Abstract
Cardiac fibrosis is strongly associated with obesity and metabolic dysfunction and may contribute to the increased incidence of heart failure, atrial arrhythmias and sudden cardiac death in obese subjects. Our review discusses the evidence linking obesity and myocardial fibrosis in animal models and human patients, focusing on the fundamental pathophysiologic alterations that may trigger fibrogenic signaling, the cellular effectors of fibrosis and the molecular signals that may regulate the fibrotic response. Obesity is associated with a wide range of pathophysiologic alterations (such as pressure and volume overload, metabolic dysregulation, neurohumoral activation and systemic inflammation); their relative role in mediating cardiac fibrosis is poorly defined. Activation of fibroblasts likely plays a major role in obesity-associated fibrosis; however, inflammatory cells, cardiomyocytes and vascular cells may also contribute to fibrogenic signaling. Several molecular processes have been implicated in regulation of the fibrotic response in obesity. Activation of the Renin-Angiotensin-Aldosterone System, induction of Transforming Growth Factor-β, oxidative stress, advanced glycation end-products (AGEs), endothelin-1, Rho-kinase signaling, leptin-mediated actions and upregulation of matricellular proteins (such as thrombospondin-1) may play a role in the development of fibrosis in models of obesity and metabolic dysfunction. Moreover, experimental evidence suggests that obesity and insulin resistance profoundly affect the fibrotic and remodeling response following cardiac injury. Understanding the pathways implicated in obesity-associated fibrosis may lead to development of novel therapies to prevent heart failure and to attenuate post-infarction cardiac remodeling in obese patients.
INTRODUCTION
The strong association between obesity and cardiovascular disease is not only due to the well-established links between obesity and the traditional coronary risk factors, but also involves direct effects on the heart, independent of the atherosclerotic process (1). A growing body of evidence suggests that obese individuals are at a higher risk for development of heart failure (2). Moreover, overweight subjects and individuals with increased abdominal adiposity also exhibit an increased risk of heart failure (3). Echocardiographic exams in healthy men and women participating in the Framingham Heart Study documented that body-mass index (BMI) was associated with increased left ventricular hypertrophy, particularly in subjects with a BMI exceeding 30.0; this association was independent of age and systemic blood pressure levels (4). The effects of obesity and its associated metabolic perturbations are not limited to effects on cardiomyocytes, but also involve the cardiac interstitium. Development of cardiac fibrosis has been extensively documented in obese patients. In 1847, William Harvey reported for the first time autopsy findings of a severely obese man, describing the heart as “large, thick and fibrous” (5). Fibrotic cardiac remodeling of the ventricle associated with evidence of diastolic dysfunction is often observed in normotensive subjects with abdominal obesity (6), highlighting the effects of increased adiposity on the cardiac matrix. Fibrosis is also a dominant pathologic alteration in animal models of obesity (7) and may increase myocardial stiffness resulting in development of diastolic dysfunction.
Although the link between obesity and cardiac fibrosis is well-established, the pathophysiologic basis for fibrotic interstitial remodeling in the hearts of obese subjects remains poorly understood. Whether the observed cardiac alterations represent direct effects of increased adiposity, or reflect consequences of the many pathophysiologic “companions” of obesity (such as hypertension, volume overload, insulin resistance, etc.) remains unknown. Our review manuscript presents the evidence documenting interstitial myocardial changes in obese subjects and discusses the cellular effectors and molecular pathways responsible for development of fibrosis in obesity.
THE CARDIAC INTERSTITIUM IN THE NORMAL HEART
Preservation of cardiac architecture and transmission of contractile force are dependent on an intricate network of extracellular matrix, comprised primarily of fibrillar collagen (8). In addition to collagen I (that forms thick fibers and confers tensile strength) and collagen III (that typically forms thin fibers and maintains elasticity) (9), (10), the interstitial cardiac extracellular matrix also contains glycosaminoglycans, glycoproteins and proteoglycans. Large amounts of proteases and growth factors are bound to the cardiac extracellular matrix; their activation following injury plays an important role in cardiac remodeling. Cardiomyocytes and interstitial cells (including fibroblasts, vascular cells and immune cells) are enmeshed into the collagen-based matrix and continuously interact with the matrix, thus sensing alterations in their microenvironment. Cardiac fibroblasts are the most abundant interstitial cells in the adult mammalian myocardium and are responsible for formation and preservation of the matrix network (11). During the neonatal period, as the heart transitions from the fetal to the neonatal circulation, elevation of left ventricular pressures results in marked expansion of the cardiac fibroblast population (12). In young adult hearts, cardiac fibroblasts appear to maintain quiescence, exhibiting limited inflammatory or proliferative activity. In aging hearts, cardiomyocyte loss is associated with expansion of the interstitium and increased collagen content (13), (14), (15). Extensive evidence, derived from animal models and human studies, suggests that obesity is associated with acceleration and accentuation of fibrotic myocardial changes.
CARDIAC FIBROSIS IN ANIMAL MODELS OF OBESITY
Development of cardiac fibrosis has been documented in most experimental models of obesity and is often associated with diastolic dysfunction. The severity of cardiac fibrosis is dependent on the species, strain and age of the animals, the underlying mechanism of obesity, and on the presence and severity of concomitant pathophysiologic conditions (such as hypertension and metabolic dysfunction). db/db mice harbor a mutation resulting in expression of a non-functional truncated long-form of the leptin receptor. These animals have hypothalamic resistance to leptin, and develop a voracious appetite, marked obesity and overt diabetes at a young age. Cardiac interstitial fibrosis has been consistently documented in db/db animals using both histological and biochemical techniques at the age of 4–6 months (16), (7); fibrotic changes in db/db mice are associated with diastolic dysfunction (17). Much like db/db mice, leptin-deficient ob/ob animals develop severe obesity, insulin resistance and cardiac hypertrophy at a young age (18); however, evidence documenting cardiac fibrosis in ob/ob mice is less consistent. Zaman et al showed that ob/ob mice exhibit significant perivascular cardiac fibrosis associated with elevated expression of Transforming Growth Factor (TGF)-β1 and Plasminogen activator inhibitor (PAI)-1, suggesting activation of matrix-preserving pathways (19). In contrast, Van den Bergh and co-workers demonstrated that at 36 weeks of age, ob/ob mice exhibit cardiac hypertrophy and diastolic dysfunction, but have comparable collagen content with lean WT animals (20). Differences in strain, age and gender of the ob/ob animals studied in various investigations, and the relative sensitivity of various techniques used to assess fibrosis may explain the discordant findings. Evidence suggests that db/db mice may be more susceptible to the development of fibrosis due to intact leptin signaling in peripheral cells (including fibroblasts) through the short-form leptin receptor; these leptin-mediated actions may promote a fibrogenic environment (21), (22). Obese hyperinsulinemic Zucker rats also develop perivascular cardiac fibrosis, associated with cardiac hypertrophy (23) and diastolic dysfunction (24).
In contrast to the early cardiac fibrotic remodeling observed in genetic models of obesity, diet-induced obesity in rodents has more subtle effects on the cardiac interstitium. In mice fed a high-fat, or a high-fat/high-sugar diet, development of cardiac fibrosis occurs late and requires prolonged feeding for 6–16 months. Administration of a high-fat high-carbohydrate diet in male C57BL6J mice induced left ventricular hypertrophy, interstitial fibrosis and diastolic dysfunction after 6–8 months of feeding (25), (26). Fibrotic changes in this model were associated with increased collagen cross-linking and upregulation of lysyl-oxidase (25). High-fat diets may be even less effective in induction of cardiac fibrotic changes. Calligaris and co-workers found that male C57/BL6J mice required feeding with a high fat diet for 16 months to develop significant cardiac hypertrophy and myocardial fibrosis (27).
The pro-fibrotic effects of obesity are not limited to the ventricle, but also involve the atria. In sheep fed a high-fat diet, increased adiposity was associated with atrial fibrosis and dilation accompanied by accentuated expression of fibrogenic mediators (28). These findings may explain the association of obesity with atrial dysrhythmias (29). Obesity may also augment the effects of other fibrogenic pathophysiologic conditions. In models of hypertensive fibrosis obesity increases myocardial hypertrophy and collagen deposition (30), (31).
EFFECTS OF OBESITY ON REPARATIVE CARDIAC FIBROSIS
Obesity and metabolic dysfunction may also alter fibrotic responses due to other myocardial insults. In models of reperfused myocardial infarction in the mouse, both genetic and diet-induced obesity accentuated dilative remodeling (32), (33). Enhanced post-infarction ventricular dilation in insulin-resistant obese mice may be due to defective scar formation and reduced function of reparative cells, resulting in decreased deposition of collagen into the infarct and reduced tensile strength of the scar. Thus, although obesity may accentuate myocardial interstitial fibrosis, it may also reduce the reparative reserve, leading to impaired fibroblast function and less effective healing.
OBESITY-ASSOCIATED CARDIAC FIBROSIS IN HUMAN PATIENTS
Documentation of cardiac fibrosis in isolated human obesity is challenging considering the common association of obesity with other comorbid conditions with known effects on the cardiac interstitium (such as hypertension and diabetes) and the practical difficulties in obtaining representative histopathologic samples. Recent advances in imaging strategies and development of circulating biomarkers that reflect collagen turnover have provided evidence suggesting fibrotic remodeling of the ventricle in obese and overweight individuals.
Many studies have demonstrated that isolated obesity in human subjects is associated with abnormal diastolic function; in contrast, impairment of systolic function is not consistently observed (34), (35), (36). Higher BMI and prolonged duration of obesity are generally associated with worse functional impairment (36). Subclinical functional alterations have also been reported in healthy overweight subjects. Tissue Doppler imaging in overweight and mildly obese human patients without clinical heart disease suggested reduced systolic and diastolic ventricular function, despite a preserved ejection fraction (37); these functional alterations correlated with fasting insulin levels (37). In addition to the functional alterations, these subjects also exhibited increased tissue density (evidenced by increased calibrated myocardial backscatter). Although such findings likely reflect underlying cardiac fibrosis, direct histopathologic evidence of cardiac fibrosis in overweight subjects is lacking.
To what extent the fibrotic alterations observed in obese subjects reflect the presence of metabolic dysregulation remains unclear. Patients with overt type 2 diabetes develop myocardial fibrosis (38), exhibiting accumulation of collagen type III in perimysial and perivascular areas (39). Normotensive non-diabetic subjects with abdominal obesity exhibited concentric hypertrophy, associated with diastolic dysfunction and evidence of increased collagen III turnover (6), suggested by increased levels of circulating biomarkers reflecting collagen synthesis. However, in these patient populations, increased levels of collagen turnover biomarkers are associated with insulin resistance (40).
THE PATHOPHYSIOLOGIC BASIS OF OBESITY-ASSOCIATED CARDIAC FIBROSIS
Obesity is accompanied by a wide range of pathophysiologic alterations that represent consequences of the increased body weight and adiposity, and may be responsible for the development of cardiac fibrosis. The high prevalence of these conditions hampers dissection of the direct effects of isolated obesity on the myocardium.
Volume and pressure overload
Hypertension is highly prevalent in obese subjects; the increased left ventricular hypertrophy and fibrosis observed in obese subjects may be in part due to the cardiac pressure overload caused by elevation of systemic blood pressures. Obesity is also associated with hypervolemia (41) resulting in volume overload. Pressure and volume overload have distinct effects on the cardiac interstitium: pressure loads cause concentric hypertrophy associated with increased deposition of collagen (42) and diastolic dysfunction, whereas volume overload induces dilation accompanied by matrix degradation (43). The relative contribution of these pathophysiologic alterations to the remodeling process in obese subjects is unknown.
Metabolic dysfunction
Obesity is often associated with metabolic dysfunction; fasting hyperglycemia, insulin resistance and dyslipidemias are highly prevalent in obese subjects and may be critically involved in fibrotic and hypertrophic cardiac remodeling. High glucose activates fibrogenic pathways in many cell types, inducing TGF-β synthesis and stimulating Smad-dependent signaling (44). Dissecting the contribution of metabolic dysregulation to cardiac remodeling in obese subjects remains a major challenge.
Inflammation
A large body of evidence from experimental models and human patients suggests that obesity is associated with a systemic inflammatory response (45). Because inflammatory pathways are critically involved in the pathogenesis of fibrosis and cardiac remodeling (46), activation of inflammatory signaling in obese subjects may promote both fibrogenic and hypertrophic responses.
THE CELLULAR EFFECTORS OF FIBROSIS IN OBESITY
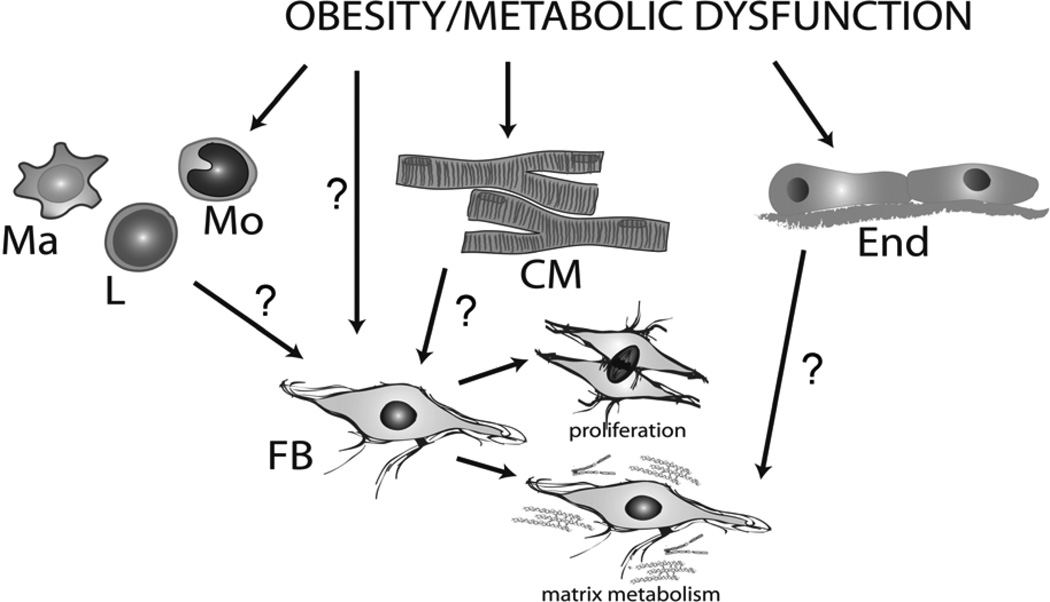
Fibrotic changes in the remodeling myocardium are driven by both cardiomyocytes and interstitial cells. The cell biology of the fibrotic response has been extensively studied in models of replacement fibrosis and in the interstitial and perivascular fibrosis associated with pressure overload and overactive angiotensin II signaling (46). In contrast, the cellular alterations implicated in the pathogenesis of cardiac fibrosis in obesity and metabolic dysfunction have not been systematically investigated (Figure 1).
Figure 1.
The cell biology of the fibrotic response in obesity and metabolic dysfunction remains underexplored. Although fibroblasts (FB) are the predominant effector cells in cardiac fibrosis, their involvement in fibrotic myocardial remodeling in obesity and metabolic dysfunction is poorly documented. Other cell types, including cardiomyocytes (CM), endothelial cells (End) and monocuclear cells (macrophages/Ma, monocytes/M and lymphocytes/L) may play an important role in obesity-associated fibrosis by activating fibroblasts through secretion of soluble mediators or through contact-dependent actions.
The fibroblast
As the main matrix-producing cells in the cardiac interstitium, fibroblasts are critically involved in all cardiac fibrotic conditions. Following cardiac injury, fibroblasts undergo myofibroblast transdifferentiation, expressing contractile proteins, such as α-smooth muscle actin (α-SMA), and exhibiting stress fiber formation (47). Although cardiac fibrosis in obesity and diabetes likely involves expansion and activation of the resident fibroblast population, studies testing this hypothesis have not been performed. However, several descriptive investigations have demonstrated that obesity is associated with phenotypic alterations of the cardiac fibroblast population. Cardiac fibroblasts harvested from Zucker rat hearts exhibited greater ability to contract gels and elevated α-SMA expression, consistent with a myofibroblast phenotype (48). Moreover, cardiac fibroblasts isolated from db/db mice exhibited a matrix-preserving phenotype showing increased expression of collagen and protease inhibitors (such as PAI-1) (49). The metabolic alterations associated with obesity may be responsible for activation of interstitial fibroblasts in the myocardium of obese subjects. High glucose levels, activation of the Renin-angiotensin-aldosterone (RAAS) system, cytokine and growth factor activation may be implicated in phenotypic modulation of cardiac fibroblasts, leading to their expansion, and inducing matrix-synthetic and matrix-preserving pathways. In models of ischemic fibrosis, recruitment of fibroblast progenitors contributes to fibrotic myocardial remodeling (50). Whether obesity and metabolic dysfunction activate bone marrow-derived fibroblast progenitors predisposing to tissue fibrosis remains unknown.
Monocytes, lymphocytes and macrophages
Monocyte and lymphocyte subsets have been implicated in the pathogenesis of cardiac fibrosis in models of myocardial infarction and in cardiac remodeling induced by pressure overload (51), (52). Whether recruitment of fibrogenic subsets of monocytes and lymphocytes mediates myocardial fibrosis in obese and diabetic subjects has not been examined. It is becoming increasingly appreciated that the myocardium contains a resident macrophage population (53), (54); these cells may undergo phenotypic modulation in response to metabolic dysfunction, acquiring pro-fibrotic properties and contributing to activation of interstitial fibroblasts. Increased infiltration of the myocardium with macrophages has been reported in several distinct models of obesity. When compared with lean animals, db/db mice exhibit increased accumulation of macrophages in the cardiac interstitium (55), (56). In a rodent model of obesity associated with metabolic syndrome and hypertension, infiltration of the myocardium with macrophages was observed (57). In a porcine model, obesity associated with metabolic dysfunction induced an increase in infiltration with pro-inflammatory M1 macrophages and accentuated cardiac fibrosis in regions perfused by a stenotic coronary artery (58). Whether increased macrophage infiltration in obesity and diabetes plays a crucial role in mediating the myocardial fibrotic response, or simply represents an epiphenomenon, remains unknown.
Vascular cells
The heart contains abundant endothelial cells and significant populations of vascular smooth muscle cells and pericytes. In the pressure overloaded myocardium endothelial-mesenchymal transition (EndMT) contributes to cardiac fibrosis, by providing an additional pool of activated fibroblasts (59). In models of type 1 diabetes, experimental evidence supports the involvement of EndMT in the expansion of fibroblasts in the cardiac interstitium (60). Whether these pathways plays a role in fibrosis associated with obesity and type 2 diabetes remains unknown. Vascular cells may also contribute to the fibrotic process by secreting cytokines and growth factors that activate the fibroblast population.
Cardiomyocytes
In the remodeling myocardium, cardiomyocytes might modulate the fibrotic response through direct interactions with interstitial cells and by secreting cytokines and growth factors that regulate fibroblast phenotype (61). Although cardiomyocyte hypertrophy is consistently found in experimental models of obesity and metabolic dysfunction, the potential contribution of hypertrophic cells on the fibrotic process has not been studied. Several studies have demonstrated apoptotic loss of cardiomyocytes in experimental models of obesity (62). Accumulation of fatty acids and triglycerides in the myocardium may induce toxic effects on the cardiomyocytes, eventually leading to dysfunction and cell death (5). Whether cardiac fibrosis in obese subjects reflects replacement of dead cardiomyocytes with fibrous tissue, rather than direct activation of interstitial fibroblasts remains unknown.
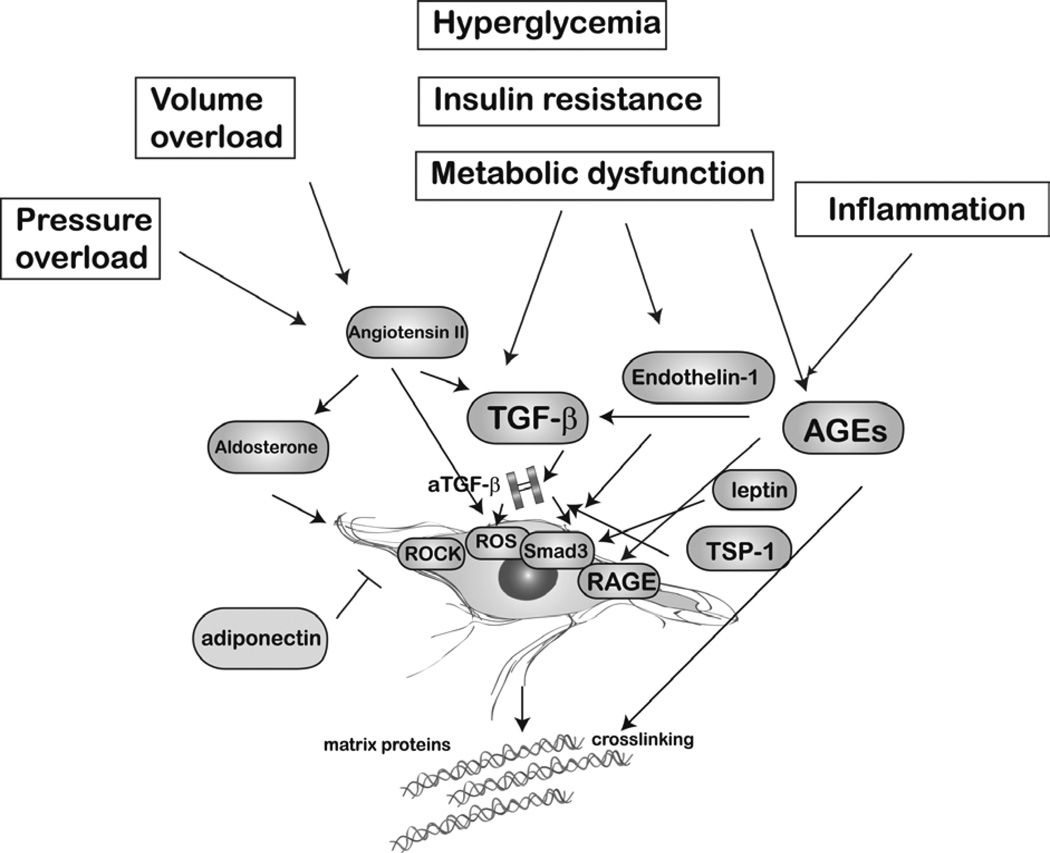
THE MOLECULAR SIGNALS REGULATING OBESITY-ASSOCIATED FIBROSIS (Figure 2)
Figure 2.
Obesity is associated with a wide range of pathophysiologic conditions (such as volume and pressure overload, hyperglycemia, insulin resistance and metabolic dysfunction and systemic inflammation) that may trigger cardiac fibrosis. The relative role of these pathophysiologic perturbations that often accompany obesity in the pathogenesis of cardiac fibrosis remains unknown. These conditions stimulate several distinct fibrogenic pathways activating resident cardiac fibroblasts. The Renin-angiotensin-aldosterone system, TGF-β/Smad3 signaling, endothelin-1, leptin, matricellular proteins (such as thrombospondin/TSP-1), reactive oxygen species, advanced glycation end-product (AGE)/Receptor for advanced glycation end products (RAGE) interactions, and Rho-kinase (ROCK) signaling have been implicated in obesity-associated fibrosis.
The RAAS
Activation of the RAAS is consistently noted in the fibrotic myocardium. In vitro, all components of the RAAS activate cardiac fibroblasts. Renin directly induces a pro-fibrotic program in cardiac fibroblasts (63). Angiotensin II activates AT1 receptors stimulating cardiac fibroblast proliferation and enhancing matrix protein synthesis (64), (65), (66). In contrast, AT2 signaling may exert anti-fibrotic actions inhibiting fibroblast proliferation and matrix synthesis (67), (68). Aldosterone enhances proliferation (69) and potently stimulates matrix protein synthesis in cardiac fibroblasts, while activating fibrogenic signaling in cardiomyocytes (70) and macrophages (71). In vivo, renin inhibition, ACE inhibition, AT1 blockade and aldosterone antagonism attenuate interstitial fibrosis in experimental models of myocardial infarction and of cardiac remodeling induced by pressure overload (72), (73), (74), (63), (75).
Activation of the RAAS has been reported in animal models of obesity and type 2 diabetes and may be due to metabolic dysregulation and hemodynamic overload. In vivo, db/db mice have increased angiotensin II levels and exhibit a significant increase in plasma angiotensin converting enzyme activity (76). Moreover, obese Dahl salt-sensitive rats (generated by crossing Dahl rats with Zucker rats in order to develop a model for the metabolic syndrome) also exhibit increased myocardial angiotensin converting enzyme (ACE) and AT1 receptor levels (77). In vitro, high glucose increases intracellular renin and angiotensin II levels in isolated cardiac fibroblasts; activation of angiotensin II signaling is implicated in glucose-induced matrix and TGF-β synthesis (78). Pharmacologic inhibition studies suggest that AT1 signaling may play an important role in the pathogenesis of obesity-associated cardiac fibrosis. In obese Zucker rats, ACE inhibition with perindopril attenuated myocardial collagen synthesis and reduced PAI-1 and TGF-β levels (79). Moreover, in ob/ob mice treatment with ACE inhibitors or AT1 blockers decreased perivascular coronary fibrosis. (19), (80) and in db/db animals ramipril attenuated myocardial fibrosis (17). In addition to attenuated TGF-β signaling, modulation of several additional pathways may explain the anti-fibrotic actions of AT1 blockade in obesity and type 2 diabetes. In db/db mice, ACE inhibition attenuated myocardial superoxide generation (17), suggesting that angiotensin signaling may enhance myocardial oxidative stress. This concept was supported by experiments in insulin-resistant rats showing that AT1 activation contributes to generation of myocardial reactive oxygen species (ROS) (81), thus promoting pro-inflammatory signaling. Moreover, ACE inhibition with captopril normalized myocardial insulin signaling and insulin-regulated metabolism (82) and attenuated autonomic dysregulation in ob/ob mice (83). Aldosterone may be an important downstream effector of angiotensin-mediated actions on the myocardium (84); however, direct evidence examining its role in animal models of obesity-associated cardiac fibrosis is lacking. Recently, a randomized controlled clinical study examined the effects of a 6-month course of spironolactone on myocardial function in patients with obesity, but without other comorbidities. Aldosterone antagonism reduced levels of serological markers of collagen synthesis and improved myocardial deformation and peak early diastolic velocity (85).
TGF-β
The link between an overactive TGF-β cascade and cardiac fibrosis is well-established (86), (87). Fibrogenic actions of TGF-β are primarily mediated through effects involving Smad signaling (88), although Smad-independent actions have also been implicated. Increased expression of myocardial TGF-β is consistently noted in experimental models of obesity and is associated with cardiac fibrosis (89), (28), (79). Upregulation of TGF-β in obesity-associated cardiomyopathy may be due to angiotensin II signaling (79), but may also involve angiotensin-independent pathways mediated through the direct stimulatory effects of high glucose and leptin on TGF-β transcription and activation (90), (91). TGF-β exerts potent pro-hypertrophic actions and promotes a matrix-preserving phenotype in cardiac fibroblasts (87). Although abundant associative evidence links obesity-associated hypertrophy and cardiac fibrosis with increased myocardial expression of TGF-β, studies examining whether the TGF-β cascade directly mediates the functional and morphological alterations observed in obesity and metabolic dysfunction have not been performed.
The role of oxidative stress
Increased oxidative stress is due to excess generation, or impaired removal of highly reactive molecules or chemical species with one unpaired electron, such as reactive oxygen species (ROS), and reactive nitrogen species. Oxidative stress is implicated in the pathogenesis of cardiac fibrosis both through direct actions and through the involvement of ROS in cytokine and growth factor signaling. The effects of cytokines, growth factors and angiotensin II on fibroblast function and matrix metabolism appear to be in part dependent on ROS.
Extensive evidence suggests that obesity and type 2 diabetes are associated with accentuated cardiac oxidative stress (1). Boudina and co-workers demonstrated that, in the myocardium of db/db mice, mitochondrial generation of ROS is markedly augmented and peroxidation of lipids and proteins is increased (92). Non-mitochondrial mechanisms may also contribute to accentuation of cardiac oxidative stress in animal models of obesity (93). Hyperglycemia and insulin resistance may directly increase myocardial ROS generation in obese animals with metabolic dysfunction. Defective scavenging of free radicals may also contribute to the increased oxidative stress levels observed in obese animals. Rats fed a high-fat diet and genetically obese Zucker rats exhibited attenuated myocardial activation of antioxidant enzymes, such as manganese superoxide dismutase and glutathione peroxidase 1 (94), (95).
The involvement of oxidative stress in the pathogenesis of cardiac fibrosis in obesity and metabolic dysfunction is suggested by pharmacologic investigations examining the effects of antioxidants. In mice fed a high-fat high-sucrose diet, treatment with the polyphenols resveratrol and S17834 (a synthetic flavonoid derivate) reduced the levels of oxidative modification and prevented cardiac hypertrophy, interstitial fibrosis and diastolic dysfunction (26). Moreover, dietary supplementation with the antioxidant coenzyme Q10 for 10 weeks reduced superoxide generation, ameliorated diastolic dysfunction and attenuated cardiomyocyte hypertrophy and fibrosis in db/db mice (17).
Advanced glycation end-products (AGE)
AGEs are a heterogeneous group of proteins and lipids, formed by non-enzymatic glycation after persistent contact with aldose sugars (96). In diabetes, obesity and metabolic dysfunction AGEs accumulate in tissues at an accelerated rate and may act as mediators of diabetic complications (96), (97). AGEs accumulate within the cells and in the extracellular milieu and may have profound effects on the structure and composition of the cardiac interstitium through two major mechanisms (98), (99). First, AGE-mediated crosslinking of extracellular matrix proteins (such as collagens and laminin) may increase cardiac stiffness contributing to the development of diastolic dysfunction. Second, AGEs may activate interstitial fibroblasts by triggering signaling through the receptors for advanced glycation end-products (RAGEs). Activation of RAGE signaling in cardiac fibroblasts may trigger pro-inflammatory responses, may stimulate proliferation and may accentuate synthesis of matrix proteins (100). The pro-fibrotic effects of RAGE may be mediated, at least in part, through TGF-β (101). In vivo experiments demonstrated that in db/db mice, RAGE blockade attenuated diastolic dysfunction and reduced myocardial collagen synthesis (102). However, whether these protective actions of RAGE inhibition reflect fibroblast deactivation remains unknown.
Endothelin-1 (ET-1)
ET-1, the best-studied member of the endothelin family, is a potent fibrogenic mediator that acts downstream of TGF-β and angiotensin (103). Experimental evidence suggests that ET-1 expression is induced in obesity-associated cardiomyopathy through actions that may involve leptin. Mice fed a high fat diet, but not ob/ob mice, exhibited significantly increased myocardial ET-1 levels suggesting that ET-1 upregulation in obesity may be mediated by leptin (104). In contrast to ob/ob mice, db/db animals had increased levels of myocardial ET-1, perhaps reflecting signaling through the short-form leptin receptor (105). Increased myocardial ET-1 levels in db/db mice are associated with accentuated expression of extracellular matrix proteins (105); however, whether ET-1 plays a crucial role for fibrotic remodeling in this model remains unknown.
Leptin
Beyond its established role as a satiety factor, leptin exerts important actions on the heart and has been implicated in the pathogenesis of cardiac remodeling in obesity and metabolic dysfunction. Elevated circulating leptin levels are associated with left ventricular hypertrophy in patients with uncomplicated obesity (106). Although some studies have suggested that disruption of leptin may be responsible for pro-hypertrophic effects (107), a large body of evidence showed that leptin directly stimulates cardiomyocyte hypertrophy (108). Whether the hypertrophic response associated with obesity is due to leptin resistance, or to overactive leptin responses remains unclear. In vivo studies examining the effects of cell-specific modulation of leptin signaling may contribute to our understanding of the role of endogenous leptin in obesity-associated cardiac remodeling. In addition to its effects on cardiomyocytes, leptin modulates fibroblast phenotype, activating a fibrogenic program (109). Whether fibroblast-specific effects of leptin mediate myocardial fibrosis in obesity has not been rigorously tested. However, exogenous leptin administration in ob/ob mice significantly increased myocardial collagen content promoting matrix-preserving actions (110).
Adiponectin
Adiponectin is an adipocyte-derived cytokine with anti-inflammatory, cardioprotective and anti-atherogenic properties (111), (112), that may also be involved in regulation of the fibrotic response. In a model of angiotensin-induced cardiac remodeling, adiponectin exerted anti-fibrotic effects, presumably mediated through Peroxisome proliferator-activated receptor (PPAR)-a activation (113). In vitro studies have demonstrated both fibrogenic and anti-fibrotic actions of adiponectin on cultured fibroblasts (114), (115); the relevance of these observations in vivo remains unclear. Shibata and co-workers (116) demonstrated that, in db/db mice, exogenous adiponectin attenuated hypertrophic signaling; whether these effects were associated with anti-fibrotic actions has not been systematically investigated.
Matricellular proteins: the role of thrombospondin (TSP)-1
Tissue remodeling involves dynamic changes in the composition of the extracellular matrix. The term “matricellular proteins” was coined to describe a family of unrelated macromolecules (such as the TSPs, tenascins-C and X, osteonectin, osteopontin, periostin and the members of the CCN family) which are upregulated following injury and deposited into the matrix, where they do not play a structural role, but interact with cell surface receptors, growth factors and proteases to modulate cell:cell and cell:matrix interactions (117). Matricellular proteins are induced in the remodeling heart and regulate inflammatory, fibrotic and angiogenic responses (118). The best-characterized matricellular protein in obesity, diabetes and metabolic dysfunction is TSP-1 (119). TSP-1 induction is consistently observed in tissue harvested from obese and diabetic subjects (120) and in animal models of obesity and metabolic dysfunction (121) Hyperglycemia potently induces TSP-1 synthesis in vascular cells (122) and may be in part responsible for increased expression of TSP-1 in obesity associated with metabolic dysfunction.
In the remodeling infarcted and pressure-overloaded myocardium TSP-1 may modulate fibrotic responses by activating TGF-β (123) and by inhibiting matrix metalloproteinase (MMP) activity (124). Our recent work demonstrated that db/db mice have increased TSP-1 expression in the cardiac interstitium, associated with collagen deposition and fibrotic remodeling (7). Genetic disruption of TSP-1 in db/db mice reduced collagen deposition while increasing chamber dimensions, suggesting that the matrix-preserving actions of TSP-1 may maintain chamber geometry (7). In addition to its effects on fibroblasts, TSP-1 is a potent angiostatic agent and is implicated in the age-associated capillary rarefaction in db/db mice through effects on angiopoietin-2 synthesis (7).
Rho-kinase signaling
Ras homolog gene family member A (RhoA) is a small GTPase involved in regulation of a diverse range of cell functions. RhoA signaling through its downstream effector, Rho-kinase (ROCK) has been implicated in the pathogenesis of cardiac fibrosis. Pharmacologic inhibition of ROCK inhibited cardiac interstitial and perivascular fibrosis in some (125) but not all (126) models of fibrotic remodeling. Genetic disruption studies demonstrated that the ROCK isoform ROCK1 mediates perivascular and interstitial fibrosis, but not cardiomyocyte hypertrophy, in models of angiotensin-induced and pressure overload-mediated cardiac remodeling (127), (128). Few studies have examined the role of ROCK signaling in cardiac remodeling associated with obesity and diabetes. In a rat model of diabetes induced by a high-fat diet in combination with alow dose streptozotocin injection, administration of the ROCK inhibitor fasudil reduced cardiac fibrosis and attenuated TGF-β/Smad signaling (129). In vitro experiments have suggested that ROCK signaling may also play a role in the hypertrophic response associated with obesity and type 2 diabetes. The pro-hypertrophic effects of leptin in cultured cardiomyocytes were attenuated by RhoA inhibitors (130).
THERAPEUTIC PERSPECTIVES
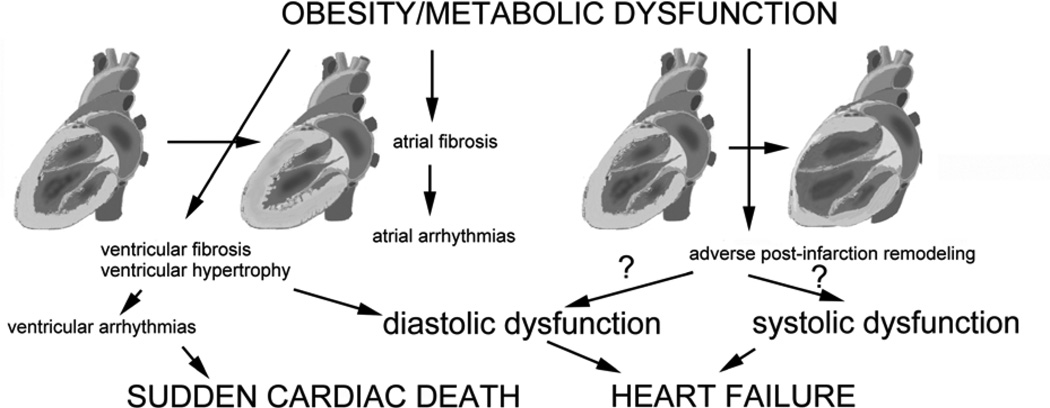
Cardiac fibrosis may play an important role in the pathogenesis of heart disease in obese patients through several distinct mechanisms (Figure 3). First, fibrotic myocardial remodeling may impair ventricular diastolic function contributing the development of heart failure with preserved ejection fraction. Second, development of atrial fibrosis may predispose obese subjects to atrial tachyarrhythmias (28). Third, collagen deposition in the ventricular myocardium may contribute to the increased incidence of ventricular arrhythmias and sudden death observed in obese individuals (131). Fourth, in some patients development of fibrotic changes in the right ventricle may perturb right ventricular function. Finally, obesity-related modulation of the reparative fibrotic response following infarction may predispose obese subjects to development of post-infarction heart failure (33). Thus, attenuation of fibrosis may have profound beneficial effects in reducing cardiac morbidity and mortality in obese subjects.
Figure 3.
The consequences of obesity-associated fibrosis. Ventricular fibrosis in obese subjects is usually accompanied by left ventricular hypertrophy and may contribute to the development of diastolic heart failure or cause ventricular arrhythmias. Atrial fibrosis may promote atrial fibrillation or other tachyarrhythmias. Obesity may also influence the response to myocardial infarction, modulating inflammatory and reparative cascades and affecting post-infarction remodeling and the fibrotic process.
Clearly, weight loss, achieved either via bariatric surgery or through diet, reverses many structural and functional cardiac abnormalities in obese patients (5). However, in several studies, despite significant weight loss, the functional consequences of obesity persist (5). Strategies aimed at inhibition and reversal of cardiac fibrosis may be particularly valuable in these patients. Anti-fibrotic measures for obese patients with significant fibrosis may include established pharmacologic agents inhibiting the RAAS (such as ACE inhibitors, angiotensin receptor blockers and aldosterone antagonists). Novel approaches targeting fibrogenic growth factors (such as TGF-β1), the ROS system, or ET-1 may be of additional value in selected cases. However, considering the important role of these pathways in homeostatic and reparative processes and the need for prolonged therapy, implementation of strategies to attenuate obesity-associated cardiac fibrosis may be challenging.
Much shorter therapeutic interventions may be effective in targeting the fibrotic response in patients with obesity and metabolic dysfunction that survive an acute myocardial infarction. Due to alterations in their metabolic and neurohumoral profile, these patients may have unique pathophysiologic defects in post-infarction repair. For example, overactive TGF-β/Smad signaling is often associated with insulin resistance, obesity and diabetes (7), (132) and may drive the reparative process towards a potent pro-fibrotic response. Identification of specific reparative defects in models of obesity and metabolic dysfunction and validation of these pathophysiologic alterations in obese patients with myocardial infarction using biomarkers or suitable imaging studies may result in design of specific therapeutic approaches to reduce adverse remodeling in these patients (133).
CONCLUSIONS AND FUTURE DIRECTIONS
Although the strong association between obesity and cardiac fibrosis is well-documented, the mechanisms of fibrotic cardiac remodeling and its impact on cardiac function and disease remain poorly understood. Most of the evidence in the field is purely associative, as robust mechanistic studies examining the role of specific pathways are lacking. Considering the increasing worldwide prevalence of obesity, there is an urgent need to understand the cellular and molecular basis of cardiac remodeling and dysfunction in obese subjects. Future research needs to focus on three main directions: a) exploration of the fundamental molecular links between metabolic dysregulation and activation of fibrogenic signaling. b) dissection of the cell biology of the fibrotic response using cell-specific targeting approaches. c) study of the significance of fibrogenic pathways in the clinical context. Although rodent models of obesity-associated cardiomyopathy are valuable tools in dissection of fundamental pathways, they cannot recapitulate the pathophysiologic complexity of human obesity.
ACKNOWLEDGMENTS
Dr Frangogiannis’ laboratory is supported by NIH grants R01 HL76246 and R01 HL85440 and by the Wilf Family Cardiovascular Research Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts to disclose. All authors have reviewed and approved the manuscript. All authors have read the journal's policy on disclosure of potential conflicts of interest and the journal's authorship agreement.
REFERENCES
- 1.Abel ED, Litwin SE, Sweeney G. Cardiac remodeling in obesity. Physiol Rev. 2008;88:389–419. doi: 10.1152/physrev.00017.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 3.Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist-to-hip ratio on the risk of heart failure. Circulation. 2010;121:237–244. doi: 10.1161/CIRCULATIONAHA.109.887893. [DOI] [PubMed] [Google Scholar]
- 4.Lauer MS, Anderson KM, Kannel WB, Levy D. The impact of obesity on left ventricular mass and geometry. The Framingham Heart Study. Jama. 1991;266:231–236. [PubMed] [Google Scholar]
- 5.Alpert MA, Omran J, Mehra A, Ardhanari S. Impact of obesity and weight loss on cardiac performance and morphology in adults. Prog Cardiovasc Dis. 2014;56:391–400. doi: 10.1016/j.pcad.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Eschalier R, Rossignol P, Kearney-Schwartz A, et al. Features of Cardiac Remodeling, Associated With Blood Pressure and Fibrosis Biomarkers, Are Frequent in Subjects With Abdominal Obesity. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.113.02419. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Quesada C, Cavalera M, Biernacka A, et al. Thrombospondin-1 induction in the diabetic myocardium stabilizes the cardiac matrix in addition to promoting vascular rarefaction through angiopoietin-2 upregulation. Circ Res. 2013;113:1331–1344. doi: 10.1161/CIRCRESAHA.113.302593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobaczewski M, de Haan JJ, Frangogiannis NG. The extracellular matrix modulates fibroblast phenotype and function in the infarcted myocardium. J Cardiovasc Transl Res. 2012;5:837–847. doi: 10.1007/s12265-012-9406-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: when is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 10.Weber KT. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 11.Eghbali M, Blumenfeld OO, Seifter S, et al. Localization of types I, III and IV collagen mRNAs in rat heart cells by in situ hybridization. J Mol Cell Cardiol. 1989;21:103–113. doi: 10.1016/0022-2828(89)91498-3. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol Heart Circ Physiol. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 13.Gazoti Debessa CR, Mesiano Maifrino LB, Rodrigues de Souza R. Age related changes of the collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- 14.de Souza RR. Aging of myocardial collagen. Biogerontology. 2002;3:325–335. doi: 10.1023/a:1021312027486. [DOI] [PubMed] [Google Scholar]
- 15.Biernacka A, Frangogiannis NG. Aging and Cardiac Fibrosis. Aging Dis. 2011;2:158–173. [PMC free article] [PubMed] [Google Scholar]
- 16.Khaidar A, Marx M, Lubec B, Lubec G. L-arginine reduces heart collagen accumulation in the diabetic db/db mouse. Circulation. 1994;90:479–483. doi: 10.1161/01.cir.90.1.479. [DOI] [PubMed] [Google Scholar]
- 17.Huynh K, Kiriazis H, Du XJ, et al. Coenzyme Q10 attenuates diastolic dysfunction, cardiomyocyte hypertrophy and cardiac fibrosis in the db/db mouse model of type 2 diabetes. Diabetologia. 2012;55:1544–1553. doi: 10.1007/s00125-012-2495-3. [DOI] [PubMed] [Google Scholar]
- 18.Sloan C, Tuinei J, Nemetz K, et al. Central leptin signaling is required to normalize myocardial fatty acid oxidation rates in caloric-restricted ob/ob mice. Diabetes. 2011;60:1424–1434. doi: 10.2337/db10-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zaman AK, Fujii S, Goto D, et al. Salutary effects of attenuation of angiotensin II on coronary perivascular fibrosis associated with insulin resistance and obesity. J Mol Cell Cardiol. 2004;37:525–535. doi: 10.1016/j.yjmcc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 20.Van den Bergh A, Vanderper A, Vangheluwe P, et al. Dyslipidaemia in type II diabetic mice does not aggravate contractile impairment but increases ventricular stiffness. Cardiovasc Res. 2008;77:371–379. doi: 10.1093/cvr/cvm001. [DOI] [PubMed] [Google Scholar]
- 21.Sahai A, Malladi P, Pan X, et al. Obese and diabetic db/db mice develop marked liver fibrosis in a model of nonalcoholic steatohepatitis: role of short-form leptin receptors and osteopontin. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1035–G1043. doi: 10.1152/ajpgi.00199.2004. [DOI] [PubMed] [Google Scholar]
- 22.Han DC, Isono M, Chen S, et al. Leptin stimulates type I collagen production in db/db mesangial cells: glucose uptake and TGF-beta type II receptor expression. Kidney Int. 2001;59:1315–1323. doi: 10.1046/j.1523-1755.2001.0590041315.x. [DOI] [PubMed] [Google Scholar]
- 23.Fredersdorf S, Thumann C, Ulucan C, et al. Myocardial hypertrophy and enhanced left ventricular contractility in Zucker diabetic fatty rats. Cardiovasc Pathol. 2004;13:11–19. doi: 10.1016/S1054-8807(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 24.Aroor AR, Sowers JR, Bender SB, et al. Dipeptidylpeptidase inhibition is associated with improvement in blood pressure and diastolic function in insulin-resistant male Zucker obese rats. Endocrinology. 2013;154:2501–2513. doi: 10.1210/en.2013-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zibadi S, Vazquez R, Moore D, Larson DF, Watson RR. Myocardial lysyl oxidase regulation of cardiac remodeling in a murine model of diet-induced metabolic syndrome. Am J Physiol Heart Circ Physiol. 2009;297:H976–H982. doi: 10.1152/ajpheart.00398.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin F, Siwik DA, Luptak I, et al. The polyphenols resveratrol and S17834 prevent the structural and functional sequelae of diet-induced metabolic heart disease in mice. Circulation. 2012;125:1757–1764. S1–S6. doi: 10.1161/CIRCULATIONAHA.111.067801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calligaris SD, Lecanda M, Solis F, et al. Mice long-term high-fat diet feeding recapitulates human cardiovascular alterations: an animal model to study the early phases of diabetic cardiomyopathy. PLoS One. 2013;8:e60931. doi: 10.1371/journal.pone.0060931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abed HS, Samuel CS, Lau DH, et al. Obesity results in progressive atrial structural and electrical remodeling: implications for atrial fibrillation. Heart Rhythm. 2013;10:90–100. doi: 10.1016/j.hrthm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Parise H, Levy D, et al. Obesity and the risk of new-onset atrial fibrillation. Jama. 2004;292:2471–2477. doi: 10.1001/jama.292.20.2471. [DOI] [PubMed] [Google Scholar]
- 30.Murase T, Hattori T, Ohtake M, et al. Cardiac remodeling and diastolic dysfunction in DahlS.Z-Lepr(fa)/Lepr(fa) rats: a new animal model of metabolic syndrome. Hypertens Res. 2012;35:186–1893. doi: 10.1038/hr.2011.157. [DOI] [PubMed] [Google Scholar]
- 31.van Bilsen M, Daniels A, Brouwers O, et al. Hypertension is a conditional factor for the development of cardiac hypertrophy in type 2 diabetic mice. PLoS One. 2014;9:e85078. doi: 10.1371/journal.pone.0085078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol. 2006;290:H146–H153. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 33.Thakker GD, Frangogiannis NG, Bujak M, et al. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am J Physiol Heart Circ Physiol. 2006;291:H2504–H2514. doi: 10.1152/ajpheart.00322.2006. [DOI] [PubMed] [Google Scholar]
- 34.Pascual M, Pascual DA, Soria F, et al. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–1156. doi: 10.1136/heart.89.10.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zarich SW, Kowalchuk GJ, McGuire MP, Benotti PN, Mascioli EA, Nesto RW. Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol. 1991;68:377–381. doi: 10.1016/0002-9149(91)90835-9. [DOI] [PubMed] [Google Scholar]
- 36.Scaglione R, Dichiara MA, Indovina A, et al. Left ventricular diastolic and systolic function in normotensive obese subjects: influence of degree and duration of obesity. Eur Heart J. 1992;13:738–742. doi: 10.1093/oxfordjournals.eurheartj.a060249. [DOI] [PubMed] [Google Scholar]
- 37.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E, Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–3087. doi: 10.1161/01.CIR.0000147184.13872.0F. [DOI] [PubMed] [Google Scholar]
- 38.Fischer VW, Barner HB, Larose LS. Pathomorphologic aspects of muscular tissue in diabetes mellitus. Hum Pathol. 1984;15:1127–1136. doi: 10.1016/s0046-8177(84)80307-x. [DOI] [PubMed] [Google Scholar]
- 39.Shimizu M, Umeda K, Sugihara N, et al. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–36. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Quilliot D, Alla F, Bohme P, et al. Myocardial collagen turnover in normotensive obese patients: relation to insulin resistance. Int J Obes (Lond) 2005;29:1321–1328. doi: 10.1038/sj.ijo.0803022. [DOI] [PubMed] [Google Scholar]
- 41.Kaltman AJ, Goldring RM. Role of circulatory congestion in the cardiorespiratory failure of obesity. Am J Med. 1976;60:645–653. doi: 10.1016/0002-9343(76)90499-x. [DOI] [PubMed] [Google Scholar]
- 42.Xia Y, Lee K, Li N, Corbett D, Mendoza L, Frangogiannis NG. Characterization of the inflammatory and fibrotic response in a mouse model of cardiac pressure overload. Histochem Cell Biol. 2009;131:471–481. doi: 10.1007/s00418-008-0541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulasova E, Gladden JD, Chen Y, et al. Loss of interstitial collagen causes structural and functional alterations of cardiomyocyte subsarcolemmal mitochondria in acute volume overload. J Mol Cell Cardiol. 2011;50:147–156. doi: 10.1016/j.yjmcc.2010.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lo CS, Chen ZH, Hsieh TJ, Shin SJ. Atrial natriuretic peptide attenuates high glucose-activated transforming growth factor-beta, Smad and collagen synthesis in renal proximal tubular cells. J Cell Biochem. 2008;103:1999–2009. doi: 10.1002/jcb.21590. [DOI] [PubMed] [Google Scholar]
- 45.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. J Clin Invest. 2011;121:2111–2117. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2014;71:549–574. doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol. 2014;70C:74–82. doi: 10.1016/j.yjmcc.2013.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fowlkes V, Clark J, Fix C, et al. Type II diabetes promotes a myofibroblast phenotype in cardiac fibroblasts. Life Sci. 2013;92:669–676. doi: 10.1016/j.lfs.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hutchinson KR, Lord CK, West TA, Stewart JA., Jr Cardiac fibroblast-dependent extracellular matrix accumulation is associated with diastolic stiffness in type 2 diabetes. PLoS One. 2013;8:e72080. doi: 10.1371/journal.pone.0072080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Haudek SB, Xia Y, Huebener P, et al. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. Proc Natl Acad Sci U S A. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kong P, Christia P, Frangogiannis NG. The pathogenesis of cardiac fibrosis. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanellakis P, Dinh TN, Agrotis A, Bobik A. CD4(+)CD25(+)Foxp3(+) regulatory T cells suppress cardiac fibrosis in the hypertensive heart. J Hypertens. 2011;29:1820–1828. doi: 10.1097/HJH.0b013e328349c62d. [DOI] [PubMed] [Google Scholar]
- 53.Pinto AR, Paolicelli R, Salimova E, et al. An abundant tissue macrophage population in the adult murine heart with a distinct alternatively-activated macrophage profile. PLoS One. 2012;7:e36814. doi: 10.1371/journal.pone.0036814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Epelman S, Lavine KJ, Beaudin AE, et al. Embryonic and Adult-Derived Resident Cardiac Macrophages Are Maintained through Distinct Mechanisms at Steady State and during Inflammation. Immunity. 2014;40:91–104. doi: 10.1016/j.immuni.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fukuda M, Nakamura T, Kataoka K, et al. Potentiation by candesartan of protective effects of pioglitazone against type 2 diabetic cardiovascular and renal complications in obese mice. J Hypertens. 2010;28:340–352. doi: 10.1097/HJH.0b013e32833366cd. [DOI] [PubMed] [Google Scholar]
- 56.Fukuda M, Nakamura T, Kataoka K, et al. Ezetimibe ameliorates cardiovascular complications and hepatic steatosis in obese and type 2 diabetic db/db mice. J Pharmacol Exp Ther. 2010;335:70–75. doi: 10.1124/jpet.110.170373. [DOI] [PubMed] [Google Scholar]
- 57.Yamaguchi T, Kitamori K, Ichihara G, et al. Serial changes in adipocytokines and cardiac function in a rat model of the metabolic syndrome. Clin Exp Pharmacol Physiol. 2013;40:443–448. doi: 10.1111/1440-1681.12107. [DOI] [PubMed] [Google Scholar]
- 58.Li ZL, Ebrahimi B, Zhang X, et al. Obesity-metabolic derangement exacerbates cardiomyocyte loss distal to moderate coronary artery stenosis in pigs without affecting global cardiac function. Am J Physiol Heart Circ Physiol. 2014 doi: 10.1152/ajpheart.00052.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zeisberg EM, Tarnavski O, Zeisberg M, et al. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 60.Widyantoro B, Emoto N, Nakayama K, et al. Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation. 2010;121:2407–2418. doi: 10.1161/CIRCULATIONAHA.110.938217. [DOI] [PubMed] [Google Scholar]
- 61.Koitabashi N, Danner T, Zaiman AL, et al. Pivotal role of cardiomyocyte TGF-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barouch LA, Gao D, Chen L, et al. Cardiac myocyte apoptosis is associated with increased DNA damage and decreased survival in murine models of obesity. Circ Res. 2006;98:119–124. doi: 10.1161/01.RES.0000199348.10580.1d. [DOI] [PubMed] [Google Scholar]
- 63.Zhi H, Luptak I, Alreja G, et al. Effects of direct Renin inhibition on myocardial fibrosis and cardiac fibroblast function. PLoS One. 2013;8:e81612. doi: 10.1371/journal.pone.0081612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin II is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 65.Sadoshima J, Izumo S. Molecular characterization of angiotensin II--induced hypertrophy of cardiac myocytes and hyperplasia of cardiac fibroblasts Critical role of the AT1 receptor subtype. Circ Res. 1993;73:413–423. doi: 10.1161/01.res.73.3.413. [DOI] [PubMed] [Google Scholar]
- 66.Crabos M, Roth M, Hahn AW, Erne P. Characterization of angiotensin II receptors in cultured adult rat cardiac fibroblasts Coupling to signaling systems and gene expression. J Clin Invest. 1994;93:2372–2378. doi: 10.1172/JCI117243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ohkubo N, Matsubara H, Nozawa Y, et al. Angiotensin type 2 receptors are reexpressed by cardiac fibroblasts from failing myopathic hamster hearts and inhibit cell growth and fibrillar collagen metabolism. Circulation. 1997;96:3954–3962. doi: 10.1161/01.cir.96.11.3954. [DOI] [PubMed] [Google Scholar]
- 68.Kurisu S, Ozono R, Oshima T, et al. Cardiac angiotensin II type 2 receptor activates the kinin/NO system and inhibits fibrosis. Hypertension. 2003;41:99–107. doi: 10.1161/01.hyp.0000050101.90932.14. [DOI] [PubMed] [Google Scholar]
- 69.Stockand JD, Meszaros JG. Aldosterone stimulates proliferation of cardiac fibroblasts by activating Ki-RasA and MAPK1/2 signaling. Am J Physiol Heart Circ Physiol. 2003;284:H176–H184. doi: 10.1152/ajpheart.00421.2002. [DOI] [PubMed] [Google Scholar]
- 70.Lavall D, Selzer C, Schuster P, et al. The mineralocorticoid receptor promotes fibrotic remodeling in atrial fibrillation. J Biol Chem. 2014 doi: 10.1074/jbc.M113.519256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Usher MG, Duan SZ, Ivaschenko CY, et al. Myeloid mineralocorticoid receptor controls macrophage polarization and cardiovascular hypertrophy and remodeling in mice. J Clin Invest. 2010;120:3350–3364. doi: 10.1172/JCI41080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol. 2013;9:459–469. doi: 10.1038/nrneph.2013.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schieffer B, Wirger A, Meybrunn M, et al. Comparative effects of chronic angiotensin-converting enzyme inhibition and angiotensin II type 1 receptor blockade on cardiac remodeling after myocardial infarction in the rat. Circulation. 1994;89:2273–2282. doi: 10.1161/01.cir.89.5.2273. [DOI] [PubMed] [Google Scholar]
- 74.Regan CP, Anderson PG, Bishop SP, Berecek KH. Pressure-independent effects of AT1-receptor antagonism on cardiovascular remodeling in aortic-banded rats. Am J Physiol. 1997;272:H2131–H2138. doi: 10.1152/ajpheart.1997.272.5.H2131. [DOI] [PubMed] [Google Scholar]
- 75.Jugdutt BI, Menon V, Kumar D, Idikio H. Vascular remodeling during healing after myocardial infarction in the dog model: effects of reperfusion, amlodipine and enalapril. J Am Coll Cardiol. 2002;39:1538–1545. doi: 10.1016/s0735-1097(02)01805-3. [DOI] [PubMed] [Google Scholar]
- 76.Senador D, Kanakamedala K, Irigoyen MC, Morris M, Elased KM. Cardiovascular and autonomic phenotype of db/db diabetic mice. Exp Physiol. 2009;94:648–658. doi: 10.1113/expphysiol.2008.046474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Takatsu M, Nakashima C, Takahashi K, et al. Calorie restriction attenuates cardiac remodeling and diastolic dysfunction in a rat model of metabolic syndrome. Hypertension. 2013;62:957–965. doi: 10.1161/HYPERTENSIONAHA.113.02093. [DOI] [PubMed] [Google Scholar]
- 78.Singh VP, Baker KM, Kumar R. Activation of the intracellular renin-angiotensin system in cardiac fibroblasts by high glucose: role in extracellular matrix production. Am J Physiol Heart Circ Physiol. 2008;294:H1675–H1684. doi: 10.1152/ajpheart.91493.2007. [DOI] [PubMed] [Google Scholar]
- 79.Toblli JE, Cao G, DeRosa G, Forcada P. Reduced cardiac expression of plasminogen activator inhibitor 1 and transforming growth factor beta1 in obese Zucker rats by perindopril. Heart. 2005;91:80–86. doi: 10.1136/hrt.2003.022707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zaman AK, Fujii S, Sawa H, et al. Angiotensin-converting enzyme inhibition attenuates hypofibrinolysis and reduces cardiac perivascular fibrosis in genetically obese diabetic mice. Circulation. 2001;103:3123–3128. doi: 10.1161/01.cir.103.25.3123. [DOI] [PubMed] [Google Scholar]
- 81.Vazquez-Medina JP, Popovich I, Thorwald MA, et al. Angiotensin receptor-mediated oxidative stress is associated with impaired cardiac redox signaling and mitochondrial function in insulin-resistant rats. Am J Physiol Heart Circ Physiol. 2013;305:H599–H607. doi: 10.1152/ajpheart.00101.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tabbi-Anneni I, Buchanan J, Cooksey RC, Abel ED. Captopril normalizes insulin signaling and insulin-regulated substrate metabolism in obese (ob/ob) mouse hearts. Endocrinology. 2008;149:4043–4050. doi: 10.1210/en.2007-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hilzendeger AM, Goncalves AC, Plehm R, et al. Autonomic dysregulation in ob/ob mice is improved by inhibition of angiotensin-converting enzyme. J Mol Med (Berl) 2010;88:383–390. doi: 10.1007/s00109-009-0569-6. [DOI] [PubMed] [Google Scholar]
- 84.Essick EE, Sam F. Cardiac hypertrophy and fibrosis in the metabolic syndrome: a role for aldosterone and the mineralocorticoid receptor. Int J Hypertens. 2011;2011:346985. doi: 10.4061/2011/346985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kosmala W, Przewlock α-Kosmala M, Szczepanik-Osadnik H, Mysiak A, Marwick TH. Fibrosis and cardiac function in obesity: a randomised controlled trial of aldosterone blockade. Heart. 2013;99:320–326. doi: 10.1136/heartjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 86.Biernacka A, Dobaczewski M, Frangogiannis NG. TGF-beta signaling in fibrosis. Growth Factors. 2011;29:196–202. doi: 10.3109/08977194.2011.595714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dobaczewski M, Chen W, Frangogiannis NG. Transforming growth factor (TGF)-beta signaling in cardiac remodeling. J Mol Cell Cardiol. 2011;51:600–606. doi: 10.1016/j.yjmcc.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dobaczewski M, Bujak M, Li N, et al. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–428. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Carroll JF, Tyagi SC. Extracellular matrix remodeling in the heart of the homocysteinemic obese rabbit. Am J Hypertens. 2005;18:692–698. doi: 10.1016/j.amjhyper.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 90.Ziyadeh FN, Sharma K, Ericksen M, Wolf G. Stimulation of collagen gene expression and protein synthesis in murine mesangial cells by high glucose is mediated by autocrine activation of transforming growth factor-beta. J Clin Invest. 1994;93:536–542. doi: 10.1172/JCI117004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kumpers P, Gueler F, Rong S, et al. Leptin is a coactivator of TGF-beta in unilateral ureteral obstructive kidney disease. Am J Physiol Renal Physiol. 2007;293:F1355–F1362. doi: 10.1152/ajprenal.00003.2007. [DOI] [PubMed] [Google Scholar]
- 92.Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–2466. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 93.Li SY, Yang X, Ceylan-Isik AF, Du M, Sreejayan N, Ren J. Cardiac contractile dysfunction in Lep/Lep obesity is accompanied by NADPH oxidase activation, oxidative modification of sarco(endo)plasmic reticulum Ca2+-ATPase and myosin heavy chain isozyme switch. Diabetologia. 2006;49:1434–1446. doi: 10.1007/s00125-006-0229-0. [DOI] [PubMed] [Google Scholar]
- 94.Ballal K, Wilson CR, Harmancey R, Taegtmeyer H. Obesogenic high fat western diet induces oxidative stress and apoptosis in rat heart. Mol Cell Biochem. 2010;344:221–230. doi: 10.1007/s11010-010-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Serpillon S, Floyd BC, Gupte RS, et al. Superoxide production by NAD(P)H oxidase mitochondria is increased in genetically obese and hyperglycemic rat heart aortab efore the development of cardiac dysfunction The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–H162. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yamazaki KG, Gonzalez E, Zambon AC. Crosstalk between the renin-angiotensin system and the advance glycation end product axis in the heart: role of the cardiac fibroblast. J Cardiovasc Transl Res. 2012;5:805–813. doi: 10.1007/s12265-012-9405-4. [DOI] [PubMed] [Google Scholar]
- 97.Asbun J, Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol. 2006;47:693–700. doi: 10.1016/j.jacc.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 98.Bodiga VL, Eda SR, Bodiga S. Advanced glycation end products: role in pathology of diabetic cardiomyopathy. Heart Fail Rev. 2014;19:49–63. doi: 10.1007/s10741-013-9374-y. [DOI] [PubMed] [Google Scholar]
- 99.Ramasamy R, Schmidt AM. Receptor for advanced glycation end products (RAGE) and implications for the pathophysiology of heart failure. Curr Heart Fail Rep. 2012;9:107–116. doi: 10.1007/s11897-012-0089-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao LM, Zhang W, Wang LP, Li GR, Deng XL. Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels. Pflugers Arch. 2012;464:613–621. doi: 10.1007/s00424-012-1165-0. [DOI] [PubMed] [Google Scholar]
- 101.Oldfield MD, Bach LA, Forbes JM, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE) J Clin Invest. 2001;108:1853–1863. doi: 10.1172/JCI11951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nielsen JM, Kristiansen SB, Norregaard R, et al. Blockage of receptor for advanced glycation end products prevents development of cardiac dysfunction in db/db type 2 diabetic mice. Eur J Heart Fail. 2009;11:638–647. doi: 10.1093/eurjhf/hfp070. [DOI] [PubMed] [Google Scholar]
- 103.Leask A. Potential therapeutic targets for cardiac fibrosis: TGFbeta, angiotensin, endothelin, CCN2, and PDGF, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 104.Adiarto S, Emoto N, Iwasa N, Yokoyama M. Obesity-induced upregulation of myocardial endothelin-1 expression is mediated by leptin. Biochem Biophys Res Commun. 2007;353:623–627. doi: 10.1016/j.bbrc.2006.12.066. [DOI] [PubMed] [Google Scholar]
- 105.Sen S, Chen S, Feng B, Iglarz M, Chakrabarti S. Renal, retinal and cardiac changes in type 2 diabetes are attenuated by macitentan, a dual endothelin receptor antagonist. Life Sci. 2012;91:658–668. doi: 10.1016/j.lfs.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 106.Perego L, Pizzocri P, Corradi D, et al. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90:4087–4093. doi: 10.1210/jc.2004-1963. [DOI] [PubMed] [Google Scholar]
- 107.Barouch LA, Berkowitz DE, Harrison RW, O'Donnell CP, Hare JM. Disruption of leptin signaling contributes to cardiac hypertrophy independently of body weight in mice. Circulation. 2003;108:754–759. doi: 10.1161/01.CIR.0000083716.82622.FD. [DOI] [PubMed] [Google Scholar]
- 108.Rajapurohitam V, Gan XT, Kirshenbaum LA, Karmazyn M. The obesity-associated peptide leptin induces hypertrophy in neonatal rat ventricular myocytes. Circ Res. 2003;93:277–279. doi: 10.1161/01.RES.0000089255.37804.72. [DOI] [PubMed] [Google Scholar]
- 109.Fukui A, Takahashi N, Nakada C, et al. Role of leptin signaling in the pathogenesis of angiotensin II-mediated atrial fibrosis and fibrillation. Circ Arrhythm Electrophysiol. 2013;6:402–409. doi: 10.1161/CIRCEP.111.000104. [DOI] [PubMed] [Google Scholar]
- 110.Zibadi S, Cordova F, Slack EH, Watson RR, Larson DF. Leptin's regulation of obesity-induced cardiac extracellular matrix remodeling. Cardiovasc Toxicol. 2011;11:325–333. doi: 10.1007/s12012-011-9124-0. [DOI] [PubMed] [Google Scholar]
- 111.Matsuzawa Y, Funahashi T, Kihara S, Shimomura I. Adiponectin and metabolic syndrome. Arterioscler Thromb Vasc Biol. 2004;24:29–33. doi: 10.1161/01.ATV.0000099786.99623.EF. [DOI] [PubMed] [Google Scholar]
- 112.Hopkins TA, Ouchi N, Shibata R, Walsh K. Adiponectin actions in the cardiovascular system. Cardiovasc Res. 2007;74:11–18. doi: 10.1016/j.cardiores.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fujita K, Maeda N, Sonoda M, et al. Adiponectin protects against angiotensin II-induced cardiac fibrosis through activation of PPAR-alpha. Arterioscler Thromb Vasc Biol. 2008;28:863–870. doi: 10.1161/ATVBAHA.107.156687. [DOI] [PubMed] [Google Scholar]
- 114.Dadson K, Chasiotis H, Wannaiampikul S, Tungtrongchitr R, Xu A, Sweeney G. Adiponectin Mediated APPL1-AMPK Signaling Induces Cell Migration, MMP Activation, and Collagen Remodeling in Cardiac Fibroblasts. J Cell Biochem. 2014;115:785–793. doi: 10.1002/jcb.24722. [DOI] [PubMed] [Google Scholar]
- 115.Cai XJ, Chen L, Li L, et al. Adiponectin inhibits lipopolysaccharide-induced adventitial fibroblast migration and transition to myofibroblasts via AdipoR1-AMPK-iNOS pathway. Mol Endocrinol. 2010;24:218–228. doi: 10.1210/me.2009-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Shibata R, Ouchi N, Ito M, et al. Adiponectin-mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. doi: 10.1038/nm1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bornstein P. Matricellular proteins: an overview. J Cell Commun Signal. 2009;3:163–165. doi: 10.1007/s12079-009-0069-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Frangogiannis NG. Matricellular proteins in cardiac adaptation and disease. Physiol Rev. 2012;92:635–688. doi: 10.1152/physrev.00008.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kong P, Cavalera M, Frangogiannis NG. The role of thrombospondin (TSP)-1 in obesity and diabetes. Adipocyte. 2014;3:81–84. doi: 10.4161/adip.26990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Varma V, Yao-Borengasser A, Bodles AM, et al. Thrombospondin-1 is an adipokine associated with obesity, adipose inflammation, and insulin resistance. Diabetes. 2008;57:432–439. doi: 10.2337/db07-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kong P, Gonzalez-Quesada C, Li N, Cavalera M, Lee DW, Frangogiannis NG. Thrombospondin-1 regulates adiposity and metabolic dysfunction in diet-induced obesity enhancing adipose inflammation and stimulating adipocyte proliferation. Am J Physiol Endocrinol Metab. 2013;305:E439–E450. doi: 10.1152/ajpendo.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Raman P, Krukovets I, Marinic TE, Bornstein P, Stenina OI. Glycosylation mediates up-regulation of a potent antiangiogenic and proatherogenic protein, thrombospondin-1, by glucose in vascular smooth muscle cells. J Biol Chem. 2007;282:5704–5714. doi: 10.1074/jbc.M610965200. [DOI] [PubMed] [Google Scholar]
- 123.Frangogiannis NG, Ren G, Dewald O, et al. The critical role of endogenous Thrombospondin (TSP)-1 in preventing expansion of healing myocardial infarcts. Circulation. 2005;111:2935–2942. doi: 10.1161/CIRCULATIONAHA.104.510354. [DOI] [PubMed] [Google Scholar]
- 124.Xia Y, Dobaczewski M, Gonzalez-Quesada C, et al. Endogenous thrombospondin 1 protects the pressure-overloaded myocardium by modulating fibroblast phenotype and matrix metabolism. Hypertension. 2011;58:902–911. doi: 10.1161/HYPERTENSIONAHA.111.175323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang YX, da Cunha V, Martin-McNulty B, et al. Inhibition of Rho-kinase by fasudil attenuated angiotensin II-induced cardiac hypertrophy in apolipoprotein E deficient mice. Eur J Pharmacol. 2005;512:215–222. doi: 10.1016/j.ejphar.2005.02.024. [DOI] [PubMed] [Google Scholar]
- 126.Satoh S, Ueda Y, Koyanagi M, et al. Chronic inhibition of Rho kinase blunts the process of left ventricular hypertrophy leading to cardiac contractile dysfunction in hypertension-induced heart failure. J Mol Cell Cardiol. 2003;35:59–70. doi: 10.1016/s0022-2828(02)00278-x. [DOI] [PubMed] [Google Scholar]
- 127.Rikitake Y, Oyama N, Wang CY, et al. Decreased perivascular fibrosis but not cardiac hypertrophy in ROCK1+/− haploinsufficient mice. Circulation. 2005;112:2959–2965. doi: 10.1161/CIRCULATIONAHA.105.584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang YM, Bo J, Taffet GE, et al. Targeted deletion of ROCK1 protects the heart against pressure overload by inhibiting reactive fibrosis. Faseb J. 2006;20:916–925. doi: 10.1096/fj.05-5129com. [DOI] [PubMed] [Google Scholar]
- 129.Zhou H, Li YJ, Wang M, et al. Involvement of RhoA/ROCK in myocardial fibrosis in a rat model of type 2 diabetes. Acta Pharmacol Sin. 2011;32:999–1008. doi: 10.1038/aps.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zeidan A, Javadov S, Karmazyn M. Essential role of Rho/ROCK-dependent processes and actin dynamics in mediating leptin-induced hypertrophy in rat neonatal ventricular myocytes. Cardiovasc Res. 2006;72:101–111. doi: 10.1016/j.cardiores.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 131.Albert CM, Chae CU, Grodstein F, et al. Prospective study of sudden cardiac death among women in the United States. Circulation. 2003;107:2096–2101. doi: 10.1161/01.CIR.0000065223.21530.11. [DOI] [PubMed] [Google Scholar]
- 132.Wang HT, Liu CF, Tsai TH, et al. Effect of obesity reduction on preservation of heart function and attenuation of left ventricular remodeling, oxidative stress and inflammation in obese mice. J Transl Med. 2012;10:145. doi: 10.1186/1479-5876-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Frangogiannis NG. Biomarkers: hopes and challenges in the path from discovery to clinical practice. Transl Res. 2012;159:197–204. doi: 10.1016/j.trsl.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]