Abstract
Topiramate, which interacts with multiple neurotransmitter and enzyme systems, is approved by the Food and Drug Administration to treat seizure disorder, prevent migraine, and (in combination with phentermine) reduce weight. Topiramate has also been shown in multiple studies to reduce heavy drinking. We found that topiramate 200 mg/day significantly reduced heavy drinking in heavy drinkers with a treatment goal of reduced drinking (Kranzler et al. 2014). Further, in the European American (EA) subsample (n=122), a single nucleotide polymorphism (rs2832407) in GRIK1, which encodes the GluK1 subunit of the kainate receptor, moderated the effect on heavy drinking days. Here we examined the effects of topiramate on body-mass index (BMI) and the moderating effect of rs2832407 in the EA subsample from Kranzler et al. (2014). Across the 12 weeks of treatment, BMI was reduced by 1.2 kg/m2 (p<0.001) in the topiramate group, but was unchanged in the placebo group. There was no evidence of moderation by rs2832407 of topiramate’s effects on BMI. Controlling for changes in drinking and other potential confounders did not alter the findings. These results suggest that the effect of topiramate on drinking behavior, in which the GluK1-containing kainate receptor appears to play a key role, can be dissociated from its effect on weight, the specific mechanism of which remains to be determined.
www.clinicaltrials.gov registration: NCT00626925
Keywords: Topiramate, Body Mass Index, Heavy Drinkers, Weight Loss, Pharmacogenetics
INTRODUCTION
Topiramate is a sulfate-substituted monosaccharide that interacts with multiple neurotransmitter and enzyme systems. It facilitates GABAergic function by interacting with a non-benzodiazepine site on the GABAA receptor (White et al. 2000), antagonizes glutamate effects at AMPA and kainate receptors (Skradski & White 2000; Gibbs et al. 2000), blocks voltage-dependent Na+ and L-type voltage-gated Ca++ channels, inhibits carbonic anhydrase, and enhances K+ conductance (McDonald and Rogawski 2006). The various effects of topiramate may contribute to its efficacy in the treatment of different disorders.
Topiramate, which was first approved by the FDA as an anticonvulsant, has also been approved to prevent migraine and, in combination with phentermine, for weight loss. Although topiramate is widely used to treat these and other disorders, its mechanism of action in producing different therapeutic effects is not fully understood. Topiramate’s effects on AMPA/kainate receptors are most potent and selective for those containing the GluK1 subunit (Gryder and Rogawski 2003; Kaminski et al. 2004), suggesting that these receptors play a key role in topiramate’s reduction of clonic seizure activity (Gryder and Rogawski 2003, Kaminski et al. 2004).
Topiramate also consistently reduces weight (Moradi et al. 2013, Rosenstock et al. 2013, Verrotti et al. 2011). Although the combination of topiramate with phentermine is more efficacious than topiramate alone in reducing weight (Aronne et al. 2013), topiramate monotherapy also produced greater weight reduction than placebo (Aronne et al. 2013). Consistent with these reports, studies of alcohol-dependent individuals (Johnson et al. 2008) and smokers (Anthenelli et al. 2008) have shown that, despite receiving treatment focused on reducing substance use, rather than on weight loss, topiramate treatment significantly reduced weight [or body mass index (BMI), which is weight divided by height squared] more than placebo treatment. Multiple mechanisms may be responsible for topiramate-induced weight reduction. Human studies show that topiramate is anorexigenic, resulting in decreased caloric intake. However, topiramate also affects the concentration of hormones such as adiponectin and glucose and lipid metabolism, any of which could also mediate its effects on weight (Verrotti et al. 2011).
Although topiramate is not approved to treat alcohol dependence, four of five placebo-controlled trials have shown it to be efficacious in reducing drinking in heavy drinkers (Johnson et al. 2003, 2007; Miranda et al. 2008; Likhitsathian et al. 2013; Kranzler et al. 2014). Thus, a growing number of practitioners prescribe the medication off-label to treat alcohol dependence (Del Re et al. 2013).
Based on the putative mechanism of topiramate’s anticonvulsant effects, we previously sought to identify a genetic moderator of the medication’s effects on drinking. On the hypothesis that the GluK1 kainate subunit (encoded by GRIK1), which was implicated in topiramate’s anticonvulsant effects, was also relevant to its effects on alcohol consumption, we examined the association to alcohol dependence of seven single nucleotide polymorphisms (SNPs) in GRIK1. Empirical p-value testing showed that only rs2832407 in intron 9 was significantly associated to alcohol dependence (Kranzler et al. 2009).
In a 12-week study of the efficacy of topiramate treatment in heavy drinkers whose goal was to reduce their drinking (Kranzler et al. 2014), we randomly assigned 138 patients to receive treatment with topiramate 200 mg/day or matching placebo, together with a brief psychosocial treatment: medical management (adapted from Pettinati et al. 2004). In a subsample of patients of self-reported European ancestry (EAs; n = 122), we found that rs2832407 interacted significantly with medication group, such that in the CC genotype group (41.8% of the EA subsample), but not in the A-allele carrier group, topiramate-treated patients significantly reduced their heavy drinking days more than placebo patients. Although the functional effects of rs2832407 remain to be determined, these findings implicate GluK1-containing kainate receptors as a key component of topiramate’s ability to reduce heavy drinking (Kranzler et al. 2014).
Based on these findings, the present analysis examined: 1) whether the patients in the EA subsample from Kranzler et al. (2014) lost weight when treated with topiramate and 2) whether rs2832407 moderated the effect. A finding that topiramate-induced weight loss was moderated by the SNP would provide preliminary evidence implicating variation in GRIK1 as playing an important role in the medication’s reduction of both heavy drinking and weight.
EXPERIMENTAL PROCEDURES
Overview
Using advertisements that sought participants with a goal of reducing drinking, we recruited 138 heavy drinkers to participate in a parallel-group, placebo-controlled trial of topiramate. The study was initiated at the University of Connecticut Health Center (UConn) and completed at the University of Pennsylvania Perelman School of Medicine (Penn). An initial telephone screening interview was followed by an in-person visit, where patients gave written, informed consent to participate and underwent a medical and psychiatric history, physical examination [which included weight, which was measured, and height, which was either measured (at the UConn site) or self-reported (at the Penn site)], clinical laboratory testing, a urine drug screen, and pregnancy testing (as appropriate). At each subsequent treatment visit, we monitored patients’ medication adherence, adverse effects, drinking behavior, and body weight. Because of the large population difference in the frequency of rs2832407 alleles, and thus to prevent confounding the pharmacogenetic analysis, we limited it to the 122 EAs from Kranzler et al. (2014).
Genotyping Procedure
DNA was extracted from whole blood using the PureGene kit (GentraSystems, Minneapolis, MN). We used a TaqMan SNP genotyping assay (Life Technologies, Grand Island, NY) to genotype rs2832407 in duplicate with consistent results.
Patients
As shown in Table 1, the sample was predominantly male, middle-aged, married or with a partner, and educated. The subjects’ mean weight was 192.9 lb (SD=37.5) and BMI was 28.8 kg/m2 (SD=4.4). Nearly half of participants (45.9%) were overweight (BMI=25.0–29.9 kg/m2) and 36.9% were obese (BMI>29.9 kg/m2). At the time of study enrollment, the medication groups were comparable on sex, age, marital status, years of education, lifetime diagnosis of major depression, Beck Depression Inventory score (BDI; Beck et al. 1961) scores, current antidepressant treatment, the percentage of days abstinent and of heavy drinking during the 90-day pretreatment period, and BMI. There was a near-significant difference in age as a function of treatment assignment (p=0.073), which, as indicated below, when included as a covariate did not affect the results of the analyses.
Table 1.
Pretreatment Demographic and Clinical Features of the Study Sample
| Medication Group | Topiramate (n=56) | Placebo (n=66) | Statistic, P-Value1 |
|---|---|---|---|
|
|
|||
| Demographics | |||
| Sex (male) | 37 (66.1%) | 38 (57.6%) | χ2=0.92, p=0.34 |
| Age2 | 50.7 (7.3) | 53.1 (7.3) | t=1.81, p=0.073 |
| Married or Partnered | 37 (66.1%) | 47 (71.2%) | χ2=0.37, p=0.54 |
| Education2 | 15.6 (2.2) | 15.2 (2.4) | t=−1.15, p=0.25 |
| Clinical Features | |||
| Lifetime Major Depression | 13 (23.2%) | 20 (30.3%) | χ2=0.77, p=0.38 |
| Beck Depression Inventory score2 | 5.9 (4.4) | 6.8 (5.2) | t=0.96, p=0.34 |
| Current Antidepressant Treatment | 10 (17.9%) | 12 (18.2%) | χ2=0.002, p=0.96 |
| 90-day pretreatment drinking | |||
| Proportion of Days Abstinent2 | 0.11 (0.15) | 0.11 (0.14) | t=−0.15, p=0.88 |
| Proportion of Heavy Drinking Days2 | 0.68 (0.27) | 0.66 (0.27) | t=−0.44, p=0.66 |
| Body Mass Index (kg/m2)2 | 29.1 (4.2) | 28.5 (4.6) | t=−0.71, p=0.48 |
for χ2 tests, df=1; for t-tests, df=120
mean (SD)
The distribution of genotypes was consistent with Hardy-Weinberg Equilibrium expectations (χ2=0.61, df=2, p=0.74). The frequency of the CC genotype in the placebo group was 45.5% and in the topiramate group it was 37.5%. As shown in Table 2, pretreatment demographics (sex, age, marital status, years of education) and clinical features (history of lifetime major depression, current antidepressant treatment, Beck Depression Inventory score, 90-day pretreatment drinking frequency and heavy drinking frequency, and BMI) did not differ as a function of the interaction of medication group by genotype group.
Table 2.
Pretreatment Demographic and Clinical Features of the Study Sample by Medication and Genotype Groups
| Genotype Group (rs2832407) | C-Allele Homozygotes | A-Allele Carriers | Test of Interaction | ||
|---|---|---|---|---|---|
| Medication Group | Topiramate (n=21) | Placebo (n=30) | Topiramate (n=35) | Placebo (n=36) | |
|
|
|||||
| Demographics | |||||
| Sex (male) | 15 (71.4%) | 14 (46.7%) | 22 (62.9%) | 24 (66.7%) | χ2(1)=2.44, p=0.12 |
| Age (yr)1 | 51.7 (8.3) | 52.5 (6.4) | 50.1 (6.7) | 53.6 (8.0) | F1,118=1.05, p=0.31 |
| Married or Partnered | 14 (66.7%) | 20 (66.7%) | 23 (65.7%) | 24 (66.7%) | χ2(1) =0.01, p=0.96 |
| Education (yr)1 | 15.7 (2.4) | 15.0 (2.2) | 15.6 (2.1) | 15.3 (2.6) | F1,118=0.22, p=0.64 |
| Clinical Features | |||||
| Lifetime Major Depression | 5 (23.8%) | 11 (36.7%) | 8 (22.9%) | 9 (25.0%) | χ2(1)=0.35, p=0.56 |
| Beck Depression Inventory score1 | 7.4 (5.1) | 6.8 (5.1) | 5.1 (3.7) | 6.8 (5.2) | F1,118=1.67, p=0.20 |
| Current Antidepressant Treatment | 2 (9.5%) | 6 (20.0%) | 8 (22.9%) | 6 (16.7%) | χ2(1)=1.45, p=0.23 |
| 90-day pretreatment drinking | |||||
| Proportion of Days Abstinent1 | 0.12 (.15) | 0.07 (.12) | 0.11 (.15) | 0.14 (.15) | F1,118=2.86, p=0.09 |
| Proportion of Heavy Drinking Days1 | 0.70 (.32) | 0.72 (.27) | 0.67 (.29) | 0.61 (.25) | F1,118=0.68, p=0.41 |
| Body Mass Index (kg/m2)1 | 28.6 (4.5) | 27.7 (5.0) | 29.4 (4.0) | 29.1 (4.1) | F1,118=0.14, p=0.71 |
mean (SD)
Patients were seen weekly for medication adjustment over the first six weeks of treatment and then biweekly for six weeks, for a total of nine visits. Treatment was initiated at a dosage of 25 mg at bedtime and was increased weekly to a maximum of 100 mg twice daily during the sixth week of treatment. We chose a maximal dosage of topiramate of 200 mg/day to limit adverse effects that could cause early discontinuation from treatment (cf. Johnson et al. 2007). Placebo and topiramate were encapsulated and could not be distinguished from one another.
Data Analysis
We used linear mixed models to test whether the change in BMI over the 12 weeks of the study differed by medication group and whether the difference was moderated by rs2832407. The model included three factors: medication group, dichotomous genotype (CC vs. A-allele carrier), and study visit (treated as a continuous variable). We chose to use the two-level genotype group to maximize statistical power, based on findings from Kranzler et al. (2014). In that study, the effects for both heavy drinking days and drinking days were more robust for the two-level than for the three-level genotype (CC/CA/AA) variable.
In the model, the 2-way and 3-way interactions were treated as fixed effects and the intercept and study visit as random effects. We repeated the analysis with topiramate dosage level, current antidepressant use, frequency of heavy drinking days, mean number of drinks/day, mean change in the number of drinks/day (and the reduction in calories associated with that reduction), sex, age, and baseline BMI as covariates to determine whether they were confounders. We calculated the size of the observed interaction effects on the change in BMI across visits using a multilevel R2 (Snijders & Bosker 2012). We also repeated the models using multiple imputation, with 10 imputed data sets, to assess the robustness of the results to missing data.
RESULTS
To test for moderation, we examined the interaction of medication group by genotype group by study visit, for which there was no effect (R2=0.003; F1,113=0.32 p=0.58). However, the conditional two-way interaction of medication group by study visit showed a highly significant effect of topiramate on the change in BMI (F1,115=24.95, p<0.001; R2=0.197, which is a moderate effect size). The other two-way interactions were not significant (genotype by visit: p=0.74 and medication group by genotype: p=0.67), nor were any of the conditional main effects (medication group: p=0.68, genotype: p=0.21, or study visit: p=0.93).
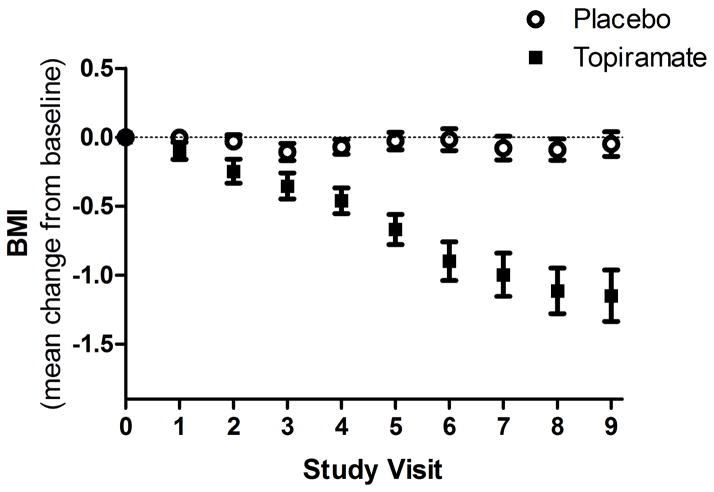
Figure 1 shows the mean change in BMI from baseline at each study visit by medication group. Within groups, the estimated change in BMI per visit was 0.001 kg/m2 for the placebo group (p=0.90) and 0.14 kg/m2 (p<0.001) for the topiramate group (which across the 12 weeks of the study was a decrease in BMI of <0.01 kg/m2 in the placebo group and 1.2 kg/m2 in the topiramate group or in weight of 0.35 lb in the placebo group and 7.74 lb in the topiramate group). Inclusion in the model of topiramate dosage level, current antidepressant use, frequency of heavy drinking days, mean number of drinks/day, mean change in the number of drinks/day (and the reduction in calories associated with that reduction), sex, age, and baseline BMI did not alter the results and none of the covariates uniquely predicted the change in BMI. Moreover, the analysis using multiple imputation produced the same results: a significant medication group by study visit interaction with no other significant effects.
Figure 1.
Mean Change in BMI (±1SEM) by Medication Group across Study Visits, which were held weekly (visits 1–6) or biweekly (visits 7–9). Although placebo-treated patients’ BMI did not change over time (mean change/per visit: 0.001 kg/m2; p=0.90), topiramate-treated patients showed a highly significant decline (mean change/per visit: 0.14 kg/m2, p<0.001).
DISCUSSION
This report focuses on the effects of topiramate on weight loss in a sample of heavy drinkers who sought treatment to reduce their drinking. More than 80% of the participants were overweight or obese. Consistent with findings from prior studies in alcohol- or nicotine-dependent individuals who received topiramate to reduce or prevent drinking or smoking and concomitantly lost weight, in this sample of heavy drinkers, we found that topiramate reduced BMI (weight) by 1.2 kg/m2 (7.74 lb) across the 12-week study period, significantly more than placebo, which reduced BMI by <0.01 kg/m2 (0.35 lb).
Although the decrease in BMI paralleled the reduction in heavy drinking days reported previously (Kranzler et al. 2014), neither a variety of drinking measures nor other potential confounders such as sex, age, or current antidepressant treatment altered the finding that topiramate significantly reduced BMI. We also found that rs2832407, which moderated the effects of topiramate on heavy drinking (Kranzler et al. 2014), did not moderate topiramate-induced weight loss. Thus, clinically, the same individuals who are likely to reduce their heavy drinking with topiramate treatment are no more likely than non-responders to reduce their weight. The pharmacogenetic effect on drinking assumes either that the variant in GRIK1 is functional or in linkage disequilibrium with a functional variant. The magnitude of the pharmacogenetic effect (see Kranzler et al. 2014) also suggests that it represents the major mechanism of topiramate’s effects on drinking. The form of the pharmacogenetic effect on heavy drinking also suggests that it is attributable to greater reductions in heavy drinking among rs2832407*CC genotype individuals rather than differences in placebo response by genotype. Assuming that rs2832407 or a linked polymorphism alters the function or expression of GluK1-containing kainate receptors, the findings reported here suggest that topiramate’s effects on alcohol consumption and BMI involve different pharmacological mechanisms.
The present study had a number of limitations. First, it focused on EAs, which limits our ability to generalize the findings to other populations. Second, subjects’ height was measured at one of the two study sites and was self-reported at the other site. Although this could have affected BMI values, as height is a key variable in the calculation of that measure, because height is a constant, misreporting it would not have substantially affected the computation of changes in BMI. Moreover, weight was measured at every visit at both sites and a parallel analysis of weight alone yielded similar findings. We chose to present data on BMI, as it may be a better indicator of health than body weight (e.g., Oliveros et al. 2014). However, despite evidence that being overweight or obese is associated with greater risk of excess mortality from a variety of causes (Guh et al. 2009), it is difficult to gauge the health consequences of the comparatively modest change in BMI observed here. Third, we did not measure weight beyond the 12 weeks of treatment, so it is unclear how durable these effects were once the medication was discontinued. Fourth, the sample size was modest, particularly for the detection of pharmacogenetic effects. However, the size of the moderator effect was so small that, even if it were demonstrable in a much larger sample (e.g., one comprised of a 1,000 subjects or more), the effect would be clinically non-significant. This is in clear contrast to the moderating effect of rs2832407 on topiramate’s ability to reduce heavy drinking (Kranzler et al. 2014). Thus, despite the limited sample size, the findings reported here argue against a common mechanism of topiramate’s reduction of heavy drinking and weight. Whereas topiramate appears to reduce heavy drinking through effects on the GluK1-containing kainate receptor, in that it reduced heavy drinking only among individuals with the CC genotype of the GRIK1 SNP rs2832407 (Kranzler et al. 2014), the findings reported here argue against a similar pharmacogenetic effect underlying weight reduction by topiramate. Replication of these findings in a larger sample is needed to support their validity. Further research is also needed to elucidate the mechanism of topiramate’s complex effects on weight and the durability and health effects of these changes in heavy drinkers, other patient groups, and in populations other than European Americans.
Acknowledgments
Role of funding source
This study was supported by NIH grants P60 AA03510 and K24 AA13736 and by the VISN 4 Mental Illness Research, Education, and Clinical Center of the Philadelphia VAMC. The funding sources had no role other than financial support.
The authors acknowledge the assistance in the conduct of this study provided by the staff of the Clinical Research and Evaluation Unit at the University of Connecticut Health Center and the Treatment Research Center of the University of Pennsylvania Perelman School of Medicine.
Footnotes
Contributors
Henry R. Kranzler (HRK), Richard Feinn (RF), Joel Gelernter (JG), Timothy Pond (TP), and Jonathan Covault (JC) had full access to the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. Study concept and design: HRK and JC designed and executed the clinical trial. Analysis and interpretation of data: HRK and RF Statistical analysis: RF and HRK. Obtained funding: HRK and JC. Study supervision: HRK and JC. All authors contributed to and have approved the final manuscript.
Conflict of Interest
RF, JG, TP, and JC have no disclosures to make. HK has been a consultant or advisory board member for the following pharmaceutical companies: Alkermes, Lilly, Lundbeck, Pfizer, and Roche. He is also a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which is supported by AbbVie, Ethypharm, Lilly, Lundbeck, and Pfizer.
References
- Anthenelli RM, Blom TJ, McElroy SL, Keck PE., Jr Preliminary evidence for gender-specific effects of topiramate as a potential aid to smoking cessation. Addiction. 2008;103:687–694. doi: 10.1111/j.1360-0443.2008.02148.x. [DOI] [PubMed] [Google Scholar]
- Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21:2163–2171. doi: 10.1002/oby.20584. [DOI] [PubMed] [Google Scholar]
- Del Re AC, Gordon AJ, Lembke A, Harris AH. Prescription of topiramate to treat alcohol use disorders in the Veterans Health Administration. Addict Sci Clin Pract. 2013;8:12. doi: 10.1186/1940-0640-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JW, 3rd, Sombati S, DeLorenzo RJ, Coulter DA. Cellular actions of topiramate: blockade of kainate-evoked inward currents in cultured hippocampal neurons. Epilepsia. 2000;41(suppl 1):S10–16. doi: 10.1111/j.1528-1157.2000.tb02164.x. [DOI] [PubMed] [Google Scholar]
- Gryder DS, Rogawski MA. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J Neurosci. 2003;23:7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361:1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM Topiramate for Alcoholism Advisory Board, Topiramate for Alcoholism Study Group. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298:1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Rosenthal N, Capece JA, Wiegand F, Mao L, Beyers K, McKay A, Ait-Daoud N, Addolorato G, Anton RF, Ciraulo DA, Kranzler HR, Mann K, O’Malley SS, Swift RM for the Topiramate for Alcoholism Advisory Board and the Topiramate for Alcoholism Study Group. Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch Int Med. 2008;168:1188–1199. doi: 10.1001/archinte.168.11.1188. [DOI] [PubMed] [Google Scholar]
- Kaminski RM, Banerjee M, Rogawski MA. Topiramate selectively protects against seizures induced by ATPA, a GluR5 kainate receptor agonist. Neuropharmacology. 2004;46:1097–1104. doi: 10.1016/j.neuropharm.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Gelernter J, Anton RF, Arias AJ, Herman A, Zhao H, Burian L, Covault J. Association of markers in the 3′ region of the GluR5 kainate receptor subunit gene to alcohol dependence. Alcohol Clin Exp Res. 2009;33:925–930. doi: 10.1111/j.1530-0277.2009.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranzler HR, Covault J, Feinn R, Armeli S, Tennen T, Arias AJ, Gelernter J, Oncken C, Pond T, Kampman KM. Topiramate treatment for heavy drinkers: Moderation by a GRIK1 polymorphism. Am J Psychiatry. 2014;171:445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhitsathian S, Uttawichai K, Booncharoen H, Wittayanookulluk A, Angkurawaranon C, Srisurapanont M. Topiramate treatment for alcoholic outpatients recently receiving residential treatment programs: a 12-week, randomized, placebo-controlled trial. Drug Alcohol Depend. 2013;133:440–446. doi: 10.1016/j.drugalcdep.2013.06.032. [DOI] [PubMed] [Google Scholar]
- McDonald R, Rogawski M. Cellular effects of antiepileptic drugs. In: Engel J Jr, Pedley TA, editors. Epilepsy: A Comprehensive Textbook. 2. Lippincott, Williams, & Wilkins; Philadelphia: 2006. pp. 1433–1446. [Google Scholar]
- Miranda R, Jr, MacKillop J, Monti PM, Rohsenow DJ, Tidey J, Gwaltney C, Swift R, Ray L, McGeary J. Effects of topiramate on urge to drink and the subjective effects of alcohol: a preliminary laboratory study. Alcohol Clin Exp Res. 2008;32:489–497. doi: 10.1111/j.1530-0277.2007.00592.x. [DOI] [PubMed] [Google Scholar]
- Moradi S, Kerman SRJ, Mollabashi M. The effect of topiramate on weight loss in patients with type 2 diabetes. J Res Med Sci. 2013;18:297–302. [PMC free article] [PubMed] [Google Scholar]
- Oliveros E, Somers VK, Sochor O, Goel K, Lopez-Jimenez F. The concept of normal weight obesity. Prog Cardiovasc Dis. 2014;56:426–33. doi: 10.1016/j.pcad.2013.10.003. [DOI] [PubMed] [Google Scholar]
- Pettinati H, Weiss R, Miller W, Donovan D, Ernst D, Rounsaville B. COMBINE Monograph Series, Volume 2. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence (DHHS Publication No. NIH 04-5289) Bethesda: NIAAA; 2004. [Google Scholar]
- Rosenstock J, Hollander P, Gadde KM, Sun X, Strauss R, Leung A OBD-202 Study Group. A randomized, double-blind, placebo-controlled, multicenter study to assess the efficacy and safety of topiramate controlled release in the treatment of obese type 2 diabetic patients. Diabetes Care. 2007;30:1480–1486. doi: 10.2337/dc06-2001. [DOI] [PubMed] [Google Scholar]
- Skradski S, White HS. Topiramate blocks kainate-evoked cobalt influx into cultured neurons. Epilepsia. 2000;41(suppl 1):S45–47. doi: 10.1111/j.1528-1157.2000.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Snijders T, Bosker R. Multilevel Analysis: An Introduction to Basic & Advanced Multilevel Modeling. Sage Publications, Inc; Thousand Oaks, CA: 2012. [Google Scholar]
- Verrotti A, Scaparrotta A, Agostinelli S, Di Pillo S, Chiarelli F, Grosso S. Topiramate-induced weight loss: A review. Epilepsy Res. 2011;95:189–99. doi: 10.1016/j.eplepsyres.2011.05.014. [DOI] [PubMed] [Google Scholar]
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate modulates GABA-evoked currents in murine cortical neurons by a nonbenzodiazepine mechanism. Epilepsia. 2000;41(suppl 1):S17–20. [PubMed] [Google Scholar]