Abstract
Aim
To test the hypothesis that there are single-nucleotide polymorphisms (SNPs) in genes of the l-arginine/nitric oxide pathway associated with pulmonary hypertension (PH) in neonates with bronchopulmonary dysplasia (BPD).
Methods
Neonates with BPD were enrolled (n = 140) and clinical characteristics compared between case (BPD + PH) and control (BPD) groups. DNA was isolated from blood leucocytes and assayed for 17 SNPs in l-arginine/nitric oxide pathway genes by Sequenom massarray. Genes included carbamoyl-phosphate synthetase, ornithine transcarbamylase, argininosuccinate synthase, nitric oxide synthase and arginase. SNPs were selected from the National Center for Biotechnology Information database for their putative functionality. Calculated minor allele frequencies (MAF) of cases and controls were compared using χ2 and logistic regression.
Results
Of the 140 patients with BPD, 26% had echocardiographic evidence of PH. Ventilation days were longer for cases than controls (mean 31 vs. 15 days, p < 0.05). Of the 17 SNPs, rs2781666 in arginase I gene was less common in cases (MAF = 0.23) than controls (MAF = 0.37, p = 0.04). The odds of PH decreased by 43% (p = 0.047) for each copy of the SNP minor allele in arginase I gene in patients with BPD.
Conclusion
Arginase I SNP (rs2781666) may be associated with protection against pulmonary hypertension in preterm neonates with BPD.
Keywords: arginine, argininosuccinate synthase, carbamoyl-phosphate synthetase, nitric oxide synthase, ornithine transcarbamylase
INTRODUCTION
Bronchopulmonary dysplasia (BPD) is the most common chronic lung disease of preterm infants, and pulmonary hypertension (PH) is a cardiovascular complication of BPD that is associated with a marked increase in morbidity and mortality. It has been estimated that 25 to 40% of infants with BPD will develop PH (1–4), but there are currently no tests for predicting which BPD patients might develop this life-threatening complication. Identifying BPD- associated PH early may be important in capturing the reversible phase of PH and thereby improving outcomes (1).
Endogenous nitric oxide synthesis is required for regulation of neonatal pulmonary vascular resistance and vasore-activity (5–7). Disruption of the l-arginine/nitric oxide pathway may alter vascular reactivity and contribute to the development of PH in patients with BPD (1, 8). Nitric oxide synthase and arginase compete for their common substrate l-arginine and have opposing effects in PH. Nitric oxide synthase produces the potent vasodilator nitric oxide, while arginase is the first step in polyamine and proline production leading to cellular proliferation and vascular remodelling. In animal models of BPD, lung levels of endothelial nitric oxide synthase are low and increasing nitric oxide production normalises patterns of lung growth and vascularisation in these models (1, 8, 9). In term infants with another form of PH, persistent pulmonary hypertension of the newborn, it has been found that the circulating levels of l-arginine are lower due to decreased production of l-arginine by the urea cycle enzymes, and this was associated with a single-nucleotide polymorphism (SNP) in the urea cycle gene carbamoyl-phosphate synthetase (10). Therefore, our objective was to test the hypothesis that in preterm infants with BPD there are SNPs in the l-arginine/ nitric oxide pathway associated with the development of PH.
DESIGN/METHODS
The Institutional Review Board at Nationwide Children's Hospital approved this study. Neonates were enrolled from the neonatal intensive care unit at Nationwide Children's Hospital after 1 September 2009. Patients were enrolled if they met National Institute of Child Health and Human Development criteria for the definition of BPD, which was defined as treatment with supplemental oxygen for at least 28 days (11). The cohort included 140 patients with BPD, of which there were 36 patients with echocardiographic evidence of elevated pulmonary arterial pressure who were classified as having PH and therefore considered cases. Elevated pulmonary arterial pressure was considered to be present on echocardiography if there was right ventricular hypertrophy, flattening of the intraventricular septum, presence of tricuspid regurgitation in the absence of pulmonary stenosis and/or elevated right ventricular pressure, as estimated by tricuspid regurgitant jet velocity (2, 12). The controls were patients with BPD who did not have PH according to these criteria. Patients with congenital heart disease, except patent ductus arteriosus or atrial septal defect, were excluded from the study. Patients with anatomical causes of PH, including diaphragmatic hernia or other causes of lung hypoplasia, were also excluded from the study.
Genotyping
After consent was obtained, blood samples were collected, maintained on ice for no more than six hours, and centrifuged. The blood cells were stored for isolation of DNA from leucocytes. SNPs were selected from the National Center for Biotechnology Information SNP database for their putative functionality (5, 13–22). DNA was isolated from blood leucocytes and assayed for 34 SNPs in genes of the l-arginine/nitric oxide pathway by Sequenom MassArray (Sequenom, San Diego, CA, USA). Subsequently, 17 SNPs had calculated minor allele frequencies (MAF) of zero and these SNPs were excluded from the study. A total of 17 SNPs were studied in six genes, including carbamoyl-phosphate synthetase (CPS1), ornithine transcarbamylase (OTC), argininosuccinate synthase (ASS), nitric oxide synthase (NOS2), arginase (ARG1, ARG2).
Statistical analysis
Demographic and clinical characteristics were compared between BPD and PH (cases) versus BPD alone (control) groups. Continuous variables were compared by Student's t-test. Categorical variables were compared using the χ2 test. Calculated MAF of cases and controls were compared using the χ2 test. An additive logistic regression model was used to estimate the adjusted odds ratio of the risk of individual genotypes in developing PH in BPD. Analysis of the distributions of genotypes was performed by χ2 analysis with 1 degree of freedom (10). A p-value <0.05 was considered to be significant. This study was designed to test a specific hypothesis and only candidate genes in the l-arginine/nitric oxide pathway were included in the study. Given the limitation of study sample size, less conservative values are also of interest, and may be hypothesis generating. Therefore, no correction was made for multiple testing. STATA/IC 12.0 (STATA Corp., College Station, TX, USA) statistical software was used to complete all of the analyses in this study.
RESULTS
Among all 140 patients with BPD, 36 (26%) patients also had echocardiographic evidence of PH. For patients with both BPD and PH, the average day of life for the first echocardiogram was 11 ± 14 days and the average day of life of PH diagnosis was 29 ± 40 days. Table 1 compares demographic and clinical characteristics between case and control groups. Overall, there were no significant differences between case and control groups, except for ventilation days and number of echocardiograms. Ventilation days were significantly longer for the case group (31 36 days) than for the control group (15 ± 27 days, p = 0.02) ± and the number of echocardiograms were also increased in the PH group (3.9 vs. 2.5, p < 0.01).
Table 1.
Demographic and clinical characteristics
| BPD and PH (n = 36) | BPD alone (n = 104) | p-value | |
|---|---|---|---|
| Gestational age (weeks) | 28 ± 4 | 28 ± 3 | 0.67 |
| Birth weight (grams) | 1060 ± 573 | 1199 ± 599 | 0.22 |
| Gender (male), % | 50 | 68 | 0.05 |
| Ethnicity (caucasian), % | 78 | 73 | 0.74 |
| Antenatal steroids, % | 82 | 81 | 0.93 |
| Small for gestational age, % | 9 | 30 | 0.06 |
| Age at admission (days) | 22 ± 43 | 34 ± 69 | 0.22 |
| APGAR at 5 minutes | 5.8 ± 2.4 | 6.4 ± 2.4 | 0.15 |
| Surfactant, % | 81 | 80 | 0.93 |
| Oxygen at discharge, % | 62 | 47 | 0.30 |
| Lasix at discharge, % | 19 | 14 | 0.56 |
| Ventilation (days) | 31 ± 36 | 15 ± 27 | 0.02 |
| Echocardiography, n | 3.9 ± 2.5 | 2.0 ± 2.4 | <0.01 |
| PDA, % | 62 | 69 | 0.46 |
| PFO/ASD, % | 79 | 81 | 0.83 |
| Post-natal steroids, % | 42 | 36 | 0.54 |
| Sepsis, % | 6 | 7 | 0.78 |
| ROP, % | 3 | 8 | 0.19 |
| Intestinal perforation, % | 9 | 6 | 0.64 |
PDA = Patent ductus arteriosus; PFO = Patent foramen ovale; ASD = Atrial septal defect; ROP = Retinopathy of prematurity.
Categorical variables presented as percentage and continuous variables presented as mean ± standard deviation.
Table 2 compares MAF for cases and controls for 17 of the SNPs studied. We found that the rs2781666 (arginase I gene) T allele was less common in cases (MAF = 0.23) than in controls (MAF = 0.37, p = 0.04). None of the other 16 SNPs showed a significant difference in MAF between cases and controls.
Table 2.
Calculated minor allele frequencies (MAF)
| Gene | SNP-rs | Minor allele | Calculated MAF: 36 cases | Calculated MAF: 104 controls | p-value |
|---|---|---|---|---|---|
| CPS1 | 7422339 | A | 0.347 | 0.330 | 0.79 |
| CPS1 | 1047883 | A | 0.439 | 0.449 | 0.89 |
| CPS1 | 1047891 | A | 0.347 | 0.330 | 0.79 |
| OTC | 1800321 | G | 0.319 | 0.427 | 0.11 |
| OTC | 5963409 | A | 0.181 | 0.279 | 0.10 |
| OTC | 1800328 | G | 0.042 | 0.015 | 0.18 |
| ASS | 7860909 | G | 0.333 | 0.359 | 0.69 |
| NOS2 | 8081248 | A | 0.386 | 0.356 | 0.66 |
| ARG1 | 2781663 | A | 0.306 | 0.404 | 0.14 |
| ARG1 | 2781665 | T | 0.306 | 0.393 | 0.19 |
| ARG1 | 2781666 | T | 0.229 | 0.365 | 0.04 |
| ARG1 | 2781667 | T | 0.306 | 0.422 | 0.08 |
| ARG1 | 60389358 | T | 0.111 | 0.107 | 0.92 |
| ARG1 | 2749935 | A | 0.597 | 0.529 | 0.32 |
| ARG1 | 2781659 | G | 0.274 | 0.365 | 0.21 |
| ARG2 | 3742879 | G | 0.250 | 0.233 | 0.77 |
| ARG2 | 17249437 | C | 0.333 | 0.392 | 0.38 |
CPS1 = Carbamoyl-phosphate synthetase; OTC = Ornithine transcarbamylase; ASS = Argininosuccinate synthase; NOS2 = Nitric oxide synthase; ARG1, ARG2 = Arginase.
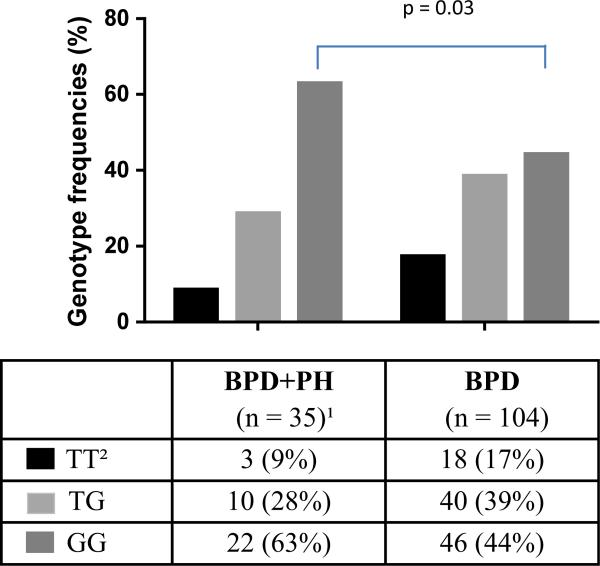
Figure 1 demonstrates the allele frequencies for the arginase I SNP rs2781666 in cases and controls. The proportion of patients who were homozygous for the G allele (wild type) was greater in the BPD and PH group than in the BPD alone group (p = 0.03). Using an additive logistic regression model, for each additional minor allele (T), having the minor allele decreases the risk of PH in BPD by 43% (OR per additional T allele 0.57 [95% CI 0.31–1.02]; p = 0.047).
Figure 1.
Genotype frequencies for the rs2781666 SNP arginase-1 gene in cases (BPD + PH) versus controls (BPD). The Y-axis is genotype frequency. Values are absolute numbers of patients with percentages in parentheses. 1One patient had unavailable data for this SNP. 2TT is the minor allele homozygote.
In this cohort of BPD patients, 27% had mild BPD and 73% had moderate/severe BPD as defined by the National Institute of Child Health and Human Development consensus workshop (11). Of the BPD patients with PH, 14% had mild BPD and 86% had moderate/severe BPD, and this was not different from patients with BPD alone (31% mild, 69% moderate/severe, p = 0.07).
DISCUSSION
The major objective of the present study was to investigate the association of SNPs in the l-arginine/nitric oxide pathway with the development of PH in patients with BPD. The major findings of this study were that the rs2781666 SNP in the arginase I gene was associated with a lower incidence of PH in patients with BPD and the odds of PH were decreased by 43% for each copy of the minor allele of the rs2781666 SNP in patients with BPD. These findings support our hypothesis that SNPs in genes involved in the l-arginine/nitric oxide pathway are associated with the development of PH in preterm infants with BPD.
The rs2781666G/T SNP is located in the promoter region of the arginase I gene. There have been reports of associated cardiovascular changes with the rs2781666G/T polymorphism of arginase I. Meroufel et al. (21) reported that the rs2781666 SNP of the arginase I gene was associated with a trend toward decreased systolic blood pressure (p = 0.067) in an adult Algerian population. Furthermore, two separate reports by Dumont et al. (23) and Sediri et al. (24) demonstrated the association of the arginase I rs2781666 SNP with the risk of myocardial infarction. In addition to cardiovascular disease, arginase genes have also been associated with childhood asthma. Salam et al. (22) reported that a particular arginase I haplotype was associated with a reduced risk of asthma.
There are both in vitro and in vivo data supporting that changes in arginase levels are associated with pulmonary vascular disease. An increase in arginase expression and activity has been shown in both human pulmonary artery smooth muscle cells and microvascular endothelial cells, resulting in greater cell proliferation (25, 26). In a murine model of PH, arginase has been shown to be elevated in the lung homogenates (27). In addition, arginase II has been shown to be elevated in adult patients with pulmonary arterial hypertension (28). In the present study, a SNP was found in the arginase I gene that differed between BPD patients with and without PH. It remains to be elucidated exactly what effects this SNP has on arginase activity, but we would speculate based on the above that the rs2781666 SNP results in lower arginase activity.
There are several limitations of this study that should be noted. Our sample size was relatively small, but using a directed gene approach we found a significant difference in the rs2781666 SNP of arginase I gene between patients with BPD and PH and those with BPD alone. We acknowledge that this study needs to be replicated in a larger cohort of patients, but emphasise that our findings provide a novel insight into a potential marker for BPD-associated PH. Another limitation of our study is the inherent difficulty of diagnosing PH in the newborn period. The gold standard for diagnosis of PH is cardiac catheterisation, but due to the invasiveness associated with cardiac catheterisation in preterm infants, 2D echocardiography is the most common approach to diagnose PH in this population (12).
Our findings suggest that genetic variations in arginase I are associated with PH in preterm infants with BPD. Our study provides important data that supports a more detailed investigation of the SNPs in the l-arginine/nitric oxide pathway genes in the BPD population. These findings also highlight that there may be genetic markers that predict the onset of PH in infants with BPD, such that therapeutic approaches might be developed to prevent PH in BPD and thereby improve outcomes for patients with BPD.
Key notes.
Pulmonary hypertension (PH) substantially increases morbidity and mortality in neonates with bronchopulmonary dysplasia (BPD), and predictors of BPD-associated PH are needed for earlier diagnosis and treatment.
In a cohort of 140 neonates with BPD, a single polymorphism (SNP) rs2781666 in arginase I gene was associated with less PH and each additional minor allele decreased the risk of PH by 43%.
PH in BPD was associated with longer need for mechanical ventilation.
ACKNOWLEDGEMENT
Clinical data and/or biospecimens used for this project were provided by Ohio Perinatal Research Network Peri-natal Research Repository institutional specimen repositories. This work was funded by an intramural grant from the Center for Clinical and Translational Research at The Research Institute at Nationwide Children's Hospital (CTSA grant UL1TR001070).
Abbreviations
- BPD
Bronchopulmonary dysplasia
- MAF
Mean allele frequency
- PH
Pulmonary hypertension
- SNP
Single-nucleotide polymorphism
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interests.
References
- 1.Berkelhamer SK, Mestan KK, Steinhorn RH. Pulmonary hypertension in bronchopulmonary dysplasia. Semin Perinatol. 2013;37:124–31. doi: 10.1053/j.semperi.2013.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slaughter JL, Pakrashi T, Jones DE, South AP, Shah TA. Echocardiographic detection of pulmonary hypertension in extremely low birth weight infants with bronchopulmonary dysplasia requiring prolonged positive pressure ventilation. J Perinatol. 2011;31:635–40. doi: 10.1038/jp.2010.213. [DOI] [PubMed] [Google Scholar]
- 3.Kim DH, Kim HS, Choi CW, Kim EK, Kim BI, Choi JH. Risk factors for pulmonary artery hypertension in preterm infants with moderate or severe bronchopulmonary dysplasia. Neonatology. 2012;101:40–6. doi: 10.1159/000327891. [DOI] [PubMed] [Google Scholar]
- 4.An HS, Bae EJ, Kim GB, Kwon BS, Beak JS, Kim EK, et al. Pulmonary hypertension in preterm infants with bronchopulmonary dysplasia. Korean Circ J. 2010;40:131–6. doi: 10.4070/kcj.2010.40.3.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Summar ML, Hall L, Christman B, Barr F, Smith H, Kallianpur A, et al. Environmentally determined genetic expression: clinical correlates with molecular variants of carbamyl phosphate synthetase I. Mol Genet Metab. 2004;81(Suppl 1):S12–9. doi: 10.1016/j.ymgme.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Lipsitz EC, Weinstein S, Smerling AJ, Stolar CJ. Endogenous nitric oxide and pulmonary vascular tone in the neonate. J Pediatr Surg. 1996;31:137–40. doi: 10.1016/s0022-3468(96)90336-x. [DOI] [PubMed] [Google Scholar]
- 7.Nelin LD, Moshin J, Thomas CJ, Sasidharan P, Dawson CA. The effect of inhaled nitric oxide on the pulmonary circulation of the neonatal pig. Pediatr Res. 1994;35:20–4. doi: 10.1203/00006450-199401000-00006. [DOI] [PubMed] [Google Scholar]
- 8.MacRitchie AN, Albertine KH, Sun J, Lei PS, Jensen SC, Freestone AA, et al. Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;281:L1011–20. doi: 10.1152/ajplung.2001.281.4.L1011. [DOI] [PubMed] [Google Scholar]
- 9.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, et al. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2003;284:L749–58. doi: 10.1152/ajplung.00334.2002. [DOI] [PubMed] [Google Scholar]
- 10.Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, et al. Neonatal pulmonary hypertension–urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001;344:1832–8. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 11.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. 2001;163:1723–9. doi: 10.1164/ajrccm.163.7.2011060. [DOI] [PubMed] [Google Scholar]
- 12.Ivy DD, Abman SH, Barst RJ, Berger RM, Bonnet D, Fleming TR, et al. Pediatric pulmonary hypertension. J Am Coll Cardiol. 2013;62:D117–26. doi: 10.1016/j.jacc.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 13.Salam MT, Bastain TM, Rappaport EB, Islam T, Berhane K, Gauderman WJ, et al. Genetic variations in nitric oxide synthase and arginase influence exhaled nitric oxide levels in children. Allergy. 2011;66:412–9. doi: 10.1111/j.1398-9995.2010.02492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lange LA, Croteau-Chonka DC, Marvelle AF, Qin L, Gaulton KJ, Kuzawa CW, et al. Genome-wide association study of homocysteine levels in Filipinos provides evidence for CPS1 in women and a stronger MTHFR effect in young adults. Hum Mol Genet. 2010;19:2050–8. doi: 10.1093/hmg/ddq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell S, Ellingson C, Coyne T, Hall L, Neill M, Christian N, et al. Genetic variation in the urea cycle: a model resource for investigating key candidate genes for common diseases. Hum Mutat. 2009;30:56–60. doi: 10.1002/humu.20813. [DOI] [PubMed] [Google Scholar]
- 16.Dumont J, Meroufel D, Bauters C, Hansmannel F, Bensemain F, Cottel D, et al. Association of ornithine transcarbamylase gene polymorphisms with hypertension and coronary artery vasomotion. Am J Hypertens. 2009;22:993–1000. doi: 10.1038/ajh.2009.110. [DOI] [PubMed] [Google Scholar]
- 17.Hozyasz KK, Mostowska A, Wojcicki P, Lianeri M, Jagodzinski PP. Polymorphic variants of genes related to arginine metabolism and the risk of orofacial clefts. Arch Oral Biol. 2010;55:861–6. doi: 10.1016/j.archoralbio.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 18.Engel K, Hohne W, Haberle J. Mutations and polymorphisms in the human argininosuccinate synthetase (ASS1) gene. Hum Mutat. 2009;30:300–7. doi: 10.1002/humu.20847. [DOI] [PubMed] [Google Scholar]
- 19.Duan QL, Gaume BR, Hawkins GA, Himes BE, Bleecker ER, Klanderman B, et al. Regulatory haplotypes in ARG1 are associated with altered bronchodilator response. Am J Respir Crit Care Med. 2011;183:449–54. doi: 10.1164/rccm.201005-0758OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Romieu I, Sienra-Monge JJ, Ramirez-Aguilar M, Estela Del Rio-Navarro B, Kistner EO, et al. Genetic polymorphisms in arginase I and II and childhood asthma and atopy. J Allergy Clin Immunol. 2006;117:119–26. doi: 10.1016/j.jaci.2005.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meroufel D, Dumont J, Mediene-Benchekor S, Benhammamouch S, Ducimetiere P, Cottel D, et al. Characterization of arginase 1 gene polymorphisms in the Algerian population and association with blood pressure. Clin Biochem. 2009;42:1178–82. doi: 10.1016/j.clinbiochem.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 22.Salam MT, Islam T, Gauderman WJ, Gilliland FD. Roles of arginase variants, atopy, and ozone in childhood asthma. J Allergy Clin Immunol. 2009;123:596–602,e1–8. doi: 10.1016/j.jaci.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dumont J, Zureik M, Cottel D, Montaye M, Ducimetiere P, Amouyel P, et al. Association of arginase 1 gene polymorphisms with the risk of myocardial infarction and common carotid intima media thickness. J Med Genet. 2007;44:526–31. doi: 10.1136/jmg.2006.047449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sediri Y, Kallel A, Ben Ali S, Omar S, Mourali MS, Elasmi M, et al. Association of rs2781666 G/T polymorphism of arginase I gene with myocardial infarction in Tunisian male population. Clin Biochem. 2010;43:106–9. doi: 10.1016/j.clinbiochem.2009.10.058. [DOI] [PubMed] [Google Scholar]
- 25.Chen B, Calvert AE, Cui H, Nelin LD. Hypoxia promotes human pulmonary artery smooth muscle cell proliferation through induction of arginase. Am J Physiol Lung Cell Mol Physiol. 2009;297:L1151–9. doi: 10.1152/ajplung.00183.2009. [DOI] [PubMed] [Google Scholar]
- 26.Toby IT, Chicoine LG, Cui H, Chen B, Nelin LD. Hypoxia-induced proliferation of human pulmonary microvascular endothelial cells depends on epidermal growth factor receptor tyrosine kinase activation. Am J Physiol Lung Cell Mol Physiol. 2010;298:L600–6. doi: 10.1152/ajplung.00122.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nelin LD, Wang X, Zhao Q, Chicoine LG, Young TL, Hatch DM, et al. MKP-1 switches arginine metabolism from nitric oxide synthase to arginase following endotoxin challenge. Am J Physiol Cell Physiol. 2007;293:C632–40. doi: 10.1152/ajpcell.00137.2006. [DOI] [PubMed] [Google Scholar]
- 28.Xu W, Kaneko FT, Zheng S, Comhair SA, Janocha AJ, Goggans T, et al. Increased arginase II and decreased NO synthesis in endothelial cells of patients with pulmonary arterial hypertension. FASEB J. 2004;18:1746–8. doi: 10.1096/fj.04-2317fje. [DOI] [PubMed] [Google Scholar]