Abstract
Background and Objective
The miR-143/145 cluster regulates VSMC specific gene expression, thus controlling differentiation, plasticity and contractile function, and promoting the VSMC phenotypic switch from a contractile/non-proliferative to a migrating/proliferative state. More recently increased miR-145 expression was observed in human carotid atherosclerotic plaques from symptomatic patients. The goal of this study was to investigate the contribution of miR-143/145 during atherogenesis by generating mice lacking miR-143/145 on an Ldlr-deficient background.
Methods and Results
Ldlr−/− and Ldlr−/−-miR-143/145−/− (DKO) were fed a Western diet (WD) for 16 weeks. At the end of the treatment, the lipid profile and the atherosclerotic lesions were assessed in both groups of mice. Absence of miR-143/145 significantly reduced atherosclerotic plaque size and macrophage infiltration. Plasma total cholesterol levels were lower in DKO and FLPC analysis showed decreased cholesterol content in VLDL and LDL fractions. Interestingly miR-143/145 deficiency per se resulted in increased hepatic and vascular ABCA1 expression. Experiments with the luciferase coding sequence fused to the ABCA1 3’UTR, Western blotting, qRT-PCR and mimicMiR confirmed the direct regulation of ABCA1 expression by miR-145.
Conclusions
miR-143/145 deficiency significantly reduces atherosclerosis in mice. Therapeutic inhibition of miR-145 might be useful for treating atherosclerotic vascular disease.
Keywords: miR-143/145, atherosclerosis, ABCA1, smooth muscle cells
INTRODUCTION
MicroRNAs (miRNAs) are short non-coding RNAs involved in the regulation of gene expression at the post-transcriptional level that have been involved in the pathogenesis of a number of cardiovascular diseases. miRNAs are necessary for vascular smooth muscle cells (VSMCs) growth, differentiation and function (1-4). Recently, we and others have shown that the miR-143/145 cluster regulates VSMC specific gene expression, differentiation, plasticity and contractile function, thus promoting the VSMC phenotypic switch from a contractile/nonproliferative to a migrating/proliferative state (5-7). Alterations of these VSMCs functions are associated with altered vascular function (8) and cardiovascular disorders including atherosclerosis. In addition to VSMCs, endothelial cells (ECs) also express miR-143/145. The expression of miR-143/145 is upregulated by transcription factors that play a central role in SMC differentiation, such as serum response factor and the coactivators, myocardin and myocardin-related transcription factors. Moreover, both miRNAs are upregulated in ECs by shear stress via a KLF2-dependent mechanism. Interestingly, EC-derived miR-143/145 is secreted in exosomes and regulates VSMC functions. Of note, extracellular microvesicles derived from KLF2-expressing ECs reduce atherosclerotic lesion formation in the aorta of apoE−/− mice. Altogether, these data suggest a potential role of miR-143/145 in modulating the progression of atherosclerosis (9, 10). Despite these observations, several questions remain unresolved, and among them the post-natal adaptation of vasculature toward miR-143/145 targeting. Furthermore, while the deficiency of this miRNA cluster is associated with an increased cell synthetic phenotype in vitro (5-7), results in vivo are less clear. In fact, in addition to decreased blood pressure (8) and reduced vascular tone (11), miR-143/145−/− mice present an increased neointimal formation in the femoral arteries(8) under normal conditions and a decrease after ligation of the carotid artery (7). Moreover increased expression of miR-145 was observed in human carotid atherosclerotic plaques from symptomatic compared to asymptomatic patients (12).
Therefore, to clarify the contribution of the miR-143/145 cluster during the progression of atherosclerosis, we generated mice lacking miR-143/145 on an Ldlr-deficient background and fed them a WD for 16 weeks. Interestingly we found that the genetic ablation of miR-143/145 markedly reduced the progression of atherosclerosis in mice. Deficiency of miR-143/145 decreased the expression of synthetic VSMC markers including alpha-actin 2 (ACTA2) and smooth muscle protein 22-alpha (SM22) and increased expression of byglican (BGL). Moreover, we also identified the cholesterol transporter ATP-binding cassette A1 (ABCA1) as a novel miR-145 target gene. Accordantly, ABCA1 expression was enhanced in the arterial wall of miR-143/145 deficient mice. Taken together, our results suggest that the reduced atherosclerosis observed in mice lacking miR-143/145 is mediated by its combined effect on VSMC functions and lipid homeostasis.
METHODS
Ldlr−/−-miR-143/145−/− (DKO) mice were generated by crossbreeding miR-143/145 deficient mice (6) with mice lacking low-density lipoprotein receptor (Ldlr−/−) (the Jackson Laboratories) for at least 8 generations. Ldlr−/− mice were used as control group. Dicerflox/flox-LyzCRE mice were generated by crossbreeding Dicerflox/flox mice and LyzCRE mice. Both strains were obtained from Jackson laboratories. The investigation conforms to the European Commission Directive 86/609/EEC and was approved by the ethical committee (Progetto di Ricerca 2009/3). At 8 weeks of age, animals were fed ad libitum with Western diet (WD) (21% fat, 0.15% cholesterol and 19.5% casein, Harlan, Bresso, Italy) for 16 weeks. Mice were sacrificed with an overdose of Avertin 2.5% (Aldrich Chemical Co.USA), followed by cervical dislocation. The heart and the arterial tree were perfused with saline solution under physiological pressure. Then, the aortas and the hearts were isolated, paraffin embedded and atherosclerosis lesions assessed as previously described (13),. A detailed description of the material and methods is provided as an online supplement.
RESULTS
MiR-143/145 deficiency protects against the progression of atherosclerosis
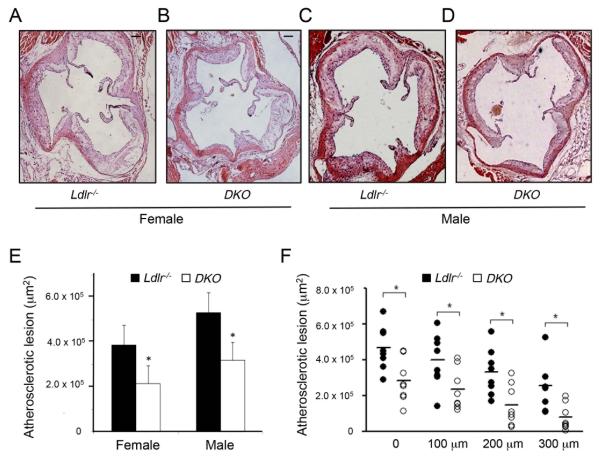
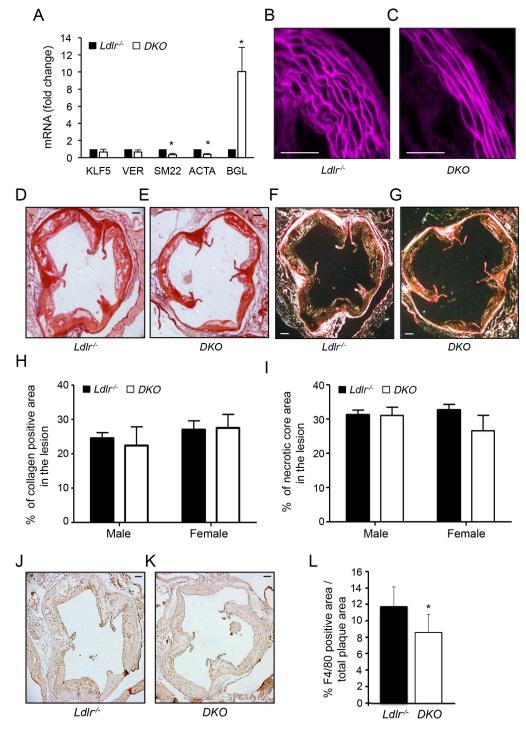
We first determined the role of miR-143/145 during the progression of atherosclerosis by feeding mice a WD diet for three months. The results showed that DKO mice developed significantly less atherosclerosis in the aortic sinus compared to Ldlr−/− mice (289970 ±143748μm2 vs. 439774 ±134843μm2; p<0.01, mean± SD is shown. N=16 for each group, 8 male and 8 female) (Fig. 1A-D, quantified in 1E). Similar results were obtained when we measured the first 300 μm of the ascending aorta; N=8 for each group, 4 male and 4 female) (Fig. 1F). Since miR-143/145 controls VSMC functions, we further analyzed the atherosclerotic plaque morphology in both groups of mice. Interestingly, aortas isolated from DKO mice presented VSMC markers associated with a synthetic phenotype characterized by reduced expression of ACTA2 and SM22 and increased expression of byglican (BGL) (14) compared to Ldlr−/− mice (Fig. 2A). In agreement with previous findings in miR-143/145−/− animals (6), histological analysis of aortas showed a thinner vessel with more distended laminas in the DKO mice compared with Ldlr−/− mice (Fig. 2B and 2C). Moreover, monomeric and fibrillar collagen distribution as well as the presence of the necrotic core did not differ between DKO and Ldlr−/− mice (Fig. 2D-2I). However, F4/80 staining analyzed from proximal aortic sections revealed a marked decrease in macrophage content in DKO mice compared to Ldlr−/− mice (Fig. 2J-2L).
Figure 1. miR-143/145−/− deficiency increase atherosclerotic plaque formation in Ldlr−/− mice.
A-D) Representative histological analysis of cross-sections of the aortic sinus from female (A and B) and male (C and D) Ldlr−/− and DKO stained with haematoxylin and eosin. E) Quantification of the lesion area in the aortic sinus. F) Quantification of the atherosclerotic lesion in the first 300 μm of the ascending aorta. Data are expressed as mean±SD, *p<0.05 vs Ldlr−/− (n=8 for each group). Scale bar indicates 100μm.
Figure 2. Atherosclerotic plaque structure and composition in Ldlr−/− and DKO mice.
A) qRT-PCR analysis of KLF5, Versican (VER), SM22, ACTA and BGL expression in aortic tissues isolated from Ldlr−/− and Ldlr−/−-miR-143/145−/− (DKO) mice. Data are expressed as mean ± S.D. * p<0.05 vs. Ldlr−/−. B and C) Representative histological images of the media structure of the thoracic aorta isolated from. Ldlr−/− and DKO mice. D-G) Representative histological analysis of cross-sections of the aortic sinus from Ldlr−/− and DKO mice stained with sirius red (D and E) and following acquisition with polarized light (F and G). H and I) Quantification of the collagen content (H) and necrotic areas (I) in the aortic sinus. Data are presented as the percentage of atherosclerotic lesion covered by collagen or the necrotic core, and are expressed as mean ± S.D. (n=8 for each group). J and K) Representative F4/80 stained aortic sinus sections from Ldlr−/− and DKO mice. L) Quantification of the F4/80 positive areas within the atherosclerotic plaques. Data are expressed as mean± S.D (n=8 for each group). Scale bar indicates 100μm.
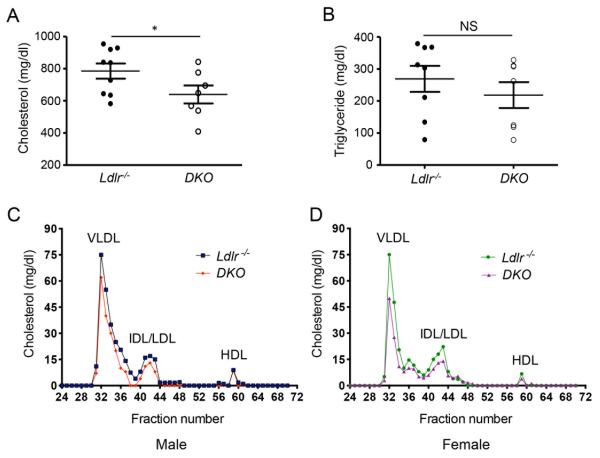
Next, we assessed whether genetic ablation of miR-143/145 cause alterations in metabolic parameters including plasma lipid levels and body weight. Surprisingly, we observed that absence of miR-143/145 results in a marked decrease of circulating cholesterol (Fig. 3A) but not plasma tryglicerides levels (Fig. 3B). Moreover, the cholesterol distribution in different lipoprotein fractions showed a decrease of very-low density lipoprotein (VLDL) and low-density lipoprotein (LDL) cholesterol in plasma from male and female DKO mice compared with Ldlr−/− mice (Fig. 3C and 3D). The change in plasma lipid levels observed in the DKO mice was independent of the hepatic expression of other miRNAs that regulates lipoprotein metabolism including miR-33 and miR-122 (Supplemental Fig. 1). Plasma high-density lipoprotein (HDL) cholesterol was similar between both groups of mice (Fig. 3C and 3D). Similarly to previous studies (15), we did not observe differences in body weight gain between both groups of mice (Supplemental Fig. 2).
Figure 3. Absence of miR-143/145−/− reduces plasma cholesterol levels.
A and B) Total plasma cholesterol (A) and triglyceride (B) levels from Ldlr−/− and in DKO mice (n=10 mice/group). * p<0.05. C and D) FPLC profiles from pooled plasma (n=4) of male (C) and female (D) Ldlr−/− and in DKO mice.
MiR-145 regulates ABCA1 expression
In order to find a potential novel mechanism by which the absence of miR-143/145 reduces the progression of atherosclerosis, we performed a bioinformatic analysis using Targetscan (http://www.targetscan.org/). Interestingly, we found that miR-145 has several binding sites in the 3’UTR of ABCA1 and the scavenger receptor BI (SR-BI) (Fig. 4A). ABCA1 promotes cellular cholesterol efflux and regulates HDL biogenesis, while SR-BI modulates the hepatic uptake of HDL particles, thereby controlling two critical steps of the reverse cholesterol transport (RCT). Thus, we hypothesized that the absence of miR-145 might increase plasma HDL levels and cholesterol efflux from macrophage accumulated in atherosclerotic plaques, thereby attenuating the progression of atherosclerosis. To directly test whether miR-145 targets ABCA1 and SR-BI, we generated reported constructs with the luciferase coding sequence fused to the 3’UTR of ABCA1 and SR-BI. As expected, miR-145 overexpression significantly repressed ABCA1 and SR-BI 3’UTR activity (Fig. 4B). Importantly, specific mutations of miR-145 target sites relieved miR-145 repression of ABCA1 and SR-BI 3’UTR activity, consistent with a direct interaction of miR-145 with these sites (Fig. 4B).
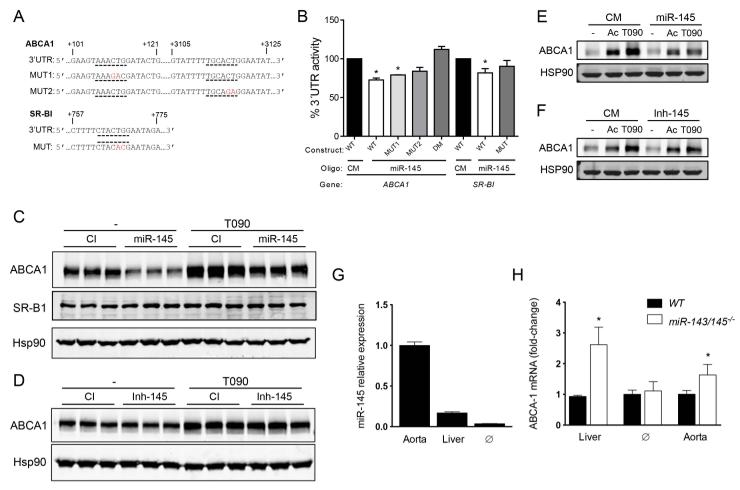
Figure 4. miR-145 regulates ABCA1 expression.
A) Human ABCA1 and SR-B1 3’-UTR sequences. Underlined indicate the mir-145 binding sites. Nucleotides highlighted in bold indicate mutations in the miR-145. B) Luciferase reporter activity in COS-7 cells transfected with non-targeting control miR (CM) or miR-145 mimic (miR-145) and the ABCA1 and SR-B1 3’UTR (wild type [WT]) or the constructs containing the indicated point mutations [MUT]). Data are expressed as mean percent of 3’UTR activity of CM±SEM and are representative of ≥3 experiments. *Significantly different from cells co-transfected with CM and wild-type (WT) 3’UTR. P≤0.05. C and D) Representative Western blot an analysis of ABCA1, ABCA1 and HSP90 in human hepatic cells (Huh7) transfected with miR-145 mimics (C) or inhibitors (D) in presence or absence of T0901317 (T090). G) qRT-PCR analysis of miR-145 expression in mouse aorta, liver and peritoneal macrophage (∅). Data represent the relative expression of miR-145 in aorta and macrophage compared to the liver. H) qRT-PCR analysis of ABCA1 expression in liver, macrophage (∅) and aorta from miR-143/145−/− mice compared to WT mice. Data are the mean ± S.D and are representative of ≥3 experiments.
We next determined whether overexpression or inhibition of miR-145 reduces or increases ABCA1 and SR-BI expression respectively. As seen in Fig. 4C, human hepatic cells (Huh7) transfected with miR-145 mimics expressed significantly reduced ABCA1 levels compared to with Huh7 cells transfected with non-targeting miRNA (CM). By contrast, antagonist miR-145 expression did not enhance the expression of ABCA1 in human hepatic cells (Fig. 4D). SR-BI expression was similar in cells transfected with CM and miR-145, suggesting that miR-145 is not targeting ABCA1 (Fig. 4C). We next assessed the effect of miR-145 overexpression and inhibition on ABCA1 expression in macrophages. To this end, we transfected thioglycollate-elicited macrophage with miR-145 mimics or inhibitors and cultured the cells in presence of acetylated low-density lipoprotein (AcLDL) (to enrich in cholesterol, thus enhancing ABCA1 expression) or the LXR ligand T0901317 (to directly stimulate ABCA1 transcription). The results showed that miR-145 overexpression markedly reduced ABCA1 levels (Fig. 4E). However, inhibition of miR-145 did not increase ABCA1 expression, suggesting that endogenous miR-145 levels are not involved in the post-transcriptional regulation of ABCA1 in macrophages (Fig. 4F). To gain insight into the function of miR-145 in regulating ABCA1 expression in vivo, we measured the relative miR-145 expression in the vessel wall (miR-145 expression is highly expressed in VSMC), liver and macrophage. qRT-PCR analysis showed that miR-145 levels were significantly higher in the vessel wall compared to the liver and macrophages (Fig. 4G). As expected by its abundant expression, ABCA1 expression was markedly increased in the aorta isolated from miR-143/145−/− mice compared to WT mice (Fig. 4H). Absence of miR-143/145−/− also increases hepatic ABCA1 expression but do not influence ABCA1 levels in macrophages (Fig. 4I). Taken together, these results identify ABCA1 as a novel miR-145 target gene and suggest that miR-145 endogenous expression controls ABCA1 expression in the artery wall and in the liver.
miR-145 is transferred from VSMCs to macrophages
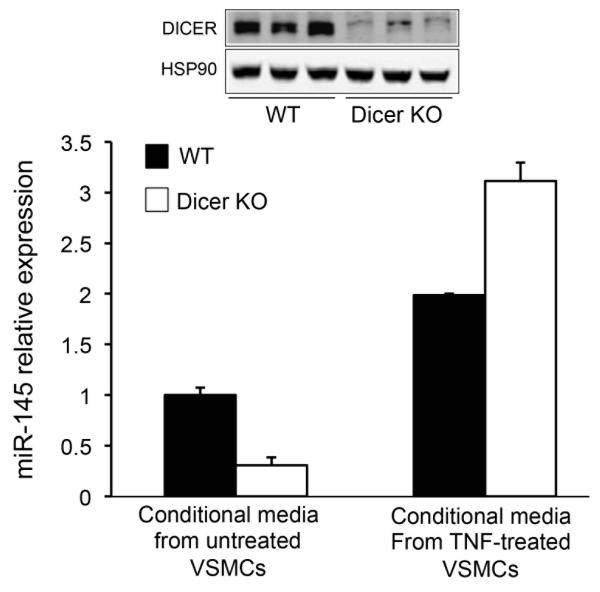
It has been recently demonstrated that miRNAs can be transferred between ECs and VSMCs via extracellular vesicles in the arterial wall. Therefore, we hypothesized that VSMCs might transfer miR-145 from VSMCs to macrophages. To test this hypothesis, we treated primary mouse macrophages with conditional media obtained from VSMC treated or not with tumor necrosis factor (TNF, a pro-atherogenic cytokine). The results showed that the incubation of macrophages with TNF-treated VSMC conditional media increases intracellular mature miR-145 levels compared to macrophages incubated with untreated VSMC conditional media (Figure 5, solid bars), suggesting that TNF enhances miR-145 transport between VSMCs and macrophages. To further rule out the possibility of TNF induces miR-145 expression in macrophages, we performed the same experiment using macrophages isolated from Dicer-deficient mice. Dicer is an endonuclease required for miRNA processing and maturation; therefore most of the mature miRNAs quantified in Dicer deficient macrophages should be acquired from the conditional media. Importantly, we found that TNF-treated VSMC conditional media markedly increases miR-145 levels compared to macrophages incubated with untreated VSMC conditional media (Figure 5, open bars). We also confirmed that Dicer expression was significantly reduced in macrophages isolated from the dicerflox/flox-LyzCRE (dicer KO) mice (Figure 5, upper panel). These results suggest that the transfer of miR-145 between vascular cells within the atherosclerotic plaques might influence gene expression in the recipient cells. This finding also suggests that the transfer of miR-145 between VSMC and macrophage in response to atherogenic cytokines might have and effect on macrophage ABCA1 expression and cellular cholesterol efflux.
Figure 5.
miR-145 is transferred from VSMCs to macrophages. qRT-PCR analysis of miR-145 expression in mouse primary macrophages (WT and dicer KO) and treated with conditional media isolated from untreated or TNF-treated VSMCs. Data are the mean ± S.D and are representative of ≥2 experiments. Representative Western blot analysis of DICER and HSP90 form WT and Dicer KO mouse primary macrophage is shown in the upper panels.
DISCUSSION
Here, we show that miR-143/145 deficiency attenuates the progression of atherosclerosis in Ldlr−/− mice. This event could be the consequence of multiple mechanisms including changes in VSMC functions and increased vascular and hepatic ABCA1 expression.
Previous studies in apoE−/− mice showed that miR143-145 expression in the vasculature was dramatically reduced during atherogenesis (6), suggesting that VSMCs-specific overexpression of miR145 in apoE−/− mice favors the shift toward a plaque morphology, which presents a more stable phenotype (16). In addition to the role VSMC derived miR-143/145, Dimmeler and colleagues demonstrated that KLF2 overexpression results in a significant increase of miR-143/145 levels in endothelial cells (ECs). These miRNAs can then be packaged in extracellular vesicles, which can directly target VMSCs (9). Of note, the injection of vesicles isolated from KLF2- transduced mouse ECs, thus enriched in miR-143/145 to apoE−/− mice results also in a marked reduction of atherosclerosis (9). All together these results suggest an anti-atherogenic role of mR-143/145 miRNA cluster. In contrast with these observations, we reported in this study that the genetic ablation of miR-143/145 attenuates the progression of atherosclerosis despite we found a dramatic switch of VSMC from contractile to a synthetic phenotype. Our findings dissect the role of miR-143/145 during atherogenesis and suggest that the proposed therapeutic approach of mimicking miR-143/145 activity in vivo (9) should be carefully investigated as this could result in increase progression of atherosclerosis.
Our study also identifies ABCA1 as a novel target for miR-145. Similar finding was recently reported during the revision of this manuscript (17). ABCA1 has a very large 3’UTR, which is regulated by numerous miRNAs including miR-33, miR-144 and miR-758. Our in vitro data demonstrate that overexpression of miR-145 markedly reduce ABCA1 expression levels in human hepatic cells and mouse macrophages. However, miR-145 endogenous inhibition did not increase ABCA1 in macrophages suggesting that the endogenous expression of miR-145 do not play a role in controlling ABCA1 levels in these cells. Even though these results argues against the physiological role of miR-145 in controlling macrophage cholesterol metabolism, the in vivo scenario could be different since miR-145 might be secreted by ECs or VSMCs in atherosclerotic plaques and influence the post-transcriptional regulation of ABCA1 in macrophages. Indeed, it has been previously reported that secretion of miR-145 by ECs impact VSMCs functions and reduce the progression of atherosclerosis (9). Here, we report that mir-145 is transferred from VSMCs to macrophages using an in vitro approach. This finding suggests that the post-transcriptional regulation of ABCA1 by miRNAs might be influenced in atherosclerotic plaques by miRNAs secreted by other vascular cells (e.g. VSMCs). Further experiments will be important to test the relevance of this mechanism in vivo.
miR-145 is also expressed in the liver and the inhibition mir-145 increases ABCA1 expression in human hepatic cells. Accordantly, miR-143/145 deficient mice have increased hepatic ABCA1 expression. However, we were unable to find differences in plasma HDL-C levels in DKO mice compared with Ldlr−/− mice. Instead, we found a significant difference in plasma VLDL and LDL levels but the mechanism behind this finding requires further investigation.
Our study also suggest that miR-145 could be an important regulator of ABCA1 expression in VSMC, where miR-145 is abundantly expressed. This is an important observation because the accumulation of cholesterol in VSMC results in transdifferentiation to macrophage-like state (18). Since ABCA1 regulates cholesterol accumulation in VSMC, absence of miR-145 might increase ABCA1 expression in these cells leading to a reduction in VSMC foam cell formation and macrophage transdifferentiation in the arterial wall.
The role of the miR-143/145 cluster in during the progression of atherosclerosis is likely to be more complex and not only the consequence of miR145 in controlling VSMC functions and lipid homeostasis. miR-143 has been also shown to be a regulator of hepatic insulin signaling and adipocyte differentiation (15, 19). Indeed, the expression of the miR-143/145 cluster is upregulated in the liver of genetic and dietary mouse models of obesity (15). Specifically, overexpression of miR-143, but not miR-145, impairs insulin stimulated Akt activation and glucose homeostasis (15). Conversely, mice deficient for the miR-143/145 cluster are protected for the development of obesity-associated insulin resistance (15). Altogether, these observations suggest that the effect of miR-145 and miR-143 in controlling lipid and glucose metabolism might be mediated by the direct regulation of ABCA1 by miR-145 and the regulatory role of miR-143 on hepatic insulin signaling and adipocyte differentiation.
In conclusion we identified that the absence of the miR-143/145 cluster protects from atherosclerosis, and that this effect could be mediated by controlling VCMS functions and lipid metabolism.
Our findings that miR-145 inhibits ABCA1 coupled to the identification of increased miR-145 levels in human carotid atherosclerotic plaques from symptomatic patients (12), supports the relevance of evaluating anti-miR145 strategies in the context of novel approaches under investigation for dyslipidemia (20, 21) and cardiovascular-related diseases, including atherosclerosis.
Supplementary Material
What is known on this topic
Recent scientific evidences have demonstrated the important role of small non-coding RNAs (miRNAs) during the progression of atherosclerosis.
Genes involved in HDL life cycle such as ABCA-1, ABCG-1 are targeted by MiRNAs.
What this paper adds
miR-143/145 deficiency is associated with reduced atherosclerosis.
miR-143/145/LDL-R double KO animals present a better plasma lipid profile.
miR-145 control the expression of ABCA1, a key transporter that regulates HDL-C biogenesis and cholesterol efflux.
ACKNOWLEDGEMENT
The study was supported by Fondazione Cariplo (2010-0768 to GDN, 2009-2582 to ALC), by the University of Milan (Piano Sviluppo B-2014 to GDN), by the National Institutes of Health (R01HL107953, R01HL106063 to CF-H) and by the Foundation Leducq Transatlantic Network of Excellence (CF-H). CMR is supported by a postdoctoral fellowship from the American Heart Association (12POST9780016). N.R is supported by a fellowship from Ministerio de Educación [Programa Nacional de Movilidad de Recursos Humanos del Plan Nacional de I-D+i 2008-2011.
REFERENCES
- 1.Albinsson S, Sessa WC. Can microRNAs control vascular smooth muscle phenotypic modulation and the response to injury? Physiological genomics. 2011 May 1;43(10):529–33. doi: 10.1152/physiolgenomics.00146.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albinsson S, Skoura A, Yu J, et al. Smooth muscle miRNAs are critical for post-natal regulation of blood pressure and vascular function. PloS one. 2011;6(4):e18869. doi: 10.1371/journal.pone.0018869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albinsson S, Suarez Y, Skoura A, et al. MicroRNAs are necessary for vascular smooth muscle growth, differentiation, and function. Arteriosclerosis, thrombosis, and vascular biology. 2010 Jun;30(6):1118–26. doi: 10.1161/ATVBAHA.109.200873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Norata GD, Sala F, Catapano AL, et al. MicroRNAs and lipoproteins: a connection beyond atherosclerosis? Atherosclerosis. 2013 Apr;227(2):209–15. doi: 10.1016/j.atherosclerosis.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cordes KR, Sheehy NT, White MP, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009 Aug 6;460(7256):705–10. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elia L, Quintavalle M, Zhang J, et al. The knockout of miR-143 and -145 alters smooth muscle cell maintenance and vascular homeostasis in mice: correlates with human disease. Cell Death Differ. 2009 Dec;16(12):1590–8. doi: 10.1038/cdd.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xin M, Small EM, Sutherland LB, et al. MicroRNAs miR-143 and miR-145 modulate cytoskeletal dynamics and responsiveness of smooth muscle cells to injury. Genes Dev. 2009 Sep 15;23(18):2166–78. doi: 10.1101/gad.1842409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boettger T, Beetz N, Kostin S, et al. Acquisition of the contractile phenotype by murine arterial smooth muscle cells depends on the Mir143/145 gene cluster. J Clin Invest. 2009 Sep;119(9):2634–47. doi: 10.1172/JCI38864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hergenreider E, Heydt S, Treguer K, et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nature cell biology. 2012 Mar;14(3):249–56. doi: 10.1038/ncb2441. [DOI] [PubMed] [Google Scholar]
- 10.Rader DJ, Parmacek MS. Secreted miRNAs suppress atherogenesis. Nature cell biology. 2012 Mar;14(3):233–5. doi: 10.1038/ncb2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norata GD, Pinna C, Zappella F, et al. MicroRNA 143-145 deficiency impairs vascular function. Int J Immunopathol Pharmacol. 2012 Apr-Jun;25(2):467–74. doi: 10.1177/039463201202500216. [DOI] [PubMed] [Google Scholar]
- 12.Cipollone F, Felicioni L, Sarzani R, et al. A unique microRNA signature associated with plaque instability in humans. Stroke; a journal of cerebral circulation. 2011 Sep;42(9):2556–63. doi: 10.1161/STROKEAHA.110.597575. [DOI] [PubMed] [Google Scholar]
- 13.Norata GD, Venu VK, Callegari E, et al. Effect of Tie-2 conditional deletion of BDNF on atherosclerosis in the ApoE null mutant mouse. Biochimica et biophysica acta. 2012 Jun;1822(6):927–35. doi: 10.1016/j.bbadis.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu-Hirota R, Sasamura H, Kuroda M, et al. Extracellular matrix glycoprotein biglycan enhances vascular smooth muscle cell proliferation and migration. Circulation research. 2004 Apr 30;94(8):1067–74. doi: 10.1161/01.RES.0000126049.79800.CA. [DOI] [PubMed] [Google Scholar]
- 15.Jordan SD, Kruger M, Willmes DM, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nature cell biology. 2011 Apr;13(4):434–46. doi: 10.1038/ncb2211. [DOI] [PubMed] [Google Scholar]
- 16.Lovren F, Pan Y, Quan A, et al. MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation. 2012 Sep 11;126(11 Suppl 1):S81–90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 17.Kang MH, Zhang LH, Wijesekara N, et al. Regulation of ABCA1 protein expression and function in hepatic and pancreatic islet cells by miR-145. Arterioscler Thromb Vasc Biol. 2013 Dec;33(12):2724–32. doi: 10.1161/ATVBAHA.113.302004. [DOI] [PubMed] [Google Scholar]
- 18.Rong JX, Shapiro M, Trogan E, et al. Transdifferentiation of mouse aortic smooth muscle cells to a macrophage-like state after cholesterol loading. Proc Natl Acad Sci U S A. 2003 Nov 11;100(23):13531–6. doi: 10.1073/pnas.1735526100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Esau C, Kang X, Peralta E, et al. MicroRNA-143 regulates adipocyte differentiation. The Journal of biological chemistry. 2004 Dec 10;279(50):52361–5. doi: 10.1074/jbc.C400438200. [DOI] [PubMed] [Google Scholar]
- 20.Norata GD, Tibolla G, Catapano AL. Gene silencing approaches for the management of dyslipidaemia. Trends in pharmacological sciences. 2013 Apr;34(4):198–205. doi: 10.1016/j.tips.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 21.Norata GD, Ballantyne CM, Catapano AL. New therapeutic principles in dyslipidaemia: focus on LDL and Lp(a) lowering drugs. Eur Heart J. 2013 Jun;34(24):1783–9. doi: 10.1093/eurheartj/eht088. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.