Abstract
Objective
Despite the fact that most obesity drugs primarily work by reducing metabolizable energy intake, elucidation of the time course of energy intake changes during long-term obesity pharmacotherapy has been prevented by the limitations of self-report methods of measuring energy intake.
Methods
We used a validated mathematical model of human metabolism to provide the first quantification of metabolizable energy intake changes during long-term obesity pharmacotherapy using body weight data from randomized, placebo-controlled trials that evaluated 14 different drugs or drug combinations.
Results
Changes in metabolizable energy intake during obesity pharmacotherapy were reasonably well-described by an exponential pattern comprising three simple parameters, with early large changes in metabolizable energy intake followed by a slow transition to a smaller persistent drug effect.
Conclusions
Repeated body weight measurements along with a mathematical model of human metabolism can be used to quantify changes in metabolizable energy intake during obesity pharmacotherapy. The calculated metabolizable energy intake changes followed an exponential time course, and therefore different drugs can be evaluated and compared using a common mathematical framework.
Keywords: Obesity Pharmacotherapy, Energy Intake, Weight Loss, Mathematical Model
Introduction
Weight loss results from an imbalance between metabolizable energy intake and energy expenditure, both of which dynamically change over time (1). Most obesity drugs work in humans by decreasing metabolizable energy intake with minor effects on energy expenditure (2). Unfortunately, current methods for measuring energy intake in free-living humans are either notoriously inaccurate (3, 4, 5) or are prohibitively expensive (6). Therefore, the time course of energy intake during long-term obesity pharmacotherapy remains to be elucidated.
Here, we used repeated mean body weight measurements as the sole model inputs to a validated mathematical model of human metabolism (7, 8, 9) and provide the first quantification of metabolizable energy intake changes during long-term obesity pharmacotherapy. We evaluated 14 different drugs or drug combinations from randomized, placebo-controlled trials (10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24). Obesity pharmacotherapy led to an early decrease in metabolizable energy intake followed by a slow exponential relaxation to a much smaller persistent effect. This universal exponential pattern suggests that drugs can be compared using a common mathematical framework comprising three simple parameters.
Methods
We searched PubMed on January 24, 2014 for randomized, placebo-controlled, obesity pharmacotherapy data with body weight time course data of at least 30 weeks in duration and at least 6 repeated body weight measurements. We found 15 studies matching our search criteria investigating 14 different drugs or drug combinations (10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24).
Several of the interventions included a prescribed lifestyle modification involving a reduced calorie diet and a modest increase in physical activity. The physical activity prescription was practically negligible in terms of its energy cost, especially considering the likely incomplete adherence. Furthermore, spontaneous physical activity typically decreases during caloric restriction (25) and this would tend to offset any voluntary activity increase. Therefore, for simplicity, we assumed that physical activity was constant.
The repeated mean body weight data were provided as the inputs to a mathematical model of human energy metabolism to quantify the underlying changes in metabolizable energy intake, ΔEI, in each group of subjects, including the placebo groups (7, 9). While repeated body weight data from individual subjects can be used to calculate confidence intervals of ΔEI (7, 9), in the present study we only had access to the mean body weight data published for each group. Therefore, we did not calculate confidence intervals for ΔEI.
All of the drugs appeared to generate a similar ΔEI pattern: a large early decrease from the zero baseline followed by a slow exponential rise towards a smaller constant persistent effect. Therefore, we fit each calculated ΔEI time course using a simple three parameter exponential model:
| [1] |
where pearly represents the initial decrease in energy intake from baseline, plate represents the long-term decrease in energy intake, τ is the exponential time constant characterizing the number of days required to transition from early to long-term metabolizable energy intake change, and t′=time−(N−1)T/2 shifts the time axis such that pearly corresponds to the earliest calculation of ΔEI, where N is the number of body weight measurements and T was the time interval between measurements used for each model-calculated ΔEI time point. We did not consider more complex functions to fit the ΔEI time courses since the introduction of additional parameters comes with the significant risk of over-fitting.
The ΔEI time courses for both the placebo and treatment groups were separately fit using equation [1]. Therefore, the placebo-subtracted ΔEI time course could be expressed as the difference between these two exponential functions. However, the calculated placebo-subtracted ΔEI did not demonstrate an obvious double exponential pattern since the characteristic time constants for the two groups were not very different. Therefore, we also fit the placebo-subtracted data using a single exponential model. The analyses were conducted using MATLAB (MathWorks Inc, Natick, MA) and the code can be downloaded as Supplementary Information.
Results
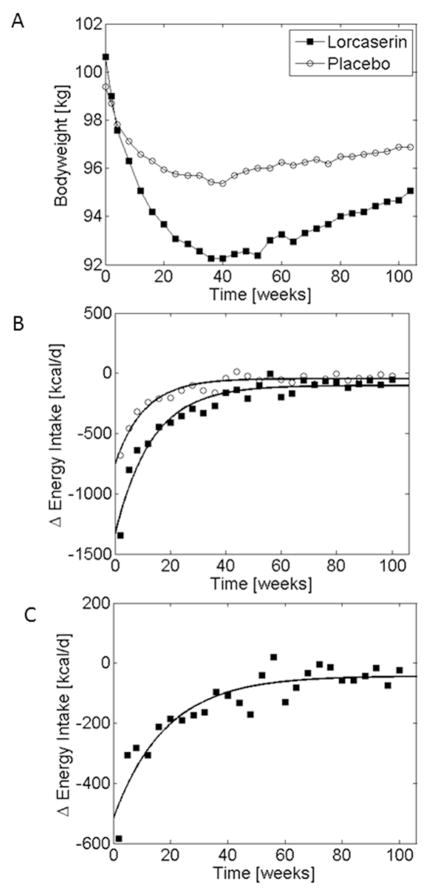
Figure 1A illustrates the mean body weight over 2 years in 1587 subjects receiving placebo and 1595 subjects receiving 10 mg of locaserin (20). Both groups were also prescribed a reduced calorie diet and instructed to exercise moderately for 30 minutes per day. Body weight decreased rapidly in both groups over the first few months and reached a plateau that is typically observed within the first year. Figure 1B shows the calculated mean metabolizable energy intake changes, ΔEI, in both groups corresponding to the body weight measurements from Figure 1A. The curves in Figure 1B correspond to the best fit exponential description of ΔEI for each group using equation 1. Interestingly, both groups were characterized by a large early reduction in energy intake from baseline followed by a slow exponential relaxation to less than 100 kcal/d below the baseline energy intake. Figure 1C illustrates the placebo subtracted effect of locaserin and demonstrates that the drug had a large initial effect on ΔEI amounting to about 500 kcal/d followed by a slow exponential relaxation towards a persistent effect of about 40 kcal/d.
Figure 1.
(A) Mean body weight changes during placebo (○) and lorcaserin (■) treatment as measured by Smith et al. (20). (B) Model-calculated changes in energy intake corresponding to the measured body weight trajectories along with the exponential best-fit curves. (C) Model-calculated placebo-subtracted effect of lorcaserin on energy intake (■) along with the exponential best-fit curve.
All the drugs investigated appeared to follow this same universal pattern (see the Supplemental Information for Figures corresponding to each intervention). Therefore, the three model parameters, pearly, τ, and plate that quantify the shape of this ΔEI curve can be used as a common framework to compare the effects of different drugs or drug doses on energy intake.
Table 1 presents the calculated best-fit exponential parameter values for placebo subtracted ΔEI for 14 drugs or drug combinations, with some studied at multiple doses. The relatively high coefficients of determination (R2 values) demonstrate that the exponential model provided a reasonably good fit to these data. The drugs produced initial decreases in energy intake, ranging between 145–1146 kcal/d (pearly), followed by an exponential relaxation with a characteristic time constant ranging between 19–501 days (τ) approaching a smaller persistent drug effect on energy intake ranging from a decrease of 600 kcal/d to a small increase (plate). Supplementary Tables 1 and 2 show the best-fit exponential parameter values and coefficients of determination separately for the placebo and treatment groups.
Table 1.
Parameter values quantifying the placebo-subtracted drug effect on changes in metabolizable energy intake.
| Drug | pearly [kcal/d] | τ [d] | plate [kcal/d] | R2 | Duration [weeks] | Dose [mg] | Ref. |
|---|---|---|---|---|---|---|---|
| Dexfenfluramine | 426 | 35 | −39 | 0.73 | 52 | 15 | (18) |
| Diethylpropion | 512 | 175 | 95 | 0.9 | 52 | 75 | (21) |
| Exenatide | 130 | 72 | 51 | 0.43 | 30 | 0.005 | (12) |
| 241 | 83 | 104 | 0.93 | 30 | 0.01 | (12) | |
| Fenproporex | 438 | 105 | 96 | 0.84 | 52 | 25 | (21) |
| Fluoxetine | 323 | 59 | −117 | 0.92 | 52 | 20 | (21) |
| Liraglutide | 823 | 17 | 225 | 0.72 | 52 | 3 | (11) |
| 676 | 45 | 143 | 0.86 | 52 | 2.4 | (11) | |
| 725 | 26 | 157 | 0.76 | 52 | 1.8 | (11) | |
| 598 | 18 | 36 | 0.86 | 52 | 1.2 | (11) | |
| Lorcaserin | 430 | 141 | 39 | 0.86 | 104 | 10 | (20) |
| Mazindol | 570 | 77 | 49 | 0.98 | 52 | 2 | (21) |
| Naltrexone + Bupropion | 458 | 69 | 76 | 0.82 | 56 | N/B 32/360 |
(16) |
| Orlistat | 240 | 273 | 46 | 0.99 | 208 | 120 | (22) |
| 149 | 499 | −2 | 0.97 | 104 | 60 | (15) | |
| 251 | 429 | −30 | 0.99 | 104 | 120 | (15) | |
| Phentermine + Fenfluramine | 882 | 53 | 571 | 0.87 | 34 | Phen/Fen 15/60 |
(23) |
| Phentermine + Topiramate | 343 | 115 | 109 | 0.82 | 56 | PHEN/TPM 3.75/23 |
(10) |
| 859 | 113 | 259 | 0.96 | 56 | PHEN/TPM 15/92 |
(10) | |
| 545 | 85 | 227 | 0.98 | 56 | PHEN/TPM 7.5/46 |
(13) | |
| 749 | 104 | 268 | 0.98 | 56 | PHEN/TPM 15/92 |
(13) | |
| 702 | 57 | 170 | 0.79 | 108 | PHEN/TPM 7.5/46 |
(14) | |
| 945 | 78 | 189 | 0.86 | 108 | PHEN/TPM 15/92 |
(14) | |
| Rimonabant | 140 | 128 | −18 | 0.82 | 52 | 5 | (19) |
| 466 | 125 | 38 | 0.93 | 52 | 20 | (19) | |
| Sibutramine | 202 | 105 | 41 | 0.99 | 260 | 10 | (17) |
| 502 | 87 | 38 | 0.96 | 48 | 15 | (24) | |
| 775 | 54 | 161 | 0.96 | 52 | 15 | (21) |
Discussion
Long-term obesity pharmacotherapy can lead to clinically meaningful long-term weight loss (26) achieved primarily via reductions in metabolizable energy intake (2). To our knowledge, the current study is the first report quantifying the long-term changes in metabolizable energy intake during obesity pharmacotherapy. To do this, we used repeated body weight measurements as the sole inputs to a validated mathematical model of human energy metabolism that quantifies the dynamic relationships between energy intake, energy expenditure, body weight, and body composition (7, 8, 9). Energy expenditure was modeled to dynamically change as a function of energy intake and body weight, but we assumed that the drugs under investigation had a negligible direct impact on human energy expenditure (2). Future studies could also include independent drug effects on energy expenditure and incorporate changes in physical activity to investigate how such parameters influence the calculated ΔEI.
We found that all drugs resulted in a universal pattern of metabolizable energy intake change characterized by a large early decrease followed by a slow exponential relaxation to a smaller persistent effect. During the first several months, weight loss is typically rapid but begins to plateau within the first year. Interestingly, following the early large reduction in energy intake at the onset of the intervention, the magnitude of ΔEI progressively decreased during the weight loss period. At the point of maximum weight loss, ΔEI has already waned to a small fraction of its initial effect. In other words, there is an apparent disconnect between the time course of ΔEI and its downstream maximum effect on body weight. This occurs because of the long characteristic time lag in humans between when a change in energy intake results in the eventual stabilization of a new steady state body weight (8). The long-term steady state body weight depends on the persistent effect of the drug (plate). Indeed, all of the weight lost will eventually be regained unless the persistent drug effect is greater than zero. However, large persistent effects on ΔEI are not necessary for clinically meaningful weight loss maintenance (8).
The placebo and treatment groups were both reasonably well-characterized by exponential functions of time. The fact that all placebo-subtracted treatment groups were also well-characterized by this universal pattern suggests that our method can be used as a common framework for comparing different drugs or drug doses. Why do changes in metabolizable energy intake follow this universal pattern? What are the mechanisms underlying the slow waning of both the placebo and the drug effects over time? Answers to these intriguing questions should be the subject of future investigations.
Supplementary Material
What is already known about this subject
In humans, most obesity drugs have little effect on energy expenditure and primarily work by decreasing metabolizable energy intake
Quantification of energy intake changes in free-living humans has never been reliably determined using self-reports or by extrapolation from short-term meal tests
What this study adds
We provide the first quantification of the changes in free-living metabolizable energy intake during obesity pharmacotherapy using 14 different drugs or drug combinations
We found that all drugs resulted in a universal exponential pattern of metabolizable energy intake changes and can be compared using a common mathematical framework.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Diabetes & Digestive & Kidney Diseases (AS, KDH). BG is supported by Sanofi.
Appendix
We have previously shown (1) that any mathematical model of human energy metabolism and body weight, BW, change can be linearized around an initial value, BW0, to yield:
| [A1] |
where ρ is the effective energy density associated with the BW change and ε is a parameter that defines how energy expenditure depends on BW. Repeated body weight measurements can be used to calculate both terms on the right side of equation A1 and thereby quantify the energy intake changes corresponding to the observed body weight time course (1).
To calculate the placebo subtracted effect of a drug on energy intake, we first define x(t) = BW(t)−BW0 as the change in body weight from baseline in the drug group and similarly y(t) as the weight change from baseline in the placebo group. Applying equation A1 to both groups implies:
| [A2] |
where the rates of weight change, dx/dt and dy/dt, are caculated using ordinary least-squares regression over each interval t = (N−1)*T, where N is the number of bodyweight measurements per interval and T is the time between measurements (in the locaserin example shown in Figure 1, N = 3 and T = 4 weeks), and (x−y) is calculated by subtracting the placebo weight change from the drug weight change data. The MATLAB code to perform these calculations is available for download as Supplementary Information.
Footnotes
Conflicts of Interest: No authors have conflicts of interest.
References
- 1.Hall KD, Heymsfield SB, Kemnitz JW, Klein S, Schoeller DA, Speakman JR. Energy balance and its components: implications for body weight regulation. Am J Clin Nutr. 2012;95:989–994. doi: 10.3945/ajcn.112.036350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray GA. Medications for weight reduction. Endocrinology and metabolism clinics of North America. 2008;37:923–942. doi: 10.1016/j.ecl.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Schoeller DA. How accurate is self-reported dietary energy intake? Nutr Rev. 1990;48:373–379. doi: 10.1111/j.1753-4887.1990.tb02882.x. [DOI] [PubMed] [Google Scholar]
- 4.Schoeller DA, Thomas D, Archer E, Heymsfield SB, Blair SN, Goran MI, et al. Self-report-based estimates of energy intake offer an inadequate basis for scientific conclusions. Am J Clin Nutr. 2013;97:1413–1415. doi: 10.3945/ajcn.113.062125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Winkler JT. The fundamental flaw in obesity research. Obes Rev. 2005;6:199–202. doi: 10.1111/j.1467-789X.2005.00186.x. [DOI] [PubMed] [Google Scholar]
- 6.Racette SB, Das SK, Bhapkar M, Hadley EC, Roberts SB, Ravussin E, et al. Approaches for quantifying energy intake and %calorie restriction during calorie restriction interventions in humans: the multicenter CALERIE study. Am J Physiol Endocrinol Metab. 2012;302:E441–448. doi: 10.1152/ajpendo.00290.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall KD, Chow CC. Estimating changes in free-living energy intake and its confidence interval. Am J Clin Nutr. 2011;94:66–74. doi: 10.3945/ajcn.111.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378:826–837. doi: 10.1016/S0140-6736(11)60812-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanghvi A, Redman LA, Martin CK, Ravussin E, Hall KD. Energy intake changes can be accurately calculated using repeated body weight measurements. Under Review. 2014 [Google Scholar]
- 10.Allison DB, Gadde KM, Garvey WT, Peterson CA, Schwiers ML, Najarian T, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring, Md. 2012;20:330–342. doi: 10.1038/oby.2011.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Astrup A, Carraro R, Finer N, Harper A, Kunesova M, Lean ME, et al. Safety, tolerability and sustained weight loss over 2 years with the once-daily human GLP-1 analog, liraglutide. International journal of obesity (2005) 2012;36:843–854. doi: 10.1038/ijo.2011.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 13.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 14.Garvey WT, Ryan DH, Look M, Gadde KM, Allison DB, Peterson CA, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. The American journal of clinical nutrition. 95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauptman J, Lucas C, Boldrin MN, Collins H, Segal KR. Orlistat in the long-term treatment of obesity in primary care settings. Archives of family medicine. 2000;9:160–167. doi: 10.1001/archfami.9.2.160. [DOI] [PubMed] [Google Scholar]
- 16.Hollander P, Gupta AK, Plodkowski R, Greenway F, Bays H, Burns C, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes. Diabetes care. 2013;36:4022–4029. doi: 10.2337/dc13-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James WP, Caterson ID, Coutinho W, Finer N, Van Gaal LF, Maggioni AP, et al. Effect of sibutramine on cardiovascular outcomes in overweight and obese subjects. The New England journal of medicine. 2010;363:905–917. doi: 10.1056/NEJMoa1003114. [DOI] [PubMed] [Google Scholar]
- 18.Pfohl M, Luft D, Blomberg I, Schmulling RM. Long-term changes of body weight and cardiovascular risk factors after weight reduction with group therapy and dexfenfluramine. Int J Obes Relat Metab Disord. 1994;18:391–395. [PubMed] [Google Scholar]
- 19.Scheen AJ, Finer N, Hollander P, Jensen MD, Van Gaal LF. Efficacy and tolerability of rimonabant in overweight or obese patients with type 2 diabetes: a randomised controlled study. Lancet. 2006;368:1660–1672. doi: 10.1016/S0140-6736(06)69571-8. [DOI] [PubMed] [Google Scholar]
- 20.Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, et al. Multicenter, placebo-controlled trial of lorcaserin for weight management. The New England journal of medicine. 2010;363:245–256. doi: 10.1056/NEJMoa0909809. [DOI] [PubMed] [Google Scholar]
- 21.Suplicy H, Boguszewski CL, Dos Santos CM, do Desterro de Figueiredo M, Cunha DR, Radominski R. A comparative study of five centrally acting drugs on the pharmacological treatment of obesity. International journal of obesity (2005) 2013 doi: 10.1038/ijo.2013.225. [DOI] [PubMed] [Google Scholar]
- 22.Torgerson JS, Hauptman J, Boldrin MN, Sjostrom L. XENical in the prevention of diabetes in obese subjects (XENDOS) study: a randomized study of orlistat as an adjunct to lifestyle changes for the prevention of type 2 diabetes in obese patients. Diabetes care. 2004;27:155–161. doi: 10.2337/diacare.27.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Weintraub M, Sundaresan PR, Madan M, Schuster B, Balder A, Lasagna L, et al. Long-term weight control study. I (weeks 0 to 34). The enhancement of behavior modification, caloric restriction, and exercise by fenfluramine plus phentermine versus placebo. Clinical pharmacology and therapeutics. 1992;51:586–594. doi: 10.1038/clpt.1992.69. [DOI] [PubMed] [Google Scholar]
- 24.Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. Jama. 2001;286:1331–1339. doi: 10.1001/jama.286.11.1331. [DOI] [PubMed] [Google Scholar]
- 25.Martin CK, Das SK, Lindblad L, Racette SB, McCrory MA, Weiss EP, et al. Effect of calorie restriction on the free-living physical activity levels of nonobese humans: results of three randomized trials. J Appl Physiol. 2011;110:956–963. doi: 10.1152/japplphysiol.00846.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanovski SZ, Yanovski JA. Long-term drug treatment for obesity: a systematic and clinical review. JAMA. 2014;311:74–86. doi: 10.1001/jama.2013.281361. [DOI] [PMC free article] [PubMed] [Google Scholar]
References
- 1.Hall KD, Chow CC. Estimating changes in free-living energy intake and its confidence interval. Am J Clin Nutr. 2011 Jul;94(1):66–74. doi: 10.3945/ajcn.111.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.