Abstract
Objective
Levels of tissue iron contribute to determining diabetes risk, but little is known about the effects of higher iron levels on weight, nor on the interaction of weight and iron overload on diabetes risk. We therefore examined the effect of iron on body mass index and diabetes in individuals with iron overload from hereditary hemochromatosis (HH), compared to non-HH siblings and historical controls.
Methods
Chart reviews were performed on a cohort of adults (age ≥40, N=101) with the common C282Y/C282Y HFE genotype, compared to wild type siblings (N=32) and comparable NHANES cohorts, with respect to body mass index and diabetes status.
Results
Males with HH have lower body mass index (BMI) than control siblings. Females had a trend toward decreased BMI that was not significant, possibly related to decreased degrees of iron overload. In both males and females, increased rates of diabetes were seen, especially in the overweight or obese.
Conclusions
High tissue iron levels may be both pro- and anti-diabetic. The prevalence of obesity and diabetes in HH is likely dependent upon the degree of iron overload, caloric intake, and other genetic and environmental factors, contributing to the observed heterogeneity in the frequency of disease-related morbidities in HH.
Keywords: hereditary hemochromatosis, diabetes, iron overload, obesity
Introduction
Iron restriction causes adiposity in rats (1), and iron deficiency is associated with obesity in humans (2). Less is known about the effects of higher levels of iron on body weight. We therefore studied individuals with hereditary hemochromatosis (HH), an autosomal recessive disorder characterized by excessive iron storage affecting 0.3-0.5% of Caucasians (3). Two missense mutations in the HFE gene account for the majority of HH: C282Y and H63D (3, 4), with C282Y homozygotes having the highest risk (5). In the absence of normal HFE, hepcidin, a peptide that regulates iron entry into the circulation, is reduced and dietary iron absorption is high (6). As iron accumulates in tissues, clinical manifestations of cirrhosis, diabetes and other organ damage may ensue.
We previously analyzed individuals with HH and found a significant prevalence of diabetes (7). A mouse model of hemochromatosis with deletion of the Hfe gene demonstrates decreased insulin secretion and loss of beta cell mass, consistent with the human phenotype (8). Before the onset of diabetes in HH, both mice and humans exhibit increased insulin sensitivity, a phenomenon that in the mouse model is caused by increased expression of the insulin sensitizing hormone adiponectin (7, 9). Additionally, the mouse model was protected from high fat diet-induced obesity (10), and we therefore hypothesized that humans with HH might also be so protected. However, if diet-induced obesity ensued despite that relative protection, we hypothesized that the inability to compensate for the insulin resistance with increased insulin secretion would cause diabetes to occur at an increased frequency compared to non-HH individuals.
Methods
Subjects
The study participants were recruited from referrals to the Hemochromatosis Research Clinic at the University of Utah from 2000 to the present. HFE genotyping was performed using allele-specific PCR primers (7). All of the referred patients agreed to serve in the study and informed consent was obtained. Siblings and other relatives of the referred subjects were screened for hemochromatosis mutations and those relatives who were found to be homozygous wild type or heterozygous wild type/mutant at the HFE locus served as controls. (Heterozygotes for HFE mutations are not iron-overloaded and do not suffer associated morbidities (11)). All participants were Europoid whites. Because of the low number of wild type sibling controls, we also used comparator groups (non-Hispanic white, age >40) from the National Health and Nutrition Examination Survey (NHANES) (12, 13)). The study was approved by the University of Utah Institutional Review Board.
Clinical studies
Chart reviews were performed on 243 charts from hemochromatosis subjects studied from 2000 to the present. Because iron overload develops over the lifespan, subjects less than 40 years of age and those with incomplete data on documentation of diabetes status or height and weight were excluded. Ages did not differ among the groups (C282Y/C282Y: Males, N=67, 53.9±1.7 years; females, N=34, 57.2±2.5 years; Wild type or heterozygotes: Males, N=15, 54.6±1.1 years; females, N=17, 56.3±1.6 years). Statistical analyses used SPSS software (Chicago, IL).
Results
Because of heterogeneity in phenotypes among different HH genotypes (5), we included only C282Y/C282Y homozygotes in the HH group, all aged ≥40y. Body mass index (BMI) was lower in males with HH (mean, 26.7±0.5 kg/m2, median 26.5), compared to wild type or heterozygote controls (mean, 30.5±1.6 kg/m2, median 29.3) (Fig. 1, p=0.014). We also compared these individuals to the 1999- 2002 NHANES cohort; most HH individuals were recruited in that interval, and we sought to minimize bias because of increasing BMI over time in the U.S. Non- Hispanic white men, age≥40 in that cohort had an average BMI of 28.7±0.3 (13), significantly greater than that of the HH group (p<0.05) but not the sibling controls. Female C282Y/C282Y homozygotes by contrast, did not have significantly lower BMI than controls (HH, mean 26.0±1.1 kg/m2, median 23.9; controls 26.7±1.1 kg/m2, median 25.7; NHANES mean 28.3±0.3 kg/m2, p=0.51). Because women have lower serum ferritin values and consequently fewer clinical manifestations of HH than men (14), we assessed ferritin values and found that they trended lower in the females than in the men (females, 508±150 ng/mL, normal 12-240 ng/mL; males 1038±207 ng/mL, normal 30-490 ng/mL, p=0.08).
Figure 1. Average body mass index (BMI) in males and females homozygous for the C282Y HFE mutation compared to wild type or heterozygous siblings.
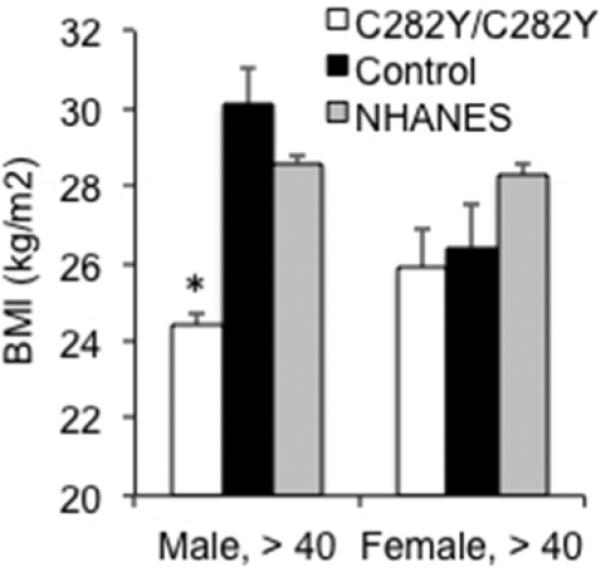
BMI (mean±SEM) was determined in subjects over age 40 (N=67 male and 34 female C282Y homozygotes; 15 male and 17 female sibling controls). Data are also shown for the 1999-2002 NHANES cohort (13). Wild type denotes the phenotype and includes C282Y heterozygotes. * p<0.05, C282Y vs. Control or NHANES, t-test
We next assessed diabetes frequency as a function of weight in HH subjects compared to control and NHANES cohorts, age≥40. The fraction of all C282Y/C282Y homozygotes with diabetes (23/92=0.25) was higher than that of non-Hispanic white adults in the 1999-2000 NHANES cohort (12)(387/3602=.11, p<0.001 by Fisher's exact test). Our non-HH controls had a similar frequency of diabetes as the NHANES cohort (0/8 with normal weight, 1/8 with overweight, and 1/9 with obesity, overall frequency .08) although because of the small number on whom we had definitive data about diabetes, the frequency was not significantly different from the C282Y/C282Y homozygotes (p=0.08). When the C282Y/C282Y homozygotes were compared to the NHANES cohort by weight category (12), the frequency of diabetes was significantly higher in all groups (p=0.025, <0.0001 and 0.0048 in normal, overweight, and obese, respectively, Fig. 2). The absolute increase in prevalence of diabetes was highest in the obese HH group, with 44.4% diabetics compared to 15.5% in controls). The overall frequency of diabetes in C282Y homozygotes did not differ between males (16/60=0.27) and females (7/32=0.22, p=0.80 by Fisher's exact test).
Figure 2. Fraction of individuals homozygous for the C282Y HFE mutation who have diabetes, by weight category, compared to the 1999-2002 study of the NHANES cohort.
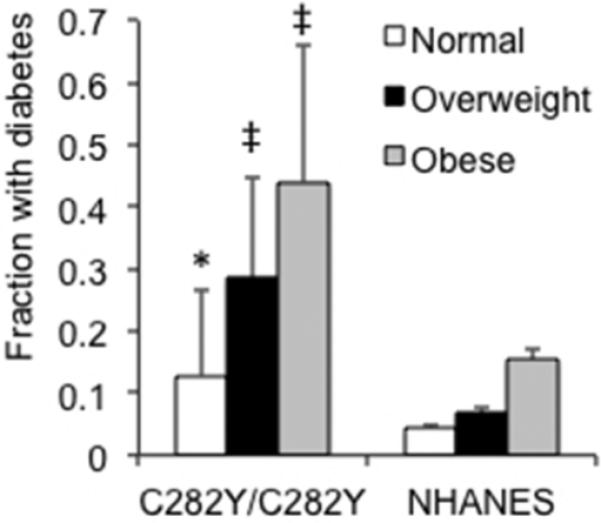
Individuals were ascertained as having diabetes by criteria of fasting blood glucose, glucose tolerance testing, or medical history and use of antidiabetic medications. Individuals whose glucose status was not known were excluded. Males and females were combined, but did not differ in overall diabetes frequency (total 16/60 males and 7/32 females). The number of sibling controls whose diabetes status was known was small but comparable to the NHANES cohort (0/8 normal weight, 1/8 overweight, 1/9=8% diabetic). The NHANES 1999-2002 cohort was chosen as the comparator because the diabetes assessment of most of the hemochromatosis cohort was performed in that time frame. (C282Y/C282Y, N=39 normal weight, 35 overweight, 18 obese; NHANES, N=5216 normal weight, 5202 overweight, 4633 obese). Error bars reflect 95% confidence intervals. * p<0.05, ‡ p<0.001 C282Y vs. NHANES
Discussion
We demonstrate that iron overload in HH is associated with decreased weight in men. In addition, overweight and obesity significantly increase the prevalence of diabetes in HH. Obesity has been previously reported to be a risk factor for diabetes in HH (15). In addition to supporting this conclusion, the current study reports an extremely high prevalence of diabetes in obese hemochromatosis subjects.
The effects of HH were dampened in women, although in all cases the differences between HH subjects and controls trended in the same direction for both sexes. Complications of HH are seen at lower frequency in women, presumably related to decreased iron overload due in part to blood loss during menstruation and child birth (14), consistent with the observed trend toward lower ferritin values in the female HH subjects.
There are conflicting reports about the frequency of morbidity in HH. In a cross- sectional study, for example, Beutler et al. found no difference in self-reported symptoms among those with HFE mutations compared to controls with the exception of homozygotes reporting a greater history of liver disorders (16). In contrast, two large studies that assessed severity of disease by objective criteria including liver biopsy found a higher frequency of morbidity (14, 17). This was true even in a clinically unselected cohort of relatives of patients with HH found to be homozygous for HFE mutations (14). The current data provide a possible explanation for these discrepancies, namely there may be competing morbid (e.g. insulin deficiency, cirrhosis) and protective (leanness) aspects of iron overload, that might be expected to be more or less present in populations with differing diets, environments, or genetic backgrounds.
There has also been controversy about the prevalence of diabetes in HH. Prior to the advent of genetic testing, there was selection bias for individuals with diabetes being identified as having hemochromatosis, but even after discovery of the HFE mutations, studies have yielded conflicting results. Some studies have detected no increase in diabetes prevalence in individuals with HFE mutations (16), although positive associations between diabetes and HFE mutations have been observed in several other studies (reviewed in (18)). The relative protection from obesity may explain some of the conflicting data on diabetes penetrance. In prior years when obesity was less prevalent, in populations with different risk for obesity, or with differing diets and prevalence of iron deficiency, the protection from obesity would vary in magnitude and thus affect the resultant overall frequency of diabetes.
The fact that diabetes risk is increased to a greater extent in HH individuals with overweight and obesity is consistent with our observations that the primary early effect of HH is loss of insulin secretory capacity (7, 8). Thus, obese individuals with HH are less able to compensate for insulin resistance with hyperinsulinemia because of less insulin secretory capacity (19). These results also speak to controversy over the relative contributions of insulin resistance and insulin secretion to diabetes in HH (18). Based on the current data, a significant number of HH diabetics would be insulin resistant based on their obesity, even though HH itself is not characterized by insulin resistance (7, 9). At the same time, it is also possible to lose almost all insulin secretory capacity in HH (7), explaining higher rates of diabetes even in lean HH subjects.
The mechanism for decreased weight in individuals with HH is not known. HH mice on a high fat diet are hypermetabolic, protected from obesity, and exhibit increased fatty acid oxidation and activation of AMPK (10, 20). Further mechanistic studies in humans are important given the conflicting effects of high iron on diabetes risk, namely protection from obesity but decreased insulin secretion. Given the broad 20-fold range of “normal” serum ferritin values, there likely exists a narrower range that supports both optimal weight and insulin secretion.
What is known:
Increased iron stores are a risk factor for diabetes
Iron deficiency is associated with increased weight, although little is known about the effects of higher iron stores on weight
What this study adds:
Iron overload in hereditary hemochromatosis is associated with lower body mass index
Despite relative protection from overweight, individuals with obesity and iron overload have significantly higher rates of diabetes
Acknowledgments
Research was supported by the NIH (NCATS, 1ULTR001067,DAM; NIDDK, DK081842, DAM) and Research Service of the VA.
Footnotes
The authors have no competing interests.
References
- 1.McClung JP, Andersen NE, Tarr TN, Stahl CH, Young AJ. Physical activity prevents augmented body fat accretion in moderately iron-deficient rats. J Nutr. 2008;138(7):1293–7. doi: 10.1093/jn/138.7.1293. Epub 2008/06/24. [DOI] [PubMed] [Google Scholar]
- 2.Pinhas-Hamiel O, Newfield RS, Koren I, Agmon A, Lilos P, Phillip M. Greater prevalence of iron deficiency in overweight and obese children and adolescents. Int J Obes Relat Metab Disord. 2003;27(3):416–8. doi: 10.1038/sj.ijo.0802224. Epub 2003/03/12. [DOI] [PubMed] [Google Scholar]
- 3.Pietrangelo A. Hereditary hemochromatosis: pathogenesis, diagnosis, and treatment. Gastroenterology. 2010;139(2):393–408. e1–2. doi: 10.1053/j.gastro.2010.06.013. Epub 2010/06/15. [DOI] [PubMed] [Google Scholar]
- 4.Feder JN, Gnirke A, Thomas W, Tsuchihashi Z, Ruddy DA, Basava A, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. Epub 1996/08/01. [DOI] [PubMed] [Google Scholar]
- 5.Neghina AM, Anghel A. Hemochromatosis genotypes and risk of iron overload--a meta-analysis. Annals of epidemiology. 2011;21(1):1–14. doi: 10.1016/j.annepidem.2010.05.013. Epub 2010/08/31. [DOI] [PubMed] [Google Scholar]
- 6.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–3. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 7.McClain D, Abraham D, Rogers J, Brady R, Gault P, Ajioka R, et al. High prevalence of abnormal glucose homeostasis secondary to decreased insulin secretion in individuals with hereditary haemochromatosis. Diabetologia. 2006;49(7):1661–9. doi: 10.1007/s00125-006-0200-0. [DOI] [PubMed] [Google Scholar]
- 8.Cooksey RC, Jouihan HA, Ajioka RS, Hazel MW, Jones DL, Kushner JP, et al. Oxidative stress, beta-cell apoptosis, and decreased insulin secretory capacity in mouse models of hemochromatosis. Endocrinology. 2004;145(11):5305–12. doi: 10.1210/en.2004-0392. [DOI] [PubMed] [Google Scholar]
- 9.Gabrielsen JS, Gao Y, Simcox JA, Huang J, Thorup D, Jones D, et al. Adipocyte iron regulates adiponectin and insulin sensitivity. J Clin Invest. 2012 doi: 10.1172/JCI44421. Epub 2012/09/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang J, Jones D, Luo B, Sanderson M, Soto J, Abel ED, et al. Iron overload and diabetes risk: a shift from glucose to Fatty Acid oxidation and increased hepatic glucose production in a mouse model of hereditary hemochromatosis. Diabetes. 2011;60(1):80–7. doi: 10.2337/db10-0593. Epub 2010/09/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Adams PC, Reboussin DM, Barton JC, McLaren CE, Eckfeldt JH, McLaren GD, et al. Hemochromatosis and iron-overload screening in a racially diverse population. N Engl J Med. 2005;352(17):1769–78. doi: 10.1056/NEJMoa041534. [DOI] [PubMed] [Google Scholar]
- 12.Gregg EW, Cadwell BL, Cheng YJ, Cowie CC, Williams DE, Geiss L, et al. Trends in the prevalence and ratio of diagnosed to undiagnosed diabetes according to obesity levels in the U.S. Diabetes Care. 2004;27(12):2806–12. doi: 10.2337/diacare.27.12.2806. Epub 2004/11/25. [DOI] [PubMed] [Google Scholar]
- 13.Ogden CL, Fryar CD, Carroll MD, Flegal KM. Mean body weight, height, and body mass index, United States 1960-2002. Advance data. 2004;(347):1–17. Epub 2004/11/17. [PubMed] [Google Scholar]
- 14.Bulaj ZJ, Ajioka RS, Phillips JD, LaSalle BA, Jorde LB, Griffen LM, et al. Disease-related conditions in relatives of patients with hemochromatosis. N Engl J Med. 2000;343(21):1529–35. doi: 10.1056/NEJM200011233432104. [DOI] [PubMed] [Google Scholar]
- 15.Barton JC, Barton JC, Acton RT. Diabetes in first-degree family members: a predictor of type 2 diabetes in 159 nonscreening Alabama hemochromatosis probands with HFE C282Y homozygosity. Diabetes Care. 2014;37(1):259–66. doi: 10.2337/dc13-0713. Epub 2013/08/31. [DOI] [PubMed] [Google Scholar]
- 16.Beutler E, Felitti VJ, Koziol JA, Ho NJ, Gelbart T. Penetrance of 845G--> A (C282Y) HFE hereditary haemochromatosis mutation in the USA. Lancet. 2002;359(9302):211–8. doi: 10.1016/S0140-6736(02)07447-0. Epub 2002/01/29. [DOI] [PubMed] [Google Scholar]
- 17.Allen KJ, Gurrin LC, Constantine CC, Osborne NJ, Delatycki MB, Nicoll AJ, et al. Iron-overload-related disease in HFE hereditary hemochromatosis. N Engl J Med. 2008;358(3):221–30. doi: 10.1056/NEJMoa073286. Epub 2008/01/18. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell TC, McClain DA. Diabetes and Hemochromatosis. Current Diabetes Reports. 2014;14:488–96. doi: 10.1007/s11892-014-0488-y. [DOI] [PubMed] [Google Scholar]
- 19.Abraham D, Rogers J, Gault P, Kushner J, McClain D. Increased insulin secretory capacity but decreased insulin sensitivity after correction of iron overload by phlebotomy in hereditary haemochromatosis. Diabetologia. 2006;49(11):2546–51. doi: 10.1007/s00125-006-0445-7. [DOI] [PubMed] [Google Scholar]
- 20.Huang J, Gabrielsen JS, Cooksey RC, Luo B, Boros LG, Jones DL, et al. Increased glucose disposal and AMP-dependent kinase signaling in a mouse model of hemochromatosis. J Biol Chem. 2007;282(52):37501–7. doi: 10.1074/jbc.M703625200. [DOI] [PubMed] [Google Scholar]


