Abstract
Objective
Investigational, near-infrared fluorescence (NIRF) lymphatic imaging was used to assess lymphatic architecture and contractile function in participants diagnosed with Dercum’s disease, a rare, poorly understood disorder characterized by painful lipomas in subcutaneous adipose tissues.
Design and Methods
After informed consent and as part of an FDA-approved feasibility study to evaluate lymphatics in diseases in which their contribution has been implicated, three women diagnosed with Dercum’s disease and four control subjects were imaged. Each participant received multiple intradermal and subcutaneous injections of indocyanine green (ICG, total dose ≤400µg) in arms, legs, and/or trunk. Immediately after injection, ICG was taken up by the lymphatics and NIRF imaging was conducted.
Results
The lymphatics in the participants with Dercum’s disease were intact and dilated, yet sluggishly propelled lymph when compared to control lymphatics. Palpation of regions containing fluorescent lymphatic pathways revealed tender, fibrotic, tubular structures within the subcutaneous adipose tissue that were associated with painful nodules, and, in some cases, masses of fluorescent tissue indicating that some lipomas may represent tertiary lymphoid tissues.
Conclusions
These data support the hypothesis that Dercum’s disease may be a lymphovascular disorder and suggest a possible association between abnormal adipose tissue deposition and abnormal lymphatic structure and function.
Keywords: Dercum’s disease, adipose dolorosa, lymphatic system, near infrared fluorescence imaging, biomedical technology
Introduction
Dercum’s disease (DD), first described in 1888 (1) and also known as adiposis dolorosa (WFS: OMIM 103200), is a rare adipose disorder that typically occurs in midlife. DD is characterized by painful subcutaneous adipose tissues (SAT), frequently with lipomas or distinct masses of SAT.(2, 3) Hansson, et al. proposed four classifications of DD including: I. generalized, diffuse form characterized by widespread pain in the fatty tissue across the body without clear lipomas; II. generalized, nodular form characterized by widespread pain in the SAT and intense pain in and around lipomas; III. localized, nodular form characterized by pain in and around lipomas; and IV. juxta-articular form where the painful lipomas are associated primarily with the joints.(4) While the presence of chronic painful lipomas is the distinguishing diagnostic criteria for DD, the majority of patients also suffer psychiatric, cardiovascular, pulmonary, endocrine, gastrointestinal, and/or rheumatologic symptoms. In addition, the patients are easily bruised and the lipomas are unaffected by weight loss.(3) The etiology of DD is not well understood and, as described in a recent review (4), a number of unsubstantiated theories have been suggested including endocrine dysfunction, nervous system dysfunction, mechanical pressure on nerves, adipose tissue dysfunction, inflammation, and trauma. The pain associated with the lipomas generally responds poorly to traditional analgesics and while numerous therapeutic approaches, including liposuction (5) and lipectomy (6, 7), have been described in the literature (see (4, 8) for review) none have been widely adopted as standard-of-care.
One non-surgical approach that has been reported to reduce the volume of SAT in individuals with DD is manual lymphatic drainage similar to that utilized to reduce swelling associated with lymphedema (9). Indeed, DD was described as a disorder of the “haemolymph” system by Dercum (10) and “a general disease of the lymphatic system” (11) suggesting that dysfunction in the hemovascular and/or lymphatic systems may contribute to the development of lipomas. In addition, when the affected tissue is diffuse, DD shares many similarities to lipedema, another adipose disorder with known lymphatic involvement (for discussion see (8)).
The lymphatic system is a secondary circulatory system that plays a role in fluid homeostasis, protein transport, and immune response and is increasingly implicated in diseases such as diabetes, asthma, and fatty disorders.(12–14) The involvement of the lymphatic system in common as well as rare diseases, however, is poorly understood due in part to our inability to readily resolve the fine lymphatic structures and subtle contractile function using clinically available imaging modalities such as lymphoscintigraphy. Recently, we employed investigational near-infrared fluorescence (NIRF) lymphatic imaging to characterize aberrant lymphatic involvement in a patient with capillary malformation-arteriovenous malformation and, in a mouse model, validated that the patient’s causative RASA1 gene variant resulted in a lympho-proliferative phenotype (15, 16). Herein, we sought to directly evaluate whether a lymphatic contribution is associated with DD in an investigational imaging study of three participants diagnosed with rare DD.
Methods
NIRF lymphatic imaging of human lymphatics in health and in lymphedema has previously been described in detail.(17, 18) Briefly, as part of a broader institutional review board-approved study of lymphatic disorders conducted under Food and Drug Administration approval (IND# 102,827) and funded by the National Heart Lung and Blood Institute of the National Institutes of Health (Clinical Trials No. NCT00833599: “Imaging lymphatic function in normal subjects and in persons with lymphatic disorders,” www.clinicaltrials.gov), the cutaneous collecting and conducting lymphatics of three participants previously diagnosed with generalized, nodular DD were imaged as well as those of four control participants. The three DD participants were females, aged 52, 56, and 45, with body mass indexes of 30.5, 31.9, and 30.9 respectively. The DD participants were not diagnosed nor treated in our clinic though a consultation was performed to confirm the presence of lipomas; their SAT was globally distributed, as is common in Dercum’s disease (3), though the hands, feet and face were spared. The DD participants reported the official diagnoses to have occurred approximately 18 months prior to imaging with symptoms being present for as long as 17 years (DD1). Self-reported treatments included bandaging, manual lymphatic drainage, and the surgical resection of lipomas. While telangiectasia was observed in the lower right leg of DD1, varicose veins were not observed in any of the participants. The control participants were not matched via recruitment, but rather were selected from an existing pool of previously imaged healthy subjects based on their BMI and the location of their injection sites. Male control participants were included as, with the exception of lymphatic pressure(19), little variation between male and female lymphatic architecture and contractile function in non-genital areas has been reported. The control participants presented herein include two females (aged 29 and 37) and two males (aged 47 and 35) with BMIs of 25.0, 29.9, 30.2, and 32.2 respectively.
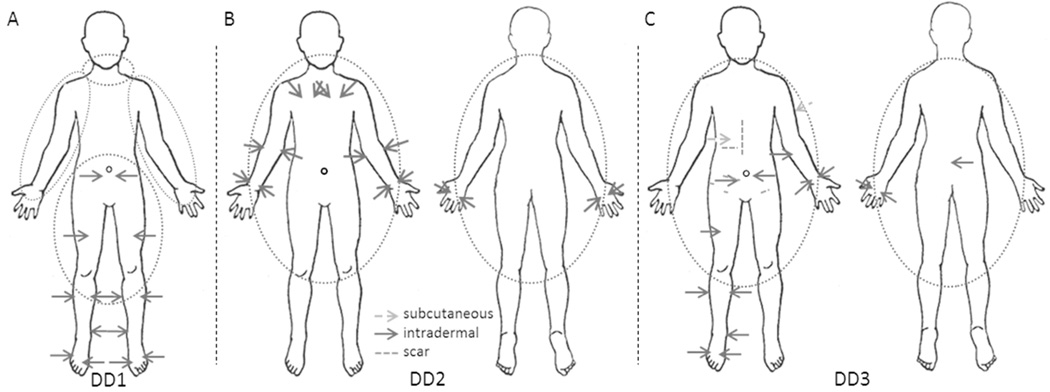
Following informed consent each participant received 12 to 16 subcutaneous or intradermal injections of 25 µg indocyanine green (ICG) in 0.1 mL saline with a maximum total dose ≤400 µg. The number and location of injections varied by participant dependent upon the sites of lipomas and SAT accumulation. As illustrated in Figure 1 the first Dercum’s participant (DD1) received six intradermal injections in each leg and two intradermal injections in the lower abdomen; the second (DD2) received 6 intradermal injections in each arm and two injections above each breast; and the third (DD3) received five intradermal injections on the left arm as well as one cutaneous injection in the upper left arm; six intradermal injections on the right leg; one subcutaneous and two intradermal injections on the abdomen; and one intradermal injection on the midline of the lower spine. The first female control participant (NC1) received injections at the same sites as DD1 plus an additional injection above and below the left buttock; the second (NC2) received six injections in each arm in the same locations as DD2 (without the injections above the breasts); the first male participant (NC3) received six injections in each leg in the same locations as DD1 (without the abdominal injections); and the second (NC4) received six injections in each arm in the same locations as DD2 (without the injections above the breasts). The injection sites were covered with small circular bandages to prevent drainage of dye onto the skin and, when needed, with black vinyl tape to prevent oversaturation of the camera due to the brightness of the ICG at the injection site. Subject DD2 had pitting lymphedema of the lower extremities as determined by the formation of an indentation in the tissue after applying pressure with the index finger for five seconds; the relationship of the lymphedema to her DD is unknown.
Figure 1.
Injection sites for participants (A) DD1, (B) DD2, and (C) DD3. Dotted circles represent areas with lipomas, solid arrows represent intradermal injections, dashed arrows represent subcutaneous injections, and dashed lines represent scars observed on the subject.
Immediately following ICG administration in the arms, legs, and/or trunk, NIRF lymphatic imaging was conducted for approximately two hours. During that time, the lymphatic channels, identified by the fluorescent signals, were gently palpated to identify whether they corresponded to lipomas or painful SAT.
Results
NIRF lymphatic imaging indicated that the lymphatics of each of the three participants with Dercum’s disease were well-defined similar to those seen in the normal participants as well as in normal subjects imaged previously (17, 18, 20). No dermal backflow or extravascular dye, as commonly observed by NIRF lymphatic imaging in lymphedema subjects (17, 18, 20, 21), was observed in these participants. However, abnormal lymphatic capillaries were observed radiating from some injection sites while other lymphatics appeared tortuous, dilated with sluggish lymph flow, and/or were associated with painful, nodular SAT as identified by palpation.
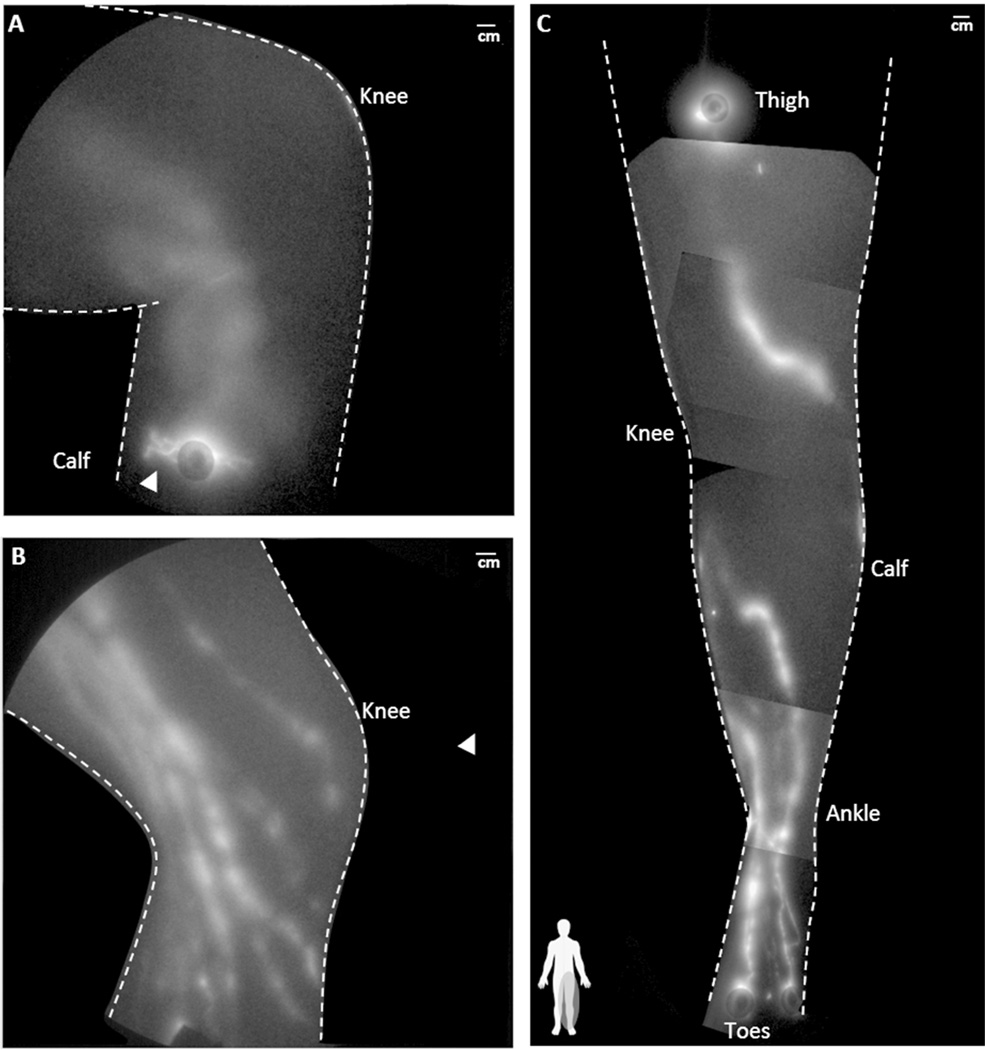
Figure 2 depicts the montage of NIRF lymphatic images demarking the lymphatics of the dorsal arm of (A) participant DD3, whose general phenotype is similar to participant DD2, normal participant (B) NC2, and (C) NC4. The flow in the dilated lymphatics vessels in the arm of DD3 (see Supplemental Video 1) was “sluggish” as compared to the flow in the arm of NC2 (see Supplemental Video 2). Abnormal fluorescent lymphatic capillaries were observed radiating from injection sites on the hand and wrists of both DD3 (arrowhead in Figure 2A)) and DD2.
Figure 2.
NIRF lymphatic imaging montages depicting dorsal arm lymphatics of participant (A) DD3 (BMI 30.9), (B) NC2 (BMI 29.9), and (C) NC4 (BMI 32.2). The arrowhead in (A) identifies abnormal lymphatic capillary structures near the injection site on the back of DD3’s hand and the arrow identifies tortuous lymphatic vasculature in the lower arm seen in both dorsal and medial views. Injection sites are covered by round bandages and/or black vinyl tape. See Supplemental Videos 1 and 2, showing the functional lymphatic drainage in NC2 and DD3 respectively.
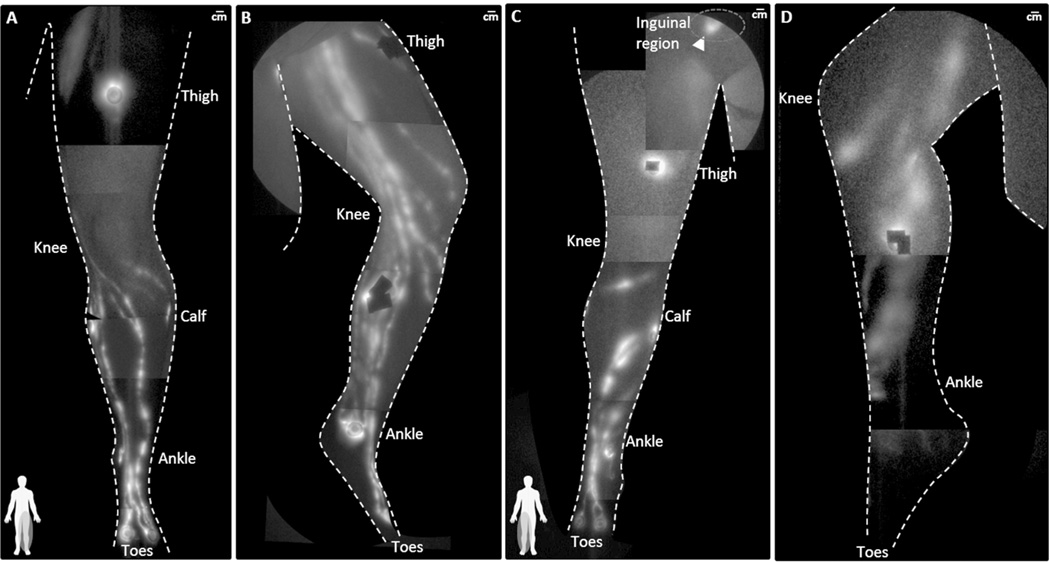
Figure 3 illustrates the anterior and medial view of leg lymphatics in normal participant NC3 (Figures 3(A) and 3(B)) and in participant DD3 (Figures 3(C) and 3(D)). Leg lymphatics in participants DD3 (see Supplemental Video 3) and DD1 were dilated with “sluggish” flow as compared to NC3 (see Supplemental Video 4). For example, Figure 4A) shows two lymphatic vessels in the medial knee of DD1 that were so enlarged (compared to those observed in participant NC3, Figure 4B)) that they initially appeared to be an elongated patch of diffuse extravascular ICG located in the SAT. Through the visualization of propagating fluorescent lymph they are distinctly identified as functioning lymphatic vessels (see Supplemental Videos 5 and 6 depicting comparison of lymphatic flow in the leg of DD1 and a normal control subject (reproduced with permission from (16))). We also observed distinct segmentations, or lymphangions in the lymphatic vessels of the lower legs that did not appear to drain into inguinal lymph nodes, but rather, in the case of participant DD3, into a painful, fluorescent, fibrotic mass in the inguinal region (arrowhead in Figure 3C)). Palpation of the lymphatics in the upper medial calf revealed fibrotic and nodular vessel-like structures which directly corresponded to the fluorescent tracks (see Supplemental Videos 7 and 8, depicting palpation of fluorescent lymphatics in the inguinal and knee regions of DD3 respectively).
Figure 3.
NIRF lymphatic imaging montages of the (A) anterior aspect and (B) medial aspect of the lymphatics in the left leg of NC3 (BMI 30.2) and (C) the anterior aspect and (D) medial aspect of the lymphatics in the right leg of DD3 (BMI 30.9). The leg lymphatics of DD3 are dilated in the anterior shin and a painful, fluorescent, fibrotic mass (arrowhead) was found in the inguinal region. The lymphatics in the medial calf are dilated and correspond with fibrotic and nodular tissues thought to be lymphatic vessels. Injection sites are covered by round bandages and/or black vinyl tape. See Supplemental Videos 3 and 4, for a comparison of functional lymphatic drainage in participants NC3 and DD3.
Figure 4.
Lymphatic vessels in the medial left knee of (A) DD1 (BMI 30.5) and (B) NC3 (BMI 30.2). (C) Montage of the lymphatics in the anterior left leg of DD1. See Supplemental Videos 5 and 6 for a comparison of functional lymphatic drainage in the medial knees of participants NC3 and DD1. (B) Reproduced with permission from [16].
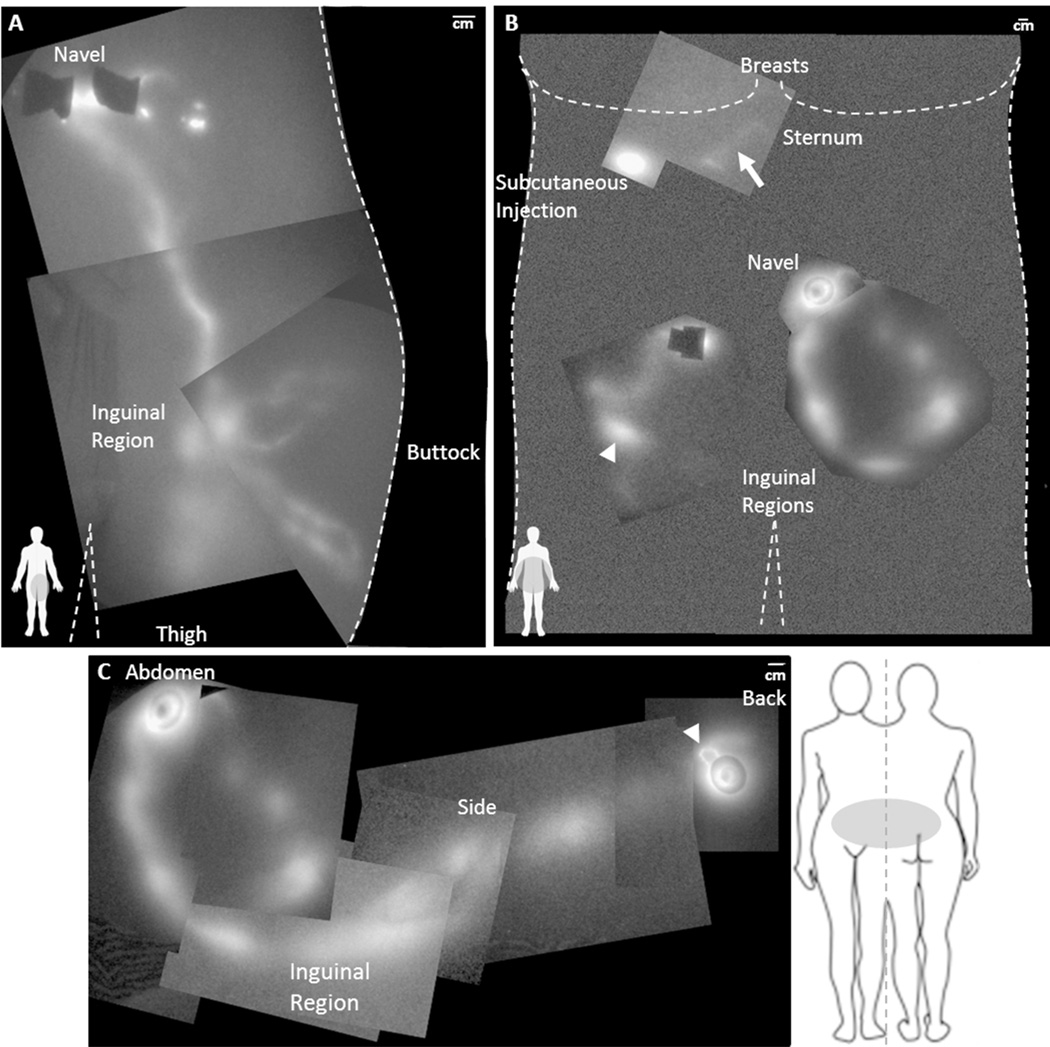
The lymphatic drainage patterns in the abdomen of normal participant (NC1) and in participant DD3 are shown in the montages in Figure 5. Figure 5A) illustrates the lymphatics that drain from the ICG injection sites near the navel to the left inguinal lymph node basin. In contrast, Figure 5B) illustrates the lymphatics in DD3 that drain from the ICG injection sites into a painful fibrotic mass located near the right inguinal lymph node basin (arrowhead in Figures (5B) and (3C)), draining irregularly toward the left inguinal lymph node basin and from the lower back of the participant (Figure 5C)). The abdominal lymphatic drainage in DD1 was also abnormal and contained tortuous vessels that drained towards the inguinal lymph nodes. Clearly the abdominal and leg drainage patterns differ in these DD participants as compared to normal participants.
Figure 5.
(A) NIRF lymphatic imaging montage of the lymphatic drainage to the inguinal region from intradermal injections near the navel, above and below the buttock, and from the leg in NC1 (BMI 25.0). (B) Montage of the lymphatic drainage of the abdomen of DD3 (BMI 30.9). The arrow identifies the location of a dim lymphatic vessel draining from the subcutaneous injection below the right breast towards the sternum. The arrowhead identifies the location of a fluorescent, painful fibrotic mass palpated in the right inguinal region and corresponds to the arrowhead in Figure 2C. (C) NIRF lymphatic imaging montage illustrating the lymphatic drainage pattern from the intradermal injections on the abdomen and lower back to the inguinal region of DD3. The arrowhead identifies abnormal lymphatic capillary structures near the injection site on the back. Note the segmented appearance of the lymphatics. Injection sites are covered by round bandages and/or black vinyl tape.
Discussion
Painful SAT-associated lipomas are a hallmark of DD. Dr. Dercum postulated that lymphatics were integral to this disease over one hundred and twenty-five years ago.(1) We confirm this assumption finding dilated, sluggish, tortuous, and tender lymphatics in three women with DD. The lymphatic vessels were large enough to be palpated and appeared to be associated with lipomas in the SAT. In one case, a mass of strongly fluorescent tissue in line with a lymphatic vessel was suggestive of the presence of tertiary lymphoid tissue. The presence of the observed lymphatic abnormalities did not appear to be associated with scarring from prior surgical procedures nor with the telangiectasia observed in DD1.
There is a close association between the lymphatics and adipose tissue in both health and disease. For example, lymph nodes and lymphatic vessels are always in close physical association with adipose tissues (22–24). In disease, lymphatic insufficiency due to inherited lymphatic abnormalities, injury, or obstruction of the lymphatic leads to lymphedema, consisting of impairment/alteration of immune responses, accumulation of lymphatic fluid and protein, as well as increased SAT. In a haplo-insufficient mouse model of lymphatic dysfunction, the loss of PROX-1 gene expression necessary for the development and maintenance of the lymphatic vasculature results in aberrant lymphatic repatterning, adult onset of obesity (13), and impaired lymph transport (25). More recently, lymphatic fluid stasis has been associated with key adipogenic factors including peroxisome proliferator-activated receptor gamma (PPAR-gamma) and CCAAT/enhanced binding protein-alpha (CEBP-alpha), as well as the metabolically active protein adiponectin (26) – all shown to be master regulators of adiogenesis (27). While this study does not imply any molecular mechanism for DD, it nonetheless points to the involvement of the lymphatics in DD lipomas and confirms the original hypothesis of Dr. Dercum as a disorder associated with the lymphatics.
Unfortunately other imaging modalities were not used in this study as they are outside the scope of the FDA approved feasibility study. However, because the lymphatics have little endogenous contrast and their small size inhibits the introduction of large volumes of exogenous contrast, the use of clinical imaging modalities such as x-ray computed tomography, ultrasound, and magnetic resonance imaging has historically been limited. Lymphoscintigraphy, following the intra- or sub-dermal administration of a radioisotope, is commonly considered the ‘gold standard’ for lymphatic imaging; however because of the limited number of gamma photons emitted, each lymphoscintigram requires minutes to acquire and thus lacks the temporal resolution required to image lymphatic contractile function. In addition, lymphoscintigrams are typically grainy and only larger lymphatic trunks and abnormalities are resolved. As we have demonstrated herein, NIRF imaging provides a unique opportunity to image the superficial lymphatics. However, the ability of NIRF imaging to resolve deep (> 4 cm) lymphatics is limited owing to the physics of light propagation in tissue. As near-infrared light travels though tissue, it undergoes multiple scattering events prior to being absorbed or exiting the tissue. Because of this scattering, fluorescent signals originating more deeply in the tissue will appear larger and more unfocused than a comparable source at the surface. Indeed, when a fluorescent source is sufficiently deep, its fluorescent signal at the tissue surface will be indistinguishable from the background. In addition, because of the broadening effect of scatter, it is difficult to determine the exact dimensions (i.e. diameters) of the lymphatics based solely on NIRF images. Despite these limitations, many of the lymphatics in humans are located in the intra- and sub-dermal tissues and as such are readily accessible by NIRF imaging. Indeed, while few direct comprehensive comparisons have been done, Mihara et al., found that NIRF imaging was superior to lymphoscintigraphy for diagnosing early stage lymphedema.(28)
To date there have been no systematic studies that evaluate the effect the time of day or other external, systemic, or local factors have on lymphatic propulsion in humans. Based on our experience, hydration may impact lymph flow and our subjects are asked to drink lots of water prior to participation, however, no systematic measure of hydration is performed prior to imaging. Animal studies in our laboratory have indicated that unhealed wounds affect both the lymphatic architecture (29) and propulsion rate (unpublished), however none of the control subjects nor the Dercums participants had local skin wounds. As mentioned above, Unno et al. noted a difference in the pumping pressure required for active lymphatic propulsion with age, but did not report the effect of pumping pressure on lymphatic propulsion. (19
NIRF lymphatic imaging has been used to characterize patients with hereditary and acquired lymphedema, demonstrating patterns of extravascular dye, dilated, non-contractile (or non-conducting) lymphatic vessels, and lack of uptake of intradermally administered ICG. Herein, a distinct NIRF lymphatic phenotype of dilated, conducting lymphatic vessels that are palpable and that are associated with painful lipomas in the subcutaneous compartment are visualized for the first time in three participants with DD. Indeed, this study may be the first to directly implicate benign subcutaneous lipomas and aberrant adipose tissue accumulation with the lymphatics in a human disease. Because this feasibility study only provided a single snapshot in time after the onset of DD and had limited numbers of subjects, it is not known if the lymphatic abnormalities observed are a result of the disease and/or contribute to its development and progression. Tissue specimens were not taken to confirm the identity of lymphatic vessel involvement in DD lipomas, but this study suggests that future characterization of the condition may include the markers of lymphatic endothelial cells, (i.e. podplanin, PROX-1, LYVE-1, CD31) and consider the interplay between adipose tissue and lymphatic vasculature. Future studies are needed to determine whether lymphatic capillary uptake rates are compromised within initial lymphatics, and whether increased lymphatic vessel tortuosity and dilation observed herein are precursive and contributory to the development of DD or a progressive sequela of abnormal SAT.
Supplementary Material
Supplemental Video 1: NIRFLI lateral view of lymphatics in arm of participant DD3 (Figure 2(B)).
Supplemental Video 2: NIRFLI lateral view of lymphatics in arm of participant NC2 (Figure 2(A)).
Supplemental Video 3: NIRFLI anterial view of lymphatics in lower leg of participant DD3.
Supplemental Video 4: NIRFLI anterial view of lymphatics in lower leg of participant NC3.
Supplemental Video 5: NIRFLI medial view of knee in participant DD1.
Supplemental Video 6: NIRFLI medial view of knee in participant NC3 (Reproduced with permission from [16]).
Supplemental Video 7: NIRFLI of clinician’s (KH) palpation of inguinal lipoma on participant DD3. The bright region represents a fibrotic mass located in the inguinal.
Supplemental Video 8: NIRFLI of clinician’s (KH) palpation of fluorescent lymphatic vessel and lipoma in leg of DD3. The fluorescent tracks corresponded to the presence of tender, nodular, tubular structures.
What is already known about this subject
Dercum’s disease is characterized by the presence of painful lipomas in the subcutaneous adipose tissues
The etiology of Dercum’s disease is poorly understood
Major lymphatic channels and many lymph nodes are located in subcutaneous adipose tissue
What this study adds
The first images of lymphatic architecture and contractile function in Dercum’s patients
Preliminary evidence that abnormal lymphatics may be seen in patients who have lipomas
Acknowledgements
Supported, in parts, by the National Institutes of Health (R01 HL092923 (ES) and U54 CA136404 (ES)).
Footnotes
Conflict of Interest:
Drs. Fife, Maus, Rasmussen and Sevick-Muraca are listed as inventors on patents related to nearinfrared fluorescence lymphatic imaging. Drs. Rasmussen and Sevick-Muraca may receive financial benefit from NIRF Imaging, Inc. a UTHSCH start-up company seeking to commercialize the imaging technology. The other authors declare no potential conflict of interest.
Author Contributions:
JR acquired and analyzed the lymphatic images and helped draft the manuscript. KH provided clinical assessment and helped draft the manuscript. MA, CD, IT, and BZ acquired lymphatic images. RG, CF, and EM provided clinical assessment and monitoring. ES conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
References
- 1.Dercum FX. University Medical Magazine. Philadelphia: 1888. A subcutaneous connective-tissue dystrophy of the arms and back, associated with symptoms resembling myxoedema; pp. 140–150. [Google Scholar]
- 2.Brorson H, Fagher B. Dercum's disease. Fatty tissue rheumatism caused by immune defense reaction? Lakartidningen. 1996;93(15):1433–1436. [PubMed] [Google Scholar]
- 3.Herbst KL, Asare-Bediako S. Adiposis Dolorosa Is More Than Painful Fat. The Endocrinologist. 2007;17(6):326–334. [Google Scholar]
- 4.Hansson E, Svensson H, Brorson H. Review of Dercum's disease and proposal of diagnostic criteria, diagnostic methods, classification and management. Orphanet J. Rare Dis. 2012;7(1):23. doi: 10.1186/1750-1172-7-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hansson E, Manjer J, Svensson H, Brorson Hk. Quality-of-life in patients with Dercum's disease - before and after liposuction. Journal of Plastic Surgery and Hand Surgery. 2012;46(3–4):252–256. doi: 10.3109/2000656X.2012.698417. [DOI] [PubMed] [Google Scholar]
- 6.Held JL, Andrew JA, Kohn SR. Surgical amelioration of Dercum's disease: a report and review. The Journal of Dermatologic Surgery and Oncology. 1989;15:1294–1296. doi: 10.1111/j.1524-4725.1989.tb03150.x. [DOI] [PubMed] [Google Scholar]
- 7.Amine B, Leguilchard F, Benhamou CL. Dercum's disease (adiposis dolorosa): a new casereport. Joint Bone Spine. 2004;71(2):147–149. doi: 10.1016/S1297-319X(03)00139-8. [DOI] [PubMed] [Google Scholar]
- 8.Herbst KL. Rare adipose disorders (RADs) masquerading as obesity. Acta Pharmacol. Sin. 2012;33(2):155–172. doi: 10.1038/aps.2011.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lange U, Oelzner P, Uhlemann C. Dercum's disease (Lipomatosis dolorosa): successful therapy with pregabalin and manual lymphatic drainage and a current overview. Rheumatol. Int. 2008;29(1):17–22. doi: 10.1007/s00296-008-0635-3. [DOI] [PubMed] [Google Scholar]
- 10.Dercum FX, McCarthy DJ. Autopsy in a Case of Adiposis Dolorosa. The American Journal of the Medical Sciences. 1902;124(6):994–1005. [Google Scholar]
- 11.Mills CK. A Case of Adeno Lipomatosis: With Some Remarks on the Differential Diagnosis of the Affection from Adiposis Dolorosa and Other Diseases. The Journal of Nervous and Mental Disease. 1909;36(2):106–108. [Google Scholar]
- 12.Baluk P, Tammela T, Ator E, Lyubynska N, Achen MG, Hicklin DJ, et al. Pathogenesis of persistent lymphatic vessel hyperplasia in chronic airway inflammation. J. Clin. Invest. 2005;115(2):247–257. doi: 10.1172/JCI22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, et al. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005;37(10):1072–1081. doi: 10.1038/ng1642. [DOI] [PubMed] [Google Scholar]
- 14.Ji RC. Characteristics of lymphatic endothelial cells in physiological and pathological conditions. Histol. Histopathol. 2005;20(1):155–175. doi: 10.14670/HH-20.155. [DOI] [PubMed] [Google Scholar]
- 15.Lapinski PE, Kwon S, Lubeck BA, Wilkinson JE, Srinivasan RS, Sevick-Muraca E, et al. RASA1 maintains the lymphatic vasculature in a quiescent functional state in mice. The Journal of Clinical Investigation. 2012;122(2):733–747. doi: 10.1172/JCI46116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burrows PE, Gonzalez-Garay ML, Rasmussen JC, Aldrich MB, Guilliod R, Maus EA, et al. Lymphatic abnormalities are associated with RASA1 gene mutations in mouse and man. Proceedings of the National Academy of Sciences. 2013;110(21):8621–8626. doi: 10.1073/pnas.1222722110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen JC, Tan IC, Marshall MV, Adams KE, Kwon S, Fife CE, et al. Human lymphatic architecture and dynamic transport imaged using near-infrared fluorescence. Transl. Oncol. 2010;3(6):362–372. doi: 10.1593/tlo.10190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rasmussen JC, Tan IC, Marshall MV, Fife CE, Sevick-Muraca EM. Lymphatic imaging in humans with near-infrared fluorescence. Curr. Opin. Biotechnol. 2009;20(1):74–82. doi: 10.1016/j.copbio.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Unno N, Tanaka H, Suzuki M, Yamamoto N, Mano Y, Sano M, et al. Influence of age and gender on human lymphatic pumping pressure in the leg. Lymphology. 2011;44(3):113–120. [PubMed] [Google Scholar]
- 20.Tan IC, Maus EA, Rasmussen JC, Marshall MV, Adams KE, Fife CE, et al. Assessment of Lymphatic Contractile Function After Manual Lymphatic Drainage Using Near-Infrared Fluorescence Imaging. Arch. Phys. Med. Rehabil. 2011;92(5):756.e1–764.e1. doi: 10.1016/j.apmr.2010.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adams KE, Rasmussen JC, Darne C, Tan I-C, Aldrich MB, Marshall MV, et al. Direct evidence of lymphatic function improvement after advanced pneumatic compression device treatment of lymphedema. Biomedical Optics Express. 2010;1(1):114–125. doi: 10.1364/BOE.1.000114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pond CM, Mattacks CA. Interactions between adipose tissue around lymph nodes and lymphoid cells in vitro. J. Lipid Res. 1995;36(10):2219–2231. [PubMed] [Google Scholar]
- 23.Pond CM, Mattacks CA. In vivo evidence for the involvement of the adipose tissue surrounding lymph nodes in immune responses. Immunol. Lett. 1998;63(3):159–167. doi: 10.1016/s0165-2478(98)00074-1. [DOI] [PubMed] [Google Scholar]
- 24.Pond CM, Mattacks CA. The source of fatty acids incorporated into proliferating lymphoid cells in immune-stimulated lymph nodes. Br. J. Nutr. 2003;89(03):375–382. doi: 10.1079/BJN2002784. [DOI] [PubMed] [Google Scholar]
- 25.Kwon S, Sevick-Muraca EM. Functional lymphatic imaging in tumor-bearing mice. J. Immunol. Methods. 2010;360(1–2):167–172. doi: 10.1016/j.jim.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aschen S, Zampell JC, Elhadad S, Weitman E, De Brot Andrade M, Mehrara BJ. Regulation of Adipogenesis by Lymphatic Fluid Stasis: Part II. Expression of Adipose Differentiation Genes. Plast. Reconstr. Surg. 2012;129(4):838–847. doi: 10.1097/PRS.0b013e3182450b47. 10.1097/PRS.0b013e3182450b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cook A, Cowan C. Adipose. In: Chien KR, editor. Stembook. Stembook; 2009. [Google Scholar]
- 28.Mihara M, Hara H, Araki J, Kikuchi K, Narushima M, Yamamoto T, et al. Indocyanine Green (ICG) Lymphography Is Superior to Lymphoscintigraphy for Diagnostic Imaging of Early Lymphedema of the Upper Limbs. PLoS ONE. 2012;7(6):e38182. doi: 10.1371/journal.pone.0038182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall MA, Robinson H, Chan W, Sevick-Muraca EM. Detection of lymphangiogenesis by near-infrared fluorescence imaging and responses to VEGF-C during healing in a mouse fulldermis thickness wound model. Wound Repair Regen. 2013;21(4):604–615. doi: 10.1111/wrr.12063. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Video 1: NIRFLI lateral view of lymphatics in arm of participant DD3 (Figure 2(B)).
Supplemental Video 2: NIRFLI lateral view of lymphatics in arm of participant NC2 (Figure 2(A)).
Supplemental Video 3: NIRFLI anterial view of lymphatics in lower leg of participant DD3.
Supplemental Video 4: NIRFLI anterial view of lymphatics in lower leg of participant NC3.
Supplemental Video 5: NIRFLI medial view of knee in participant DD1.
Supplemental Video 6: NIRFLI medial view of knee in participant NC3 (Reproduced with permission from [16]).
Supplemental Video 7: NIRFLI of clinician’s (KH) palpation of inguinal lipoma on participant DD3. The bright region represents a fibrotic mass located in the inguinal.
Supplemental Video 8: NIRFLI of clinician’s (KH) palpation of fluorescent lymphatic vessel and lipoma in leg of DD3. The fluorescent tracks corresponded to the presence of tender, nodular, tubular structures.