Abstract
Acute pancreatitis (AP), while most often a mild and self-limiting inflammatory disease, worsens to a characteristically necrotic severe acute pancreatitis (SAP) in about 20% of cases. Obesity, affecting more than a third of American adults, is a risk factor for the development of SAP, but the exact mechanism of this association has not been identified. Coincidental with chronic low-grade inflammation, activation of the NLRP3 inflammasome increases with obesity. Lean mice genetically deficient for specific components of the NLRP3 inflammasome are protected from experimentally-induced AP, indicating a direct involvement of this pathway in AP pathophysiology. We hypothesized that inhibition of the NLRP3 inflammasome with the sulfonylurea drug glyburide would reduce disease severity in obese mice with cerulein-induced SAP. Treatment with glyburide led to significantly reduced relative pancreatic mass and water content and less pancreatic damage and cell death in genetically obese ob/ob mice with SAP compared to vehicle-treated obese SAP mice. Glyburide administration in ob/ob mice with cerulein induced SAP also resulted in significantly reduced serum levels of interleukin-6, lipase and amylase, and led to lower production of LPS-stimulated IL-1β release in cultured peritoneal cells, compared to vehicle treated ob/ob mice with SAP. Together, these data indicate involvement of the NLRP3 inflammasome in obesity-associated SAP, and expose the possible utility of its inhibition in prevention or treatment of SAP in obese individuals.
INTRODUCTION
While typically a mild and self limiting disease, approximately 20% of acute pancreatitis (AP) cases worsen to severe acute pancreatitis (SAP), characterized by pancreatic tissue necrosis, high morbidity and up to a 20% mortality rate [1–3]. Obesity is an established risk factor in the progression from AP to SAP, and mortality due to SAP complications [1, 4, 5]. Despite the observed connection between obesity and SAP, the mechanisms of increased AP severity in the obese continue to remain unknown.
Both genetic and diet-induced obesity (DIO) increase disease severity in multiple AP models in rodents, including co-administration of IL-12+IL-18, pancreatic duct retrograde infusion of sodium taurocholate (NaT) and repeated injection of cerulein [2, 6–11]. Former work by our group demonstrated that, despite differences in physiology between genetically obese and DIO models, the presence of obesity, rather than leptin/leptin signaling deficiency, results in SAP [7]. Thus, while not exactly mimicking the pathophysiology of human obesity, genetically obese rodents provide relevant insight into the pathophysiological outcomes of SAP.
The nucleotide-binding domain, leucine-rich-containing family, pyrin-domain-containing-3 (NLRP3) inflammasome is an intracellular multimolecular pattern recognition receptor responsible for the caspase-1 (CASP1)-mediated activation and secretion of the pro-inflammatory cytokines interleukin (IL)-1β and IL-18 [12–14]. Obesity is associated with a state of chronic low-grade inflammation and increased circulating inflammatory cytokines, which are linked to the pathogenesis of obesity-related diseases, such as cardiovascular disease and type-2 diabetes [15–18]. Coincidental with this chronic inflammation, NLRP3 inflammasome activity is increased in obese individuals, as a result of adipocyte hypertrophy, hypoxia due to poor vascularization of expanding adipose tissue and necrotic cell death, leading to reactive oxygen species (ROS) production and the release of free fatty acids (FFAs) and inflammatory cytokines [19–21].
NLRP3 inflammasome activation has also been implicated in rodent models of AP. Hoque and colleagues have shown that NLRP3, ASC (apoptosis-associated speck-like protein containing a CARD; an adaptor molecule in the NLRP3 inflammasome structure) and CASP1 genes are required for development of pancreatic inflammation in AP in lean rodents [22]. Moreover, treatment with IL-1 receptor antagonist (IL-1RA) before or during the initiation of AP reduces pancreatic inflammation and tissue damage. Upstream of IL-1β, CASP1 inhibition in rats ameliorates disease severity and increases survival in NaT7 induced SAP [23–25]. Thus, blocking IL-1 maturation or activity effectively reduces AP severity in lean animals.
Glyburide (International Nonproprietary Name: glibenclamide), an antidiabetic sulfonylurea drug, inhibits NLRP3 inflammasome activation by several stimuli, without affecting the NLRC4 or NLRP1 inflammasomes [14]. The mechanism of action of glyburide on the NLRP3 inflammasome is not completely understood, though the drug appears to interfere with signaling events upstream of the NLRP3 subunit. Specifically, glyburide inhibits the regulatory subunit of ATP-sensitive potassium channels (KATP) on the cell membrane, which prevents a cellular efflux of K+ [14, 26]. As an intracellular drop in K+ (below 70 mM) is known to induce NLRP3 inflammasome activation, inhibition of KATP channels is a very plausible mechanism of action for glyburide [27]. Glyburide’s inhibitory effect has largely been documented using in vitro cell culture, but a handful of studies have shown glyburide to effectively inhibit the NLRP3 inflammasome in vivo as well. For example, glyburide significantly delays lipopolysaccharide (LPS)-induced lethality, prevents onset of experimental colitis and reduces the severity of ventilator-induced lung injury in rodents [14, 28, 29]. In human diabetic patients, glyburide is associated with reduced mortality during meliodosis (caused by the gram negative bacterium Burkholderia pseudomallei), which is correlated to glyburide’s anti-inflammatory effects [30].
Because the NLRP3 inflammasome is activated in obesity and has been involved in the pathophysiology of AP in lean mice, we hypothesized this pathway may be mechanistically involved in the increased AP severity of obesity. We thus used glyburide to inhibit the NLRP3 inflammasome in lean and genetically obese mice prior to induction of experimental AP with cerulein, under the expectation that inhibition of the NLRP3 inflammasome would afford increased protection in obese compared to lean animals.
METHODS
All reagents and chemicals were purchased from Sigma-Aldrich (St. Louis MO) unless otherwise noted.
Animals
Animal use was conducted in accordance with The Guide for the Care and Use of Laboratory Animals, all protocols were approved by the Animal Care Committee of the University of Illinois at Chicago, and all efforts were made to minimize animal suffering [31]. 8–10 week-old male lean WT and obese ob/ob (B6.V-Lepob/OlaHsd) C57BL/6 mice were obtained from Harlan Laboratories (Indianapolis, IN). Mice were allowed to acclimatize to the facility environment for a minimum of 1 week prior to experimentation. Mice were group-housed (5 mice/cage) in standard shoebox cages with microisolator tops and corncob bedding in an AAALAC accredited laboratory facility. Food (Harlan Teklad LM-485 7012 Rodent Diet) and water were available to animals ad libitum at all times. Mice were maintained in an environmentally controlled room on a 12 h light/dark cycle at a temperature of 72 (± 2) °F and a humidity of 30 – 70%. All experimental procedures were performed in a clean, well ventilated laboratory space.
Treatments
Individual WT and ob/ob mice were weight matched and randomized to one of four treatment groups: Vehicle + PBS, Vehicle + Cerulein , Glyburide + PBS and Glyburide + Cerulein, for a total of 8 groups (four treatment groups per strain). Glyburide was prepared for injection as previously [14, 32]. Briefly, Glyburide was dissolved to a final concentration of 33.3 mg/mL in 2.5% DMSO/97.5% 2-hydroxypropyl-β-cyclodextrin (10%). Mice received intraperitoneal (IP) injections of either vehicle (v/v) or glyburide (500 mg/kg) at −13 h, −1 h and 9 h relative to induction of AP (first injection of cerulein, beginning at the onset of the light phase of their light-dark cycle). Experimental AP was induced in mice via eight (8) hourly injections of cerulein (100 μg/kg, or v/v PBS vehicle), as has been previously established [33]. 10 h after induction of AP, mice were euthanized via lethal dose of anesthesia and cervical dislocation and tissues and body fluids collected for further analysis.
Miscellaneous measurements
Blood was collected immediately after euthanasia for serum separation. Immediately following peritoneal lavage, the peritoneal cavity was opened and pancreas removed. Whole pancreatic weight was measured to determine relative pancreatic weight (percent body weight). A portion of the pancreas was weighed before and after drying in a 95 °C oven to determine its water content as a measure of pancreatic edema. A separate portion of the pancreas was fixed in formalin, embedded in paraffin, sliced, mounted and stained with hematoxylin and eosin (H&E) or Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) for histological assessment. Histology services were provided by the Research Resources Center - Research Histology and Tissue Imaging Core at the University of Illinois at Chicago established with the support of the Vice Chancellor of Research. Histology sections were scored by a pathologist (RJC) blinded to experimental treatments, as previously described [2, 7]. Briefly, edema was scored as 0 (absent or rare), 1 (in the interlobular space), 2 (in the intralobular space), and 3 (severe edema); inflammatory cell infiltrate was scored as 0 (absent), 1 (mild), 2 (moderate), and 3 (severe); and acinar cell and intrapancreatic fat necrosis were independently graded as 0 (absent), 1 (focal), 2 (and/or sublobular), and 3 (and/or lobular). Scores for edema, inflammatory infiltrate, acinar necrosis and intrapancreatic fat necrosis were analyzed individually, as well as summed prior to statistical analysis. TUNEL-stained sections were scored by averaging TUNEL positive cells in 4 randomly selected 200× microscopic fields per sample. Peripancreatic fat necrosis was scored using an arbitrary scoring system of 0 for absence of fat necrosis or 1 for presence of fat necrosis, and averaging treatment group scores. Serum amylase and lipase were measured using kits from Teco Diagnostics (Anaheim, CA) and Trinity Biotech (Jamestown, NY), respectively. Cytokine levels were measured using ELISA kits from R&D Systems (Minneapolis, MN) or eBioscience (San Diego, CA).
Peritoneal cell LPS stimulation
To assess treatment differences on NLRP3 inflammasome activation, IL-1β release was measured in LPS-stimulated peritoneal cells collected by lavage immediately following euthanasia. 5 mL of RPMI 1640 (Gibco, Grand Island, NY), supplemented with 1% penicillin/streptomycin, were injected into the peritoneal sac, lightly jostled and drawn out using a sterile syringe and 18 gauge needle. Cells were subsequently washed, counted and plated at 2.5 × 106 cells/mL in sterile 24-well plates. Cells were then stimulated with LPS (Escherichia coli O111:B1; 1 μg/mL). After overnight (18 h) incubation, culture supernatants were collected for assessment of IL-1β release by ELISA.
Statistical analysis
Data are expressed as mean ± s.e.m. Data were analyzed using SAS 9.2 (SAS Institute, Inc., Cary NC). Statistically significant differences between treatment groups were determined by ANOVA as appropriate. Statistical significance was denoted at P < 0.05 for all comparisons.
RESULTS
Glyburide reduces pancreatic damage in response to cerulein in obese ob/ob mice
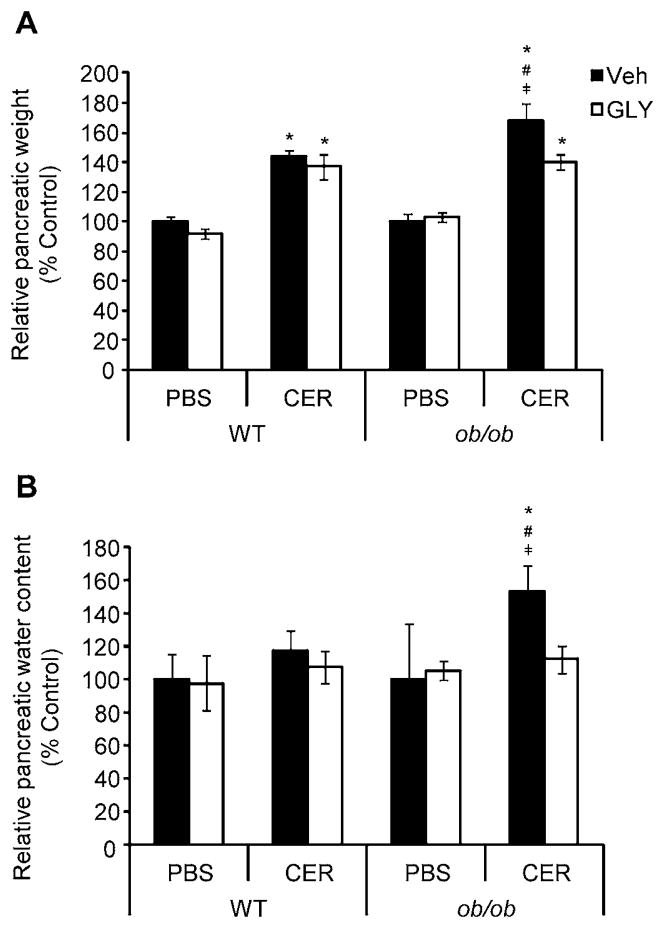
As expected, ob/ob mice weighed significantly more than did lean WT mice prior to experimentation (24.877 ± 0.319 g v. 43.521 ± 0.813 g, WT v. ob/ob, respectively; P < 0.05). The ratio of whole pancreas to-body weight is a common indicator of AP-associated pancreatic edema, and was used as such in our study. As shown in Figure 1a, WT mice exhibited a significant increase in relative pancreatic weight over control following AP induction. Ob/ob mice showed a similar response to AP induction, with a significant increase in relative pancreatic weight compared to both WT mice with AP as well as ob/ob controls. While glyburide treatment had no effect on reducing the increase in relative pancreatic mass over control in WT mice receiving cerulein, glyburide-treated ob/ob mice showed a significant reduction in pancreatic weight compared to vehicle-treated ob/ob mice with AP. As an additional measure of pancreatic edema, we estimated water content by weighing a portion of the pancreas before and after drying in a 95 °C oven. Figure 1b shows that, compared to all respective treatment groups, ob/ob mice had significantly greater increase in relative pancreatic water content following AP induction. WT AP showed only a slight, but non-statistically significant, increase in relative pancreatic water content, which differs in comparison to the significant increase in relative pancreatic weight over control in WT AP mice shown in Figure 1a. This difference could be attributed to the fact that the relative pancreatic weight (Figure 1a) made use of the whole pancreas during analysis, while only a portion of the pancreas was dried in an oven for determination of relative pancreatic water content. As WT developed a milder AP than did obese ob/ob mice, it is possible that not all of the pancreas was affected by cerulein in lean mice, leading to the differences observed in the two estimations of pancreatic edema. Glyburide treatment in ob/ob mice with AP significantly reduced pancreatic water content, to a level on par with both lean and obese non-AP control mice.
Figure 1. Relative pancreatic weight and water content in lean WT and obese ob/ob mice following cerulein-induced AP, with or without glyburide treatment.
AP was induced in lean WT and obese ob/ob via eight (8) hourly IP injections of cerulein (CER; 100 μg/kg or PBS (v/v) control) following glyburide (GLY; 500 mg/kg) or vehicle (Veh; v/v) treatment. (a) Following sacrifice, pancreata were removed and their relative weight determined as a percentage of total body weight. (b) A portion of each pancreas was subsequently weighed before and after drying in at 95 °C to determine water content. Results are expressed as percent control (within respective strains), mean ± s.e.m. n = 3–14 mice per group. *P < 0.05 vs. respective PBS-treated group. #P < 0.05 vs. respective WT group. ╪P < 0.05 vs. respective glyburide-treated group.
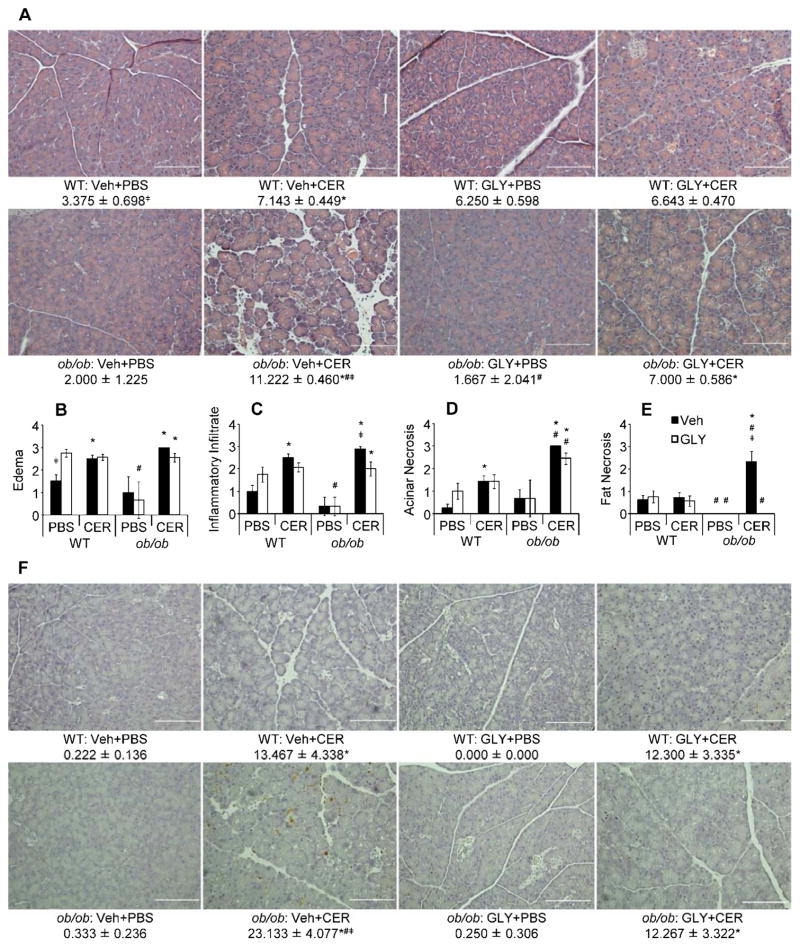
Scoring of H&E-stained pancreatic sections revealed significantly greater pancreatic tissue damage in cerulein-treated ob/ob mice compared to WT mice (Representative H&E stained pancreatic tissue sections shown in Figure 2a). Individually, histology scores for edema (Figure 2b), inflammatory infiltrate (Figure 2c), acinar necrosis (Figure 2d) and intrapancreatic fat necrosis (Figure 2e) generally showed increases in severity of AP-associated pancreatic damage over their respective controls. Glyburide significantly reduced scores only for pancreatic inflammatory infiltrate and fat necrosis in ob/ob AP mice (Figure 2c and 2e, respectively), whereas non-significant reductions in edema and acinar necrosis occurred with glyburide treatment in ob/ob AP mice (Figure 2b and 2d, respectively). Lean WT mice with AP had increases over their respective PBS-injected controls in all scored criteria except fat necrosis, but glyburide treatment failed to reduce scores significantly (Figure 2b-e). To get a broader picture of how the scoring criteria reflected the overall pancreatic damage as a whole, individual scores were summed, as we have done in previously [7]. Summed histology scoring revealed that ob/ob AP mice had significantly greater pancreatic damage, over both WT AP mice and PBS injected ob/ob control mice (Figure 2a). Glyburide administration yielded a significant reduction in pancreatic damage severity in ob/ob mice, to a level comparable to that of WT mice with AP. However, no such reduction was observed in lean mice with milder AP (Figure 2a). To determine the degree of pancreatic acinar cell death, TUNEL staining of pancreatic tissue sections was performed and revealed that both lean and obese mice with AP had increased acinar cell death versus their respective non-AP controls, and that cerulein-injected ob/ob mice had a higher level of acinar death compared to WT mice (Figure 2f). Remarkably, injection of glyburide significantly reduced acinar cell death in ob/ob mice but had no effect in lean animals (Figure 2f).
Figure 2. Pancreatic alterations and cell death in WT and ob/ob mice following cerulein-induced AP with or without glyburide treatment.
AP was induced in lean WT and obese ob/ob mice via eight (8) hourly IP injection of cerulein (CER; 100 μg/kg or PBS (v/v) control) following glyburide (GLY; 500 mg/kg) or vehicle (Veh; v/v) treatment. Following sacrifice, pancreata were removed and a portion fixed in formalin for paraffin embedding, slicing and staining with H&E or TUNEL. (a) Representative micrographs (200X) of H&E stained pancreatic sections, and summed H&E scores. (b–e) Individual H&E scores for (b) edema, (c) inflammatory infiltrate, (d) acinar necrosis and (e) intrapancreatic fat necrosis. (f) Representative micrographs (200X) of TUNEL stained pancreatic sections, and mean TUNEL scores (see methods section for scoring criteria/procedure). Summed H&E (a) results are expressed as total individual scores for edema, inflammatory infiltrate, acinar cell death and fat necrosis, mean ± s.e.m. n = 3–14 mice per group. Individual H&E score (b–e) results are expressed as mean ± s.e.m. n = 3–14 mice per group. TUNEL (f) results are expressed as mean number of TUNEL positive cells in 4 scored 200X microscopic fields, mean ± s.e.m. n = 3–5 mice per group. *P < 0.05 vs. respective PBS-treated group. #P < 0.05 vs. respective WT group. ╪P < 0.05 vs. respective glyburide-treated group.
Intra- and peri-pancreatic fat necrosis, both hallmarks of SAP [27], were significantly increased over WT AP and ob/ob PBS-injected control mice in ob/ob mice injected with cerulein (Figure 2e and Figure 3) [7, 34]. Peripancreatic fat necrosis occurs when pancreatic enzymes (namely lipase) extravasate from the pancreas and damage surrounding adipose tissue. Increased AP severity is associated with increased peripancreatic fat necrosis, which causes saponification of FFAs with calcium, leading to distinct opaque white foci on the affected adipose tissue [7, 35, 36]. Of note, peripancreatic fat necrosis was observed in cerulein-treated ob/ob mice, but not other treatment groups (Figure 3). Importantly, glyburide prevented development of both types of fat necrosis in ob/ob mice (Figure 2e and Figure 3).
Figure 3. Peripancreatic fat necrosis in ob/ob mice following cerulein-induced AP with or without glyburide pretreatment.
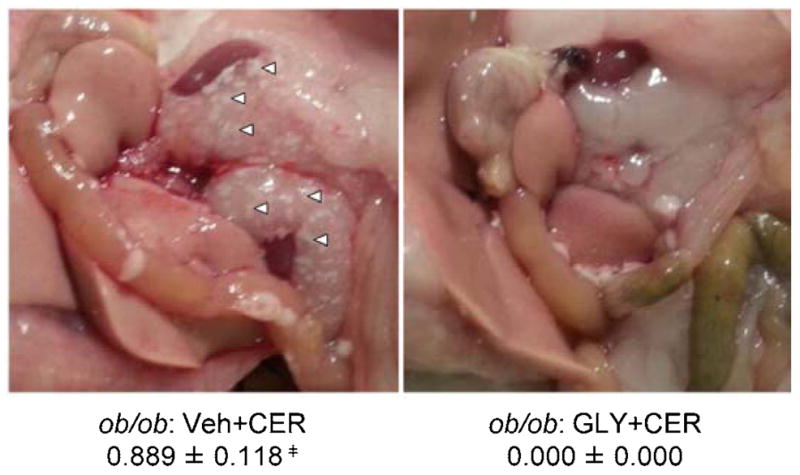
AP was induced in obese ob/ob mice via eight (8) hourly IP injection of cerulein (CER; 100 μg/kg or PBS (v/v) control) following glyburide (GLY; 500 mg/kg) or vehicle (Veh; v/v) treatment. Following sacrifice, the peritoneal cavity was exposed and presence or absence of peripancreatic adipose tissue saponification determined. Representative photographs showing peripancreatic fat saponification (white arrows: representative indication of fat necrosis), and scores in vehicle treated (left) and glyburide treated ob/ob AP mice. Results are expressed as scores (presence = 1 or absence = 0), mean ± s.e.m. n = 9 mice per group. ╪P < 0.05, vs. respective glyburide treated group.
In summary, obese mice developed more severe AP in response to cerulein, with glyburide restoring pathology to a level comparable to that of lean mice.
Obese mice with AP have elevated serum lipase, amylase and IL-6, which are reduced by glyburide
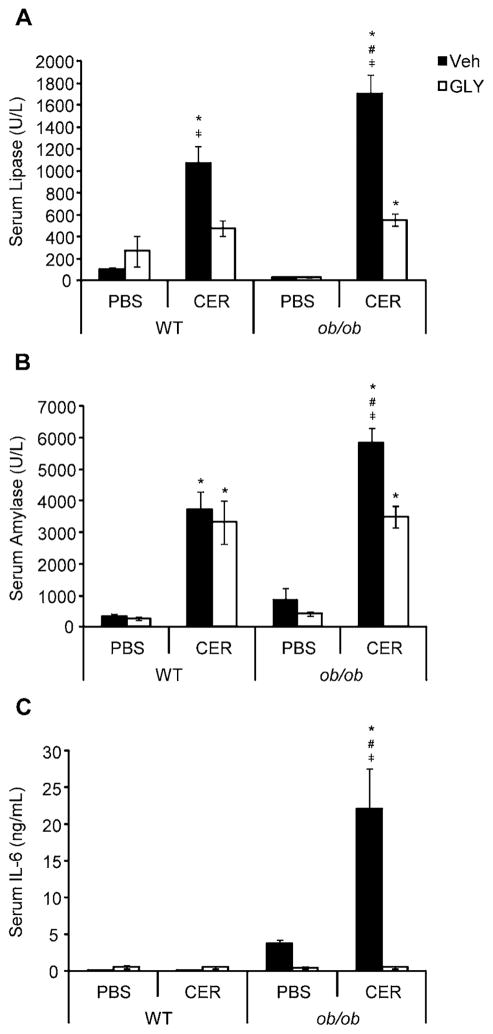
Elevation of serum lipase and amylase are used as diagnostic indicators of AP presence [7, 37]. In our experiments, cerulein-injected ob/ob mice exhibited significantly elevated serum lipase and amylase compared to both lean mice with AP and ob/ob mice without AP (Figure 4a–b). Glyburide dramatically reduced serum levels of both enzymes in ob/ob mice, down to levels equivalent to those of lean mice (Figure 4a–b). Glyburide also significantly reduced lipase – though not amylase – levels in lean mice with AP. The magnitude of elevation in serum IL-6, an indication of the degree of AP severity [10, 38], was markedly increased in cerulein-injected ob/ob mice compared to all relevant treatment groups, whereas cerulein did not significantly increase IL-6 in lean mice (Figure 4c). Glyburide significantly reduced serum IL-6 in ob/ob mice (Figure 4c). Concordant with our histopathological findings, glyburide’s ability to reduce serum levels of lipase, amylase and IL-6 in obese mice to that of lean AP animals supports the hypothesis that the NLRP3 inflammasome is a major contributor to obesity-associated SAP development.
Figure 4. Serum levels of pancreatic enzymes and IL-6 in WT and ob/ob mice following cerulein-induced AP with or without glyburide treatment.
AP was induced in lean WT and obese ob/ob mice via eight (8) hourly IP injection of cerulein (CER; 100 μg/kg or PBS (v/v) control) following glyburide (GLY; 500 mg/kg) or vehicle (Veh; v/v) treatment. Following sacrifice, blood was drawn for serum separation and analysis of (a) serum lipase, (b) serum amylase and (c) serum IL-6 levels. Results are expressed as mean ± s.e.m. n = 3–14 mice per group. *P < 0.05 vs. respective PBS-treated group. #P < 0.05 vs. respective WT group. ╪P < 0.05 vs. respective glyburide-treated group.
Glyburide reduces IL-1β release from peritoneal cells
As an indication of glyburide’s ability to inhibit NLRP3 inflammasome activity, peritoneal cells were collected via lavage for ex vivo culture and LPS stimulation immediately following sacrifice. LPS was selected as a stimulus since release of IL-1β in response to this compound is NLRP3-dependent [14, 39]. Figure 5 shows that LPS-stimulated peritoneal cells from ob/ob mice released significantly more IL-1β than cells from lean mice. As expected, cells from glyburide-treated mice released lower amounts of IL-1β, particularly in obese mice. In summary, Figure 5 provides two important pieces of information: first, that the NLRP3 inflammasome is more active in obese mice, as indicated by a more robust IL-1β response; and second, that glyburide effectively inhibits the NLRP3 inflammasome, as evidenced by a significant reduction in IL-1β release. Serum levels of IL-1β, a potential additional marker of inflammasome activity, were below detection level in all treatment groups.
Figure 5. LPS-stimulated IL-1β production by ex vivo peritoneal cells from WT and ob/ob mice following cerulein-induced AP with or without glyburide treatment.
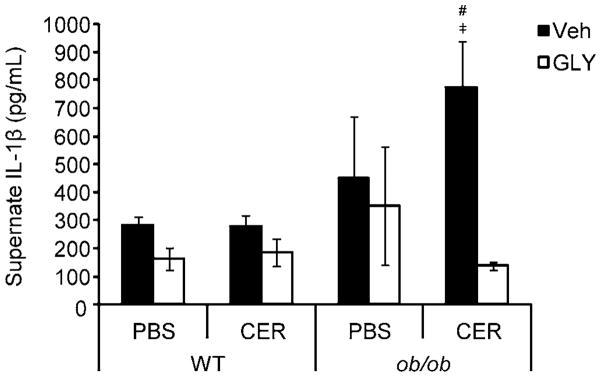
AP was induced in lean WT and obese ob/ob mice via eight (8) hourly IP injection of cerulein (CER; 100 μg/kg or PBS (v/v) control) following glyburide (GLY; 500 mg/kg) or vehicle (Veh; v/v) treatment. Following sacrifice, peritoneal cells were collected via lavage, washed, plated, stimulated with LPS (1 μg/mL) and incubated overnight. Supernatents were collected for analysis of IL-1β concentration by ELISA. Results are expressed as mean ± s.e.m. n = 3–14 mice per group. #P < 0.05 vs. respective WT group. ╪P < 0.05 vs. respective glyburide treated group.
DISCUSSION
In agreement with previously reported findings, our results show that, compared to lean mice, obese ob/ob mice developed more severe cerulein-induced AP pathology, co-occurring with increased relative pancreatic weight and serum levels of lipase, amylase and IL-6 [9]. The observed SAP in ob/ob mice not only supports an increasing number of animal studies demonstrating a connection between obesity and SAP, but also echoes clinical findings in humans in which obesity is an accepted risk factor for the development of SAP [1, 2, 7, 9, 40, 41]. Administration of glyburide reduced AP severity in ob/ob mice, while it had little-to-no effect on the milder AP seen in lean WT mice. While not exactly mimicking the findings shown by Hoque et al., in which genetic deletion completely ablated NLRP3 inflammasome functionality in NLRP3, ASC and CASP1 knockout mice, our approach is different in that we pharmacologically inhibited the NLRP3 inflammasome in mice with functional NLRP3 inflammasome machinery. Glyburide, therefore, may exert its inhibitory effects and reduced disease severity only in mice with SAP (obese ob/ob in this study), while having little-to-no utility in mice with milder AP (lean WT in this study). This is the first study demonstrating that experimental SAP in obese rodents can be mitigated using glyburide, which has been shown to inhibit the NLRP3 inflammasome.
Histopathology scoring revealed significantly greater cerulein-induced pancreatic tissue damage in ob/ob than lean mice. Whereas glyburide had little effect in reducing the mild pancreatic pathology of WT mice, it significantly reduced histology scores in obese mice to the level of lean mice. Intrapancreatic fat necrosis, in particular, was markedly pronounced in ob/ob mice with AP compared to all other relevant treatment groups. Complementing the increased fat necrosis within the pancreas, peripancreatic fat saponification was exclusively observed in obese mice with SAP, and was eliminated in ob/ob AP mice treated with glyburide. Peripancreatic fat necrosis leading to saponification is considered an important factor in the severity of SAP [27, 42]. While previously reported in other models of experimental AP, ours is the first study to show an obesity-associated occurrence of cerulein-induced peripancreatic fat saponification, and that glyburide administration led to its prevention, presumably via inhibition of the NLRP3 inflammasome [2, 7, 43, 44].
As we expected following induction of cerulein AP, we observed significantly greater increase in serum levels of lipase and amylase in obese compared to lean AP mice. Elevations in serum lipase and amylase are generally indicative of AP, and are in accord with previous studies using cerulein and other experimental AP models [7, 37]. The magnitude of enzyme increase can vary, however, and is not necessarily indicative of AP severity [2, 5, 9]. Similar to the relative pancreatic weight and water content findings (Figure 1a and 1b, respectively), glyburide had mixed effects on reducing serum pancreatic enzymes in WT AP mice, with a reduction in lipase, but not amylase. These results further support other evidence that lean WT mice in our study have mild cerulein-induced AP, which is generally unaffected by glyburide administration, indicating minimal involvement of the NLRP3 inflammasome in its pathophysiology. As with its ability to reduce the observed histopathological indices of AP severity, glyburide treatment also reduced serum levels of these pancreatic enzymes in obese mice. In particular, because of its role in digestion of lipids, the reduction in lipase levels by glyburide may ultimately lead to reduced digestion of triglycerides to FFAs [27]. Increased FFAs may be a critical determinant of AP severity and have been shown to exacerbate disease in obese ob/ob mice with IL-12+IL-18-induced SAP [5]. However, our data show that glyburide treatment in ob/ob AP mice leads to significantly reduced serum lipase concentrations, indicating that inflammasome activation may occur upstream of FFA generation. Further studies are needed to determine the individual contributions of NLRP3 inflammasome activation versus increased FFA in AP severity, especially within the context of obesity.
At variance with serum amylase and lipase levels, serum IL-6 levels are good predictors of AP severity [10, 38]. Our findings show that ob/ob mice with AP had significantly greater serum IL-6 levels than lean AP mice, which was expected based on previous animal and clinical findings [2, 45]. As IL-6 release is correlated to disease severity in AP, and given the mild nature of AP in lean mice, we expected to see little, if any, increase in serum IL-6 in lean mice. In obese ob/ob mice, however, the stark and significant increase in serum IL-6 was as we predicted and in support of the histological findings, given the greater severity of AP in the ob/ob mice. Glyburide significantly reduced serum IL-6 in ob/ob mice with AP, pointing to the potential important role of the NLRP3 inflammasome in mediating aberrant release of this cytokine during SAP, likely as a result of increased levels of IL-1β. While not included as a part of our study, glyburide’s effect on lowering serum IL-6 may lead to a more rapid resolution of AP, because IL-6 is known to participate in the heightened acute-phase response and to sustain inflammation during experimental AP [10].
We expected to see a greater concentration of IL-1β released from peritoneal cells of obese mice, since it had previously been shown that: a) NLRP3 inflammasome activity is increased in obesity, and b) the NLRP3 inflammasome has a role in the development and pathology of AP in lean mice [18, 22]. Indeed, following stimulation with LPS, peritoneal cells from ob/ob mice released a significantly higher concentration of IL-1β than did peritoneal cells from lean mice. In ob/ob mice, administration of glyburide lead to a significant reduction in LPS-induced IL-1β release, confirming inhibition of the inflammasome by glyburide, since release of IL-1β in response to LPS is dependent on inflammasome activation [46, 47]. We observed a larger-than expected standard error in PBS-treated ob/ob mouse IL-1β release in response to LPS stimulation ex-vivo. This likely occurred due to a limited number of mice used in these treatment groups (n = 3). PBS-treated control mice were included as negative AP controls in each strain to verify cerulein’s functionality within each mouse strain, but our principal focus was on glyburide’s effects on AP severity, so we centered much of our investigation and analyses on lean and obese AP mice with or without glyburide treatment.
Based on our work and that of others, the aberrant nature of NLRP3 inflammasome activity during obesity likely plays an important role in the progression from AP to SAP in obese mice. A steadily increasing volume of work has linked NLRP3 inflammasome activation to a variety of metabolic danger signals, including ROS, ATP, extracellular calcium, ceramides, cholesterol crystals, islet amyloid polypeptide and saturated FFAs [19, 46–49]. Although macrophages have been the focus of the majority of NLRP3 inflammasome research, NLRP3 mRNA expression occurs at low levels in adipocytes as well, indicating the importance of these cells for future research in the fields of metabolism, inflammation and disease [21]. Currently, the general consensus is that obese adipocytes and adipose tissue immune cells, especially macrophages, engage in crosstalk with each other via adipokines and cytokines, which then initiate NLRP3 inflammasome assembly and activation, thus leading to a chronic state of low-grade inflammation [19–21]. As the NLRP3 inflammasome is involved in the pathogenesis of AP in lean rodents, it seems reasonable that the already hyperactive NLRP3 inflammasome of the obese state results in a greater inflammatory onslaught to the pancreas during AP in obesity, and remains a threat throughout disease course, as lipase-mediated fat digestion and necrotic cell death generate additional danger signals, such as saturated FFAs and ATP. On the other hand, the basally less active NLRP3 inflammasome in lean WT mice has less of an impact on experimental AP severity. Therefore, while little effect in reducing disease severity was observed in lean mice, especially compared to obese mice in our study, additional dose-response and time-response studies will yield greater insight into NLRP3 inflammasome activity in lean AP mice.
While no direct measurements of the NLRP3 inflammasome itself were conducted in our study, Lamkanfi et al. have shown that IL-1β production following stimulation of NLRC4 or NLRP1 inflammasomes with their respective activators (S. typhimurium infection and anthrax lethal toxin, respectively) is unaffected in the presence of glyburide. This, in addition to the findings by the same group showing glyburide inhibits the inflammasome upstream of the NLRP3 subunit (likely through inhibition of KATP channel-mediated efflux of K+), provides strong evidence that glyburide is indeed specifically inhibiting NLRP3 inflammasome activation [14]. However, further study investigating the potential role of other inflammasomes (e.g., NLRP6, NLRP12) in AP pathophysiology are warranted, if only to rule out their involvement.
SPECULATIONS
To conclude, we have shown that pretreatment with the sulfonylurea drug glyburide leads to reduced severity of cerulein-induced AP in obese ob/ob mice, likely through inhibition of the NLRP3 inflammasome. Future work to better determine the mechanisms of NLRP3 inflammasome involvement in AP and SAP is needed, as well as studies designed to determine whether glyburide has a therapeutic effect on relieving AP severity after disease induction.
BACKGROUND
During acute pancreatitis (AP), obesity is a risk factor for developing severe acute pancreatitis (SAP). However, the mechanism(s) linking obesity and increased SAP risk are unknown. Concomitant with chronic inflammation, NLRP3 inflammasome hyperactivity also occurs during obesity. NLRP3 inflammasome functionality is required for development of AP in lean mice, indicating its potential role in AP pathophysiology.
TRANSLATIONAL SIGNIFICANCE
We show inhibition of the NLRP3 inflammasome with the sulfonylurea drug glyburide reduces the severity of experimental AP in genetically obese mice. As this drug is already approved for use in diabetic patients, our findings indicate glyburide’s therapeutic potential in human patients with SAP.
Acknowledgments
Funding for this work was provided by grants from the National Institutes of Health (DK082238 to G.F.), and by the Diabetes, Nutrition and Obesity Research Training Program (5T32DK080674-03; PI: Terry G Unterman) (Postdoctoral Fellowship to J.M.Y.).
ABBREVIATIONS IN TEXT/FIGURE LEGENDS
- AP
Acute pancreatitis
- SAP
Severe acute pancreatitis
- NaT
sodium taurocholate
- DIO
Diet-induced obesity
- NLRP3
nucleotide-binding domain, leucine-rich-containing family, pyrin-domain-containing-3
- CASP1
Caspase-1
- IL-12
Interleukin-12
- IL-18
Interleukin-18
- IL-1β
Interleukin-1beta
- IL-6
Interleukin-6
- IL-1RA
Interleukin-1 receptor antagonist
- ASC
apoptosis-associated speck-like protein containing a CARD
- ROS
Reactive oxygen species
- FFA
Free fatty acid
- LPS
Lipopolysaccharide
- WT
Wild type
- DMSO
Dimethyl Sulfoxide
- H&E
hematoxylin and eosin
- TUNEL
Terminal deoxynucleotidyl transferase dUTP nick end labeling
- ELISA
Enzyme-Linked Immunosorbent Assay
- RPMI
Roswell Park Memorial Institute medium
- ANOVA
Analysis of Variance
- CER
Cerulein
- GLY
Glyburide
- PBS
Phosphate buffered saline
- Veh
Vehicle
- ATP
Adenosine triphosphate
- IP
Intraperitoneal
- KATP
ATP-sensitive potassium channels
Footnotes
Conflicts of Interest: The authors confirm they have read the journal’s policy on disclosure of potential conflicts of interest and have none to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jason M York, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago IL, USA.
Karla J Castellanos, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago IL, USA.
Robert J Cabay, Department of Pathology, University of Illinois at Chicago, Chicago IL, USA.
Giamila Fantuzzi, Department of Kinesiology and Nutrition, University of Illinois at Chicago, Chicago IL, USA.
References
- 1.Al-Azzawi HH, Wade TE, Swartz-Basile DA, Wang S, Pitt HA, Zyromski NJ. Acute pancreatitis in obesity: adipokines and dietary fish oil. Digestive diseases and sciences. 2011 Aug;56(8):2318–25. doi: 10.1007/s10620-011-1626-x. [DOI] [PubMed] [Google Scholar]
- 2.Pini M, Sennello JA, Cabay RJ, Fantuzzi G. Effect of Diet-induced Obesity on Acute Pancreatitis Induced by Administration of Interleukin-12 Plus Interleukin-18 in Mice. Obesity. 2010;18(3):476–81. doi: 10.1038/oby.2009.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhatia M. Acute pancreatitis as a model of SIRS. Frontiers in bioscience (Landmark edition) 2009;14:2042–50. doi: 10.2741/3362. [DOI] [PubMed] [Google Scholar]
- 4.Martinez J, Johnson CD, Sanchez-Paya J, de Madaria E, Robles-Diaz G, Perez-Mateo M. Obesity is a definitive risk factor of severity and mortality in acute pancreatitis: an updated meta-analysis. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al] 2006;6(3):206–9. doi: 10.1159/000092104. [DOI] [PubMed] [Google Scholar]
- 5.Navina S, Acharya C, DeLany JP, et al. Lipotoxicity causes multisystem organ failure and exacerbates acute pancreatitis in obesity. Science translational medicine. 2011 Nov 2;3(107):107ra10. doi: 10.1126/scitranslmed.3002573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Segersvard R, Tsai JA, Herrington MK, Wang F. Obesity alters cytokine gene expression and promotes liver injury in rats with acute pancreatitis. Obesity (Silver Spring, Md) 2008 Jan;16(1):23–8. doi: 10.1038/oby.2007.27. [DOI] [PubMed] [Google Scholar]
- 7.Sennello JA, Fayad R, Pini M, et al. Interleukin-18, together with interleukin-12, induces severe acute pancreatitis in obese but not in nonobese leptin-deficient mice. Proceedings of the National Academy of Sciences. 2008 Jun 10;105(23):8085–90. doi: 10.1073/pnas.0804091105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Segersvard R, Sylvan M, Herrington M, Larsson J, Permert J. Obesity increases the severity of acute experimental pancreatitis in the rat. Scandinavian journal of gastroenterology. 2001 Jun;36(6):658–63. [PubMed] [Google Scholar]
- 9.Zyromski NJ, Mathur A, Pitt HA, et al. A murine model of obesity implicates the adipokine milieu in the pathogenesis of severe acute pancreatitis. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2008 Sep 05;295(3):G552-G8. doi: 10.1152/ajpgi.90278.2008. 17:12:09. [DOI] [PubMed] [Google Scholar]
- 10.Pini M, Rhodes DH, Castellanos KJ, et al. Role of IL-6 in the resolution of pancreatitis in obese mice. Journal of leukocyte biology. 2012 Jun;91(6):957–66. doi: 10.1189/jlb.1211627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Araki H, Nishihara T, Matsuda M, et al. Adiponectin plays a protective role in caerulein-induced acute pancreatitis in mice fed a high-fat diet. Gut. 2008 Oct 1;57(10):1431–40. doi: 10.1136/gut.2007.135665. [DOI] [PubMed] [Google Scholar]
- 12.Schroder K, Tschopp J. The inflammasomes. Cell. 2010 Mar 19;140(6):821–32. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 13.Horng T, Hotamisligil GS. Linking the inflammasome to obesity-related disease. Nature medicine. 2011 Feb;17(2):164–5. doi: 10.1038/nm0211-164. [DOI] [PubMed] [Google Scholar]
- 14.Lamkanfi M, Mueller JL, Vitari AC, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. The Journal of cell biology. 2009 Oct 5;187(1):61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speaker KJ, Fleshner M. Interleukin-1 beta: a potential link between stress and the development of visceral obesity. BMC physiology. 2012;12:8. doi: 10.1186/1472-6793-12-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emanuela F, Grazia M, Marco de R, Maria Paola L, Giorgio F, Marco B. Inflammation as a Link between Obesity and Metabolic Syndrome. Journal of nutrition and metabolism. 2012;2012:476380. doi: 10.1155/2012/476380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lumeng CN, Saltiel AR. Inflammatory links between obesity and metabolic disease. The Journal of clinical investigation. 2011 Jun;121(6):2111–7. doi: 10.1172/JCI57132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell metabolism. 2012 Jan 4;15(1):10–8. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 19.Gregor MF, Hotamisligil GS. Inflammatory Mechanisms in Obesity. Annual review of immunology. 2011;29(1):415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 20.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nature reviews Immunology. 2006 Oct;6(10):772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 21.Vandanmagsar B, Youm YH, Ravussin A, et al. The NLRP3 inflammasome instigates obesity induced inflammation and insulin resistance. Nature medicine. 2011 Feb;17(2):179–88. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011 Jul;141(1):358–69. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makhija R, Kingsnorth AN. Cytokine storm in acute pancreatitis. Journal of hepato-biliary pancreatic surgery. 2002;9(4):401–10. doi: 10.1007/s005340200049. [DOI] [PubMed] [Google Scholar]
- 24.Paszkowski AS, Rau B, Mayer JM, Moller P, Beger HG. Therapeutic application of caspase 1/interleukin-1beta-converting enzyme inhibitor decreases the death rate in severe acute experimental pancreatitis. Annals of surgery. 2002 Jan;235(1):68–76. doi: 10.1097/00000658-200201000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Norman J, Yang J, Fink G, et al. Severity and mortality of experimental pancreatitis are dependent on interleukin-1 converting enzyme (ICE) Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1997 Feb;17(2):113–8. doi: 10.1089/jir.1997.17.113. [DOI] [PubMed] [Google Scholar]
- 26.Serrano-Martin X, Payares G, Mendoza-Leon A. Glibenclamide, a blocker of K+(ATP) channels, shows antileishmanial activity in experimental murine cutaneous leishmaniasis. Antimicrobial agents and chemotherapy. 2006 Dec;50(12):4214–6. doi: 10.1128/AAC.00617-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death & Differentiation. 2007;14(9):1583–9. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Liu L, Ye M, Yang J, Joosse M, Marohn M, editors. JOURNAL OF IMMUNOLOGY. AMER ASSOC IMMUNOLOGISTS; 9650 ROCKVILLE PIKE, BETHESDA, MD 20814 USA: 2013. Spontaneous and persistent activation of NLRP3/NALP3 inflammasome plays a critical pathological role in colitis of IL-10 deficient (IL-10-/-) mice. [Google Scholar]
- 29.Kuipers MT, Aslami H, Janczy JR, et al. Ventilator-induced Lung Injury Is Mediated by the NLRP3 Inflammasome. Anesthesiology. 2012;116(5):1104–15. doi: 10.097/ALN.0b013e3182518bc0. [DOI] [PubMed] [Google Scholar]
- 30.Koh GCKW, Maude RR, Schreiber MF, et al. Glyburide Is Anti-inflammatory and Associated with Reduced Mortality in Melioidosis. Clinical Infectious Diseases. 2011 Mar 15;52(6):717–25. doi: 10.1093/cid/ciq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guide for the Care and Use of Laboratory Animals: Eighth Edition. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 32.Hirota JA, Hirota SA, Warner SM, et al. The airway epithelium nucleotide-binding domain and leucine-rich repeat protein 3 inflammasome is activated by urban particulate matter. The Journal of allergy and clinical immunology. 2012 Apr;129(4):1116–25. e6. doi: 10.1016/j.jaci.2011.11.033. [DOI] [PubMed] [Google Scholar]
- 33.Tsai MJ, Chen C, Chen SH, Huang YT, Chiu TH. Pomalidomide suppresses cerulein-induced acute pancreatitis in mice. Journal of gastroenterology. 2011 Jun;46(6):822–33. doi: 10.1007/s00535-011-0394-x. [DOI] [PubMed] [Google Scholar]
- 34.Merkle E, Görich J. Imaging of acute pancreatitis. Eur Radiol. 2002 Aug 01;12(8):1979–92. doi: 10.1007/s00330-001-1235-8. [DOI] [PubMed] [Google Scholar]
- 35.Abou-Assi S, O’Keefe SJD. Nutrition support during acute pancreatitis. Nutrition. 2002;18(11–12):938–43. doi: 10.1016/s0899-9007(02)00991-7. [DOI] [PubMed] [Google Scholar]
- 36.Balthazar EJ. Complications of acute pancreatitis: clinical and CT evaluation. Radiologic clinics of North America. 2002 Dec;40(6):1211–27. doi: 10.1016/s0033-8389(02)00043-x. [DOI] [PubMed] [Google Scholar]
- 37.Papachristou GI, Clermont G, Sharma A, Yadav D, Whitcomb DC. Risk and markers of severe acute pancreatitis. Gastroenterology clinics of North America. 2007 Jun;36(2):277–96. viii. doi: 10.1016/j.gtc.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Aoun E, Chen J, Reighard D, Gleeson FC, Whitcomb DC, Papachristou GI. Diagnostic accuracy of interleukin-6 and interleukin-8 in predicting severe acute pancreatitis: a meta-analysis. Pancreatology : official journal of the International Association of Pancreatology (IAP) [et al] 2009;9(6):777–85. doi: 10.1159/000214191. [DOI] [PubMed] [Google Scholar]
- 39.Bauernfeind FG, Horvath G, Stutz A, et al. Cutting Edge: NF-κB Activating Pattern Recognition and Cytokine Receptors License NLRP3 Inflammasome Activation by Regulating NLRP3 Expression. The Journal of Immunology. 2009 Jul 15;183(2):787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Evans AC, Papachristou GI, Whitcomb DC. Obesity and the risk of severe acute pancreatitis. Minerva gastroenterologica e dietologica. 2010 Jun;56(2):169–79. [PubMed] [Google Scholar]
- 41.Frossard JL, Lescuyer P, Pastor CM. Experimental evidence of obesity as a risk factor for severe acute pancreatitis. World journal of gastroenterology : WJG. 2009 Nov 14;15(42):5260–5. doi: 10.3748/wjg.15.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beger HG, Rau BM. Severe acute pancreatitis: Clinical course and management. World journal of gastroenterology : WJG. 2007 Oct 14;13(38):5043–51. doi: 10.3748/wjg.v13.i38.5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pini M, Rhodes DH, Castellanos KJ, Cabay RJ, Grady EF, Fantuzzi G. Rosiglitazone improves survival and hastens recovery from pancreatic inflammation in obese mice. PloS one. 2012;7(7):e40944. doi: 10.1371/journal.pone.0040944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereda J, Perez S, Escobar J, et al. Obese rats exhibit high levels of fat necrosis and isoprostanes in taurocholate-induced acute pancreatitis. PloS one. 2012;7(9):e44383. doi: 10.1371/journal.pone.0044383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Štimac D, Fišic E, Milic S, Bilic-Zulle L, Peric R. Prognostic values of IL-6, IL-8, and IL-10 in acute pancreatitis. Journal of clinical gastroenterology. 2006;40(3):209–12. doi: 10.1097/00004836-200603000-00007. [DOI] [PubMed] [Google Scholar]
- 46.Schroder K, Zhou R, Tschopp J. The NLRP3 Inflammasome: A Sensor for Metabolic Danger? Science. 2010 Jan 15;327(5963):296–300. doi: 10.1126/science.1184003. [DOI] [PubMed] [Google Scholar]
- 47.De Nardo D, Latz E. NLRP3 inflammasomes link inflammation and metabolic disease. Trends Immunol. 2011 Aug;32(8):373–9. doi: 10.1016/j.it.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rossol M, Pierer M, Raulien N, et al. Extracellular Ca2+ is a danger signal activating the NLRP3 inflammasome through G protein-coupled calcium sensing receptors. Nature communications. 2012;3:1329. doi: 10.1038/ncomms2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.L'Homme L, Esser N, Riva L, et al. Unsaturated fatty acids prevent activation of NLRP3 inflammasome in human monocytes/macrophages. Journal of lipid research. 2013 Nov;54(11):2998–3008. doi: 10.1194/jlr.M037861. [DOI] [PMC free article] [PubMed] [Google Scholar]