes were ≤0.2.
Discussion
The main results of this study showed that women generally reported greater pain at all KL grades compared with men, especially in the presence of PFOA. These sex differences also were found prior to the onset of radiographic knee OA (TFOA KL grade <2) and were present regardless of adjustment for covariates in knees without PF or TFOA. Indeed, Maleki-Fischbach and Jordan26 suggested that more studies need to specifically examine sex differences in various assessments of OA, and whether risk factors for OA act similarly in both men and women, rather than simply controlling for sex.
Our results are consistent with previous findings. In one large cohort study, a greater proportion of women had symptomatic knee OA than men, when stratifying by knee OA grade27. Similarly, in a Korean population, symptom severity was greater in women compared with men at the same KL grade, with the exception of KL grade 328. However, that particular KL grade comparison was likely underpowered. In a study conducted in the Netherlands, Schiphof et al found being female was a significant risk factor for knee pain, but the increased risk for women was not KL grade specific29. In a study involving adults scheduled for knee replacement surgery, women were found to have greater pain intensity during movement and pain sensitivity compared with men6. Lastly, in a study of older adults with knee pain, men were found to have a higher incidence of radiographic disease30, suggesting women had similar pain despite less severe disease. Our findings further expand on these previous studies by considering PFOA and covariates that may account for sex differences, radiographic status of the contralateral knee, and study of a larger sample size. While gender-related pain changes have been observed when considering either TFOA or PFOA, few studies have attempted to consider both variables in the same study. Thus, in addition to the large sample size, this study uniquely considered the presence of PFOA in addition to KL grade when evaluating sex differences.
Results from the main analyses in our study in knees with radiographic TFOA showed that sex differences in knee pain severity were largely explained by BMI, depressive symptoms, comorbid conditions, socioeconomic status, and, especially, presence of widespread pain. However, significant differences generally remained following adjustment for all covariates for KL grades <4, suggesting additional unmeasured factors may contribute to knee pain severity differences between men and women at these particular grades. For example, KL grade 2 corresponds with presence of osteophytes while KL grade 3 corresponds with the onset of joint space narrowing which was found to have a stronger association with knee pain than osteophytes in a large cohort study.31. Though not detectable by radiography, joint space narrowing may reflect loss of cartilage as well as features associated with knee pain such as meniscal extrusion32, 33. However, we found estimates for differences in knee pain severity between women and men were slightly lower for KL grade 3 than for KL grade 2. In our study, sex differences were typically the least pronounced at the highest KL grade (KL grade 4). In those cases, the underlying nociceptive input from the damaged knees may over-ride other factors contributing to the heterogeneous pain experience, such as multiple psychosocial, experimental, genetic, and neurochemical variables that can influence sex differences in both clinical and experimental pain perception34.
The largest differences we observed for pain severity were in knees with PFOA. This is consistent with previous data that show PFOA is an important contributor to knee pain35, 36. It has also been suggested that risk factors for TFOA and PFOA may differ37, 38, which could explain why widespread pain had a more significant effect on pain severity differences between men and women with TFOA than in knees with both PFOA and TFOA. Thus, our findings support evaluation of the PF joint in addition to the more commonly evaluated TF joint in studies of knee OA and knee pain.
The reasons for the sex differences in knee OA and knee OA symptoms have yet to be fully elucidated. It has been suggested that sex differences in hormones, body composition, psychosocial characteristics, knee structure and neural processing may play a role. Several studies have examined the role of estrogens on knee OA. While results support a contribution to knee OA39–41, the evidence is not definitive. BMI has been found to be highly predictive of both knee OA42, 43 and knee pain44 and, similar to our study, BMI is generally greater in women than in men. Psychosocial characteristics such as depression may also contribute. For example, it has been reported that women have higher rates of depression45, which has also been associated with pain46. In addition, structural differences between men and women, including cartilage thickness, volume, and joint surface area have been documented47–49.
The evidence for the role of central sensitization in various pain conditions is growing. While widespread pain is not a measure of central sensitivity, it is believed to involve centrally-mediated processes50. Our hypothesis, that presence of widespread pain would be associated with greater knee pain for each radiographic knee OA severity level was supported. We also found a higher proportion of women than men with some degree of widespread pain at baseline. Since women may be more susceptible to centrally-mediated pain than men13–15, a proportion of the observed sex differences in those with OA could be a result of centrally-mediated mechanisms. However, presence of widespread pain did not appear to explain sex differences in pain severity in knees with PFOA.
Our findings should be interpreted in light of the following limitations. First, we categorized individuals by radiographic tibiofemoral KL grades and presence/absence of PFOA, though these grades cannot fully represent differences in peripheral joint disease, such as synovitis or other pathologies, that may further contribute to sex differences. In addition, categorization of knees by presence or absence of PFOA was less precise than grading structural changes at the PF joint and it is possible that women may have had more severe PFOA than men in our study, but this could not be determined from the available data. Despite our less precise assessment of the PF joint, we found differences in pain severity between men and women with PFOA were clinically significant for all KL grades for VAS pain and all KL grades <4 for WOMAC pain. We found the magnitude of the sex-differences in pain, though significant, to be relatively small for TFOA. In particular, these differences required relatively large sample sizes to be adequately powered, especially for knees with KL grades <2. This is likely one of the reasons this issue has not been well characterized in previous studies. Lastly, this analysis evaluated sex differences in knee pain, controlling for radiographic severity, but did not include measures of central sensitization. Future studies further examining whether these observed sex differences could be explained by sex differences in central pain modulation would be desirable. However, this information advances our general understanding of sex differences in knee pain due to OA, which may assist in the care of individual patients despite the relatively small overall mean effect size. That is, numerous factors likely contribute to pain variability, but greater pain in women, even after controlling for numerous confounding variables, was consistently observed.
In summary, women report greater knee pain than men despite similar levels of radiographic knee OA. Thus, a disparity in disease impact for knee OA between men and women was observed, and exists prior to the onset of radiographic knee OA. However, sex differences were in part ameliorated by considering the presence of patellofemoral knee OA; supporting that the PF joint, in addition to the TF joint, should be included in future studies of knee pain. The strong association between pain severity and presence of widespread pain suggests that central sensitivity may be one component contributing to the observed sex differences.
Acknowledgments
Role of the funding source
The study sponsor had no involvement in the study design, collection, analysis and interpretation of data, writing of the manuscript and decision to submit the manuscript for publication. This study was supported by NIH grants to: Boston University (David Felson, MD - AG18820);The University of Iowa (James Torner, PhD - AG18832); The University of Iowa (Laura Frey Law, PhD – AR056134); University of Alabama at Birmingham (Cora E. Lewis, MD MSPH - AG18947); University of California San Francisco (Michael Nevitt, PhD - AG19069).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Responsibility for the integrity of the work as a whole: N. Glass
Study concept and design: L. Frey Law, N. Glass, N. A. Segal
Acquisition of data: J. C. Torner, M. Nevitt, D. T. Felson, C. E. Lewis
Analysis and interpretation of data: L. Frey Law, N. Glass
Drafting of the manuscript: L. Frey Law, N. Glass
Critical revision of the manuscript for important intellectual content: all authors
Statistical Expertise: N. Glass
Obtained funding: J. C. Torner, M. D. Nevitt, D. T. Felson, C. E. Lewis
Administrative, technical, or material support: J. C. Torner, M. Nevitt, D. T. Felson, C. E. Lewis
Competing Interest Statement
The authors have no professional relationships with companies or manufacturers who will benefit from the results of the present study.
References
- 1.Felson DT, Zhang Y, Hannan MT, Naimark A, Weissman B, Aliabadi P, et al. Risk factors for incident radiographic knee osteoarthritis in the elderly: the Framingham Study. Arthritis Rheum. 1997;40:728–733. doi: 10.1002/art.1780400420. [DOI] [PubMed] [Google Scholar]
- 2.Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995;38:1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 3.Ebrahimpour PB, Do HT, Bornstein LJ, Westrich GH. Relationship between demographic variables and preoperative pain and disability in 5945 total joint arthroplasties at a single institution. The Journal of arthroplasty. 2011;26:133–137. e1. doi: 10.1016/j.arth.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Hawker GA, Wright JG, Coyte PC, Williams JI, Harvey B, Glazier R, et al. Differences between men and women in the rate of use of hip and knee arthroplasty. N Engl J Med. 2000;342:1016–1022. doi: 10.1056/NEJM200004063421405. [DOI] [PubMed] [Google Scholar]
- 5.Elbaz A, Debbi EM, Segal G, Haim A, Halperin N, Agar G, et al. Sex and body mass index correlate with Western Ontario and McMaster Universities Osteoarthritis Index and quality of life scores in knee osteoarthritis. Archives of physical medicine and rehabilitation. 2011;92:1618–1623. doi: 10.1016/j.apmr.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Tonelli SM, Rakel BA, Cooper NA, Angstom WL, Sluka KA. Women with knee osteoarthritis have more pain and poorer function than men, but similar physical activity prior to total knee replacement. Biology of sex differences. 2011;2:12. doi: 10.1186/2042-6410-2-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srikanth VK, Fryer JL, Zhai G, Winzenberg TM, Hosmer D, Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 8.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 9.Fillingim RB, King CD, Ribeiro-Dasilva MC, Rahim-Williams B, Riley JL., 3rd Sex, gender, and pain: a review of recent clinical and experimental findings. The journal of pain : official journal of the American Pain Society. 2009;10:447–485. doi: 10.1016/j.jpain.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yunus MB. Fibromyalgia and overlapping disorders: the unifying concept of central sensitivity syndromes. Seminars in arthritis and rheumatism. 2007;36:339–356. doi: 10.1016/j.semarthrit.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Staud R. Evidence for shared pain mechanisms in osteoarthritis, low back pain, and fibromyalgia. Current rheumatology reports. 2011;13:513–520. doi: 10.1007/s11926-011-0206-6. [DOI] [PubMed] [Google Scholar]
- 12.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 13.Fillingim RB, Maixner W, Kincaid S, Silva S. Sex differences in temporal summation but not sensory-discriminative processing of thermal pain. Pain. 1998;75:121–127. doi: 10.1016/S0304-3959(97)00214-5. [DOI] [PubMed] [Google Scholar]
- 14.Frey Law LA, Sluka KA, McMullen T, Lee J, Arendt-Nielsen L, Graven-Nielsen T. Acidic buffer induced muscle pain evokes referred pain and mechanical hyperalgesia in humans. Pain. 2008;140:254–264. doi: 10.1016/j.pain.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arendt-Nielsen L, Sluka KA, Nie HL. Experimental muscle pain impairs descending inhibition. Pain. 2008;140:465–471. doi: 10.1016/j.pain.2008.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Segal NA, Torner JC, Felson D, Niu J, Sharma L, Lewis CE, et al. Effect of thigh strength on incident radiographic and symptomatic knee osteoarthritis in a longitudinal cohort. Arthritis and Rheumatism. 2009;61:1210–1217. doi: 10.1002/art.24541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Radloff LS. The CES-D scale: a self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 19.Lewinsohn PM, Seeley JR, Roberts RE, Allen NB. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 20.Segal NA, Felson DT, Torner JC, Zhu Y, Curtis JR, Niu J, et al. Greater trochanteric pain syndrome: epidemiology and associated factors. Archives of Physical Medicine and Rehabilitation. 2007;88:988–992. doi: 10.1016/j.apmr.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Felson DT, McAlindon TE, Anderson JJ, Naimark A, Weissman BW, Aliabadi P, et al. Defining radiographic osteoarthritis for the whole knee. Osteoarthritis Cartilage. 1997;5:241–250. doi: 10.1016/s1063-4584(97)80020-9. [DOI] [PubMed] [Google Scholar]
- 22.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. The Journal of rheumatology. 2002;29:131–138. [PubMed] [Google Scholar]
- 23.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis and rheumatism. 2001;45:384–391. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 24.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and rheumatism. 1990;33:160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioral sciences. 2nd Edition. Hillsdale, N.J.: L. Erlbaum Associates; 1988. [Google Scholar]
- 26.Maleki-Fischbach M, Jordan JM. New developments in osteoarthritis. Sex differences in magnetic resonance imaging-based biomarkers and in those of joint metabolism. Arthritis research & therapy. 2010;12:212. doi: 10.1186/ar3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–918. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 28.Cho HJ, Chang CB, Yoo JH, Kim SJ, Kim TK. Gender differences in the correlation between symptom and radiographic severity in patients with knee osteoarthritis. Clinical orthopaedics and related research. 2010;468:1749–1758. doi: 10.1007/s11999-010-1282-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schiphof D, Kerkhof HJ, Damen J, de Klerk BM, Hofman A, Koes BW, et al. Factors for pain in patients with different grades of knee osteoarthritis. Arthritis Care & Research. 2013;65:695–702. doi: 10.1002/acr.21886. [DOI] [PubMed] [Google Scholar]
- 30.Lacey RJ, Thomas E, Duncan RC, Peat G. Gender difference in symptomatic radiographic knee osteoarthritis in the Knee Clinical Assessment--CAS(K): a prospective study in the general population. BMC musculoskeletal disorders. 2008;9:82. doi: 10.1186/1471-2474-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neogi T, Felson D, Niu J, Nevitt M, Lewis CE, Aliabadi P, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Adams JG, McAlindon T, Dimasi M, Carey J, Eustace S. Contribution of meniscal extrusion and cartilage loss to joint space narrowing in osteoarthritis. Clinical radiology. 1999;54:502–506. doi: 10.1016/s0009-9260(99)90846-2. [DOI] [PubMed] [Google Scholar]
- 33.Hunter DJ, Zhang YQ, Tu X, Lavalley M, Niu JB, Amin S, et al. Change in joint space width: hyaline articular cartilage loss or alteration in meniscus? Arthritis and rheumatism. 2006;54:2488–2495. doi: 10.1002/art.22016. [DOI] [PubMed] [Google Scholar]
- 34.Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nature reviews. Neuroscience. 2012;13:859–866. doi: 10.1038/nrn3360. [DOI] [PubMed] [Google Scholar]
- 35.Hunter DJ, March L, Sambrook PN. The association of cartilage volume with knee pain. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2003;11:725–729. doi: 10.1016/s1063-4584(03)00160-2. [DOI] [PubMed] [Google Scholar]
- 36.Kornaat PR, Bloem JL, Ceulemans RY, Riyazi N, Rosendaal FR, Nelissen RG, et al. Osteoarthritis of the knee: association between clinical features and MR imaging findings. Radiology. 2006;239:811–817. doi: 10.1148/radiol.2393050253. [DOI] [PubMed] [Google Scholar]
- 37.Cicuttini FM, Spector T, Baker J. Risk factors for osteoarthritis in the tibiofemoral and patellofemoral joints of the knee. J Rheumatol. 1997;24:1164–1167. [PubMed] [Google Scholar]
- 38.Hinman RS, Crossley KM. Patellofemoral joint osteoarthritis: an important subgroup of knee osteoarthritis. Rheumatology. 2007;46:1057–1062. doi: 10.1093/rheumatology/kem114. [DOI] [PubMed] [Google Scholar]
- 39.Wluka AE, Davis SR, Bailey M, Stuckey SL, Cicuttini FM. Users of oestrogen replacement therapy have more knee cartilage than non-users. Annals of the rheumatic diseases. 2001;60:332–336. doi: 10.1136/ard.60.4.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richette P, Corvol M, Bardin T. Estrogens, cartilage, and osteoarthritis. Joint, bone, spine : revue du rhumatisme. 2003;70:257–262. doi: 10.1016/s1297-319x(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 41.Riancho JA, Garcia-Ibarbia C, Gravani A, Raine EV, Rodriguez-Fontenla C, Soto-Hermida A, et al. Common variations in estrogen-related genes are associated with severe large-joint osteoarthritis: a multicenter genetic and functional study. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2010;18:927–933. doi: 10.1016/j.joca.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 42.Felson DT, Anderson JJ, Naimark A, Walker AM, Meenan RF. Obesity and knee osteoarthritis. The Framingham Study. Ann Intern Med. 1988;109:18–24. doi: 10.7326/0003-4819-109-1-18. [DOI] [PubMed] [Google Scholar]
- 43.Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. Int J Obes Relat Metab Disord. 2001;25:622–627. doi: 10.1038/sj.ijo.0801585. [DOI] [PubMed] [Google Scholar]
- 44.Jinks C, Jordan K, Croft P. Disabling knee pain--another consequence of obesity: results from a prospective cohort study. BMC public health. 2006;6:258. doi: 10.1186/1471-2458-6-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, et al. The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) JAMA : the journal of the American Medical Association. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 46.Bair MJ, Robinson RL, Katon W, Kroenke K. Depression and pain comorbidity: a literature review. Archives of Internal Medicine. 2003;163:2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 47.Otterness IG, Eckstein F. Women have thinner cartilage and smaller joint surfaces than men after adjustment for body height and weight. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2007;15:666–672. doi: 10.1016/j.joca.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Hanna FS, Teichtahl AJ, Wluka AE, Wang Y, Urquhart DM, English DR, et al. Women have increased rates of cartilage loss and progression of cartilage defects at the knee than men: a gender study of adults without clinical knee osteoarthritis. Menopause. 2009;16:666–670. doi: 10.1097/gme.0b013e318198e30e. [DOI] [PubMed] [Google Scholar]
- 49.Cicuttini F, Forbes A, Morris K, Darling S, Bailey M, Stuckey S. Gender differences in knee cartilage volume as measured by magnetic resonance imaging. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 1999;7:265–271. doi: 10.1053/joca.1998.0200. [DOI] [PubMed] [Google Scholar]
- 50.Meeus M, Nijs J. Central sensitization: a biopsychosocial explanation for chronic widespread pain in patients with fibromyalgia and chronic fatigue syndrome. Clinical rheumatology. 2007;26:465–473. doi: 10.1007/s10067-006-0433-9. [DOI] [PMC free article] [PubMed] [Google Scholar]