Abstract
Background
Selective serotonin-reuptake inhibitors (SSRIs) are the most commonly prescribed anti depressants. Previous studies have suggested that SSRIs may increase the risk of birth defects, including clubfoot. Using data from a population-based case-control study, we evaluated whether SSRI use increased the risk of clubfoot.
Methods
Mothers were interviewed within one year after delivery about sociodemographic factors, pregnancy events and exposures. They were specifically asked if they experienced depression or anxiety or if they took any of the following SSRIs: citalopram, escitalopram, fluvoxamine, paroxetine, sertraline or fluoxetine. We used logistic regression models to calculate odds ratios (ORs) and 95% confidence intervals.
Results
We included a total of 622 clubfoot cases and 2002 non-malformed controls born between 2006 and 2011 in Massachusetts, New York, and North Carolina. For the second or third lunar month of pregnancy (the relevant gestational period), SSRI use for a period of more than 30 days was higher in case mothers (5%) than control mothers (3%). After adjustment for maternal smoking and body mass index, the OR for any SSRI use and clubfoot was 1.8 (95% confidence interval=1.1–2.8). When individual SSRIs were examined, ORs were elevated for sertraline (1.6 [0.8–3.2]), paroxetine (9.2 [0.7–484.6]) and escitalopram (2.9 [1.1–7.2]).
Conclusion
Our data suggest an increased risk of clubfoot occurrence in relation to SSRI use. Drug-specific risks varied widely and some estimates were unstable.
In the United States, approximately 5%–13% of pregnant women use antidepressants for depression.1–3 The most commonly prescribed class of antidepressants is selective serotonin re-uptake inhibitors (SSRIs).1,2 However, in recent years, concern has been raised that SSRIs may increase the risk of birth defects when taken during pregnancy.1,4–8 Talipes equinovarus, or clubfoot, is one such birth defect that has been linked with SSRI use.4,7
The lower limbs begin to form their adult positions in the early weeks of gestation. Initially, the soles of the feet are facing each other and the knees extend outward. Beginning in the 9th week after the last menstrual period, the legs begin to rotate inward almost 90 degrees, bringing the big toe into a medial position.9,10 Clubfoot is a structural malformation that occurs when the lower limbs fail to rotate correctly; it results in the feet remaining in their early fetal position, with one or both feet turning inward and downward. Structural clubfoot is distinguished from positional clubfoot in that the foot cannot be moved into a normal position; additionally, positional clubfoot is believed to result from uterine constraint.11,12 The prevalence of structural clubfoot is approximately 1 per 1,000 births.13,14
To date, two studies have assessed the risk of clubfoot in relation to SSRI use.4,7 The first reported a two-fold increase in the risk when SSRIs were used in the first trimester, and the risk was highest with paroxetine use.4 The second study observed a 50% increase in risk and similarly found an elevated risk with paroxetine use.7
The objective of our study was to evaluate whether SSRI use in early pregnancy increased the risk of structural clubfoot in the fetus. In addition, we assessed the role of depression and the risk of clubfoot, independent of SSRI use.
METHODS
Study design
A population based case-control study of structural clubfoot was conducted between 2006 and 2011 by the Slone Epidemiology Center at Boston University. Its objective was to identify risk factors for clubfoot. The study has been previously described in detail.15 Briefly, case infants who were less than 1 year of age were identified from birth defect registries in Massachusetts, North Carolina, and New York. Cases were eligible for the study if they had a diagnosis of talipes equinovarus (“clubfoot”) without a known inherited syndrome, chromosomal anomaly, Potter syndrome, bilateral renal agenesis, amniotic bands, arthrogryposis, or neural tube defect, as reported by either the mother, the state birth defects registry, or the medical record. Diagnosis of structural clubfoot was confirmed primarily by orthopedic records (77%); when medical records were not available, maternal report of 3 or more castings for the clubfoot was used to confirm a true structural clubfoot (23%). The sensitivity of self-reported clubfoot was high in our study, with case mothers reporting 3 or more casts in 98% of orthopedic-confirmed clubfoot cases. This analysis was restricted to the 95% of cases with only clubfoot and no other major structural malformations. Eligible controls consisted of infants with no major malformations or foot problems, drawn from the same birth population as cases and selected from either birth certificates (MA, NC) or hospital medical records (NY). The institutional review boards at Boston University and the state health departments in Massachusetts, North Carolina, and New York approved the study protocol.
Telephone interviews were conducted by trained nurses within one year after delivery. A translation service with medical interpreters was used by the nurses when interviewing mothers who did not speak English. The interview consisted of questions on sociodemographic factors, behaviors during pregnancy, dietary history, reproductive and medical history, and reported illnesses and medications. A tiered approach was used in obtaining information on illnesses and medications. Women were first asked if they experienced any occurrences of “depression or anxiety” in the month prior to their pregnancy through the end of pregnancy. If a mother responded positively, the dates of depression or anxiety and any medications used, including type and timing, were recorded. Independent of their responses to the illness questions, mothers were questioned about any medications they took and were prompted with names of specific medications, including “Celexa” (citalopram), “Lexapro” (escitalopram), “Prozac” (fluoxetine), “Luvox” (fluvoxamine), “Paxil” (paroxetine), and “Zoloft” (sertraline). If a mother reported using any medications, the timing and indication for use were noted.
Exposure
For this analysis, the exposure window of interest was the second and third lunar months after the last menstrual period, as this is the etiologically-relevant time period for the development of clubfoot. We created two exposure definitions; the primary exposure group consisted of women who reported using an SSRI for a period of more than 30 days in in the 2nd and 3rd lunar months, while the secondary exposure group consisted of women who reported SSRIs for a period of ≤30 days in the exposure window.
To incorporate maternal reports of depression or anxiety (henceforth referred to as “depression”) that may have occurred anytime in the month before or during pregnancy, we created the following mutually exclusive categories: 1) no depression was reported and no anti-depressant medication was used at any time in pregnancy (reference group); 2) depression was reported and an SSRI treatment was used for a >30 day period in lunar months 2 – 3; 3) depression was reported and an SSRI treatment was used for a period of ≤30 days in the exposure window; 4) depression was reported and SSRI treatment occurred only outside the lunar month 2 – 3 window; 5) depression was reported and a non-SSRI medication was used in lunar months 2 – 3; and 6) depression was reported and no medications were used to treat the depression during pregnancy. If a mother reported taking both an SSRI and a non-SSRI for depression (n=36), she was included in category 2, 3, or 4 depending on timing and duration of use. We excluded 42 women who took non-SSRIs outside of the lunar month 2 – 3 window and did not take an SSRI during pregnancy, because their patterns of use and indications varied widely. Also, mothers who reported only herbal treatments for depression (e.g., St John’s wort) or taking anti-depressants for reasons other than depression (e.g., smoking cessation or attention deficit disorder) were excluded from the analysis.
Statistical analysis
Sociodemographic and behavioral factors were obtained from the interview and were assessed for cases and controls. We used logistic regression models to calculate odds ratios (ORs) and 95% confidence intervals (CIs). Potential confounders that were assessed included maternal race, education, age, smoking, alcohol intake, body mass index (BMI), parity, infant sex, and study center. We added covariates to the model one at a time, and factors that changed the crude estimate by more than 10% were kept in the final model. A sub-analysis was conducted restricting cases and controls to those with no family history of clubfoot in a first-degree relative. While all infants with major structural malformations other than clubfoot were removed from the analysis, some infants did have unconfirmed “heart murmurs” reported; therefore, due to previous reports of an association between SSRIs and heart defects,4,7,16 a separate analysis was conducted removing cases and controls with a reported heart murmur.
RESULTS
Mothers of 3,298 eligible controls and 1,323 eligible cases were approached for the study. Among controls, 954 could not be contacted due to missing telephone numbers or non-response, 2,091 were interviewed, 243 refused participation, and 10 were ineligible due to death, incarceration, living abroad, lack of interpreter, or having previously participated in a similar study. In addition, 54 control mothers reported their child had a congenital anomaly or foot problem and were consequently excluded from the study, resulting in 2,037 controls. Among the cases, 286 could not be contacted, 955 were interviewed, and 82 refused participation. Of the interviewed cases, 269 were excluded due to ineligible diagnoses (arthrogryposis, amniotic bands, syndromes, severe oligohydramnios, surrogate mother, and questionable clubfoot) and 8 with ineligible age. The overall response rates for cases and controls were 72% and 64%, respectively. After restriction to cases with only clubfoot and no other major structural malformations, a total of 646 confirmed cases were eligible.
Of the 646 cases and 2037 controls, mothers of 6 cases and 8 controls took anti-depressants for reasons other than depression/anxiety and were excluded (only 4 of these women reported use in the exposure window); mothers of 3 controls took only herbal remedies for depression/anxiety and were also excluded. In addition, 42 women who took non-SSRIs outside of the lunar month 2 – 3 window were excluded. Thus, 622 case and 2002 controls were included in this analysis.
Compared with control mothers, mothers of cases were more likely to be overweight, nulliparous, and to have smoked during pregnancy, and these infants were more likely to be male (Table 1). When sociodemographic and behavioral factors were considered among control mothers according to exposure status, smokers were more likely to report depression (Table 2). In addition, among controls with depression, SSRI treatment was more common in women who were White non-Hispanic, multiparous, and obese. The use of SSRIs among control mothers declined during pregnancy, starting in the third lunar month (Figure 1). The specific SSRIs reported by control mothers varied over the course of the study; paroxetine was reported only in the first study year (Figure 2).
Table 1.
Demographic and behavioral characteristics of clubfoot cases and controls, Massachusetts, New York, and North Carolina, 2006 – 2011.
| Clubfoot Cases |
Controls | |
|---|---|---|
| (n=622) | (n=2002) | |
|
|
||
| No. (%) | No. (%) | |
| Maternal race/ethnicity | ||
| White non-Hispanic | 450 (72) | 1307 (65) |
| Black non-Hispanic | 80 (13) | 334 (17) |
| Hispanic | 70 (11) | 241 (12) |
| Other/Unknown | 22 (4) | 120 (6) |
| Maternal age at conception (years) | ||
| <25 | 190 (31) | 600 (30) |
| 25–29 | 183 (29) | 558 (28) |
| 30–34 | 153 (25) | 516 (26) |
| ≥35 | 96 (15) | 328 (16) |
| Maternal education (years) | ||
| < 12 | 77 (12) | 278 (14) |
| 12 | 158 (25) | 448 (22) |
| > 12 | 387 (62) | 1276 (64) |
| Parity | ||
| Nulliparous | 304 (49) | 802 (40) |
| Multiparous | 318 (51) | 1200 (60) |
| Maternal cigarette smoking in lunar months 2 or 3 | ||
| Smoked | 164 (26) | 353 (18) |
| Did not smoke | 449 (72) | 1632 (82) |
| Missing data | 9 (1) | 17 (1) |
| Maternal alcohol use in lunar months 2 or 3 | ||
| Used alcohol | 55 (9) | 122 (6) |
| Did not use alcohol | 536 (86) | 1798 (90) |
| Missing data | 31 (5) | 82 (4) |
| Maternal body mass index (kg/m2) | ||
| < 18.5 | 21 (3) | 85 (4) |
| 18.5– < 24.9 | 289 (46) | 1062 (53) |
| 25– <30 | 168 (27) | 445 (22) |
| ≥ 29.9 | 127 (20) | 351 (18) |
| Missing data | 17 (3) | 59 (3) |
| Infant sex | ||
| Male | 452 (73) | 993 (50) |
| Female | 170 (27) | 1009 (50) |
| First degree family history of clubfoot | ||
| None | 551 (89) | 1987 (99) |
| Yes | 71 (11) | 15 (1) |
| Study center | ||
| North Carolina | 254 (41) | 990 (49) |
| New York | 199 (32) | 560 (28) |
| Massachusetts | 169 (27) | 452 (23) |
Table 2.
Maternal sociodemographic factors by use of antidepressants status among controls, Massachusetts, New York, and North Carolina, 2006 – 2011.
| No depression
|
Depression
|
|||||
|---|---|---|---|---|---|---|
| SSRI Use
|
Non-SSRI use in Lunar Months 2–3 (n=17) |
No Drugs (n=230) |
||||
| (n=1,650)
|
In Lunar Months 2–3 for >30 days (n=58) |
In Lunar Months 2–3 for ≤30 days (n=24) |
Outside Lunar Months 2–3 (n=23) |
|||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |
| Maternal race/ethnicity | ||||||
| White non-Hispanic | 1060 (64) | 51 (88) | 19 (79) | 21 (91) | 13 (76) | 143 (62) |
| Black non-Hispanic | 280 (17) | 3 (5) | 4 (17) | 0 (0) | 2 (12) | 45 (20) |
| Hispanic | 200 (12) | 4 (7) | 1 (4) | 1 (4) | 2 (12) | 33 (14) |
| Other/Unknown | 110 (7) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 9 (4) |
| Maternal age at conception (years) | ||||||
| <25 | 479 (29) | 12 (21) | 4 (17) | 5 (22) | 4 (24) | 96 (42) |
| 25–29 | 464 (28) | 14 (24) | 7 (29) | 8 (35) | 6 (35) | 59 (26) |
| 30–34 | 441 (27) | 13 (22) | 7 (29) | 8 (35) | 6 (35) | 41 (18) |
| ≥35 | 266 (16) | 19 (33) | 6 (25) | 2 (9) | 1 (6) | 34 (15) |
| Maternal education (years) | ||||||
| < 12 | 221 (13) | 5 (9) | 1 (4) | 2 (9) | 1 (6) | 48 (21) |
| 12 | 372 (23) | 11 (19) | 7 (29) | 4 (17) | 4 (24) | 50 (22) |
| > 12 | 1057 (64) | 42 (72) | 16 (67) | 17 (74) | 12 (71) | 132 (57) |
| Parity | ||||||
| Nulliparous | 672 (41) | 15 (26) | 5 (21) | 5 (22) | 8 (47) | 97 (42) |
| Multiparous | 978 (59) | 43 (74) | 19 (79) | 18 (78) | 9 (53) | 133 (58) |
| Maternal cigarette smoking in lunar months 2 or 3 | ||||||
| Smoked | 252 (15) | 16 (28) | 11 (46) | 6 (26) | 7 (41) | 61 (27) |
| Did not smoke | 1386 (84) | 42 (72) | 13 (54) | 16 (70) | 10 (59) | 165 (72) |
| Missing data | 12 (1) | 0 (0) | 0 (0) | 1 (4) | 0 (0) | 4 (2) |
| Maternal alcohol use in lunar months 2 or 3 | ||||||
| Used alcohol | 89 (5) | 11 (19) | 8 (33) | 1 (4) | 0 (0) | 13 (6) |
| Did not use alcohol | 1495 (91) | 45 (78) | 16 (67) | 19 (83) | 17 (100) | 206 (90) |
| Missing data | 66 (4) | 2 (3) | 0 (0) | 3 (13) | 0 (0) | 11 (5) |
| Maternal body mass index (kg/m2) | ||||||
| < 18.5 | 70 (4) | 3 (5) | 0 (0) | 1 (4) | 2 (12) | 9 (4) |
| 18.5– < 24.9 | 888 (54) | 25 (43) | 9 (38) | 7 (30) | 9 (53) | 124 (54) |
| 25– <29.9 | 357 (22) | 14 (24) | 6 (25) | 7 (30) | 5 (29) | 56 (24) |
| ≥ 30 | 282 (17) | 15 (26) | 9 (38) | 8 (35) | 1 (6) | 36 (16) |
| Missing data | 53 (3) | 1 (2) | 0 (0) | 0 (0) | 0 (0) | 5 (2) |
| Infant sex | ||||||
| Male | 819 (50) | 27 (47) | 12 (50) | 9 (39) | 8 (47) | 118 (51) |
| Female | 831 (50) | 31 (53) | 12 (50) | 14 (61) | 9 (53) | 112 (49) |
| Study center | ||||||
| North Carolina | 814 (49) | 32 (55) | 6 (25) | 11 (48) | 9 (53) | 118 (51) |
| New York | 460 (28) | 16 (28) | 12 (50) | 7 (30) | 6 (35) | 59 (26) |
| Massachusetts | 376 (23) | 10 (17) | 6 (25) | 5 (22) | 2 (12) | 53 (23) |
Figure 1.
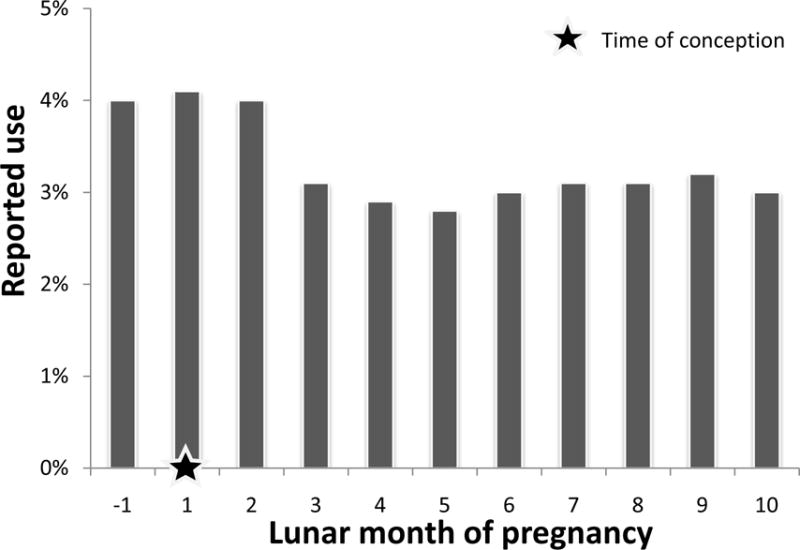
Frequency of selective serotonin reuptake inhibitor use among control mothers, Massachusetts, New York, and North Carolina, 2006 – 2011. The denominator for each lunar month includes only control mothers who were pregnant in that lunar month
Figure 2.
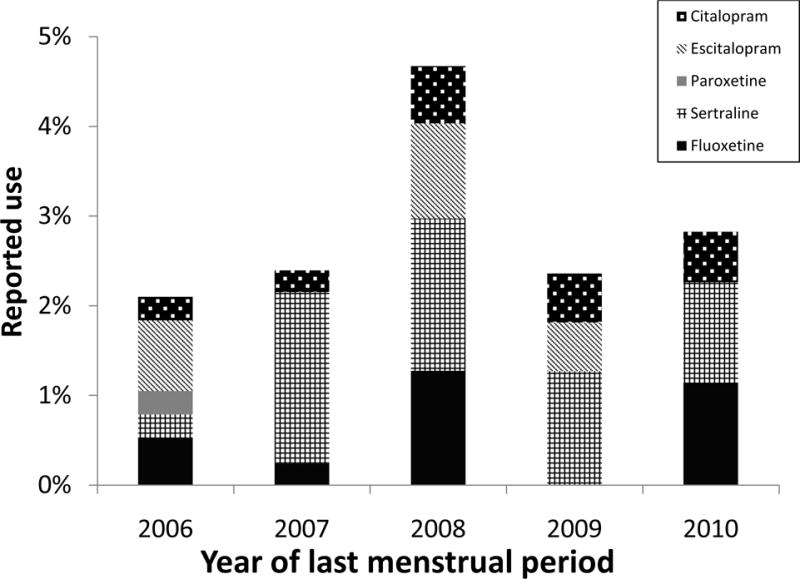
Frequency of selective serotonin reuptake inhibitor use in the 2nd and 3rd lunar months among control mothers by year of last menstrual period, Massachusetts, New York, and North Carolina, 2006 – 2011.
More mothers of cases (24%) reported depression, independent of treatment, than did mothers of controls (17%) (Table 3). Among the women who reported depression, SSRI use for a period of more than 30 days was higher among cases (5%) than controls (3%) (Table 3). SSRI use for a period of ≤30 days in the exposure window was also slightly more common in cases (2%) than controls (1%). In contrast, the use of SSRI medications outside the lunar month 2–3 window was similar for cases and controls (1% each), as was the use of non-SSRI medications in the exposure window (1% each). The following medications were reported by mothers who used a non-SSRI medication for depression/anxiety: alprazolam, buprenorphine, bupropion, buspirone, clonazepam, duloxetine, gabapentin, haloperidol, lithium, lorazepam, paliperidone, quetiapine, venlafaxine, zolpidem, and a not-otherwise-specified sleep or sedative medication.
Table 3.
The association of depression and medication use with clubfoot, Massachusetts, New York, and North Carolina, 2006 – 2011.
| Depression | Treatment | Cases | Controls | Crude | Adjusteda |
|---|---|---|---|---|---|
|
| |||||
| No. (%) | No. (%) | OR (95% CI) | OR (95% CI) | ||
| No | Nob | 477 (77) | 1650 (82) | 1.0 | 1.0 |
| SSRI lunar month 2–3 | |||||
| Yes | for >30 day period | 33 (5) | 58 (3) | 2.0 (1.3–3.1) | 1.8 (1.1–2.8) |
| Yes | for ≤30 day period | 11 (2) | 24 (1) | 1.6 (0.8–3.3) | 1.0 (0.4–2.2) |
| Yes | SSRI outside lunar month 2–3 | 5 (1) | 23 (1) | 0.8 (0.3–2.0) | 0.8 (0.3–2.2) |
| Yes | Non-SSRI use in lunar month 2–3 | 5 (1) | 17 (1) | 1.0 (0.4–2.8) | 1.0 (0.4–2.7) |
| Yes | No | 91 (15) | 230 (11) | 1.4 (1.1–1.8) | 1.3 (1.0–1.7) |
Adjusted for maternal smoking, alcohol use, and body mass index
Reference category
We assessed which covariates changed exposure ORs by >10%. Maternal alcohol consumption changed the OR for SSRI use for a ≤30 day period in lunar months 2–3; maternal BMI changed the OR for SSRI use outside the lunar month 2–3 window; and maternal smoking changed the OR for non-SSRI medication in lunar months 2–3. Therefore, smoking (referent group: non-smoker in lunar months 2–3), alcohol (referent: no use in lunar months 2–3), and BMI (referent group: 18.5–24.9 kg/m2) were included in all final models.
As noted in Table 3, the adjusted OR for clubfoot and maternal depression with SSRI use for a >30 day period in lunar months 2–3 was 1.8 (95% CI=1.1–2.8). On the other hand, use of an SSRI for a period of ≤30-day was not associated with an elevated risk of clubfoot (OR=1.0 [0.4–2.2]). For depression with SSRI use outside the lunar month 2–3 window, the adjusted OR was close to the null (0.8 [0.3–2.2]). Similarly, the adjusted ORs were not elevated when non-SSRI drugs were used to treat depression in lunar months 2–3 (1.0 [0.4–2.7]). The risk for women reporting depression with no drug treatment was modestly elevated (1.3 [1.0–1.7]).
The most commonly-reported SSRI drug was sertraline, with cases (2%) reporting more use than controls (1%) (Table 4 and Figure 2). Adjusted ORs were: sertraline 1.6 (13 exposed cases [95% CI=0.8–3.2); paroxetine 9.2 (3 exposed cases; [CI=0.7–484.6]); and escitalopram, 2.9 (9 exposed cases; [CI=1.1–7.2]).
Table 4.
The association of clubfoot and depression treated with specific selective serotonin reuptake inhibitors, Massachusetts, New York, and North Carolina, 2006 – 2011.
| Medication | Cases | Controls | Crude | Adjusteda |
|---|---|---|---|---|
|
| ||||
| No. (%) | No. (%) | OR (95% CI) | OR (95% CI) | |
| No medication or depressionb | 477 (77) | 1650 (82) | 1.0 | 1.0 |
| Fluoxetine | 4 (1) | 11 (1) | 1.3 (0.4–4.0) | 1.3 (0.4–4.2) |
| Sertraline | 13 (2) | 26 (1) | 1.7 (0.9–3.4) | 1.6 (0.8–3.2) |
| Paroxetine | 3 (1) | 1 (0) | 10.4 (0.8–545.1)c | 9.2 (0.7–484.6)c |
| Citalopram | 4 (1) | 9 (0) | 1.5 (0.5–5.0) | 0.9 (0.2–3.4) |
| Escitalopram | 9 (1) | 11 (1) | 2.8 (1.2–6.9) | 2.9 (1.1–7.2) |
Adjusted for maternal smoking, alcohol use, and body mass index
Reference category
Exact logistic regression was used
When the data were restricted to the 551 cases and 1987 controls with no family history of clubfoot, the results did not change substantially (for SSRI use for >30 days in lunar months 2–3, adjusted OR=1.7 [CI=1.0–2.7]). In addition, the results did not change when the data were restricted to the 596 cases and 1996 controls without heart murmurs (for SSRI use for >30 days in lunar months 2–3, adjusted OR=1.8 [CI=1.1–2.9]).
DISCUSSION
Overall, our data suggest an increased risk of clubfoot in the offspring of women who use SSRIs for a substantial period of time in the second and third lunar months of pregnancy, but no increased risk for use for shorter durations or outside that critical developmental window. Drug-specific risks varied widely, but where numbers were sufficient to produce stable estimates, we observed increased risks for sertraline and for escitalopram; the large risk observed for paroxetine was based on only 3 exposed cases and 1 exposed control.
Our findings support those previously reported for SSRI use and the risk of clubfoot. Louik and colleagues4 observed a 2.2-fold (95% CI=1.4–3.6) increased risk of clubfoot with any SSRI use in the first trimester. Similar to our findings, they found elevated risks with use of sertraline (adjusted OR=2.4 [95% CI=0.9–6.2) and paroxetine (5.8 [2.6, 13.1]), while no elevated risk was associated with fluoxetine (0.8 [0.2–2.5]). Their finding of an increased risk with citalopram (2.7 [0.5–13.1]) was higher than our observed finding (0.9 [0.2–3.4]). Their study was similar to ours in that it used a multicenter case-control design and exposure information came from maternal reports. One key difference was the timing of the exposure window; because our study focused solely on clubfoot, we narrowed the window to the second and third lunar months, while Louik et al. used a window of 28 days before conception through the fourth lunar month. In addition, our study data was population-based, with cases coming from registries, while their data used a combination of hospital based and population-based sources for cases. Our cases had structural clubfoot confirmed by an orthopedist or information on treatment, whereas the Louik et al study used maternal reports with or without medical record diagnoses for case confirmation.
Colvin and colleagues7 investigated the effects of SSRI medications in the first trimester using pharmaceutical claims databases and the birth defects registry in Western Australia from 2002 to 2005. They found a modestly increased risk with any SSRI use and clubfoot (OR=1.5 [95% CI=0.8–3.1]). For specific SSRI medications, a modest elevation in risk was identified for paroxetine (OR=1.8 [0.4–7.2]) but not for citalopram (1.3 [0.3–5.3]) or sertraline (1.1 [0.3–4.5]). Their exposure window was broader than ours and included the first trimester. In addition, because Colvin at al. used administrative databases to obtain exposure information, it is possible that mothers who received a prescription for an SSRI medication may not have taken it. Thus, use of a prescription database to obtain exposure information may have led to some exposure misclassification, which would likely bias findings toward the null.
In the current and previous studies, the SSRI with the strongest association with clubfoot has been paroxetine, where large but unstable risks estimates were observed. It is of note that use of paroxetine in pregnancy has been decreasing since the US Food and Drug Administration issued a warning about such use in 2005.3
The increased risks we identified when SSRIs were used for a period of more than 30 days, combined with the lack of an association for use 30 days or less, suggests that consistent exposure in the etiologic window may be required to increase the risk of clubfoot. When we examined SSRI use outside the etiologically relevant time window, no increased effect was observed, adding biologic plausibility to our finding. Further, since the effect was confined to the etiologically relevant time period, there is less concern for recall bias because it would likely be present for use reported at any time in pregnancy. The lack of an effect for non-SSRI medications in the etiologically relevant window and the small risk for untreated depression suggest that the underlying depression is likely not responsible for the increased risk observed with SSRI use.
If SSRIs do indeed increase the risk of clubfoot, the mechanism may involve the vasoconstrictive properties of serotonin.17–20 SSRIs function by blocking the reuptake of serotonin, thereby increasing serotonin levels. Animal studies have found that serotonin is a uterine vasoconstrictor that leads to contractions of the umbilical artery,17,20 which results in a decrease of uterine artery blood flow in a dose-dependent fashion.21 Additionally, a study in pregnant ewes found that, while serotonin infusions in the third trimester led to uterine vasoconstriction, no systemic cardiovascular response was observed; therefore, serotonin may have a selective effect on the uterine vasculature.22 Given that clubfoot has been hypothesized to be the result of a vascular disruption,23–25 a possible pathway for the pathogenesis of clubfoot may be through the vasoconstriction from higher levels of circulating serotonin due to SSRI use.
The present study is the largest population-based study of idiopathic clubfoot cases to date. The use of orthopedic medical records and information on treatment ensured that the cases were structural clubfoot, rather than positional-type clubfoot. When orthopedic records were not available, we used the maternal report of 3 or more castings, which we found to have a high sensitivity when compared with orthopedic records. Further, this study was designed specifically to assess medication use in relation to clubfoot, and one of its strengths is the detailed information on illness and medication history collected by the study nurses within one year of the infant’s birth. One limitation was the small numbers of mothers exposed to some specific SSRIs, which resulted in unstable OR estimates for those drugs. In addition, we asked mothers about “depression or anxiety” as one question in our questionnaire and therefore were not able to determine whether the medications they reported were used specifically for depression or specifically for anxiety. However, not only can it be difficult to differentiate between depression and anxiety in general, they often occur as comorbidities.26
While we controlled for a large number of variables, we cannot rule out the possibility of confounding from unmeasured factors. Of particular concern is confounding by the underlying condition. To assess this possibility we considered both untreated depression and depression treated by drugs other than SSRIs and found little evidence of confounding by indication. Inaccurate recall of medications or depression histories may also be of concern, though it is less likely that mothers would forget using anti-depressant medications since they are typically used on a regular basis; while moderate to severe depression is likely to be reported, less severe forms may not have been recognized or diagnosed. Denial of exposure is a possibility, but for such under-reporting to explain the observed positive associations would require that a higher proportion of control mothers deny use compared to case mothers; this situation seems unlikely.
In conclusion, our data support and expand previous suggestions that SSRI use in the second and third lunar months of pregnancy increases the risk of clubfoot, independent of maternal report of depression. While there was a suggestion of an elevated risk for sertraline, escitalopram, and particularly paroxetine, the estimate for paroxetine was unstable due to small numbers of exposed subjects.
Acknowledgments
We thank Lisa Crowell and Mary Beth Pender, Interviewers; Michelle Heinz and Eileen Mack, Research Assistants; Michael Bairos, Oleg Starobinets, and Elie Sirotta, Database Analysts; and the mothers who participated in the study.
Source of funding: Support for this work was provided by Eunice Kennedy Shriver National Institute for Child Health and Human Development grant RO1-HD051804.
Footnotes
Conflicts of interest: MMW holds consultancy contracts with Amgen, Abbott, and UCB; no financial relationships with any organizations that might have an interest in this work; and no other relationships or activities that could appear to have influenced these analyses. AAM and CL received GlaxoSmithKline support for an analysis of bupropion in relation to cardiac defects. AAM serves as a member of advisory committees for Biogen-Idec’s pregnancy registries for biologics used to treat multiple sclerosis. MMY states no known conflicts of interest.
REFERENCES CITED
- 1.Alwan S, Reefhuis J, Rasmussen SA, Friedman JM, Study NBDP Patterns of Antidepressant Medication Use Among Pregnant Women in a United States Population. The Journal of Clinical Pharmacology. 2011;51(2):264–270. doi: 10.1177/0091270010373928. [DOI] [PubMed] [Google Scholar]
- 2.Cooper WO, Willy ME, Pont SJ, Ray WA. Increasing use of antidepressants in pregnancy. American Journal of Obstetrics and Gynecology. 2007;196(6):544. e1–544. e5. doi: 10.1016/j.ajog.2007.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Wichman CL, Fothergill A, Moore KM, Lang TR, Heise RH, Jr, Watson WJ. Recent trends in selective serotonin reuptake inhibitor use in pregnancy. Journal of Clinical Psychopharmacology. 2008;28(6):714–716. doi: 10.1097/JCP.0b013e31818b53fd. [DOI] [PubMed] [Google Scholar]
- 4.Louik C, Lin AE, Werler MM, Hernández-Díaz S, Mitchell AA. First-trimester use of selective serotonin-reuptake inhibitors and the risk of birth defects. New England Journal of Medicine. 2007;356(26):2675–2683. doi: 10.1056/NEJMoa067407. [DOI] [PubMed] [Google Scholar]
- 5.Pedersen LH, Henriksen TB, Vestergaard M, Olsen J, Bech BH. Selective serotonin reuptake inhibitors in pregnancy and congenital malformations: population based cohort study. BMJ: British Medical Journal. 2009;339 doi: 10.1136/bmj.b3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bakker MK, Kerstjens-Frederikse WS, Buys CHCM, de Walle HEK, de Jong-van den Berg LTW. First-trimester use of paroxetine and congenital heart defects: A population-based case-control study. Birth Defects Research Part A: Clinical and Molecular Teratology. 2010;88(2):94–100. doi: 10.1002/bdra.20641. [DOI] [PubMed] [Google Scholar]
- 7.Colvin L, Slack-Smith L, Stanley FJ, Bower C. Dispensing patterns and pregnancy outcomes for women dispensed selective serotonin reuptake inhibitors in pregnancy. Birth Defects Research Part A: Clinical and Molecular Teratology. 2011;91(3):142–152. doi: 10.1002/bdra.20773. [DOI] [PubMed] [Google Scholar]
- 8.Malm H, Artama M, Gissler M, Ritvanen A. Selective serotonin reuptake inhibitors and risk for major congenital anomalies. Obstetrics and Gynecology. 2011;118(1):111–120. doi: 10.1097/AOG.0b013e318220edcc. [DOI] [PubMed] [Google Scholar]
- 9.Moore KL, Persaud TVN, Torchia MG, Persaud T. The developing human: clinically oriented embryology. Saunders/Elsevier; Philadelphia: 2008. [Google Scholar]
- 10.Sadler TW. Langman’s medical embryology. Wolters Kluwer Health; 2011. [Google Scholar]
- 11.Anand A, Sala DA. Clubfoot: Etiology and treatment. Indian journal of orthopaedics. 2008;42(1):22. doi: 10.4103/0019-5413.38576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter R. Congenital talipes equinovarus: I. Resolving and resistant deformities. Journal of Bone & Joint Surgery, British Volume. 1987;69-B(5):822–825. doi: 10.1302/0301-620X.69B5.3680351. [DOI] [PubMed] [Google Scholar]
- 13.Parker SE, Mai CT, Strickland MJ, Olney RS, Rickard R, Marengo L, Wang Y, Hashmi SS, Meyer RE. Multistate study of the epidemiology of clubfoot. Birth Defects Research Part A: Clinical and Molecular Teratology. 2009;85(11):897–904. doi: 10.1002/bdra.20625. [DOI] [PubMed] [Google Scholar]
- 14.Wynne-Davies R. Family studies and aetiology of club foot. Journal of Medical Genetics. 1965;2(4):227. doi: 10.1136/jmg.2.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werler MM, Yazdy MM, Mitchell AA, Meyer RE, Druschel CM, Anderka M, Kasser JR, Mahan ST. Descriptive epidemiology of idiopathic clubfoot. American Journal of Medical Genetics Part A. 2013;161(7):1569–1578. doi: 10.1002/ajmg.a.35955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alwan S, Reefhuis J, Rasmussen SA, Olney RS, Friedman JM. Use of selective serotonin-reuptake inhibitors in pregnancy and the risk of birth defects. New England Journal of Medicine. 2007;356(26):2684–2692. doi: 10.1056/NEJMoa066584. [DOI] [PubMed] [Google Scholar]
- 17.Altura BM, Malaviya D, Reich CF, Orkin LR. Effects of vasoactive agents on isolated human umbilical arteries and veins. American Journal of Physiology. 1972;222(2):345–355. doi: 10.1152/ajplegacy.1972.222.2.345. [DOI] [PubMed] [Google Scholar]
- 18.Bjoro K, Stray-Pedersen S. In vitro perfusion studies on human umbilical arteries. I. Vasoactive effects of serotonin, PGF2 alpha and PGE2. Acta Obstetricia et Gynecologica Scandinavica. 1986;65(4):351–5. doi: 10.3109/00016348609157359. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez C, Cruz MA, Gallardo V, Albornoz J, Bravo I. Serotonin-induced vasoconstriction in human placental chorionic veins: interaction with prostaglandin F2 alpha. Gynecologic and Obstetric Investigation. 1993;35(2):86–90. doi: 10.1159/000292671. [DOI] [PubMed] [Google Scholar]
- 20.Mohammad-Zadeh LF, Moses L, Gwaltney-Brant SM. Serotonin: a review. Journal of Veterinary Pharmacology and Therapeutics. 2008;31(3):187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 21.Clark KE, Mills EG, Otte TE, Stys SJ. Effect of serotonin on uterine blood flow in pregnant and nonpregnant sheep. Life Sciences. 1980;27(25):2655–2661. doi: 10.1016/0024-3205(80)90556-1. [DOI] [PubMed] [Google Scholar]
- 22.Lang U, Prada J, Clark KE. Systemic and uterine vascular response to serotonin in third trimester pregnant ewes. European Journal of Obstetrics & Gynecology and Reproductive Biology. 1993;51(2):131–138. doi: 10.1016/0028-2243(93)90025-8. [DOI] [PubMed] [Google Scholar]
- 23.Philip J, Silver RK, Wilson RD, Thom EA, Zachary JM, Mohide P, Mahoney MJ, Simpson JL, Platt LD, Pergament E. Late first-trimester invasive prenatal diagnosis: results of an international randomized trial. Obstetrics and Gynecology. 2004;103(6):1164–1173. doi: 10.1097/01.AOG.0000128049.73556.fb. [DOI] [PubMed] [Google Scholar]
- 24.Stoler JM, McGuirk CK, Lieberman E, Ryan L, Holmes LB. Malformations reported in chorionic villus sampling exposed children: A review and analytic synthesis of the literature. Genetics in Medicine. 1999;1(7):315–322. doi: 10.1097/00125817-199911000-00001. [DOI] [PubMed] [Google Scholar]
- 25.Van Allen M. Structural anomalies resulting from vascular disruption. Pediatric Clinics of North America. 1992;39(2):255. doi: 10.1016/s0031-3955(16)38294-3. [DOI] [PubMed] [Google Scholar]
- 26.Miller K, Massie MJ. Depression and anxiety. The Cancer Journal. 2006;12(5):388. doi: 10.1097/00130404-200609000-00008. [DOI] [PubMed] [Google Scholar]


