Abstract
Drosophila suzukii recently invaded North America and Europe. Populations in Hawaii, California, New York and Nova Scotia are polymorphic for Wolbachia, typically with <20% infection frequency. The Wolbachia in D. suzukii, denoted wSuz, is closely related to wRi, the variant prevalent in continental populations of D. simulans. wSuz is also nearly identical to Wolbachia found in D. subpulchrella, plausibly D. suzukii's sister species. This suggests vertical Wolbachia transmission through cladogenesis (“cladogenic transmission”). The widespread occurrence of 7-20% infection frequencies indicates a stable polymorphism. wSuz is imperfectly maternally transmitted, with wild infected females producing on average 5-10% uninfected progeny. As expected from its low frequency, wSuz produces no cytoplasmic incompatibility (CI), i.e., no elevated embryo mortality when infected males mate with uninfected females, and no appreciable sex-ratio distortion. The persistence of wSuz despite imperfect maternal transmission suggests positive fitness effects. Assuming a balance between selection and imperfect transmission, we expect a fitness advantage on the order of 20%. Unexpectedly, Wolbachia-infected females produce fewer progeny than do uninfected females. We do not yet understand the maintenance of wSuz in D. suzukii. The absence of detectable CI in D. suzukii and D. subpulchrella makes it unlikely that CI-based mechanisms could be used to control this species without transinfection using novel Wolbachia. Contrary to their reputation as horizontally transmitted reproductive parasites, many Wolbachia infections are acquired through introgression or cladogenesis and many cause no appreciable reproductive manipulation. Such infections, likely to be mutualistic, may be central to understanding the pervasiveness of Wolbachia among arthropods.
Keywords: endosymbiont, mutualism, reproductive manipulation, fecundity, transmission
Introduction
Wolbachia are maternally transmitted, intracellular endosymbionts estimated to occur in nearly half of all insect species (Zug & Hammerstein 2012) and in many other arthropods (Bouchon et al. 1998) and filarial nematodes (Taylor et al. 2013). Wolbachia belong to the Rickettsiales order of α-Proteobacteria, whose members include the arthropod-vectored pathogens Ehrlichia and Rickettsia (Werren et al. 2008). Much Wolbachia research has focused on their ability to manipulate host reproduction to favor Wolbachia spread (Werren et al. 2008). Four types of reproductive manipulation are known: cytoplasmic incompatibility (CI), in which embryos produced by matings between infected males and uninfected females (or males and females with incompatible Wolbachia) suffer increased mortality (Hoffmann & Turelli 1997); male killing (MK), where infected females produce female-biased sex ratios (Hurst et al. 2000); feminization of genetic males (Rousset et al. 1992; Rigaud & Juchault 1993); and parthenogenesis induction (Rousset et al. 1992; Stouthamer et al. 1993). Only CI and MK are known in Drosophila. Transinfection experiments have established that both the nature and intensity of reproductive manipulation depend on host genetics and Wolbachia strain (Braig et al. 1994; Jaenike 2007; Zabalou et al. 2008; Veneti et al. 2012).
Despite the emphasis on reproductive effects, some Wolbachia infections cause little or no reproductive manipulation, including wMel in D. melanogaster (Hoffmann 1988) and wAu in D. simulans (Hoffmann et al. 1996; Kriesner et al. 2013). Both infections exhibit imperfect maternal transmission, which should systematically reduce their frequency. Yet the wMel-D. melanogaster association is minimally thousands of years old (Richardson et al. 2012; Chrostek et al. 2013); and wAu has been observed in Australia for over 20 years (approximately 200 fly generations), including a relatively rapid rise to an apparently stable equilibrium frequency near 0.6 (Kriesner et al. 2013). Presumably both infections persist by increasing host fitness (Hoffmann & Turelli 1997; Kriesner et al. 2013). Because Wolbachia are maternally transmitted, even when significant CI occurs, natural selection focuses on increasing fitness benefits for hosts rather than increasing reproductive manipulation (Turelli 1994; Haygood & Turelli 2009). Consistent with this expectation, Wolbachia have become critical to the survival and reproduction of several hosts. Wolbachia have been coevolving with filarial nematodes for millions of years; and removal causes various deleterious effects, including the inhibition of embryogenesis and larval development, reduced motility and adult viability, and stunted adult growth (Taylor et al. 2005). Within insects, Wolbachia are essential for female fertility in the parasitic wasp Asobara tabida (Dedeine et al. 2001). For several Drosophila paulistorum semispecies, Wolbachia have persisted through cladogenesis and removal is lethal (Miller et al. 2010). In D. mauritiana, infected females produce four times as many eggs as uninfected females (Fast et al. 2011).
Wolbachia can enhance host fitness in more subtle ways, including metabolic provisioning (Brownlie et al. 2009) and protection from other microbes (Hedges et al. 2008; Teixeira et al. 2008). This recently discovered anti-microbial effect has revitalized efforts to use Wolbachia for disease control, an idea that goes back to the 1960s (Laven 1967; McGraw & O'Neill 2013). The disease-vector mosquito Aedes aegypti has been transinfected with two Wolbachia strains from D. melanogaster (McMeniman et al. 2009; Walker et al. 2011), and two natural Australian Ae. aegypti populations have been transformed with wMel to suppress dengue virus transmission (Hoffmann et al. 2011). Recently Anopheles stephensi was transinfected with Wolbachia, making them less able to transmit the malarial parasite (Bian et al. 2013). These disease-suppression applications motivate additional analyses of Wolbachia in nature.
Although hundreds of papers concerning Wolbachia have appeared in the past decade, very few Wolbachia-host interactions have been studied intensively in nature. For fewer than 20 species do we have estimates of infection frequencies for multiple populations, analyses of transmission efficiency in nature, or analyses of reproductive manipulation or other phenotypes in the wild that might explain Wolbachia persistence and prevalence. Indeed, relatively complete scenarios explaining natural infection frequencies are available in very few cases, including: (1) the wRi infection in D. simulans, which is maintained at a high level (about 93%) in most populations by a balance between fairly intense CI but imperfect maternal transmission (Turelli & Hoffmann 1991, 1995; Carrington et al. 2011; Kriesner et al. 2013); (2) the infections in the mosquitoes Culex pipiens (Barr 1980; Rasgon & Scott 2003) and Ae. albopictus (Kittayapong et al. 2002) that produce complete CI and exhibit essentially perfect maternal infection, so that almost all individuals in nature are infected; (3) the monomorphic infections in the D. paulistorum species complex, which cause CI, contribute to assortative mating and have evolved to obligate mutualism while persisting through cladogenesis (Miller et al. 2010); (4) the imperfectly transmitted male-killing (MK) strain in D. innubila that confers a selective advantage of about 5% and is maintained at ∼35% infection frequency (Dyer et al. 2004); and (5) the MK infection in the butterfly Hypolimnas bolina, which shows both high infection frequency and transmission efficiency (Charlat et al. 2009). In other species, such as D. melanogaster and D. yakuba, we know that Wolbachia persists despite imperfect maternal transmission and no appreciable reproductive manipulation (Harcombe & Hoffmann 2004; Charlat et al. 2004; but the fitness benefits maintaining the infection are not known with certainty, although plausible candidates exist (Teixeira et al. 2008; Brownlie et al. 2009).
Within host species, Wolbachia are typically maternally transmitted. In contrast, phylogenetic discordance between distantly related insect hosts and their Wolbachia (O'Neill et al. 1992; Werren et al. 1995) suggests that Wolbachia may be generally horizontally transmitted between species (Stahlhut et al. 2010; Jaenike 2012), unlike the co-speciation typical of some insect-endosymbiont mutualisms, such as aphids and Buchnera (Moran & Baumann 1994). Although coalescent analyses are consistent with some Wolbachia infections of Drosophila being relatively young, on the order of a few thousand years (Richardson et al. 2012), some infections have persisted for hundreds of thousands of years (Jaenike & Dyer 2008), including through cladogenesis (Miller et al. 2010; Stahlhut et al. 2010). Because so few sister species have been examined for infection status, the frequency with which species acquire Wolbachia via descent (cladogenic transmission) or introgression (Rousset & Solignac 1995) versus horizontal transmission (O'Neill et al. 1992) is essentially unknown.
D. suzukii, the spotted wing Drosophila, is an invasive pest in North America and Europe that has spread rapidly, grows under a wide range of conditions (Tochen et al. 2014), and damages marketable fruit (Goodhue et al. 2011; Hauser 2011). Since its detection in coastal California in 2008, D. suzukii has spread through over half of the United States, Mexico and Canada (Fig. 1; Walsh 2011; Burrack et al. 2012; Freda & Braverman 2013). Since its discovery in Spain in 2008, it has also spread through most of continental Europe (Calabria et al. 2012; Cini et al. 2012). Given its economic importance and widespread distribution, D. suzukii has become a popular research organism (Hauser 2011), with an annotated genome (Chiu et al. 2013). Wolbachia was initially found in Japanese D. suzukii (Cordaux et al. 2008). Based on wsp sequencing, the infection was identified as wRi (Bennett et al. 2012), the strain prevalent in most populations of D. simulans (Ballard 2000; Kriesner et al. 2013). While identical to wRi at many commonly sequenced loci, a draft genome of Wolbachia from D. suzukii revealed several differences (Siozios et al. 2013), leading to the designation wSuz. Here we document wSuz infection in several populations of D. suzukii and describe initial attempts to better understand the population biology of wSuz and elucidate its prevalence, effects and origin, as such information might guide possible control measures. We also examined the Wolbachia infection in laboratory stocks of D. subpulchrella, plausibly D. suzukii's sister species.
Fig. 1.
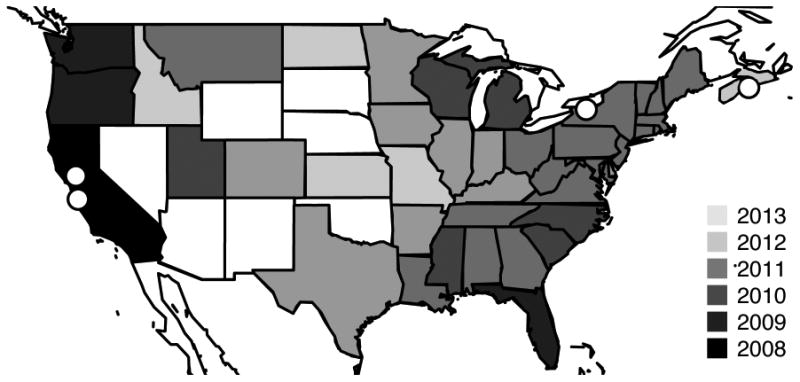
Map of D. suzukii samples used in this study (open circles) and timing of first detection by state (modified with permission from Burrack et al. 2012).
Materials and methods
Wolbachia detection and prevalence in natural populations
To determine Wolbachia prevalence in D. suzukii, in 2012 and/or 2013 we sampled one natural population in New York, two in California, and one in Nova Scotia (Fig. 1; Table 1). We also assayed two laboratory lines of D. subpulchrella and one of D. biarmipes. We used two concurrent PCR assays to determine the infection status of individual flies (Turelli & Hoffmann 1995). One reaction targeted the Wolbachia-specific 16S rDNA locus (Zhou et al. 1998; Werren & Windsor 2000), while the second targeted the arthropod-specific 28S rDNA (Folmer et al. 1994; Morse et al. 2009; primers used for all PCR experiments listed in Supplementary Material 1). Positive controls using single-copy nuclear genes are essential because failure to detect a Wolbachia PCR product could be due to: absence of Wolbachia, too much DNA, failure to extract DNA from the single-fly prep, or low-titer Wolbachia infection (e.g., Miller et al. 2010; Schneider et al. 2014).
Table 1.
Sampling locations by date with sample sizes (N), number infected (I) and infection frequency, with 95% confidence intervals (CI).
| Location | Date | I/N | Frequency (95% CI) |
|---|---|---|---|
| Rochester, NY | August 2012 | 10/109 | 0.092 (0.045, 0.162) |
| Rochester, NY | September 2012 | 12/178 | 0.067 (0.035, 0.115) |
| Nova Scotia, CAN | September 2013 | 4/34 | 0.117 (0.033, 0.275) |
| Winters, CA | June 2012 | 7/38 | 0.184 (0.077, 0.343) |
| Winters, CA | May 2013 | 41/71 | 0.577 (0.454, 0.694) |
| Watsonville, CA | September 2012 | 32/192 | 0.167 (0.117, 0.227) |
| Watsonville, CA | October 2012 | 7/40 | 0.175 (0.073, 0.327) |
| Watsonville, CA | August 2013 | 13/57 | 0.228 (0.123, 0.358) |
| Watsonville, CA | October 2013 | 33/210 | 0.157 (0.111, 0.214) |
We extracted DNA following the “squish” buffer protocol (Gloor et al. 1993) or used a DNeasy Blood and Tissue kit (Qiagen). PCR reaction concentrations followed Hamm et al. (2014) and profiles were derived from Duron et al. (2008). PCR products were visualized on 1% agarose gels alongside a standard. We considered an individual infected when both the Wolbachia-specific primers and nuclear controls produced fragments of the appropriate size. In population samples, Wolbachia infection frequency was estimated using only individuals with control-confirmed positive or negative infection status. We estimated exact 95% confidence intervals assuming a binomial distribution.
Of the California D. suzukii samples that failed to produce a Wolbachia band, 45 (15%) were randomly subjected to 1/10 and 1/100 dilutions of DNA and re-assayed. These samples were also assayed using quantitative real-time PCR (qPCR) to avoid false negative that might be produced by low-titer infections (Arthofer et al. 2009). These controls are important because highly variable infection titers have been reported within populations (Clark et al. 2005; Unckless et al. 2009). Using qPCR, we examined the titer of Wolbachia by amplifying a short segment of the wsp gene and comparing its abundance to that of Rps17, a reference nuclear gene (Osborne et al. 2009). All DNA utilized in qPCR experiments was extracted using a DNeasy kit. Each sample was assayed with four technical replicates per locus on an Illumina Eco™ real-time PCR machine. The concentrations and thermocycler profiles for qPCR followed Osborne et al. (2009) and relative Wolbachia density was estimated using the ΔCt method.
Wolbachia identification
To identify the Wolbachia strain(s) infecting D. suzukii, we randomly selected five infected females from Watsonville, California for multilocus sequence typing (MLST) (Baldo et al. 2006). We also typed the Wolbachia in D. subpulchrella line 201. Following Baldo et al. (2006), we sequenced five MLST protein-coding genes (gatB, coxA, hcpA, ftsZ, and fbpA) as well as wsp. Each gene was sequenced in both directions on an ABI 3730 DNA Analyzer (Applied Biosytems™) at the University of California, Davis DNA Sequencing Facility. The resulting chromatograms were assembled into contigs and visually inspected to ensure both reads were in agreement. These contigs were used as queries for a BLASTn search (Altschul et al. 1990) using the NCBI “nr” database to confirm that orthologous genes were amplified. Contigs were also used to search the Wolbachia MLST database (http://pubmlst.org/wolbachia/) using the “multiple locus query” feature. The allelic profiles were used to identify the Wolbachia strain. All chromatograms and sequences were deposited in the MLST database, and all data, accession numbers and statistical code were deposited on the DataDryad website (doi:10.5061/dryad.0pg63).
Given that new Wolbachia introductions should be associated with greatly reduced mitochondrial DNA variation (Turelli et al. 1992), we amplified ∼1300 bp of mitochondrial cytochrome oxidase I (COI) with PCR to estimate haplotype frequencies using 30 wild-caught individuals from California, 24 from New York, and from three lines of D. subpulchrella using a combination of primers (Folmer et al. 1994; Simon et al. 1994; Simon et al. 2006). Conditions for COI PCR followed the hcpA protocol from Baldo et al. (2006) or followed Haselkorn et al. (2013). To visualize the relationships among haplotypes, we generated a neighbor-joining tree using PAUP* (Swofford 2003) and asked if haplotypes were randomly associated with Wolbachia infection status, using Fisher's exact test.
Host phylogenies
Both D. subpulchrella (Takamori et al. 2006) and D. biarmipes have been proposed as sister to D. suzukii (van der Linde & Houle 2008; Yang et al. 2012). To reexamine these relationships, we used DNA from 13 protein-coding genes (3 mtDNA, 10 nDNA, downloaded from Genbank) for 10 members of the melanogaster species group (Supplementary Material 2). We conducted a partitioned Bayesian phylogenetic analysis using MrBayes 3.2.1 (Ronquist & Huelsenbeck 2003) under the GTR substitution model with Γ-distributed rate heterogeneity. We ran this analysis using 10 chains for at least 1 million generations (with sampling every 5,000 generations) and until the standard deviation of split frequencies was below 0.05.
Maternal transmission
Wild female D. suzukii were collected in September 2012 from Rochester, NY. Flies were allowed five days for oviposition. Individual females were maintained in vials on 8 mL of banana medium (Drosophila Species Stock Center recipe), supplemented with pieces of strawberry as needed. Cultures were kept at 22°C, 70% RH, and a 12:12 light:dark cycle. Following the oviposition period, females were frozen for PCR Wolbachia screening. For Wolbachia-infected females, we screened all daughters, up to a maximum of 20. A second assay used wild-caught female D. suzukii collected in November 2013 from Watsonville, CA. Individual females were maintained as above in vials with 10 mL of “Bloomington” standard medium fly food and ½ a blueberry to stimulate oviposition. Females were allowed to oviposit for two days before being transferred to a fresh vial, and the experiment was continued for 10 days. We screened all offspring of Wolbachia-infected females for infection. We estimated the rate of maternal transmission and used a bias corrected and accelerated (BCa) bootstrap to obtain 95% confidence intervals based on 10,000 pseudoreplicates (Efron & Tibshirani 1993).
Cytoplasmic incompatibility assays
Pairs of naturally infected and uninfected isofemale lines of D. suzukii were used for these experiments. Lines were established from wild-caught females from Rochester, NY (August 2012) and Central California (July 2009). Flies were reared as described above. Upon emergence, virgins were isolated and maintained on medium for five days. Immediately prior to mating a small piece of strawberry or ½ blueberry was provided. All four pairwise crosses between infection states were performed using single-pair matings. Flies were allowed to oviposit for 24 hours, and then transferred to fresh vials daily for four days. Following each transfer the eggs were counted, and after 48 hours the unhatched eggs were counted. We calculated the mean and standard error of hatch rate for each crossing type, averaging over the hatch rates associated with individual pairs. We did not distinguish between unfertilized eggs and dead embryos, and only crosses that generated at least 10 eggs were used in statistical analysis. Homogeneity among the four groups was examined using a Kruskal-Wallis rank-sum test. The infection status of males and females from each cross were verified by PCR. Similarly, CI assays were conducted using infected and uninfected D. subpulchrella laboratory strains, though here we compared the number of adult progeny produced.
Male killing (MK) assay
To investigate whether Wolbachia induced MK in D. suzukii or D. subpulchrella, we conducted reciprocal crosses between infected and uninfected males and females (derived from isofemale lines). For these experiments we used D. suzukii lines MTY3 (stock E-15003 from the Ehime, Japan Drosophila stock center), PacO (a stock previously available from Ehime), and MBW (a multi-female line established from Monterey Bay/Watsonville area by the Begun lab) and D. subpulchrella lines E-15201 (Ehime) and NGN5 (Ehime E-15203). Single pairs were placed in vials and maintained as above. Adults were transferred to fresh vials every two days. After the experiment, the parents were screened for Wolbachia to confirm infection status. Progeny from each cross were counted 14 days after the parents were removed. The total numbers of males and females for each experiment from infected lines were compared using a binomial test with p = 0.5.
Fecundity assays
Wild-caught D. suzukii were collected at an organic raspberry farm (Garroutte Farms) in Watsonville, California in October 2012 and October 2013. These flies were immediately taken to the laboratory, and individual females were reared as above with ½ a blueberry in each vial. Females were allowed to oviposit for two days before being transferred to a fresh vial, and the experiment was continued for 10 days. All ovipositing females were assayed for Wolbachia. The numbers of adult offspring from infected versus uninfected females were compared using a Wilcoxon signed-rank test.
We also compared the fecundity of infected versus uninfected females from laboratory stocks. Single pairs were placed in food vials and transferred to fresh vials every two days for a total of 25 days, after which the infection status of the pair was tested by PCR. After two weeks, the adult progeny were counted. We tested the infection status of five progeny from each cross. The numbers of offspring for Wolbachia infected and uninfected females were compared using a Wilcoxon test.
Because Wolbachia density can be influenced by many factors, including diet and rearing density, we conducted a fecundity experiment in which the parents were reared under more controlled conditions. We informally controlled density by placing three males and three females in food vials for three days; replicates were established for both infected and uninfected lines. From these offspring we collected virgins and held them for three days before performing reciprocal crosses (U♂ ⊥ I♀ and I♂ ⊥ U♀), placing one male and female in each vial. We transferred the pairs daily to fresh vials for five days. After two weeks the offspring from each cross were counted.
For D. subpulchrella, we controlled for nuclear effects on fecundity with reciprocal crosses between infected and uninfected lines. We informally controlled density by placing three males and three females in standard food vials with ½ blueberry for two days. Single pairs of virgin F1 females and uninfected F1 males were placed in holding vials with ½ blueberry for one day, then transferred to fresh food vials with ½ blueberry every day for five days. We counted emerged adults after 14 days, but excluded counts from females that produced fewer than 10 offspring.
Desiccation assay
To determine whether Wolbachia modified desiccation resistance, wild-caught D. suzukii males (collected from Watsonville, CA in October 2013) were placed individually in small test tubes, then transferred to a 0.04 m3 glass aquarium with 200 gm of desiccant (Drierite). The tubes were checked every hour and dead flies removed. After 24 hours, the experiment was terminated and the flies screened for Wolbachia.
Starvation assay
To determine whether Wolbachia modified starvation resistance, we placed groups of five wild-caught D. suzukii females in standard Drosophila vials filled with 15 mL of 1% agar and placed the vials in an incubator. The vials were checked every 12 hours and dead flies were removed. After 72 hours, the experiment was terminated and the flies screened for Wolbachia.
Results
Wolbachia detection and prevalence
We found D. suzukii infected with Wolbachia throughout North America (Table 1). All 929 D. suzukii surveyed for Wolbachia produced a visible control band of the appropriate size for 28S rDNA. Of these, 159 were PCR-positive for Wolbachia. Of the 475 California flies that produced a 28S rDNA product but failed to generate a Wolbachia product, we assayed 45 using serial dilution, low-titer primers for the SMArTR and 12S rDNA loci (Schneider et al. 2014), and qPCR. These more sensitive assays also failed to detect Wolbachia infection.
In eight of our nine samples, infection frequencies ranged from 7 to 23% among the four populations surveyed. One sample from Winters, CA produced an outlier frequency of 58% (Table 1). The infection frequencies were not equal across all samples (χ2 = 103.3, df = 8, P < 0.0001). However, the eastern samples (New York and Nova Scotia) were statistically homogeneous (χ2 = 1.2, df = 2, P = 0.55), with an overall infection frequency of 0.08 and 95% confidence interval of (0.05, 0.12). Similarly, without the outlier, the California samples were homogeneous (χ2 = 2.0, df = 4, P = 0.74), with an overall infection frequency of 0.17 (0.14, 0.21). Even without the anomalous May 2013 sample from Winters, CA, the pooled eastern versus western samples showed significantly different frequencies (χ2 = 16.5 P = 0.02). The anomalous Winters sample corresponded to a statistically significant infection frequency increase between June 2012 and May 2013 (χ2 = 15.3, P < 0.001). Using each collection as a single observation of Wolbachia infection prevalence, the data suggest that the mean prevalence was greater in California than in eastern populations (Kruskal-Wallis χ2 test; Z = 5.4, P = 0.02). Strain E-15201 of D. subpulchrella carried Wolbachia, whereas strain NGN5 of D. subpulchrella and the genome strain of D. biarmipes were uninfected.
Wolbachia identification and mtDNA diversity
Using the Wolbachia MLST protocol on infected D. suzukii, we identified the following alleles with 100% identity to previously reported alleles for each of the five protein-coding genes sequenced: gatB 22, coxA 23, hcpA 24, ftsZ 3, and fbpA 23. This allelic profile corresponded with strain 17 in the MLST database, the wRi strain found in D. simulans (Hoffmann et al. 1986). We observed the same allelic profile (100% identity) in D. subpulchrella. Similarly, D. suzukii and D. subpulchrella showed 100% sequence identity with wsp allele 16 found in wRi of D. simulans.
We sequenced over 1200bp of mitochondrial COI for 23 female D. suzukii from Watsonville, CA and three D. subpulchrella lines. We identified five mtDNA haplotypes and found three haplotypes shared between infected and uninfected individuals. In contrast to the identity of the Wolbachia genotypes, we observed 11 fixed differences between the COI haplotypes of D. suzukii and D. subpulchrella (Fig. 2). The same five COI haplotypes were found in D. suzukii from Rochester. As expected with imperfect maternal transmission of Wolbachia and an infection that has approached its equilibrium frequency (Turelli et al. 1992), the infection was randomly associated with D. suzukii mtDNA haplotypes (Fisher's exact test, P = 0.29 based on 10,000 Monte Carlo simulations).
Fig. 2.
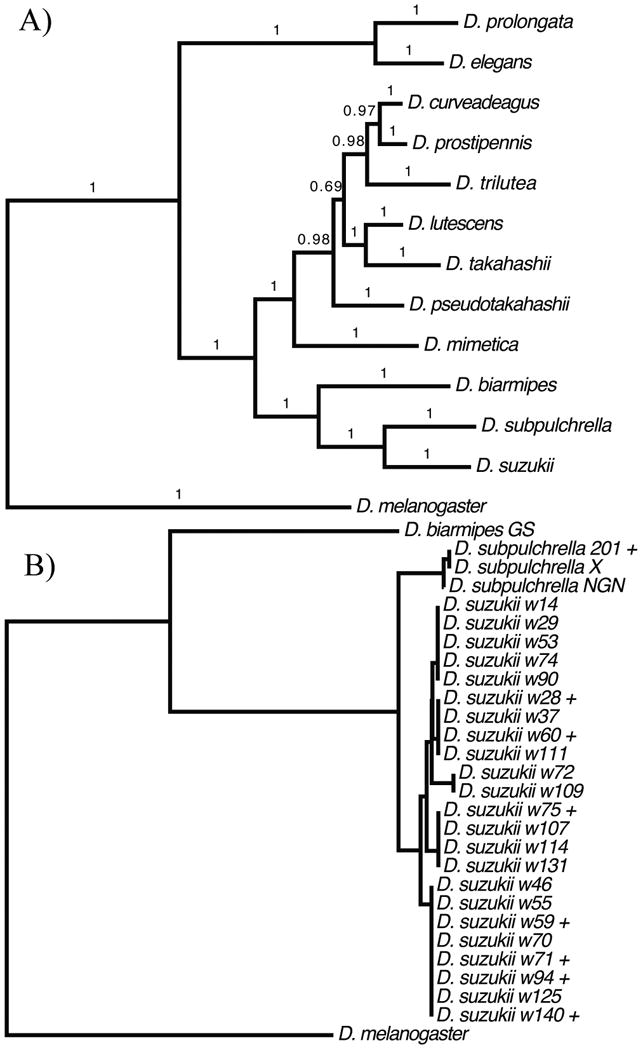
Phylogenetic trees: A) Bayesian phylogeny depicting the relationships among relatives of D. suzukii; B) Neighbor-joining tree of mtDNA haplotypes for D. suzukii and relatives. Wolbachia-infected individuals denoted (+).
Host phylogenetics
To understand the evolutionary history of Wolbachia infection in D. suzukii and D. subpulchrella, we estimated the phylogeny of the suzukii subgroup, using 11,382 bp from 13 protein-coding loci. The fully resolved Bayesian tree placed the members of the D. suzukii subgroup in a monophyletic clade with D. subpulchrella sister to D. suzukii (a result concordant with the mtDNA tree) with high posterior support (Fig. 2).
Maternal transmission
We screened the offspring of wild-caught D. suzukii females infected with Wolbachia to estimate transmission frequency. From Rochester, NY samples we screened the female offspring of 14 Wolbachia-infected females that produced at least 20 offspring. We screened male and female progeny from six infected females from Watsonville, CA. Most females perfectly transmitted Wolbachia to both male and female offspring (Fig. 3), though six exhibited imperfect transmission with transmission rates varying from 95% to 20% (Fig. 3). The mean transmission rate was 0.86 with a 95% BCa bootstrap confidence interval of (0.73, 0.96). Given this variation, many infected females would have to be screened to accurately assess the fraction of “low transmitters”; but maternal transmission of Wolbachia is clearly imperfect in natural populations of D. suzukii.
Fig. 3.
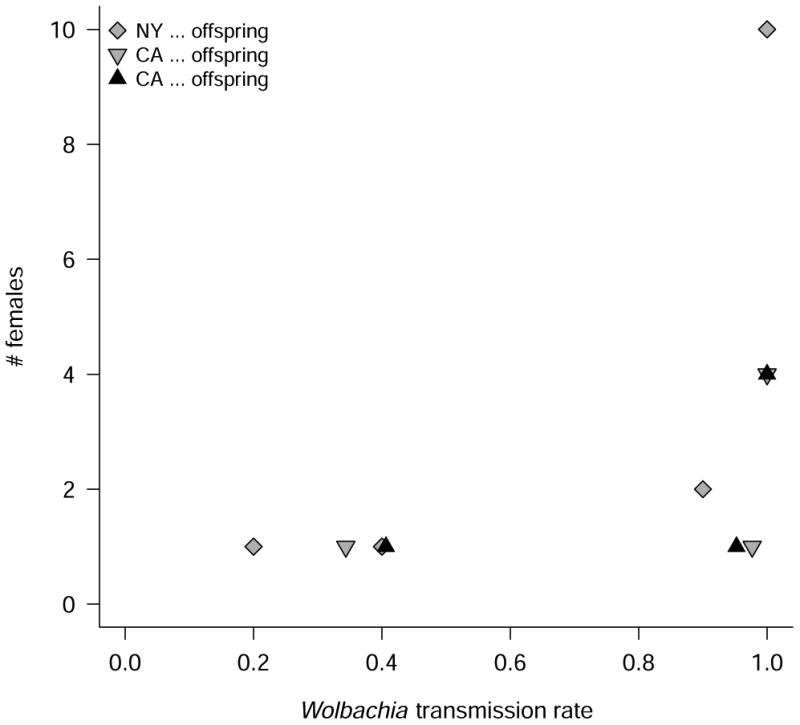
Wolbachia transmission by wild-caught D. suzukii females.
Cytoplasmic incompatibility (CI)
With CI, we expect reduced egg hatch when infected males mate with uninfected females. We found no evidence for CI in D. suzukii (Table 2). For our California analysis, hatch rates were homogeneous among all four classes of crosses (Kruskal-Wallis test, P = 0.7). In contrast, the hatch rates among crosses of New York D. suzukii were not homogeneous (Kruskal-Wallis test, P = 0.005). However, this was due to an unexpected low hatch rate from crosses between uninfected males and infected females, the opposite of what would be expected with CI. Similarly, we found no evidence of CI in D. subpulchrella (Table 3). Although the numbers of progeny were not homogeneous among groups (Kruskal-Wallis test, P = 0.04; Table 3) (Table 3), there was no significant difference between the potential CI cross and its reciprocal (Wilcoxon test P = 0.82). In principle, CI might be observed with males of different ages (Hoffmann et al. 1986; Hoffmann 1988).
Table 2.
Cytoplasmic incompatibility assays for D. suzukii from New York and California. Mean egg hatch rates (for females that laid ≥ 10 eggs) ± standard error (se), and sample sizes (N). U denotes Wolbachia-uninfected, I denotes infected. There was a difference among group hatch rates for New York flies (Kruskal-Wallis test, P = 0.005), but not California (Kruskal-Wallis test, P = 0.687).
| New York (block 1) | New York (block 2) | California | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Female | Male | Mean hatch rate (± se) | N | Mean hatch rate (± se) | N | Mean hatch rate (± se) | N |
| U | U | 0.30 ± 0.06 | 9 | 0.625 ± 0.03 | 19 | 0.588 ± 0.05 | 23 |
| U | I | 0.51 ± 0.06 | 5 | 0.474 ± 0.05 | 19 | 0.538 ± 0.07 | 15 |
| I | U | 0.38 ± 0.04 | 11 | 0.307 ± 0.09 | 9 | 0.596 ± 0.03 | 28 |
| I | I | 0.38 ± 0.04 | 11 | 0.600 ± 0.07 | 15 | 0.547 ± 0.05 | 23 |
Table 3.
Cytoplasmic incompatibility assay for D. subpulchrella. Mean adult numbers (for females that produced ≥ 10 progeny) ± standard error (se), and sample sizes (N). U denotes Wolbachia-uninfected, I denotes infected. There was a difference among hatch rates of all groups (Kruskal-Wallis test, P = 0.036); however there was no difference between CI cross and its reciprocal (two-tailed Wilcoxon test, P = 0.65)
| Female | Male | Mean emerged adults (± se) | N |
|---|---|---|---|
| U | U | 15.5 (± 1.56) | 6 |
| U | I | 19.7 (± 2.46) | 10 |
| I | U | 20.14 (± 2.25) | 7 |
| I | I | 25.24 (± 2.12) | 17 |
Male killing (MK)
With MK, we expect biased sex ratios from infected mothers. Reciprocal crosses between infected and uninfected lines of D. suzukii and D. subpulchrella revealed no evidence of female-biased sex ratio in either D. suzukii (P = 0.37; N = 179) or D. subpulchrella (P = 0.47; N = 507) (Fig. 4).
Fig. 4.
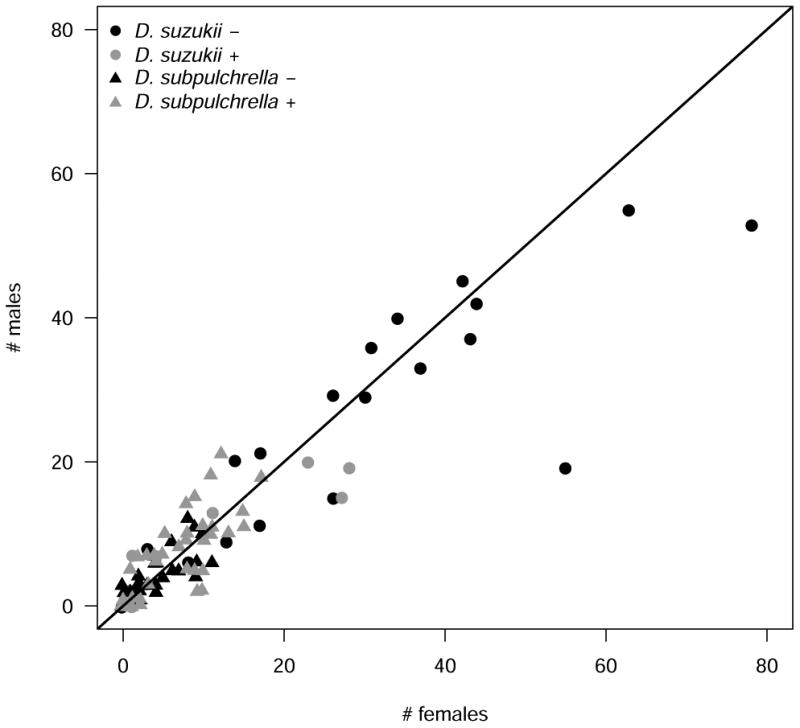
Scatterplot of the number of male and female offspring produced by Wolbachia-infected (+) and uninfected (–) females (mated to uninfected and infected males, respectively) of D. suzukii and D. subpulchrella. The line denotes 1:1 offspring sex ratios. Female offspring did not occur at a higher rate than males in infected D. suzukii + (binomial test p = 0.5, P = 0.37; N = 179) or D. subpulchrella + (P = 0.47; N = 507), indicating no male killing (or feminization).
Fecundity assays
To offset imperfect maternal transmission, we expected a fecundity advantage for Wolbachia-infected females. We collected 40 D. suzukii females from Watsonville, CA in 2012 and 66 in 2013. In both 2012 and 2013, we detected a significant fecundity disadvantage for infected females [2012: I 10.3 ± 8.4 (N = 7); U 51.6 ± 8.3 (N = 29), two-tailed Wilcoxon test, P < 0.005; 2013: I 19.9 ± 6.5 (N = 20); U 54.5 ± 8.3 (N = 9), Wilcoxon test, P = 0.02]. In principle, these differences could have been caused by host genotype differences rather than infection status. However, with imperfect maternal transmission, infection status is rapidly randomized over both nuclear and mitochondrial genotypes (Turelli et al. 1992).
We complemented these field assays by crossing infected and uninfected laboratory lines of D. suzukii in both New York and California. Again we detected a significant fecundity disadvantage associated with Wolbachia (Table 4, Wilcoxon tests, P < 0.01). When repeated with density control, the infected females produced slightly fewer offspring than uninfected (I 7.6 ± 1.7 (N = 8); U 9.6 ± 3.4 (N = 14) but the difference was not statistically significant (Wilcoxon test, P = 0.41). Our density-controlled fecundity experiment for D. subpulchrella showed no statistically significant difference in the number of offspring produced by infected versus uninfected females produced from reciprocal crosses (I 30.9 ± 2.8 [N = 15]; U 33.7 ± 2.9 [N = 11]; Wilcoxon test, P = 0.58).
Table 4.
Mean number of adult D. suzukii (± standard error, sample size) generated by Wolbachia-infected (I) versus uninfected (U) laboratory-reared females.
| State | Block | I | U |
|---|---|---|---|
| NY | 1 (October 2012) | 3.84 (± 0.70, 60) | 9.24 (± 1.10, 56) |
| 2 (March 2013) | 14.25 (± 2.62, 60) | 9.73 (± 2.30, 48) | |
| CA | 19.89 (± 6.49, 9) | 54.45 (± 6.49, 20) |
Although several tests indicated a Wolbachia-associated reduction in fecundity, some infected females had apparently normal numbers of offspring. We used qPCR to compare Wolbachia titer among females and found no significant association between the level of infection and fecundity (linear regression, P = 0.31, df = 1,8).
Desiccation assay
We assayed 76 male D. suzukii for resistance to desiccation, 12 of which were Wolbachia-infected. The mean survival time for infected flies was 14.25 hours [95% confidence (12.9, 15.5; N = 12)], while the mean survival time for uninfected flies was 13.16 hours (9.9, 16.4; N = 64). Our data provided no evidence that Wolbachia increases desiccation resistance (Wilcoxon test, P = 0.67). Only males were available for the desiccation assay due the requirements for females in other experiments.
Starvation assay
We assayed 70 female D. suzukii for resistance to starvation, 11 of which were Wolbachia-infected. Mortality rates were fairly high within the first 12 hours of the experiment because many flies stuck to the agar. Excluding these flies, the mean survival times for infected and uninfected flies were 26.6 (20.5, 32.8; N = 9) and 22.7 (18.8, 25.7; N = 55) hours, respectively. We detected no Wolbachia effect on survival (Wilcoxon test, P = 0.205).
Discussion
What maintains wSuz in D. suzukii?
Wolbachia are best known for reproductive manipulations that increase the representation of infected cytoplasms. Yet, we found no evidence of cytoplasmic incompatibility (CI) or male killing (MK) in D. suzukii or D. subpulchrella. Reciprocal crosses between infected and uninfected lineages do not show CI (Tables 2 and 3). Furthermore, Wolbachia-infected D. suzukii and D. subpulchrella females produce 1:1 offspring sex ratios, indicating neither MK nor feminization (Fig. 4). Thus, efforts to control or manipulate D. suzukii by Wolbachia-induced reproductive manipulation, if possible at all, would require transinfection with non-native Wolbachia. CI or MK may be observed with males and females of different ages than we have used (Hoffmann et al. 1986; Hoffmann 1988). This will be explored in future analyses.
With no apparent reproductive manipulation, wSuz also shows imperfect maternal transmission in D. suzukii. This should systematically reduce its frequency. Yet, the widespread geographic distribution of 7-20% infection frequencies, including our 2012-13 North American samples (Table 1) and an earlier Hawaiian sample (Bennett et al. 2013), strongly suggests that wSuz is stably maintained, as does the random association between Wolbachia infection and mtDNA haplotypes. The simplest explanation is that wSuz produces a fitness advantage that balances its imperfect transmission (Hoffmann & Turelli 1997). Under this scenario, if a fraction μ of the ova produced by infected females are uninfected, wSuz should persist only if it enhances the relative fitness, F, of infected females sufficiently that F(1 – μ) > 1. With constant parameter values, the predicted equilibrium frequency is p = 1 – [μF/(F – 1)]. Hence, to maintain frequencies on the order of 5-20% in the face of μ ≅ 15%, the selective advantage, F – 1, associated with wSuz should be appreciable, on the order of 20%.
This prediction motivated our experiments examining relative fecundity, starvation tolerance and desiccation resistance. None of our assays revealed a beneficial effect of wSuz. We may have assayed the wrong phenotypes, our experiments (apart from fecundity) may have been too small, or fitness benefits may be context-dependent. Wolbachia strains closely related to wSuz confer resistance to RNA viruses that are otherwise virulent to Drosophila (Hedges et al.2008; Teixeira et al. 2008; Osborne et al. 2009; Unckless & Jaenike 2012). D. suzukii has a relatively robust immune response, associated with increased hemocyte loads relative to D. melanogaster (Kacsoh & Schlenke 2012); and its largest gene-family expansions and contractions are related to immune response (Chiu et al. 2013). Hence, effects of Wolbachia on immune response are natural candidates for the expected fitness benefits we have not yet discovered.
Temporal and spatial heterogeneity of infection frequencies
If wSuz confers resistance to microparasites, which often occur as epidemics, substantial fluctuations in prevalence may be expected. Thus, the significant increase in Wolbachia infection frequency in the Winters, CA population from 18% to 58% (P < 0.001) in less than one year may reflect a cryptic epidemic. Anomalous frequency changes have also been observed in D. simulans (Turelli & Hoffmann 1995), and Unckless et al. (2009) documented seasonal changes in Wolbachia titer in D. innubila that may alter fitness effects. The frequency differences observed in eastern and western samples may reflect systematic environmental differences. Alternatively, because D. suzukii has been detected in California longer than in northeastern US (Burrack et al. 2012), frequency differences may reflect increased build up in pathogen loads in the west. These conjectures will be tested in future studies, along with the effects of wSuz on pathogen resistance, development time, longevity and mating. For now, the apparent persistence of wSuz remains a mystery.
Although we have sampled only a handful of populations, we found a significantly lower frequency of wSuz infection in eastern (New York and Nova Scotia) than western D. suzukii populations. Continued sampling will reveal whether the difference persists or is a consequence of chance effects associated with recent invasion. Fig. 1 shows that D. suzukii had a disjunct distribution across the United States in 2013. Because this species was first discovered in California and Florida, it is likely that northeast populations were derived from the west or south, plausibly as a result of shipping strawberries or raspberries, favored D. suzukii breeding resources (Goodhue et al. 2011). If the lower eastern frequencies are founder events, infection prevalence might increase to the level seen in California. Given the inferred selective advantage of carrying Wolbachia, such frequency increases may be detectable within a few years.
Master manipulator or helpful guest?
The Wolbachia strains present in the sister taxa D. suzukii and D. subpulchrella are very closely related (identical at the five MLST loci and wsp), whereas their mtDNA show significant differences. Moreover, in D. suzukii the same mtDNA haplotypes are found among infected and uninfected individuals. These facts indicate that the association of wSuz with D. suzukii is old. The lack of differentiation between Wolbachia in D. suzukii and D. subpulchrella, combined with appreciable interspecific mtDNA differences, suggests that an infection in their most recent common ancestor may have persisted through cladogenesis. Cladogenic transmission has also been proposed for Wolbachia shared by D. simulans and D. sechellia (Rousset & Solignac 1995), for three species of the D. testacea group (Jaenike et al. 2010) and for several of the reproductively isolated D. paulistorum semispecies (Miller et al. 2010).
Although horizontal transmission of Wolbachia is proven by phylogenetic discordance between Wolbachia and distantly related hosts (O'Neill et al. 1992), only comparisons of sister species can reveal the relative frequencies of horizontal transmission versus cladogenic transmission or introgression. Horizontal transmission is clearly indicated when closely related hosts harbor distantly related Wolbachia. In contrast, when closely related hosts harbor closely related Wolbachia, cladogenic transmission or introgression seems more plausible. Recent introgression can be ruled out if mtDNA and nuclear loci show concordant phylogenies. Hence, we have inferred cladogenic transmission of the closely related Wolbachia in D. suzukii and D. subpulchrella. Table 5 provides a preliminary summary of what is known within the genus Drosophila about Wolbachia reproductive manipulation and mode of acquisition. Except where noted, we have accepted the conclusions of the original investigators. When closely related hosts harbor very similar Wolbachia, we have inferred cladogenic transmission. What is most striking from Table 5 is how few infections have been characterized and how often transmission has been via cladogenesis or introgression (as evidenced by mtDNA comparisons, cf. Rousset & Solignac 1995). Table 5 proposes several new examples of cladogenic transmission among Hawaiian Drosophila. Additional mtDNA analyses may support introgression in some of these cases; however, our preliminary analysis suggests that fewer than half of Wolbachia-infected Drosophila received their infections via horizontal transmission (9 out of 31 cases in Table 5).
Table 5.
Summary of published naturally occurring Wolbachia infections in Drosophila, indicating the Wolbachia strain designation, the reproductive manipulation phenotype (CI = cytoplasmic incompatibility, MK = male killing, N = very weak or none), and the mode of acquisition of the Wolbachia infection (C = cladogenic transmission, H = horizontal transmission, I = introgression). Empty cells indicate no information. Species in small clades in which cladogenic or introgressive inheritance of Wolbachia is suggested are grouped.
| Species | Strain | Phenotype | Origin | References |
|---|---|---|---|---|
| cardini group | ||||
| arawakana | wWil | 1 | ||
| arawakana | wSpt | 1 | ||
| arawakana | 1 | |||
| neocardini | 2 | |||
| Hawaiian species | ||||
| bristle tarsus subgroup | ||||
| nr basimacula | wBas | 3,4 | ||
| prodita | wDas | C | 3,4 | |
| redunca | wDas | C | 3,4 | |
| split tarsus subgroup | ||||
| ancyla | wGin | C | ||
| fundita | wFun | C | 3,4 | |
| nr fundita | wGin | C | 3,4 | |
| forficata | wFor | Ha | 3,4 | |
| nr dorsigera | wFor | 3,4 | ||
| spoon tarsus subgroup | ||||
| dasycnemia | wDas | 3,4 | ||
| setiger subgroup | ||||
| eurypeza | wEur | 3,4 | ||
| tetraspilota | wTet | 3,4 | ||
| melanogaster group | ||||
| ananassae subgroup | ||||
| ananassae | wRi | CI | 5 | |
| ananassae | wSpt | 1 | ||
| pseudoananassae | wPana | 1 | ||
| melanogaster subgroup | ||||
| melanogaster | wMel | CI (weak) | Ha | 6 |
| simulans | wRi | CI | Ha | 7 |
| simulans | wAu | N | Ha | 8 |
| sechellia | wHa | CI | C | 9 |
| sechellia | wNo&wHa | CI | C | 9 |
| simulans | wHa | CI | C | 9, 10 |
| simulans | wNo&wHa | CI | C | 9, 11, 12 |
| simulans | wNo/wMab | CI | C | 9, 11, 12 |
| mauritiana | wMa | N | I | 9 |
| santomea | wSty | N | I or Cc | 13, 14 |
| teissieri | wSty | N | I or Cc | 13, 14, 15 |
| yakuba | wSty | N | I or Cc | 13, 14, 15 |
| montium subgroup | ||||
| auraria | CI | 16 | ||
| baimaii | wBai | 1 | ||
| bicornuta | wBic | 1 | ||
| kikkawai | wKik | 3 | ||
| nikananu | wNik | 1 | ||
| triauraria/quadrariad | wRi | 1, 17 | ||
| suzukii subgroup | ||||
| subpulchrella | wSuz | N | C | this study |
| suzukii | wSuz | N | C | 17, 18 |
| takahashii subgroup | ||||
| pseudotakahashii | wPse | 1 | ||
| mitchellii group | ||||
| nigrocirrus | wEla | 3 | ||
| obscura group | ||||
| ambigua | ?e | 19 | ||
| tristis | ?e | 19 | ||
| bifasciata | wBif | MK | 20 | |
| quinaria group | ||||
| innubila | wInn | MK | H | 21 |
| munda | wMun | 22 | ||
| quinaria | H | 23 | ||
| recens | CI | H | 24 | |
| saltans group | ||||
| prosaltans | wPro | C | 25 | |
| septentriosaltans | wSpt | C | 25 | |
| sturtevanti | wStv | 1 | ||
| semieuscata group | ||||
| apicipuncta | wApi | 3 | ||
| testacea group | ||||
| orientacea | wOri | C | 26 | |
| neotestacea | wNeo | C | 26 | |
| testacea | wTes | C | 27 | |
| virilis group | ||||
| borealis | MK | 22 | ||
| willistoni group | ||||
| paulistorum semispecies | wAu | C | 28 | |
| tropicalis | wTro | H | 29 | |
| willistoni | wWil | H | 25, 29 |
Based on the observed molecular differences between the Wolbachia in this species and its closest relatives, horizontal transmission seems most plausible.
These infections may be identical (Ballard 2004)
Lachaise et al. (2000) conjecture that the Wolbachia were transmitted by introgression. However, although strong evidence exists for mtDNA introgression (Bachtrog et al. 2006; Llopart et al. 2014), there is insufficient resolution of the Wolbachia differentiation to rule out cladogenic transmission.
D. quadraria is a junior synonym of D. triauraria (Watada et al. 2011).
Haine et al. (2005) conjecture that these obscura species may have experienced horizontal transmission; but given the very close relationship of the hosts and the lack of mtDNA data, cladogenic or introgressive transmission seem equally plausible.
References:
The lack of detectable reproductive manipulation by the Wolbachia in D. suzukii and D. subpulchrella may reflect a long history of coevolution (Hoffmann & Turelli 1997; Hornett et al. 2006). As Table 5 shows, lack of detectable CI or MK is not uncommon. Prior to PCR assays, there was an ascertainment bias favoring the discovery of Wolbachia that manipulate reproduction. With so few natural Wolbachia infections characterized for their effects, we know relatively little about the frequency or intensity of reproductive manipulation. For very few hosts do we know Wolbachia frequencies in natural populations. Finding persistent low-frequency infections rules out significant CI, which produces stable equilibrium frequencies above 0.5 (Turelli & Hoffmann 1995). Mutualistic effects may prove more common and/or more important for Wolbachia prevalence than reproductive manipulation (cf. Jaenike & Brekke 2011).
Combining infection studies of sister species with tests of reproductive manipulation will help us understand Wolbachia biology and patterns of coevolution with its hosts. In addition to providing a model for host-symbiont coevolution, Wolbachia hold significant promise for the control of vector-borne diseases (McGraw & O'Neill 2013). Although we do not yet understand what maintains wSuz in D. suzukii, it is clear that Wolbachia infections need not manipulate host reproduction to persist or spread (Hoffmann 1988; Hoffmann et al. 1996; Charlat et al. 2004; Kriesner et al. 2013).
Supplementary Material
Acknowledgments
We thank Ary Hoffmann, Wolfgang Miller and an anonymous reviewer for constructive comments on an earlier versions. We thank Emma Dietrich, Wyatt Hanft, Derek Leale, and Andrew Tremain for assistance with the experiments and Artyom Kopp for providing D. subpulchrella stocks. This research was supported in part by NIH grant R01 GM104325 to MT, NIH grant R01 GM084056 to DJB and NSF grant DEB-1144581 to JJ. CAH was supported by USDA-NIFA SCRI Competitive Research Grant 2010-51181-21167 to DJB.
Footnotes
The authors declare no conflicts of interest
Data accessibility: All chromatograms and sequences generated from the Wolbachia MLST protocol have been archived on the Wolbachia MLST database.
All data, accession numbers and statistical code have been deposited on Dryad: doi:10.5061/dryad.0pg63.
Author contributions: MT, JJ, CAH and DJB designed the research.
CAH, AV, CCRS, PS and AOS performed the research.
CAH, JJ, and MT analyzed the data.
MT and CAH wrote the paper.
References
- Altschul SF, Gish W, Miller W, et al. Basic local alignment search tool. Journal of Molecular Biology. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Arthofer W, Riegler M, Schneider D, et al. Hidden Wolbachia diversity in field populations of the European cherry fruit fly, Rhagoletis cerasi (Diptera, Tephritidae) Molecular Ecology. 2009;18:3816–3830. doi: 10.1111/j.1365-294X.2009.04321.x. [DOI] [PubMed] [Google Scholar]
- Bachtrog D, Thornton K, Clark A, et al. Extensive introgression of mitochondrial DNA relative to nuclear DNA in the Drosophila yakuba species group. Evolution. 1980;60:292–302. [PubMed] [Google Scholar]
- Barr AR. Cytoplasmic incompatibility in natural populations of mosquito, Culex pipiens L. Nature. 1980;283:71–72. doi: 10.1038/283071a0. [DOI] [PubMed] [Google Scholar]
- Baldo L, Hotopp JCD, Jolley KA, et al. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Applied and Environmental Microbiology. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard JWO. Comparative genomics of mitochondrial DNA in Drosophila simulans. Journal of Molecular Evolution. 2000;51:64–75. doi: 10.1007/s002390010067. [DOI] [PubMed] [Google Scholar]
- Ballard JWO. Sequential evolution of a symbiont inferred from the host: Wolbachia and Drosophila simulans. Molecular Biology and Evolution. 2004;21:428–442. doi: 10.1093/molbev/msh028. [DOI] [PubMed] [Google Scholar]
- Bennett GM, Pantoja NA, O'Grady PM. Diversity and phylogenetic relationships of Wolbachia in Drosophila and other native Hawaiian insects. Fly. 2012;6:1–11. doi: 10.4161/fly.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bian G, Joshi D, Dong Y, et al. Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 2013;340:748–751. doi: 10.1126/science.1236192. [DOI] [PubMed] [Google Scholar]
- Bouchon D, Rigaud T, Juchault P. Evidence for widespread Wolbachia infection in isopod crustaceans: molecular identification and host feminization. Proceedings of the Royal Society of London B. 1998;265:1081–1090. doi: 10.1098/rspb.1998.0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtzis K, Nirgianaki A, Markakis G, et al. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics. 1996;144:1063–1073. doi: 10.1093/genetics/144.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braig HR, Guzman H, Tesh RB, et al. Replacement of the natural Wolbachia symbiont of Drosophila simulans with a mosquito counterpart. Nature. 1994;367:453–455. doi: 10.1038/367453a0. [DOI] [PubMed] [Google Scholar]
- Brownlie JC, Cass BN, Riegler M, et al. Evidence for metabolic provisioning by a common invertebrate endosymbiont, Wolbachia pipientis, during periods of nutritional stress. PLoS Pathogens. 2009;5:e1000368. doi: 10.1371/journal.ppat.1000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrack HJ, Smith JP, Pfeiffer DG, et al. Using volunteer-based networks to track Drosophila suzukii (Diptera: Drosophilidae) an invasive pest of fruit crops. Journal of Integrated Pest Management. 2012;4 2012 DOI: http://dx.doi.org/10.1603/IPM12012. [Google Scholar]
- Calabria G, Máca J, Bächli G, et al. First records of the potential pest species Drosophila suzukii (Diptera: Drosophilidae) in Europe. Journal of Applied Entomology. 2012;136:139–147. [Google Scholar]
- Carrington LB, Lipkowitz JR, Hoffmann AA, et al. A re-examination of Wolbachia-induced cytoplasmic incompatibility in California Drosophila simulans. PLoS ONE. 2011;6:e22565. doi: 10.1371/journal.pone.0022565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlat S, Ballard JWO, Mercot H. What maintains noncytoplasmic incompatibility inducing Wolbachia in their hosts: a case study from a natural Drosophila yakuba population. J Evolutionary Biology. 2004;17:322–330. doi: 10.1046/j.1420-9101.2003.00676.x. [DOI] [PubMed] [Google Scholar]
- Charlat S, Duplouy A, Hornett EA, et al. The joint evolutionary histories of Wolbachia and mitochondria in Hypolimnas bolina. BMC Evolutionary Biology. 2009;9:64. doi: 10.1186/1471-2148-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu JC, Jiang X, Zhao L, et al. Genome of Drosophila suzukii, the Spotted Wing Drosophila. G3. 2013;3:2257–2271. doi: 10.1534/g3.113.008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrostek E, Marialva MSP, Esteves SS, et al. Wolbachia variants induce differential protection to viruses in Drosophila melanogaster: a phenotypic and phylogenetic analysis. PLoS Genetics. 2013;9:e1003896. doi: 10.1371/journal.pgen.1003896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cini A, Ioriatti C, Anfora G. A review of the invasion of Drosophila suzukii in Europe and a draft research agenda for integrated pest management. Bulletin of Insectology. 2012;65:149–160. [Google Scholar]
- Clark ME, Anderson CL, Cande J, et al. Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics. 2005;170:1667–1675. doi: 10.1534/genetics.104.038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux R, Pichon S, Ling A, et al. Intense transpositional activity on insertion sequences in an ancient obligate endosymbiont. Molecular Biology and Evolution. 2008;25:1889–1896. doi: 10.1093/molbev/msn134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeine F, Vavre F, Fleury F. Removing symbiotic Wolbachia specifically inhibits oogenesis in a parasitic wasp. Proceedings of the National Academy of Sciences. 2001;98:6247–6252. doi: 10.1073/pnas.101304298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duron O, Bouchon D, Boutin S, et al. The diversity of reproductive parasites among arthropods: Wolbachia do not walk alone. BMC Biology. 2008;6:27. doi: 10.1186/1741-7007-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer K, Jaenike J. Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: Molecular evidence from the host and parasite genomes. Genetics. 2004;168:1443–1455. doi: 10.1534/genetics.104.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer KA, Burke C, Jaenike J. Wolbachia-mediated persistence of mtDNA from a potentially extinct species. Molecular Ecology. 2011;2:2805–2817. doi: 10.1111/j.1365-294X.2011.05128.x. [DOI] [PubMed] [Google Scholar]
- Efron B, Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993. p. 436. [Google Scholar]
- Fast EM, Toomey ME, Panaram K, et al. Wolbachia enhance Drosophila stem cell proliferation and target the germline stem cell niche. Science. 2011;334:990–992. doi: 10.1126/science.1209609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folmer O, Black M, Hoeh W, et al. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology. 1994;3:294–299. [PubMed] [Google Scholar]
- Freda PJ, Braverman JM. Drosophila suzukii, or Spotted Wing Drosophila, Recorded in Southeastern Pennsylvania, U.S.A. Entomological News. 2013;123:71–75. [Google Scholar]
- Gloor GB, Preston CR, Johnson-Schlitz DM, et al. Type I repressors of P element mobility. Genetics. 1993;135:81–95. doi: 10.1093/genetics/135.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhue RE, Bolda M, Farnsworth D, et al. Spotted wing drosophila infestation of California strawberries and raspberries: economic analysis of potential revenue losses and control costs. Pest Management Science. 2011;67:1396–1402. doi: 10.1002/ps.2259. [DOI] [PubMed] [Google Scholar]
- Haine ER, Pickup NJ, Cook JM. Horizontal transmission of Wolbachia in a Drosophila community. Ecological Entomology. 2005;30:464–472. [Google Scholar]
- Hamm CA, Handley CA, Pike A, et al. Wolbachia infection and Lepidoptera of conservation concern. Journal of Insect Science. 2014;14:6. doi: 10.1093/jis/14.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harcombe W, Hoffmann AA. Wolbachia effects in Drosophila melanogaster: in search of fitness benefits. Journal of Invertebrate Pathology. 2004;87:45–50. doi: 10.1016/j.jip.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Haselkorn TS, Cockburn SN, Hamilton PT, et al. Infectious adaptation: potential host range of a defensive endosymbiont in Drosophila. Evolution. 2013;67:934–945. doi: 10.1111/evo.12020. [DOI] [PubMed] [Google Scholar]
- Hauser M. A historic account of the invasion of Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) in the continental United States, with remarks on their identification. Pest Management Science. 2011;67:1352–1357. doi: 10.1002/ps.2265. [DOI] [PubMed] [Google Scholar]
- Haygood R, Turelli M. Evolution of incompatibility-inducing microbes in subdivided host populations. Evolution. 2009;63:432–447. doi: 10.1111/j.1558-5646.2008.00550.x. [DOI] [PubMed] [Google Scholar]
- Hedges LM, Brownlie JC, O'Neill SL, et al. Wolbachia and virus protection in insects. Science. 2008;322:702–702. doi: 10.1126/science.1162418. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA. Partial cytoplasmic incompatibility between two Australian populations of Drosophila melanogaster. Entomologia Experimentalis et Applicata. 1988;48:61–67. [Google Scholar]
- Hoffmann AA, Turelli M. Cytoplasmic incompatibility in insects. In: O'Neill SL, Hoffmann AA, Werren JH, editors. Influential Passengers: Inherited microorganisms and arthropod reproduction. Oxford University Press; New York, NY: 1997. pp. 42–80. [Google Scholar]
- Hoffmann AA, Clancy D, Duncan J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity. 1996;76:1–8. doi: 10.1038/hdy.1996.1. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Turelli M, Simmons GM. Unidirectional incompatibility between populations of Drosophila simulans. Evolution. 1986:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Montgomery BL, Popovici J, et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature. 2011;476:454–457. doi: 10.1038/nature10356. [DOI] [PubMed] [Google Scholar]
- Hornett EA, Charlat S, Duplouy AMR, et al. Evolution of male killer suppression in a natural population. PLoS Biology. 2006;4:e283. doi: 10.1371/journal.pbio.0040283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst GD, Jiggins FM. Male-killing bacteria in insects: mechanisms, incidence, and implications. Emerging Infectious Diseases. 2000;6:329–336. doi: 10.3201/eid0604.000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenike J. Spontaneous emergence of a new Wolbachia phenotype. Evolution. 2007;61:2244–2252. doi: 10.1111/j.1558-5646.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J. Population genetics of beneficial heritable symbionts. Trends in Ecology and Evolution. 2012;27:226–232. doi: 10.1016/j.tree.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Brekke TD. Defensive endosymbionts: a cryptic trophic level in community ecology. Ecology Letters. 2011;14:150–155. doi: 10.1111/j.1461-0248.2010.01564.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Dyer KA. No resistance to male-killing Wolbachia after thousands of years of infection. Journal of Evolutionary Biology. 2008;21:1570–1577. doi: 10.1111/j.1420-9101.2008.01607.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Stahlhut JK, Boello LM, et al. Association between Wolbachia and Spiroplasma within Drosophila neotestacea: an emerging symbiotic mutualism. Molecular Ecology. 2010;19:414–425. doi: 10.1111/j.1365-294X.2009.04448.x. [DOI] [PubMed] [Google Scholar]
- James AC, Ballard JWO. Expression of cytoplasmic incompatibility in Drosophila simulans and its impact on infection frequencies and distribution of Wolbachia pipientis. Evolution. 2000;54:1661–1672. doi: 10.1111/j.0014-3820.2000.tb00710.x. [DOI] [PubMed] [Google Scholar]
- Kacsoh BZ, Schlenke T. High hemocyte load is associated with increased resistance against parasitoids in Drosophila suzukii, a relative of D. melanogaster. PLoS ONE. 2012;7:e34721. doi: 10.1371/journal.pone.0034721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittayapong P, Mongkalangoon P, Baimai V, et al. Host age effect and expression of cytoplasmic incompatibility in field populations of Wolbachia-superinfected Aedes albopictus. Heredity. 2002;88:270–274. doi: 10.1038/sj.hdy.6800039. [DOI] [PubMed] [Google Scholar]
- Kriesner P, Hoffmann AA, Lee SF, et al. Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathogens. 2013;9:e1003607. doi: 10.1371/journal.ppat.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaise D, Harry M, Solignac M, et al. Evolutionary novelties in islands: Drosophila santomea, a new melanogaster sister species from São Tomé. Proceedings of the Royal Society of London B. 2000;267:1487–1495. doi: 10.1098/rspb.2000.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laven H. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature. 1967;216:383–384. doi: 10.1038/216383a0. [DOI] [PubMed] [Google Scholar]
- Llopart A, Herrig D, Brud E, et al. Sequential adaptive introgression of the mitochondrial genome in Drosophila yakuba and Drosophila santomea. Molecular Ecology. 2014;23:1124–1136. doi: 10.1111/mec.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos M, Castrezana SJ, Nankivell BJ, et al. Heritable endosymbionts in Drosophila. Genetics. 2006;174:363–376. doi: 10.1534/genetics.106.058818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw EA, O'Neill SL. Beyond insecticides: new thinking on an ancient problem. Nature Reviews Microbiology. 2013;11:181–193. doi: 10.1038/nrmicro2968. [DOI] [PubMed] [Google Scholar]
- McMeniman CJ, Lane RV, Cass BN, et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science. 2009;323:141–144. doi: 10.1126/science.1165326. [DOI] [PubMed] [Google Scholar]
- Merçot H, Llorente B, Jacques M, et al. Variability within the Seychelles cytoplasmic incompatibility system in Drosophila simulans. Genetics. 1995;141:1015–1023. doi: 10.1093/genetics/141.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WJ, Riegler M. Evolutionary dynamics of wAu-like Wolbachia variants in neotropical Drosophila spp. Applied and Environmental Microbiology. 2006;72:826–835. doi: 10.1128/AEM.72.1.826-835.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WJ, Ehrman L, Schneider D. Infectious speciation revisited: impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLoS Pathogens. 2010;6:e1001214. doi: 10.1371/journal.ppat.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro H, Hatadani LM, Medeiros HF, et al. Male killing in three species of the tripunctata radiation of Drosophila (Diptera: Drosophilidae) Journal of Zoological Systematics. 2006;44:130–135. [Google Scholar]
- Moran N, Baumann P. Phylogenetics of cytoplasmically inherited microorganisms of arthropods. Trends in Ecology and Evolution. 1994;9:15–20. doi: 10.1016/0169-5347(94)90226-7. [DOI] [PubMed] [Google Scholar]
- Morse JG, Rugman-Jones PF, Watson GW, et al. High levels of exotic armored scales on imported avocados raise concerns regarding USDA–APHIS' phytosanitary risk assessment. Journal of Economic Entomology. 2009;102:855–867. doi: 10.1603/029.102.0303. [DOI] [PubMed] [Google Scholar]
- Müller MJ, Dörr NCD, Deprá M, et al. Reevaluating the infection status by the Wolbachia endosymbiont in Drosophila Neotropical species from the willistoni subgroup. Infection, Genetics, and Evolution. 2013;19:232–239. doi: 10.1016/j.meegid.2013.07.022. [DOI] [PubMed] [Google Scholar]
- O'Grady PM, Lapoint RT, Bonacum J, et al. Phylogenetic and ecological relationships of the Hawaiian Drosophila inferred from mitochondrial DNA analysis. Molecular Phylogenetics and Evolution. 2011;58:244–256. doi: 10.1016/j.ympev.2010.11.022. [DOI] [PubMed] [Google Scholar]
- O'Neill SL, Karr TL. Bidirectional incompatibility between conspecific populations of Drosophila simulans. Nature. 1990;348:178–180. doi: 10.1038/348178a0. [DOI] [PubMed] [Google Scholar]
- O'Neill SL, Giordano R, Colbert AM, et al. 16S rRNA phylogenetic analysis of the bacterial endosymbionts associated with cytoplasmic incompatibility in insects. Proceedings of the National Academy of Sciences. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne SE, Leong YS, O'Neill SL, et al. Variation in antiviral protection mediated by different Wolbachia strains in Drosophila simulans. PLoS Pathogens. 2009;5:e1000656. doi: 10.1371/journal.ppat.1000656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon JL, Scott TW. Wolbachia and cytoplasmic incompatibility in the California Culex pipiens mosquito species complex: parameter estimates and infection dynamics in natural populations. Genetics. 2003;165:2029–2038. doi: 10.1093/genetics/165.4.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MF, Weinert LA, Welch JJ, et al. Population genomics of the Wolbachia endosymbiont in Drosophila melanogaster. PLoS Genetics. 2012;8:e1003129. doi: 10.1371/journal.pgen.1003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud T, Juchault P. Conflict between feminizing sex ratio distorters and an autosomal masculinizing gene in the terrestrial isopod Armadillidium vulgare Latr. Genetics. 1993;133:247–252. doi: 10.1093/genetics/133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rousset F, Bouchon D, Pintureau B, et al. Wolbachia endosymbionts responsible for various alterations of sexuality in arthropods. Proceedings of the Royal Society of London B. 1992;250:91–98. doi: 10.1098/rspb.1992.0135. [DOI] [PubMed] [Google Scholar]
- Rousset F, Solignac M. Evolution of the single and double Wolbachia symbioses during speciation in the Drosophila simulans complex. Proceedings of the National Academy of Sciences. 1995;92:6389–6393. doi: 10.1073/pnas.92.14.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DI, Klasson L, Lind AE, et al. More than fishing in the dark: PCR of a dispersed sequence produces simple but ultrasensitive Wolbachia detection. BMC Microbiology. 2014;14:121. doi: 10.1186/1471-2180-14-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon C, Buckley T, Frati F, et al. Incorporating molecular evolution into phylogenetic analysis, and a new compilation of conserved polymerase chain reaction primers for animal mitochondrial DNA. Annual Review of Ecology and Systematics. 2006;37:545–579. [Google Scholar]
- Simon C, Frati F, Beckenbach A, et al. Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Annals of the Entomological Society of America. 1994;87:651–701. [Google Scholar]
- Siozios S, Cestaro A, Kaur R, et al. Draft genome sequence of the Wolbachia endosymbiont of Drosophila suzukii. Genome Announcements. 2013;1:e00032–13. doi: 10.1128/genomeA.00032-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahlhut JK, Desjardins CA, Clark ME, et al. The mushroom habitat as an ecological arena for global exchange of Wolbachia. Molecular Ecology. 2010;19:1940–1952. doi: 10.1111/j.1365-294X.2010.04572.x. [DOI] [PubMed] [Google Scholar]
- Sheeley SL, McAllister BF. Mobile male-killer: similar Wolbachia strains kill males of divergent Drosophila hosts. Heredity. 2009;102:286–292. doi: 10.1038/hdy.2008.126. [DOI] [PubMed] [Google Scholar]
- Stouthamer R, Breeuwert JA, Luck RF, et al. Molecular identification of microorganisms associated with parthenogenesis. Nature. 1993;361:66–68. doi: 10.1038/361066a0. [DOI] [PubMed] [Google Scholar]
- Swofford DL. Phylogenetic analysis using parsimony (*and other methods) version 4. Sinauer Associates; Sunderland, MA, USA: 2003. [Google Scholar]
- Takamori H, Watabe H, Fuyama Y, et al. Drosophila subpulchrella, a new species of the Drosophila suzukii species subgroup from Japan and China (Diptera: Drosophilidae) Entomological Science. 2006;9:121–128. [Google Scholar]
- Taylor MJ, Bandi C, Hoerauf A. Wolbachia bacterial endosymbionts of filarial nematodes. Advances in Parasitology. 2005;60:245–284. doi: 10.1016/S0065-308X(05)60004-8. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Voronin D, Johnston KL, et al. Wolbachia filarial interactions. Cellular Microbiology. 2013;15:520–526. doi: 10.1111/cmi.12084. [DOI] [PubMed] [Google Scholar]
- Teixeira L, Ferreira Á, Ashburner M. The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biology. 2008;6:e2. doi: 10.1371/journal.pbio.1000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tochen S, Dalton DT, Wiman N, et al. Temperature-related development and population parameters for Drosophila suzukii (Diptera: Drosophilidae) on cherry and blueberry. Environmental Entomology. 2014;43:501–510. doi: 10.1603/EN13200. [DOI] [PubMed] [Google Scholar]
- Turelli M. Evolution of incompatibility-inducing microbes and their hosts. Evolution. 1994;48:1500–1513. doi: 10.1111/j.1558-5646.1994.tb02192.x. [DOI] [PubMed] [Google Scholar]
- Turelli M, Hoffmann AA. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature. 1991;353:440–442. doi: 10.1038/353440a0. [DOI] [PubMed] [Google Scholar]
- Turelli M, Hoffmann AA. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics. 1995;140:1319–1338. doi: 10.1093/genetics/140.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Hoffmann AA, McKechnie SW. Dynamics of cytoplasmic incompatibility and mtDNA variation in natural Drosophila simulans populations. Genetics. 1992;132:713–723. doi: 10.1093/genetics/132.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unckless RL, Boelio LM, Herren JK, et al. Wolbachia as populations within individual insects: causes and consequences of density variation in natural populations. Proceedings of the Royal Society B. 2009;276:2805–2811. doi: 10.1098/rspb.2009.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unckless RL, Jaenike J. Maintenance of a male-killing Wolbachia in Drosophila innubila by male-killing dependent and male-killing independent mechanisms. Evolution. 2012;66:678–689. doi: 10.1111/j.1558-5646.2011.01485.x. [DOI] [PubMed] [Google Scholar]
- Van Der Linde K, Houle D. A supertree analysis and literature review of the genus Drosophila and closely related genera (Diptera, Drosophilidae) Insect Systematics & Evolution. 2008;39:241–267. [Google Scholar]
- Veneti Z, Zabalou S, Papafotiou G, et al. Loss of reproductive parasitism following transfer of male-killing Wolbachia to Drosophila melanogaster and Drosophila simulans. Heredity. 2012;109:306–312. doi: 10.1038/hdy.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker T, Johnson PH, Moreira LA, et al. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 2011;476:450–453. doi: 10.1038/nature10355. [DOI] [PubMed] [Google Scholar]
- Walsh DB, Bolda MP, Goodhue RE, et al. Drosophila suzukii (Diptera: Drosophilidae): invasive pest of ripening soft fruit expanding its geographic range and damage potential. Journal of Integrated Pest Management. 2011;2:1–7. [Google Scholar]
- Watada M, Matsumoto M, Kondo M, et al. Taxonomic study of the Drosophila auraria species complex (Diptera: Drosophilidae) with description of a new species. Entomological Science. 2011;14:392–398. [Google Scholar]
- Werren JH, Jaenike J. Wolbachia and cytoplasmic incompatibility in mycophagous Drosophila and their relatives. Heredity. 1995;75:320–326. doi: 10.1038/hdy.1995.140. [DOI] [PubMed] [Google Scholar]
- Werren JH, Windsor DM. Wolbachia infection frequencies in insects: evidence of a global equilibrium? Proceedings of the Royal Society of London B. 2000;267:1277–1285. doi: 10.1098/rspb.2000.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology. 2008;6:741–751. doi: 10.1038/nrmicro1969. [DOI] [PubMed] [Google Scholar]
- Werren JH, Zhang W, Guo LR. Evolution and phylogeny of Wolbachia: reproductive parasites of arthropods. Proceedings of the Royal Society of London B. 1995;261:55–63. doi: 10.1098/rspb.1995.0117. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hou ZH, Qian YH, et al. Increasing the data size to accurately reconstruct the phylogenetic relationships between nine subgroups of the Drosophila melanogaster species group (Drosophilidae, Diptera) Molecular Phylogenetics and Evolution. 2012;62:214–223. doi: 10.1016/j.ympev.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Zabalou S, Charlat S, Nirgianaki A, et al. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics. 2004;167:826–834. doi: 10.1534/genetics.103.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalou S, Apostolaki A, Pattas S, et al. Multiple rescue factors within a Wolbachia strain. Genetics. 2008;178:2145–2160. doi: 10.1534/genetics.107.086488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Rousset F, O'Neill S. Phylogeny and PCR-based classification of Wolbachia strains using wsp gene sequences. Proceedings of the Royal Society of London B. 1998;265:509–515. doi: 10.1098/rspb.1998.0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zug R, Hammerstein P. Still a host of hosts for Wolbachia: analysis of recent data suggests that 40% of terrestrial arthropod species are infected. PLoS ONE. 2012;7:e38544. doi: 10.1371/journal.pone.0038544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


