Abstract
In schizophrenia, diminished vocal expressivity is associated with lower quality of life. Studies using computerized acoustic analysis of speech have found no evidence of diminished vocal prosody related to categorically-defined schizotypy, a subclinical analog of schizophrenia. However, existing studies have not examined the interaction between schizotypy and sex with vocal prosody measures. The current study examined 44 young adults (50% male) who were recruited to represent a continuous range of schizotypy. Speech samples were digitally recorded during autobiographical narratives and analyzed for prosody. In male participants, variability of fundamental frequency and variability of intensity were each negatively related to Schizotypal Personality Questionnaire (SPQ) Ideas of Reference subscale, while SPQ Suspiciousness was related to a greater number of utterances, and SPQ Odd Behavior was related to a greater number of pauses. As the relationships were restricted to males, and not significant in females, results may explain earlier negative findings with schizotypy.
Keywords: schizotypy, schizotypal, schizophrenia, speech, prosody
Introduction
The negative symptoms of schizophrenia are related to a wide range of adverse outcomes, including lower subjective psychological well-being (Strauss et al., 2012b), reduced likelihood of illness remission (Diaz et al., 2013), and impairments in social and vocational functioning (Strauss et al., 2012a; Tsang et al., 2010). As compared to positive symptoms, the negative symptoms remain relatively resistant to current forms of treatment for schizophrenia (Moller, 2012). A better understanding of negative symptoms will improve empirical and clinical assessments techniques and guide new treatment development.
Replicated evidence supports a two-factor structure of negative symptoms that separates this category into diminished expressivity (e.g., flattened affect, blunted musculature response to affective stimuli) and asociality (e.g., social withdrawal, social avolition) domains that are likely related to different underlying neural dysfunction (Blanchard and Cohen, 2006; Liemburg et al., 2013). Diminished expressivity (i.e., flattened affect) is associated with a lower quality of life and worse outcome (Gur et al., 2006; Hoekert et al., 2007). Much of the research regarding flattened affect in schizophrenia has focused on reduced facial musculature response to emotionally-valenced stimuli (Kring & Moran, 2008). However, the diminished expressivity domain encompasses a broader range of emotion expression that includes affective, linguistic, and paralinguistic indicators (Messinger, 2011). In particular, diminished vocal communication has been reported in a subset of individuals with schizophrenia (for meta-analysis, see: Hoekert et al., 2007), and there is evidence that this involves separate channels of speech production (i.e., alogia) and speech variability (i.e., flattened vocal affect; Blanchard and Cohen, 2006). However, much of this early work relied on subjective ratings of prosody. A more objective method involves employing computerized analysis of behavior, for example, though acoustic analysis of natural speech to understand pause and utterance length, and variability in amplitude and fundamental frequency (i.e., “pitch”). Using this technique, diminished vocal prosody in schizophrenia has been associated with basic neurocognitive abnormalities (Cohen et al., 2013a) and poor social functioning (Cohen et al., 2012b). Not surprisingly, acoustic-based measures of speech have been associated with clinically-rated negative symptoms (Cohen et al., 2013a), though associations with other symptoms have been inconsistent.
Schizophrenia appears to exist on a spectrum that begins with normality, proceeds towards schizotypy (a latent personality construct related to schizophrenia), then schizotypal personality disorder (SPD), and ends with the more impairing disorders of schizoaffective disorder and schizophrenia (Asai et al., 2011; Esterberg et al., 2009). The study of schizotypy provides an analog for examining neurocognitive aspects of schizophrenia in the relative absence of confounds such as unrelated illness consequences (e.g., hospitalization) and pharmacological interventions (Cochrane et al., 2012). However, variability in the measurement of schizotypy poses a problem in this area of research, as particular self-report measures of schizotypal traits emphasize different symptoms. For instance, while the Schizotypal Personality Questionnaire (SPQ; Raine, 1991) does not have a significant emphasis on the subjective experience of anhedonia (particularly in non-social domains), the Chapman Scales of Psychosis Proneness (Chapman et al., 1995) include both physical and social anhedonia scales, but do not capture all components of the SPQ. However, both of these instruments are typically used in isolation. While each measure offers an efficient and effective tool for identifying individuals with schizotypal traits, each only represents a portion of the full theoretical symptom profile.
Similar to schizophrenia, individuals with schizotypy tend to display limited affective expression, although findings are mixed. Studies that have defined schizotypy through social anhedonia measures found reduced behavioral ratings of facial expressivity (Collins et al., 2005; Kring et al., 1994; Leung et al., 2010) and verbal expressivity (Collins et al., 2005). In contrast, one recent SPQ study using computerized facial expression analysis did not find a relationship between facial expressivity and schizotypy (Cohen et al., 2013b), although some facial abnormalities were associated with severity of psychotic experiences more generally. Similarly, three studies that used computerized prosodic analysis of speech in SPQ-defined schizotypy did not find an overall group deficit in prosody during conditions which allowed for self-directed speech without additional cognitive demands (Cohen et al., 2009; Cohen et al., 2011; Cohen et al., 2012a). Only one of these three studies examined correlations between the speech variables and dimensional symptom ratings of schizotypy under a relatively natural speaking condition (Cohen et al., 2011). This study found that higher scores on Ideas of Reference, Unusual Perceptual Experiences, and Suspiciousness subscales of the SPQ related to greater overall speech output in reaction to stimuli, while the SPQ subscale of Constricted Affect showed a negative relationship with speech inflection (i.e., variability in intonation). In addition, a recent study reported reduced variability in intonation during self-generated speech in individuals formally diagnosed with SPD, which has similar features as schizotypy but adds the requirement of a clinical level of functional impairment or distress (Dickey et al., 2012).
Notably, the studies examining prosody differences in samples of individuals with schizotypy and SPD did not examine potential interactions of sex. Examining this interaction is important for several reasons. First, sex differences have been reported in the symptomology of schizotypy, with males reporting more negative traits, and females reporting more positive traits (Bora et al., 2009; Fonseca-Pedrero et al., 2012; Miller et al., 1995; Raine, 1992). In addition, there have been several reports of schizotypy by sex interactions on a variety of other neurocognitive measures (Bedwell et al., 2011; Johnston et al., 2008; Sumich et al., 2008). Finally, there is evidence that within individuals with schizophrenia, males are more likely to show flat affect (Gur et al., 2006). Therefore, a potential schizotypy by sex interaction may account for the lack of group differences in speech prosody reported with samples of individuals with schizotypy (Cohen et al., 2009; Cohen et al., 2011; Cohen et al., 2012a).
The aims of this study were to examine the relationship between a broad range of dimensional symptoms of schizotypy (assessed by three scales of schizotypy), sex, and the interactions with computerized prosodic analysis of speech samples. As schizotypy provides insight into the neurocognitive functioning of schizophrenia without many of the related confounds, this study aimed to inform assessment efforts and understanding of the underlying mechanisms in schizophrenia-spectrum disorders. Given the finding that flat affect may be more common in males with schizophrenia (Gur et al., 2006), we hypothesized that statistically significant relationships between speech variables and features of schizotypy would be limited to the male participants. As flat affect is less common in females with schizophrenia, and in females in general, we hypothesized that this would translate to a weaker relationship between prosody and the related construct of schizotypy, which was not expected to reach statistical significance. The examination of specific dimensional symptoms of schizotypy in relation to the speech variables was largely exploratory, given the paucity of research that has examined symptoms at the level of specificity present in this study. However, based on this limited research, we hypothesized that the number of utterances and variability in both volume (i.e., intensity) and pitch (i.e., prosody) would be negatively correlated with one or more subscales reflecting negative schizotypy, particularly the Constricted Affect subscale. We also included an exploratory analysis of how performance on measures of verbal declarative memory and verbal and visual working memory related to the speech measures. These neurocognitive measures were available from a larger related study and were not chosen based on theoretical associations with speech prosody.
Methods
Participants
The sample consisted of undergraduate students enrolled in classes offered by the Psychology Department of a large U.S. university who received academic credit in return for participation. A large number of potential participants were initially screened with an online administration of the SPQ (N > 2,000). Participants were then selectively invited to the in-person speech prosody assessment based on their total SPQ score from the screening, with an attempt to recruit a wide range of SPQ total scores. Following recruitment efforts, 44 individuals agreed to participate, whose in-person re-administration of the SPQ resulted in the desired wide continuous range of total scores (mean = 16.86; SD = 14.51; range = 0 to 48; sample was 50% male; mean age = 20.00 years; SD = 4.83; range = 18 to 47). Eight participants (4 males and 4 females) met diagnostic criteria for schizotypal personality disorder based on the Structured Clinical Interview for DSM-IV Axis II Disorders (First et al., 1997). These eight participants had a notably higher mean SPQ total score than the total sample (mean = 33.50; SD = 11.40; range = 20 to 48). All participants denied a personal or family history of schizophrenia or schizoaffective disorder.
Measures
Schizotypal Personality Questionnaire (SPQ; Raine et al., 1991)
The SPQ is a 74-item self-report measure of schizotypal features, which yields a total score along with nine subscales: Ideas of Reference, Magical Thinking, Unusual Perceptual Experiences, Suspiciousness, Social Anxiety, No Close Friends, Constricted Affect, Odd Behavior, and Odd Speech.
Physical Anhedonia Scale (PAS; Chapman & Chapman, 1978)
The Physical Anhedonia scale is a 61 item self-report (true/false) measure that assesses deficits in the ability to experience pleasure derived from sensory and aesthetic stimuli (e.g., food, environment). The PAS has been shown to be both reliable and valid (Chapman & Chapman, 1978), and detect the physical components of anhedonia independent of state anxiety and depression (Bailey et al., 1993).
Revised Social Anhedonia Scale (SAS; Chapman et al., 1976; Eckblad et al., 1982)
The SAS is 40-item true/false measure assessing the ability to experience pleasure from interpersonal interactions. The SAS has shown good reliability, appears to be relatively independent of other measures of psychosis-proneness (including the PAS), and identifies individuals exhibiting significant social maladjustment (Chapman et al., 1985; Merritt et al., 1993).
Portions of the Wechsler Memory Scales – 3rd Edition (WMS-III; Wechsler, 1997)
Three subtests from the WMS-III were administered: Logical Memory (Parts I and II; used combined total score) for assessing verbal declarative memory; Letter-Number Sequencing for assessing verbal working memory; and Symbol Span for assessing visual working memory.
Procedures
The study was approved by the university’s Institutional Review Board. Participants provided informed consent and completed the self-report scales (SPQ, PAS, and SAS) and the three WMS-III subscales. Speech samples were obtained from autobiographical narrative prompts (Autobiographical Memory Questionnaire; Rubin et al., 2003), which was administered as part of a larger study (Deptula & Bedwell, unpublished data). Autobiographical memories (AMs) were elicited by employing 15 cue words, each prompting the first AM that comes to mind. Participants orally reported each memory into a digital audio recorder, in as much detail to sufficiently “tell a story.” Participants were encouraged to take as much time as necessary.
Speech Sample Processing
The digital audio recordings were first spliced to reduce any pauses in speech greater than 5 s, to be approximately 50 ms, as there were often long pauses in between cue words in the AM task. Following the splicing, the first 100 s of the resulting speech files were analyzed consistent with prior studies of acoustic analysis of speech in samples of individuals with schizotypy (Cohen et al., 2009; Cohen et al., 2011; Cohen et al., 2012a). Only two participants had less than 100 s of speech in the resulting speech file (69.1 and 79.5 s respectively), and were retained in the analyses. The digitized recordings were analyzed using Praat (Boersma et al., 2006), a program that has been used extensively in acoustic analysis. The Praat system organizes sound files into “frames” for analysis, which for the present study was set at a rate of 100 per second. During each of these frames, frequency and volume was quantified. Four particular indices of speech characteristics of interest were chosen for analysis: 1) mean number of utterances > 150 ms (an index of speech production), 2) number of pauses (> 10 ms); 3) intonation – defined as the variability in the “pitch” of speech, calculated as the average of standard deviations of the fundamental frequency computed separately from each utterance (i.e., lower values represent more monotone speech), and 4) variability in volume of speech, calculated as the average of standard deviations of the volume computed separately from each utterance (i.e., lower values represent more monotone speech). The first two variables were chosen to represent the production of speech (tapping into the negative symptom construct of “alogia”), while the second two variables were chosen to represent two different aspects of blunted or monotone speech (variability of fundamental frequency and volume).
Results
Descriptive statistics by sex for all features of schizotypy and speech variables are listed in Table 1. As noted in Table 1, there were sex differences for only two of the 11 features of schizotypy, as males had higher scores on the SPQ subscale of Constricted Affect and the SAS. For the speech variables, the only significant difference was that females had greater variability in intonation than males. Age was not related to any of the schizotypy or speech variables (all p’s < .49). Pearson correlations of the four speech variables revealed relative independence of each measure across the sample (all r’s < .62). See Table 2 for intercorrelations of all measures included in the study within each sex.
Table 1.
Descriptive Statistics by Sex
| Measure | Males | Females |
|---|---|---|
| SPQ Ideas of Reference | 2.77 (2.96) | 1.95 (2.40) |
| SPQ Social Anxiety | 3.23 (2.71) | 2.55 (1.92) |
| SPQ Odd Beliefs | 1.64 (2.19) | 1.23 (1.77) |
| SPQ Unusual Perceptual Experiences | 1.50 (2.28) | 1.69 (2.09) |
| SPQ Eccentric Behavior | 2.41 (2.48) | 1.82 (2.40) |
| SPQ No Close Friends | 1.82 (2.11) | 1.09 (2.00) |
| SPQ Odd Speech | 2.95 (2.84) | 2.95 (2.98) |
| SPQ Constricted Affect | 1.64 (1.56)** | 0.55 (0.67)** |
| SPQ Suspiciousness | 0.91 (1.60) | 1.14 (1.58) |
| PAS (Physical Anhedonia) | 12.68 (7.25) | 8.95 (6.31) |
| SAS (Social Anhedonia) | 9.64 (7.59)* | 5.29 (5.95)* |
| WMS-III Logical Memory Total (Raw) | 45.91 (14.50) | 50.45 (11.68) |
| WMS-III Symbol Span (Raw) | 25.45 (8.90) | 26.77 (7.21) |
| WMS-III Letter Number Sequencing (Raw) | 20.64 (4.38) | 20.76 (2.91) |
| Speech - Number of Utterances | 86.36 (11.68) | 89.05 (15.37) |
| Speech – Length of Utterances (ms) | 1174.51 (178.10) | 1149.85 (224.86) |
| Speech – Variability of Pitch | 0.022 (0.009)*** | 0.041 (0.014)*** |
| Speech - Variability of Volume | 0.717 (0.112) | 0.704 (0.123) |
p < .05,
p < .01,
p < .001
SPQ = Schizotypal Personality Questionnaire; PAS = Physical Anhedonia Scale; SAS = Revised Social Anhedonia Scale; WMS-III = Wechsler Memory Scales –3rd edition.
Table 2.
Zero-order Pearson Correlation Table by Sex (Males in Upper Quadrant; Females in Lower Quadrant)
| Measure | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. SPQ Ideas of Reference | -- | .16 | .76*** | .69*** | .27 | .41 | .51* | .28 | .44* | −.48* | .34 | .02 | .40 | .16 | .49* | .05 | −.50* | −.47* |
| 2. SPQ Social Anxiety | .12 | -- | .26 | .51* | .42 | .64** | .33 | .63** | −.04 | .12 | .47* | −.07 | −.24 | −.33 | −.04 | −.05 | .12 | −.39 |
| 3. SPQ Odd Beliefs | .48* | .61** | -- | .73*** | .38 | .52* | .59** | .42 | .41 | −.38 | .55** | .07 | .31 | .14 | .41 | .21 | −.36 | -.27 |
| 4. SPQ Unusual Perceptual Experiences | .60** | .48* | .65** | -- | .59** | .69*** | .56** | .48* | .43* | −.27 | .76*** | −.13 | .07 | −.05 | .35 | .33 | −.35 | −.30 |
| 5. SPQ Eccentric Behavior | .61** | .25 | .60** | .59** | -- | .49* | .59** | .48* | .44* | −.02 | .60** | −.58** | −.17 | −.28 | .32 | .56** | .13 | −.14 |
| 6. SPQ No Close Friends | .42 | .40 | .52* | .56** | .59** | -- | .64** | .75*** | .39 | −.12 | .79*** | .07 | .07 | −.01 | .32 | .24 | .04 | −.24 |
| 7. SPQ Odd Speech | .59** | .42 | .64** | .66** | .86*** | .62** | -- | .58** | .59** | −.42 | .56** | −.06 | .41 | .26 | .36 | .30 | −.37 | −.25 |
| 8. SPQ Constricted Affect | .05 | .50* | .21 | .44* | .42 | .67** | .44* | -- | .41 | −.15 | .57** | −.16 | −.05 | −.09 | .24 | .31 | .14 | −.22 |
| 9. SPQ Suspiciousness | .67** | .19 | .69*** | .58** | .65** | .73*** | .65** | .29 | -- | −.29 | .38 | −.22 | .32 | .19 | .54** | .51* | −.14 | −.18 |
| 10. PAS (Physical Anhedonia) | .07 | −.20 | <.001 | −.06 | .05 | .34 | −.003 | .08 | .35 | -- | .11 | −.23 | −.46* | −.13 | −.18 | −.22 | .39 | −.08 |
| 11. SAS (Social Anhedonia) | .64** | .23 | .53* | .64** | .53* | .77*** | .57** | .41 | .82*** | .46* | -- | −.17 | .03 | −.05 | .23 | .33 | −.12 | −.06 |
| 12. WMS-III Logical Memory Total | −.02 | −.08 | −.05 | −.07 | −.10 | −.41 | −.20 | −.45* | −.22 | .13 | −.11 | -- | .38 | .31 | .02 | −.32 | −.02 | −.22 |
| 13. WMS-III Symbol Span | .16 | −.27 | −.08 | .01 | .06 | −.38 | −.09 | −.26 | −.14 | −.03 | −.25 | .47* | -- | .71*** | .23 | −.10 | −.39 | .05 |
| 14. WMS-III Letter-Number Sequencing | .10 | −.01 | .22 | −.01 | .22 | −.15 | .17 | −.23 | .12 | .05 | −.03 | .16 | .29 | -- | .16 | −.11 | −.44* | .01 |
| 15. Speech – Number of Utterances | −.07 | .24 | .15 | −.16 | .17 | .04 | .06 | .26 | .11 | .03 | .05 | .08 | .17 | .05 | -- | .62** | .07 | −.42* |
| 16. Speech – Number of Pauses | −.13 | .04 | .06 | −.24 | .18 | −.11 | .12 | −.11 | −.09 | −.10 | −.10 | .25 | −.10 | −.04 | .57** | -- | .20 | .11 |
| 17. Speech – Variability of Pitch | .03 | −.16 | .01 | −.05 | .16 | .02 | .15 | −.14 | .05 | −.10 | −.08 | .28 | .21 | −.29 | −.02 | .29 | -- | .07 |
| 18. Speech - Variability of Volume | .11 | .07 | .003 | .18 | .34 | .14 | .37 | .22 | .09 | −.06 | −.10 | .01 | −.02 | −.11 | −.31 | −.10 | .38 | -- |
p < .05,
p < .01,
p < .001
SPQ = Schizotypal Personality Questionnaire; PAS = Physical Anhedonia Scale; SAS = Revised Social Anhedonia Scale; WMS-III = Wechsler Memory Scales – 3rd edition.
As a result of main effects of sex, along with previous research suggesting interactions of sex and schizotypy on a range of neurocognitive variables, all analyses were conducted separately within each sex. Consistent with the dimensional model of schizotypy (Nelson et al., 2013; Schurhoff et al., 2007), stepwise regressions were conducted to examine which of 11 schizotypal features (the nine SPQ subscales along with the total scores from SAS and PAS) account for the most unique variance in relation to each of the four speech variables of interest.
In the male participants (N = 22), for number of utterances, there was a positive relationship with Suspiciousness, β = .54, t(20) = 2.90, p = .009, adjusted R2 = .26. For number of pauses, there was a positive relationship with Odd Behavior, β = .56, t(20) = 3.03, p = .007, adjusted R2 = .28 (see Figure 1). For variability of pitch, there was a negative relationship with Ideas of Reference, β = −.50, t(20) = 2.57, p = .02, adjusted R2 = .21 (see Figure 2). For variability of intensity, there was a similar negative relationship with Ideas of Reference, β = −.47, t(20) = 2.41, p = .03, adjusted R2 = .19. All of these relationships remained statistically significant after controlling for the percentage of time in silence (using partial correlations), suggesting that these relationships were relatively independent of the overall amount of speech produced.
Figure 1.
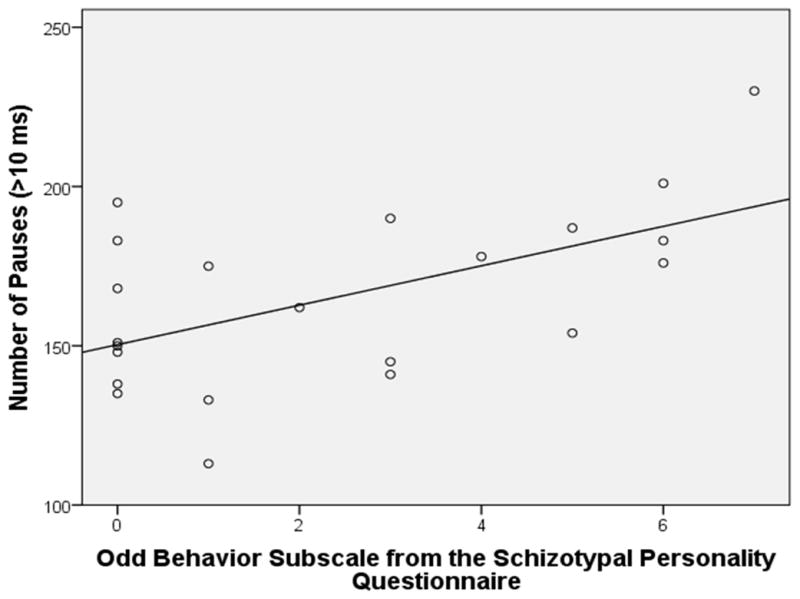
Scatterplot of the Odd Behavior Subscale from the Schizotypal Personality Questionnaire by Number of Pauses in Male Participants (N =22)
Figure 2.
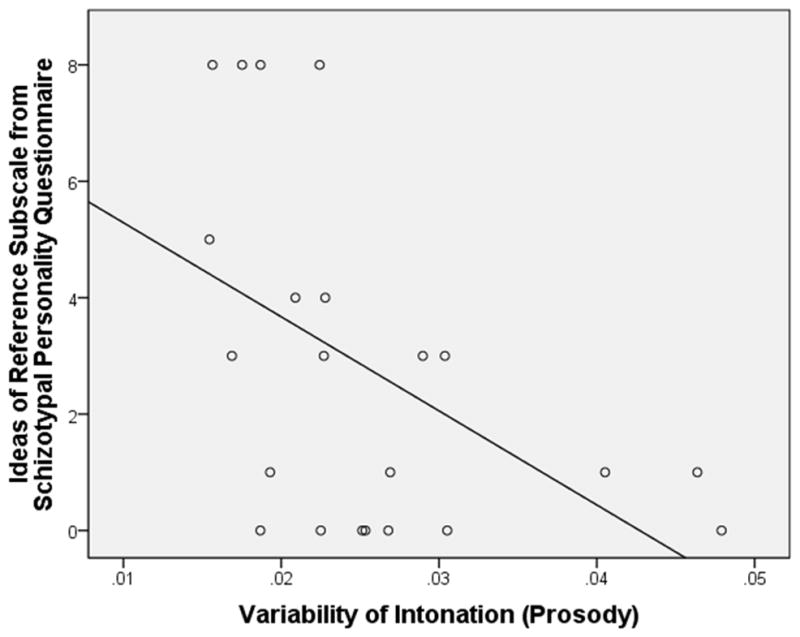
Scatterplot of the Ideas of Reference Subscale from the Schizotypal Personality Questionnaire by Variability of Intonation in Male Participants (N =22)
In the female participants (N = 22), no features of schizotypy entered the model for any of the four speech variables.
Interestingly, as can be seen in Table 2, there were no significant correlations between self-reported Constricted Affect from the SPQ with any of the speech variables within either sex.
Examination of the relationships between the three subscales of the WMS-III and the speech variables (see Table 2) revealed that males showed a negative relationship between Letter-Number Sequencing and the variability in pitch from the speech sample. When covarying for Letter-Number Sequencing performance on the relationship between variability in pitch and the Ideas of Reference scale in the male participants, the relationship remained statistically significant, r(19) = -.49, p = .03, suggesting that this relationship was not substantially accounted for by variability in verbal working memory. There were no significant relationships between the cognitive and speech variables in female participants.
Discussion
Our hypothesis was partially supported as we found that the relationships between features of schizotypy and speech variables were limited to male participants. This is consistent with a previous study which found that flat affect was more common in males with schizophrenia (Gur et al., 2006), and consistent with an emerging body of literature suggesting that neurocognitive abnormalities in schizotypy often show interactions with sex (Bedwell et al., 2011; Johnston et al., 2008; Krabbendam et al., 2005; Lubow et al., 2002; Sumich et al., 2008). However, contrary to our hypothesis, the male participants who produced reduced variability in pitch and volume reported higher scores on Ideas of Reference, which is a positive, rather than negative, feature. In addition, we found additional relationships in only the male participants, as Suspiciousness was positively related to the number of utterances, and Odd Behavior was positively related to number of pauses. In the female participants, there was no relationship between any feature of schizotypy and any of the speech variables.
In addition to the finding that females with schizophrenia are less likely to show flat affect than males with schizophrenia (Gur et al. 2006), females are also likely to show reduced severity of negative symptoms in general, and have a later age of onset, better premorbid functioning, and greater affective and positive symptoms (reviewed by Mendrek & Stip, 2011). A similar pattern of females showing less negative features and greater positive features has also been reported in schizotypy (Bora et al., 2009; Fonseca-Pedrero et al., 2012; Miller et al., 1995; Raine, 1992). A recent review of the literature explored the theory that estrogen may play a protective role in the schizophrenia in females, which may explain why females tend to have a later age of onset, better premorbid functioning, and reduced negative symptoms; however, does not account for the increased positive symptoms (Begemann et al., 2012). This meta-analysis found that supplementation of estrogens in females with schizophrenia reduced both negative and positive symptoms (Begemann et al., 2012). This raises the interesting possibility that the higher level of naturally-occuring estrogens in females with schizophrenia or schizotypy, may explain the reduced severity of some negative symptoms such as reduced speech prosody, as compared with males.
This schizotypy by sex interaction may explain why three previous studies did not find speech prosody differences related to overall schizotypy, as none of those studies examined potential interactions with sex (Cohen et al., 2009; Cohen et al., 2011; Cohen et al., 2012a). In addition, only one of those previous studies examined the dimensional relationship of specific features of schizotypy with speech variables (Cohen et al., 2011). Although that study did not examine sex interactions, the finding of increased speech production related to SPQ Suspiciousness was consistent with findings in the current study, as, at least in our male participants, we found that Suspiciousness was related to an increase in the number of utterances. However, contrary to that study, the self-reported Constricted Affect scale was not related to objective measures of speech prosody in either males or females in our sample. One explanation for the lack of consistency across our subjective and objective assessment is that vocal prosody only reflects a single component assessed by the broader SPQ Constricted Affect subscale. In addition, participants may lack sufficient self-awareness of vocal prosody.
We found a negative relationship between a measure of verbal working memory (Letter-Number Sequencing) and variability of pitch across all male participants, while no cognitive measures related to speech variables in female participants. As we did not have a basis for a specific hypothesis with these exploratory correlations, this relationship remains preliminary and in need of replication. However, this measure of verbal working memory did not account for significant variance in the relationship between the SPQ Ideas of Reference subscale and variability of pitch in the male participants.
The study was limited to college students; therefore, findings may not generalize to the larger population. In addition, we had a limited sample size within each sex. However, we did have an equal number of males and females (N = 22 of each), which makes it unlikely that reduced statistical power was primarily responsible for the observed difference in findings within each sex. Finally, the speech samples were collected from autobiographical narrative prompts, which may bias the findings, particularly as autobiographical memory has been shown to be impaired in some individuals with schizophrenia (reviewed in Watson et al., 2012).
Overall, this study was novel in that it appears to be the first study to examine specific features of schizotypy and the interactions with sex in relation to objectively-assessed speech prosody measures. The primary finding that these relationships were limited to the male participants may explain the lack of overall findings in speech prosody studies examining schizotypy to date. These preliminary findings suggest the need to examine schizotypy by sex interactions when examining speech prosody, and along with past studies, suggest that schizotypy by sex interactions should be routinely examined for any neurocognitive measure in schizotypy. Sex interactions are also rarely examined in studies of neurocognition in schizophrenia samples, and future studies on these clinical populations may be better informed by examination of potential sex interactions as well. As diminished expressivity is associated with a lower quality of life and worse outcome in schizophrenia (Gur et al., 2006; Hoekert et al., 2007), further knowledge of particular features of diminished expressivity and sex interactions will inform both assessment and treatment efforts.
Conclusions
The relationships between dimensional features of schizotypy and abnormal aspects of speech prosody appear to be limited to male participants, as no significant relationships were found in the female participants. In male participants, variability of pitch and variability of intensity were each negatively related to an Ideas of Reference subscale, while Suspiciousness was related to a greater number of utterances, and Odd Behavior was related to a greater number of pauses.
Acknowledgments
This study was not funded.
The authors would like to thank Mr. Carlos Puentes for assistance in processing the digital audio files, and Mr. Ian Vann-Campbell for assistance with recruitment and data collection.
Footnotes
The authors have no conflict of interest to report. This study was not funded.
References
- Asai T, Sugimori E, Bando N, Tanno Y. The hierarchic structure in schizotypy and the five-factor model of personality. Psychiatry Res. 2011;185:78–83. doi: 10.1016/j.psychres.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Bailey B, West KY, Widiger TA, Freiman K. The convergent and discriminant validity of the Chapman Scales. Journal of personality assessment. 1993;61:121–35. doi: 10.1207/s15327752jpa6101_9. [DOI] [PubMed] [Google Scholar]
- Bedwell JS, Hernandez DC, Ranieri AY. The backward masking red light effect and schizotypy: the influence of sex. Psychiatry Res. 2011;189:228–32. doi: 10.1016/j.psychres.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Begemann MJ, Dekker CF, van Lunenburg M, Sommer IE. Estrogen augmentation in schizophrenia: a quantitative review of current evidence. Schizophr Res. 2012;141:179–84. doi: 10.1016/j.schres.2012.08.016. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Cohen AS. The structure of negative symptoms within schizophrenia: implications for assessment. Schizophr Bull. 2006;32:238–45. doi: 10.1093/schbul/sbj013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boersma P, Weenink D. Praat: Doing phonetics by computer. 4.4.05 2006. [Google Scholar]
- Bora E, Baysan Arabaci L. Effect of age and gender on schizotypal personality traits in the normal population. Psychiatry and clinical neurosciences. 2009;63:663–9. doi: 10.1111/j.1440-1819.2009.02011.x. [DOI] [PubMed] [Google Scholar]
- Chapman JP, Chapman LJ, Kwapil TR. Scales for the measurement of schizotypy. In: Raine A, Lencz T, Mednick SA, editors. Schizotypal Personality. New York, NY: Cambridge University Press; 1995. pp. 79–106. [Google Scholar]
- Chapman LJ, Chapman JP. Psychosis proneness. In: AM, editor. Controversies in schizophrenia: Changes and consistencies. New York: Guilford; 1985. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for physical and social anhedonia. J Abnorm Psychol. 1976;85:374–82. doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Cochrane M, Petch I, Pickering AD. Aspects of cognitive functioning in schizotypy and schizophrenia: evidence for a continuum model. Psychiatry Res. 2012;196:230–4. doi: 10.1016/j.psychres.2012.02.010. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Hong SL. Understanding constricted affect in schizotypy through computerized prosodic analysis. Journal of personality disorders. 2011;25:478–91. doi: 10.1521/pedi.2011.25.4.478. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Iglesias B, Minor KS. The neurocognitive underpinnings of diminished expressivity in schizotypy: what the voice reveals. Schizophr Res. 2009;109:38–45. doi: 10.1016/j.schres.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Kim Y, Najolia GM. Psychiatric symptom versus neurocognitive correlates of diminished expressivity in schizophrenia and mood disorders. Schizophr Res. 2013a;146:249–53. doi: 10.1016/j.schres.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Morrison SC, Brown LA, Minor KS. Towards a cognitive resource limitations model of diminished expression in schizotypy. J Abnorm Psychol. 2012a;121:109–18. doi: 10.1037/a0023599. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Morrison SC, Callaway DA. Computerized facial analysis for understanding constricted/blunted affect: Initial feasibility, reliability, and validity data. Schizophr Res. 2013b doi: 10.1016/j.schres.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Kim Y, Dinzeo TJ. On the boundaries of blunt affect/alogia across severe mental illness: implications for Research Domain Criteria. Schizophr Res. 2012b;140:41–5. doi: 10.1016/j.schres.2012.07.001. [DOI] [PubMed] [Google Scholar]
- Collins LM, Blanchard JJ, Biondo KM. Behavioral signs of schizoidia and schizotypy in social anhedonics. Schizophr Res. 2005;78:309–22. doi: 10.1016/j.schres.2005.04.021. [DOI] [PubMed] [Google Scholar]
- Diaz I, Pelayo-Teran JM, Perez-Iglesias R, Mata I, Tabares-Seisdedos R, Suarez-Pinilla P, Vazquez-Barquero JL, Crespo-Facorro B. Predictors of clinical remission following a first episode of non-affective psychosis: sociodemographics, premorbid and clinical variables. Psychiatry Res. 2013;206:181–7. doi: 10.1016/j.psychres.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Dickey CC, Vu MT, Voglmaier MM, Niznikiewicz MA, McCarley RW, Panych LP. Prosodic abnormalties in schizotypal personality disorder. Schizophr Res. 2012;142:20–30. doi: 10.1016/j.schres.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad ML, Chapman LJ, Chapman JP, Mishlove M. The revised social anhedonia scale. University of Wisconsin; Madison: 1982. [Google Scholar]
- Esterberg ML, Compton MT. The psychosis continuum and categorical versus dimensional diagnostic approaches. Curr Psychiatry Rep. 2009;11:179–84. doi: 10.1007/s11920-009-0028-7. [DOI] [PubMed] [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- Fonseca-Pedrero E, Lemos-Giraldez S, Paino M, Sierra-Baigrie S, Muniz J. Phenotypic expression of schizotypal traits in an adolescent population. Journal of personality disorders. 2012;26:539–50. doi: 10.1521/pedi.2012.26.4.539. [DOI] [PubMed] [Google Scholar]
- Gur RE, Kohler CG, Ragland JD, Siegel SJ, Lesko K, Bilker WB, Gur RC. Flat affect in schizophrenia: relation to emotion processing and neurocognitive measures. Schizophr Bull. 2006;32:279–87. doi: 10.1093/schbul/sbj041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekert M, Kahn RS, Pijnenborg M, Aleman A. Impaired recognition and expression of emotional prosody in schizophrenia: review and meta-analysis. Schizophr Res. 2007;96:135–45. doi: 10.1016/j.schres.2007.07.023. [DOI] [PubMed] [Google Scholar]
- Johnston AE, Rossell SL, Gleeson JF. Evidence of semantic processing abnormalities in schizotypy using an indirect semantic priming task. J Nerv Ment Dis. 2008;196:694–701. doi: 10.1097/NMD.0b013e318183f882. [DOI] [PubMed] [Google Scholar]
- Krabbendam L, Myin-Germeys I, Hanssen M, van Os J. Familial covariation of the subclinical psychosis phenotype and verbal fluency in the general population. Schizophr Res. 2005;74:37–41. doi: 10.1016/j.schres.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Kring AM, Smith DA, Neale JM. Individual differences in dispositional expressiveness: development and validation of the Emotional Expressivity Scale. Journal of personality and social psychology. 1994;66:934–49. doi: 10.1037//0022-3514.66.5.934. [DOI] [PubMed] [Google Scholar]
- Leung WW, Couture SM, Blanchard JJ, Lin S, Llerena K. Is social anhedonia related to emotional responsivity and expressivity? A laboratory study in women. Schizophr Res. 2010;124:66–73. doi: 10.1016/j.schres.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liemburg E, Castelein S, Stewart R, van der Gaag M, Aleman A, Knegtering H. Two subdomains of negative symptoms in psychotic disorders: established and confirmed in two large cohorts. J Psychiatr Res. 2013;47:718–25. doi: 10.1016/j.jpsychires.2013.01.024. [DOI] [PubMed] [Google Scholar]
- Lubow RE, De la Casa G. Latent inhibition as a function of schizotypality and gender: implications for schizophrenia. Biol Psychol. 2002;59:69–86. doi: 10.1016/s0301-0511(01)00124-7. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Stip E. Sexual dimorphism in schizophrenia: is there a need for gender-based protocols? Expert Rev Neurother. 2011;11:951–9. doi: 10.1586/ern.11.78. [DOI] [PubMed] [Google Scholar]
- Merritt RD, Balogh DW, DeVinney SE. Use of the MMPI to assess the construct validity of the revised Social Anhedonia Scale as an index of schizotypy. Journal of personality assessment. 1993;60:227–38. doi: 10.1207/s15327752jpa6002_2. [DOI] [PubMed] [Google Scholar]
- Miller LS, Burns SA. Gender differences in schizotypic features in a large sample of young adults. J Nerv Ment Dis. 1995;183:657–61. doi: 10.1097/00005053-199510000-00007. [DOI] [PubMed] [Google Scholar]
- Moller HJ. Pharmacotherapy of schizophrenic patients: achievements, unsolved needs, future research necessities. Current pharmaceutical biotechnology. 2012;13:1476–89. doi: 10.2174/138920112800784907. [DOI] [PubMed] [Google Scholar]
- Nelson MT, Seal ML, Pantelis C, Phillips LJ. Evidence of a dimensional relationship between schizotypy and schizophrenia: a systematic review. Neuroscience and biobehavioral reviews. 2013;37:317–27. doi: 10.1016/j.neubiorev.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Raine A. The schizotypal personality questionnaire (SPQ): A scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Raine A. Sex differences in schizotypal personality in a nonclinical population. J Abnorm Psychol. 1992;101:361–4. doi: 10.1037//0021-843x.101.2.361. [DOI] [PubMed] [Google Scholar]
- Rubin DC, Schrauf RW, Greenberg DL. Belief and recollection of autobiographical memories. Memory & cognition. 2003;31:887–901. doi: 10.3758/bf03196443. [DOI] [PubMed] [Google Scholar]
- Schurhoff F, Szoke A, Chevalier F, Roy I, Meary A, Bellivier F, Giros B, Leboyer M. Schizotypal dimensions: an intermediate phenotype associated with the COMT high activity allele. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2007;144B:64–8. doi: 10.1002/ajmg.b.30395. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Allen DN, Miski P, Buchanan RW, Kirkpatrick B, Carpenter WT., Jr Differential patterns of premorbid social and academic deterioration in deficit and nondeficit schizophrenia. Schizophr Res. 2012a;135:134–8. doi: 10.1016/j.schres.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Sandt AR, Catalano LT, Allen DN. Negative symptoms and depression predict lower psychological well-being in individuals with schizophrenia. Comprehensive psychiatry. 2012b;53:1137–44. doi: 10.1016/j.comppsych.2012.05.009. [DOI] [PubMed] [Google Scholar]
- Sumich A, Kumari V, Gordon E, Tunstall N, Brammer M. Event-related potential correlates of paranormal ideation and unusual experiences. Cortex; a journal devoted to the study of the nervous system and behavior. 2008;44:1342–52. doi: 10.1016/j.cortex.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Tsang HW, Leung AY, Chung RC, Bell M, Cheung WM. Review on vocational predictors: a systematic review of predictors of vocational outcomes among individuals with schizophrenia: an update since 1998. Aust N Z J Psychiatry. 2010;44:495–504. doi: 10.3109/00048671003785716. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Memory Scales. 3. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Watson A, Barker V, Hall J, Lawrie SM. Remembering the self in schizophrenia. Br J Psychiatry. 2012;201:423–4. doi: 10.1192/bjp.bp.112.110544. [DOI] [PubMed] [Google Scholar]


