Abstract
The touch dome is an innervated structure in the epidermis of mammalian skin. Composed of specialized keratinocytes and neuroendocrine Merkel cells, the touch dome has distinct molecular characteristics compared to the surrounding epidermal keratinocytes. Much of the research on Merkel cell function has focused on their role in mechanosensation, specifically light-touch. Recently, more has been discovered about Merkel cell molecular characteristics and their cells of origin. Here we review Merkel cell and touch dome biology, and discuss potential functions beyond mechanosensation.
Keywords: Merkel cell, touch dome, mechanosensation, immune, endocrine
Merkel cells and touch domes in mammalian skin
In the time since their discovery in 1875 by Friedrich Merkel (1), Merkel cells (MCs) have garnered attention for their distinct morphology and putative sensory function. MCs are described in many species including reptiles, fish, and mammals, where they are found in the basal layer of hairy skin, glabrous skin, and some mucosal epithelia (2). Ultrastructurally, MCs are characterized by dense-core granules, indented nuclei, and desmosomal connections to neighboring keratinocytes (KCs). On their basal surface, MCs associate with sensory nerve endings. In some parts of the skin, MCs are surrounded by morphologically distinct columnar KCs at the base of a stratified squamous epithelium that is thicker than the surrounding epidermis. This specialized epidermal structure was named a touch dome (TD, or haarscheibe) based on its speculated function (3). Keratin 8 (K8) and Keratin 18 (K18) are established markers for MCs in late fetal and adult skin. Keratin 20 (K20) is also a highly specific marker for MCs in the skin. The hair follicle keratin, Keratin 17 (K17), is a marker for TD KCs (4, 5). Table 1 summarizes reported markers for MC and TD KCs.
Table 1.
Molecular characters of touch dome keratinocyte and Merkel cell.
| Marker | Note | Ref |
|---|---|---|
| K18 | A type I cytokeratin expressed by MCs. Usually expressed in single layer epithelial tissues with its partner K8. | (9) |
| K20 | A type I cytokeratin expressed by MCs. K20+ MCs cells are negative for K17. | (25) |
| K8 | At E15, K8 is restricted to scattered immature MCs in developing tylotrich follicles and the adjacent epidermis. By E16-E17, the K8+ MCs form a disc-shaped TD. | (21) |
| Math1/Atoh1 | A basic helix-loop-helix transcription factor expressed by MCs and necessary for MC specification. | (17) |
| FM 1-43 | Small fluorescent styryl dye taken up by MCs after systemic administration. | (17) |
| VGLUT2 | Presynaptic protein. VGLUT2 immunoreactivity is most intense on the side of the MCs that abut sensory nerve terminals. The ionotropic receptor GluR2 that monitors glutamate release is also enriched in MCs. | (57) |
| Cav2.1 | MCs have functional L-type and P/Q–type voltage-gated calcium channels. | (57) |
| Rab3c, SNAP25, piccolo, CCK8, synaptotagmin13, cholecystokinin 26-33, Synaptic vesicle protein | Among 362 MC-enriched transcripts, this list is confirmed by immunostaining. These features demonstrate that MCs are excitable cells. | (57) |
| Vasoactive intestinal peptide (VIP) | A neuropeptide hormone expressed by MCs. VIP can enhance keratinocyte proliferation and reduce inflammation. | (32, 38, 52) |
| Serotonin | A monoamine hormone expressed by MCs. Serotonin plays important roles in many biological process including neurotransmission, stress responses, and the regulation of inflammation. | (35, 36) |
| Sox2 | All MCs express Sox2, a transcription factor expressed in adult stem cells that plays a crucial role in tissue regeneration in various organs. | (22) |
| CD200 | A membrane glycoprotein expressed in TD KCs and MCs. CD200 attenuates inflammatory reactions and promotes immune tolerance. | (12, 58) |
| Tbc1d10c | Marks TD KCs. | (12) |
| K17 | A type I cytokeratin. TD KCs prominently express K17 and show reduced K15 expression. K17 can regulate cutaneous inflammation. | (5, 55) |
Merkel cell and touch dome keratinocyte progenitors originate from epidermal cells
Where MCs originate is important to MC biology. The identification of MCs in subepidermal embryonic mesenchyme initially led to the hypothesis that they arise from migrating neural crest progenitors (6). This hypothesis was widely accepted after a transgenic model utilizing Wnt1-Cre to fate map neural crest derived cells described labeled MCs in mouse vibrissae (7). However, conditional knockout of Atoh1, a transcription factor essential in MC development, using Wnt1-Cre resulted in no effect on MCs. In contrast, a K14-Cre driven deletion of Atoh1 in epidermal keratinocyte derivatives led to a loss of MCs, suggesting MCs are derived from a keratinocyte lineage rather than a neural crest origin (8). Early studies characterizing cytokeratin expression in human embryonic skin also suggested that MCs arise from epidermal epithelial precursors (4, 9), and xenografts of human embryonic skin onto nude mice further supported the idea that MCs rise from epidermal precursor cells (10). Recently, lineage-tracing experiments showed that MCs originate from K14-expressing epidermal progenitors (11). Consistent with an epidermal origin for MC, CD200+ basal cells sorted from touch domes in dissociated mouse skin were able to reconstitute touch domes when grafted onto a host mouse (12). Additional studies showed that K17+ epidermal progenitors maintain both MCs and KCs during TD homeostasis (13). The identification of MC and TD progenitors has greatly advanced our understanding of TD biology. However, the question still remains as to whether a single, multipotent progenitor gives rise to both MCs and TD KCs, or if there are distinct lineage-specific K17+ progenitors maintaining the two TD cell types.
Merkel cells are required for light-touch sensation
Many types of cutaneous mechanoreceptors endow human skin with a tactile discrimination approaching 10 nm (14). Since their discovery, a sensory function for MCs has been inferred based on their synapse-like contact with nerve endings. Merkel cells in vitro and in vivo are activated by mechanical stimuli (15), including hypotonic-induced cell swelling, supporting the hypothesis that MCs function as mechanoreceptors (16). Genetic ablation of MCs in the context of maintained TD morphology and innervation, determined that MCs are essential for light-touch responses in isolated skin preparations (17). Additional studies revealed that MCs express presynaptic molecules essential for synaptic vesicle release, as well as neuroactive substances. A recent review discusses the numerous potential neurotransmitters found in MCs (18). The details of sensory transduction by the MC-neurite complex are still unclear, and the involvement of individual neurotransmitters in MC touch-evoked responses remains to be elucidated. Now that lineage specific Cre lines are available, conditional knockout studies to determine the critical factors should be forthcoming.
A role for Merkel cells in peripheral nerve development
Studies in human embryonic plantar skin showed most MCs initially lack innervation, and the MC-neurite complex appears later in development, suggesting that MCs attract the nerves that innervate them (19). Similar studies in developing mice confirmed that MCs are specified days prior to being innervated, further suggesting MCs may have a target role for certain type I sensory nerve fibers during embryogenesis (20). Other studies further established that both the location and early differentiation of MCs in the epidermis are independent of nerves, but are instead linked to the development of tylotrich (guard) hair follicles (21). The fact that MCs express neuroactive substances that can modulate neuronal cell fate determination also supports the notion that MCs play a regulatory role for peripheral nerves (18). However, even though MCs are the ultimate target for innervation, there is some evidence that the MCs themselves may not be necessary to attract incoming nerve branches to the TD. When MCs are ablated via Atoh1 deletion, TD morphology is maintained and innervation appears unaffected (17). Moreover, in K14-Cre;Sox2floxed/floxed mice where MC number is decreased because of ablation of Sox2 in MC progenitors, TD innervation is not affected (22). Thus, factors in the TD, independent of differentiated MCs, may provide the necessary trophic signals to attract sensory nerves.
Heterogeneity of the Merkel cell population
Early morphologic observations and more recent molecular studies both suggest that subpopulations exist among MCs. Studies in early human embryonic skin found mainly round to oval MCs, with infrequent MCs having short dendrites (4). It has also been observed that dendritic MCs (DMCs) are usually not innervated but most oval MCs (OMC) are innervated (23). Later studies showed that the number of OMCs per TD remains steady over time, whereas the number of DMCs fluctuates concomitantly with the hair cycle (24, 25). Although it is intriguing to speculate that non-innervated DMCs may be a dynamic population of functionally distinct non-sensory MCs, it may be that DMCs are newly differentiated MCs that have yet to be innervated and take on an oval morphology. Interestingly, immunostaining studies observe that MCs do not all share the same molecular signatures. Differential expression of villin, N-CAM, NGF-R, neurofilaments, chromogranin A, and pancreastatin are found among MCs, demonstrating molecularly distinct populations of MCs coexist in the skin (26-28). Presently, the functional implications of the different morphological and molecular subtypes of MCs remain unknown.
Merkel cells in the skin neuroendocrine system
Merkel cells form complexes with sensory nerve afferents, and they possess typical neuroendocrine features including dense core granules and the expression of chromogranin A (29), chromogranin B (30), and synaptophysin (31). Importantly, they synthesize an array of neuroactive polypeptides/hormones such as vasoactive intestinal peptide (VIP), calcitonin gene-related peptide (CGRP) (32), neuroendocrine protein B2 (33), prepro-orexin and orexin receptors (34), serotonin (35, 36), and somatostatin (37). The presence of these signal ligands strongly suggests some form of paracrine or autocrine signaling within the skin. MCs directly contact TD KCs, and VIP is known to enhance keratinocyte proliferation (38), thus MCs may be endocrine regulators of the surrounding epidermis. MCs may also affect dermal fibroblasts by producing substance P, a peptide that can activate skin fibroblast proliferation (30, 39). However, the neuropeptides produced by MCs have diverse functions in other systems, and it remains to be seen how they function and what their targets are in the skin. It is likely they influence the local fibroblast, keratinocytes, immune cells and vasculature, in addition to their partner neurons. This would be similar to the diverse functions of neuroendocrine cells in other organs. Intriguingly, neuropeptide release from MCs can be calcium independent (40), suggesting it may be a separate process from the Ca2+-dependent neurotransmitter release thought to be involved in mechanosensation.
Merkel cells in the cutaneous immune system
Potential immunoregulatory functions of MCs and TDs have not been rigorously studied. The finding of robust CD200 expression in MC and TD KCs (12) raises a question about an unrevealed role in immunity. CD200 is a transmembrane protein that signals through the CD200 receptor (CD200R) to attenuate inflammatory reactions and promote immune tolerance. CD200 is normally expressed on thymocytes, T and B lymphocytes, neurons, and endothelial cells (41, 42), and CD200R is expressed on cells of the monocyte/macrophage lineage and T lymphocytes (43). CD200 is also highly expressed on KCs of the murine hair follicle outer root sheath (ORS) (44), and skin lacking CD200 is highly susceptible to hair follicle-associated inflammation and immune-mediated alopecia (45). Significant perifollicular inflammation is also observed in syngeneic grafts of CD200-deficient mouse skin (46). Thus, CD200 expression in the TD is likely to represent a second immune privileged compartment in the skin.
A further roll for MCs in regulating the immune system is implicated by their relationship with Langerhans cells (LC), dendritic antigen presenting cells that reside in the epidermis (47). MCs are known to interact with LCs in the skin (48). Hair follicles normally function as portals for LCs to enter the epidermis (49), however the portals for LCs into glabrous skin are unknown. It is possible that the dense clusters of MCs in acral skin allow LC to enter the hairless epidermis. At the same time, MCs produce CGRP, a neuropeptide that can inhibit antigen presentation by LCs (50).
MCs may also be involved in modulating inflammatory responses in the skin. Normal MCs produce met-enkephalin, which can enhance immune responses at low doses and suppress responses at high doses (20, 51). In addition, MCs can react to histamine or activation of the osmoreceptor TRPV4 by releasing VIP (40), which can decrease the production of pro-inflammatory cytokines (52). In psoriasis, MC numbers are higher in lesional skin than in normal skin (53). Moreover, MC expression levels of neuropeptides such as somatostatin, which is thought to be involved in skin immunology, are also different between psoriatic lesions and controls (54). The MC hyperplasia observed in psoriasis implies that MCs respond to, and possibly regulate, pathological skin inflammation. Occasionally, leukocytes are observed closely abutting DMCs, suggesting another potential functional linkage with the immune system (23). TD KCs also potentially contribute to regulating epidermal immunity by expression of K17, which can function to polarize the cutaneous inflammatory response (55). Taken together, these features of MCs and TD KCs predicate their potential involvement in cutaneous immunity.
Other hypothetical functions
Although little has been done to substantiate non-sensory functions of MCs and TDs in the skin, it seems probable that some additional functions will be found. In addition to the proposed functions discussed above, some highly speculative roles for MCs have been put forward (56). These include: (1) Involvement in magnetoreception, as MCs occasionally contain transferred melanosome which magnetite. (2) Involvement in fingerprint formation, as MCs cluster at the base of epidermal ridges in glabrous skin. (3) Proposing that efferent neural signals drive MCs to generate electromagnetic fields for telekinesis. (4) Suggesting that the their broad distribution and possible multimodality sensing allows MCs to transmit environmental information to oocytes to alter epigenetic imprinting. (5) Determination of hair form, as MCs are found in hair follicles and proliferate synchronously with the hair cycle. It should be noted that some of the observations upon which these fanciful hypotheses are based have not been widely accepted.
Conclusions and perspectives
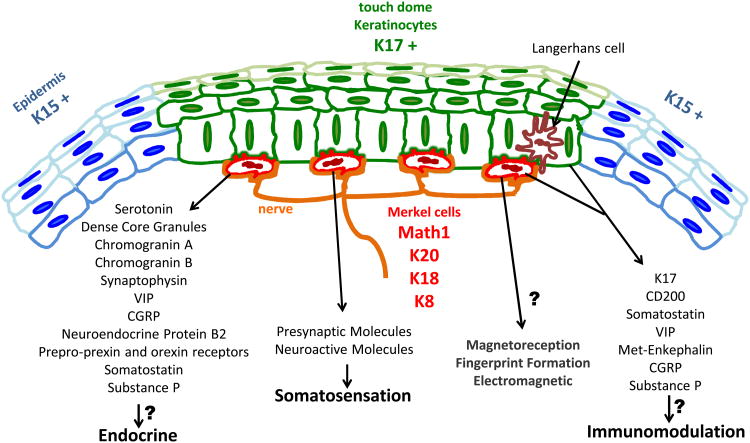
Early studies of MC and TD ultrastructure predicted a role in somatosensation for the innervated neuroendocrine cells and their specialized epidermal niche. Since then, MC involvement in light touch sensation has been demonstrated in a number of experimental systems. Modern molecular characterizations have furthered our understanding of MC and TD biology (Figure 1). Recently, transgenic mice have allowed for the identification of epithelial MC progenitors, and now offer genetic tools that will allow for mechanistic studies of MC function.
Figure 1.
Schematic of the mouse touch dome. Markers reported to be expressed in Merkel cells and touch dome keratinocytes that suggest function.
A number of MC characteristics suggest functions beyond mechanosensation. Apparent molecular and structural heterogeneity among MCs is consistent with functionally distinct subpopulations of MCs. As MCs produce a number of secreted signaling factors, it is likely that they act as endocrine regulators in the skin. The precise nature and targets of such endocrine regulation remain to be elucidated. It is also likely that MCs interact with the cutaneous immune system. MCs and TD KCs express a number of known immunoregulators, and it will be interesting to learn how MCs contribute to immune responses.
Investigating MCs is an important aspect of cutaneous biology. There is also tremendous clinical and translational interest in Merkel cell carcinoma (MCC), a highly aggressive skin cancer that might originate from mutant MCs or MC progenitors. Although knowledge of MC and TD biology could contribute to understanding MCC, the inability to culture normal MCs currently limits their in vitro study. The identification of MC precursors in vivo will hopefully accelerate our understanding of TD homeostasis, and will provide insight into the conditions needed to culture MCs. Thus, research into MC biology contributes to our understanding of mechanosensation and the neuro-cutaneous interface, and could also provide insights into the endocrine regulation of the skin, the cutaneous immune system, and the formation of skin cancer.
Acknowledgments
We thank Dr. Amy Coxon for critical reading of the manuscript. This manuscript was supported by the National Institutes of Health Intramural Research Program, Center of Cancer Research, National Cancer Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. YX performed the literature review and wrote the paper, JW made the figure and revised the paper, IB revised the paper.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Merkel FS. Tastzellen und Taskörperchen bei den Hausthieren und beim Menschen. Arch Mikr Anat. 1875;11 [Google Scholar]
- 2.Halata Z, Grim M, Bauman KI. Friedrich Sigmund Merkel and his “Merkel cell”, morphology, development, and physiology: review and new results. The anatomical record Part A, Discoveries in molecular, cellular, and evolutionary biology. 2003 Mar;271:225. doi: 10.1002/ar.a.10029. [DOI] [PubMed] [Google Scholar]
- 3.Pinkus F. Zur Kenntnis des Anfangsstadiums des Liehen ruber planus. Arch f Dermat Syph. 1902;60:163. [Google Scholar]
- 4.Moll I, Moll R. Early development of human Merkel cells. Experimental dermatology. 1992 Nov;1:180. doi: 10.1111/j.1600-0625.1992.tb00186.x. [DOI] [PubMed] [Google Scholar]
- 5.Moll I, Troyanovsky SM, Moll R. Special program of differentiation expressed in keratinocytes of human haarscheiben: an analysis of individual cytokeratin polypeptides. The Journal of investigative dermatology. 1993 Jan;100:69. doi: 10.1111/1523-1747.ep12354535. [DOI] [PubMed] [Google Scholar]
- 6.Hashimoto K. The ultrastructure of the skin of human embryos. X. Merkel tactile cells in the finger and nail. Journal of anatomy. 1972 Jan;111:99. [PMC free article] [PubMed] [Google Scholar]
- 7.Szeder V, Grim M, Halata Z, Sieber-Blum M. Neural crest origin of mammalian Merkel cells. Developmental biology. 2003 Jan 15;253:258. doi: 10.1016/s0012-1606(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 8.Morrison KM, Miesegaes GR, Lumpkin EA, Maricich SM. Mammalian Merkel cells are descended from the epidermal lineage. Developmental biology. 2009 Dec 1;336:76. doi: 10.1016/j.ydbio.2009.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moll I, Moll R, Franke WW. Formation of epidermal and dermal Merkel cells during human fetal skin development. The Journal of investigative dermatology. 1986 Dec;87:779. doi: 10.1111/1523-1747.ep12458993. [DOI] [PubMed] [Google Scholar]
- 10.Moll I, Lane AT, Franke WW, Moll R. Intraepidermal formation of Merkel cells in xenografts of human fetal skin. The Journal of investigative dermatology. 1990 Mar;94:359. doi: 10.1111/1523-1747.ep12874488. [DOI] [PubMed] [Google Scholar]
- 11.Van Keymeulen A, et al. Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. The Journal of cell biology. 2009 Oct 5;187:91. doi: 10.1083/jcb.200907080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woo SH, Stumpfova M, Jensen UB, Lumpkin EA, Owens DM. Identification of epidermal progenitors for the Merkel cell lineage. Development. 2010 Dec;137:3965. doi: 10.1242/dev.055970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Doucet YS, Woo SH, Ruiz ME, Owens DM. The touch dome defines an epidermal niche specialized for mechanosensory signaling. Cell reports. 2013 Jun 27;3:1759. doi: 10.1016/j.celrep.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skedung L, et al. Feeling small: exploring the tactile perception limits. Scientific reports. 2013;3:2617. doi: 10.1038/srep02617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gottschaldt KM, Vahle-Hinz C. Merkel cell receptors: structure and transducer function. Science. 1981 Oct 9;214:183. doi: 10.1126/science.7280690. [DOI] [PubMed] [Google Scholar]
- 16.Haeberle H, Bryan LA, Vadakkan TJ, Dickinson ME, Lumpkin EA. Swelling-activated Ca2+ channels trigger Ca2+ signals in Merkel cells. PloS one. 2008;3:e1750. doi: 10.1371/journal.pone.0001750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maricich SM, et al. Merkel cells are essential for light-touch responses. Science. 2009 Jun 19;324:1580. doi: 10.1126/science.1172890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maksimovic S, Baba Y, Lumpkin EA. Neurotransmitters and synaptic components in the Merkel cell-neurite complex, a gentle-touch receptor. Annals of the New York Academy of Sciences. 2013 Mar;1279:13. doi: 10.1111/nyas.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Narisawa Y, Hashimoto K. Immunohistochemical demonstration of nerve-Merkel cell complex in fetal human skin. Journal of dermatological science. 1991 Sep;2:361. doi: 10.1016/0923-1811(91)90030-2. [DOI] [PubMed] [Google Scholar]
- 20.Cheng Chew SB, Leung PY. Ultrastructural study of the Merkel cell and its expression of met-enkephalin immunoreactivity during fetal and postnatal development in mice. Journal of anatomy. 1994 Dec;185(Pt 3):511. [PMC free article] [PubMed] [Google Scholar]
- 21.Vielkind U, Sebzda MK, Gibson IR, Hardy MH. Dynamics of Merkel cell patterns in developing hair follicles in the dorsal skin of mice, demonstrated by a monoclonal antibody to mouse keratin 8. Acta anatomica. 1995;152:93. doi: 10.1159/000147688. [DOI] [PubMed] [Google Scholar]
- 22.Lesko MH, Driskell RR, Kretzschmar K, Goldie SJ, Watt FM. Sox2 modulates the function of two distinct cell lineages in mouse skin. Developmental biology. 2013 Oct 1;382:15. doi: 10.1016/j.ydbio.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tachibana T, et al. Polymorphism of Merkel cells in the rodent palatine mucosa: immunohistochemical and ultrastructural studies. Archives of histology and cytology. 1997 Oct;60:379. doi: 10.1679/aohc.60.379. [DOI] [PubMed] [Google Scholar]
- 24.Nakafusa J, et al. Changes in the number of Merkel cells with the hair cycle in hair discs on rat back skin. The British journal of dermatology. 2006 Nov;155:883. doi: 10.1111/j.1365-2133.2006.07441.x. [DOI] [PubMed] [Google Scholar]
- 25.Moll I, Paus R, Moll R. Merkel cells in mouse skin: intermediate filament pattern, localization, and hair cycle-dependent density. The Journal of investigative dermatology. 1996 Feb;106:281. doi: 10.1111/1523-1747.ep12340714. [DOI] [PubMed] [Google Scholar]
- 26.Eispert AC, et al. Evidence for distinct populations of human Merkel cells. Histochemistry and cell biology. 2009 Jul;132:83. doi: 10.1007/s00418-009-0578-0. [DOI] [PubMed] [Google Scholar]
- 27.W E, Horsch D, Muller S, Hancke E. Distribution and coexistence of chromogranin A-, serotonin- and pancreastatin-like immunoreactivity in endocrine-like cells of the human anal canal. Cell Tissue Res. 1992;268:109. doi: 10.1007/BF00338059. [DOI] [PubMed] [Google Scholar]
- 28.Fantini F, Johansson O. Neurochemical markers in human cutaneous Merkel cells. An immunohistochemical investigation. Experimental dermatology. 1995 Dec;4:365. doi: 10.1111/j.1600-0625.1995.tb00061.x. [DOI] [PubMed] [Google Scholar]
- 29.Hartschuh W, Weihe E, Egner U. Chromogranin A in the mammalian Merkel cell: cellular and subcellular distribution. The Journal of investigative dermatology. 1989 Nov;93:641. doi: 10.1111/1523-1747.ep12319788. [DOI] [PubMed] [Google Scholar]
- 30.Hartschuh W, Weihe E, Yanaihara N. Immunohistochemical analysis of chromogranin A and multiple peptides in the mammalian Merkel cell: further evidence for its paraneuronal function? Archives of histology and cytology. 1989;52 Suppl:423. doi: 10.1679/aohc.52.suppl_423. [DOI] [PubMed] [Google Scholar]
- 31.Ortonne JP, et al. Normal Merkel cells express a synaptophysin-like immunoreactivity. Dermatologica. 1988;177:1. doi: 10.1159/000248491. [DOI] [PubMed] [Google Scholar]
- 32.Cheng-Chew SB, Leung PY. Localisation of VIP-and CGRP-like substances in the skin and sinus hair follicles of various mammalian species. Histochemistry and cell biology. 1996 Jun;105:443. doi: 10.1007/BF01457657. [DOI] [PubMed] [Google Scholar]
- 33.Garcia-Caballero A, et al. Cellular and subcellular distribution of 7B2 in porcine Merkel cells. The Anatomical record. 1997 Jun;248:159. doi: 10.1002/(SICI)1097-0185(199706)248:2<159::AID-AR2>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 34.Beiras-Fernandez A, et al. Merkel cells, a new localization of prepro-orexin and orexin receptors. Journal of anatomy. 2004 Feb;204:117. doi: 10.1111/j.1469-7580.2004.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tachibana T, Endoh M, Fujiwara N, Nawa T. Receptors and transporter for serotonin in Merkel cell-nerve endings in the rat sinus hair follicle. An immunohistochemical study. Archives of histology and cytology. 2005;68:19. doi: 10.1679/aohc.68.19. [DOI] [PubMed] [Google Scholar]
- 36.Nordlind K, Azmitia EC, Slominski A. The skin as a mirror of the soul: exploring the possible roles of serotonin. Experimental dermatology. 2008 Apr;17:301. doi: 10.1111/j.1600-0625.2007.00670.x. [DOI] [PubMed] [Google Scholar]
- 37.Vockel M, Breitenbach U, Kreienkamp HJ, Brandner JM. Somatostatin regulates tight junction function and composition in human keratinocytes. Experimental dermatology. 2010 Oct;19:888. doi: 10.1111/j.1600-0625.2010.01101.x. [DOI] [PubMed] [Google Scholar]
- 38.Granoth R, Fridkin M, Gozes I. VIP and the potent analog, stearyl-Nle(17)-VIP, induce proliferation of keratinocytes. FEBS letters. 2000 Jun 16;475:78. doi: 10.1016/s0014-5793(00)01628-8. [DOI] [PubMed] [Google Scholar]
- 39.Kahler CM, Herold M, Wiedermann CJ. Substance P: a competence factor for human fibroblast proliferation that induces the release of growth-regulatory arachidonic acid metabolites. Journal of cellular physiology. 1993 Sep;156:579. doi: 10.1002/jcp.1041560318. [DOI] [PubMed] [Google Scholar]
- 40.Boulais N, et al. Merkel cells as putative regulatory cells in skin disorders: an in vitro study. PloS one. 2009;4:e6528. doi: 10.1371/journal.pone.0006528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barclay AN, Clark MJ, McCaughan GW. Neuronal/lymphoid membrane glycoprotein MRC OX-2 is a member of the immunoglobulin superfamily with a light-chain-like structure. Biochemical Society symposium. 1986;51:149. [PubMed] [Google Scholar]
- 42.Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology. 2001 Feb;102:173. doi: 10.1046/j.1365-2567.2001.01163.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barclay AN, Wright GJ, Brooke G, Brown MH. CD200 and membrane protein interactions in the control of myeloid cells. Trends in immunology. 2002 Jun;23:285. doi: 10.1016/s1471-4906(02)02223-8. [DOI] [PubMed] [Google Scholar]
- 44.Bertolini M, et al. The immune system of mouse vibrissae follicles: cellular composition and indications of immune privilege. Experimental dermatology. 2013 Sep;22:593. doi: 10.1111/exd.12205. [DOI] [PubMed] [Google Scholar]
- 45.Rosenblum MD, Yancey KB, Olasz EB, Truitt RL. CD200, a “no danger” signal for hair follicles. Journal of dermatological science. 2006 Mar;41:165. doi: 10.1016/j.jdermsci.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 46.Rosenblum MD, et al. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? The Journal of investigative dermatology. 2004 Nov;123:880. doi: 10.1111/j.0022-202X.2004.23461.x. [DOI] [PubMed] [Google Scholar]
- 47.Merad M, Ginhoux F, Collin M. Origin, homeostasis and function of Langerhans cells and other langerin-expressing dendritic cells. Nature reviews Immunology. 2008 Dec;8:935. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- 48.Taira K, Narisawa Y, Nakafusa J, Misago N, Tanaka T. Spatial relationship between Merkel cells and Langerhans cells in human hair follicles. Journal of dermatological science. 2002 Dec;30:195. doi: 10.1016/s0923-1811(02)00104-4. [DOI] [PubMed] [Google Scholar]
- 49.Nagao K, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nature immunology. 2012 Aug;13:744. doi: 10.1038/ni.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosoi J, et al. Regulation of Langerhans cell function by nerves containing calcitonin gene-related peptide. Nature. 1993 May 13;363:159. doi: 10.1038/363159a0. [DOI] [PubMed] [Google Scholar]
- 51.Plotnikoff NP, Faith RE, Murgo AJ, Herberman RB, Good RA. Methionine enkephalin: a new cytokine--human studies. Clinical immunology and immunopathology. 1997 Feb;82:93. doi: 10.1006/clin.1996.4287. [DOI] [PubMed] [Google Scholar]
- 52.Fernandez-Martin A, et al. VIP prevents experimental multiple sclerosis by downregulating both inflammatory and autoimmune components of the disease. Annals of the New York Academy of Sciences. 2006 Jul;1070:276. doi: 10.1196/annals.1317.026. [DOI] [PubMed] [Google Scholar]
- 53.Wollina U, Karsten U. Immunohistochemical demonstration of cytokeratin 19-positive basal cells in psoriatic plaques. Archives of dermatological research. 1988;280:257. doi: 10.1007/BF00513966. [DOI] [PubMed] [Google Scholar]
- 54.Wollina U, Mahrle G. Epidermal Merkel cells in psoriatic lesions: immunohistochemical investigations on neuroendocrine antigen expression. Journal of dermatological science. 1992 May;3:145. doi: 10.1016/0923-1811(92)90028-a. [DOI] [PubMed] [Google Scholar]
- 55.Depianto D, Kerns ML, Dlugosz AA, Coulombe PA. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nature genetics. 2010 Oct;42:910. doi: 10.1038/ng.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irmak MK. Multifunctional Merkel cells: their roles in electromagnetic reception, finger-print formation, Reiki, epigenetic inheritance and hair form. Medical hypotheses. 2010 Aug;75:162. doi: 10.1016/j.mehy.2010.02.011. [DOI] [PubMed] [Google Scholar]
- 57.Haeberle H, et al. Molecular profiling reveals synaptic release machinery in Merkel cells. Proceedings of the National Academy of Sciences of the United States of America. 2004 Oct 5;101:14503. doi: 10.1073/pnas.0406308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kretz-Rommel A, Bowdish KS. Rationale for anti-CD200 immunotherapy in B-CLL and other hematologic malignancies: new concepts in blocking immune suppression. Expert opinion on biological therapy. 2008 Jan;8:5. doi: 10.1517/14712598.8.1.5. [DOI] [PubMed] [Google Scholar]