Abstract
Objective
Determine the association of prenatal and neonatal infections with neurodevelopmental outcomes in very preterm infants.
Study Design
Secondary retrospective analysis of 155 very preterm infants at a single tertiary referral center. General linear or logistic regression models were used to evaluate the association with hospital factors; brain injury, growth, and development; and neurobehavioral outcome.
Result
Necrotizing enterocolitis with sepsis was associated with reduced transcerebellar diameter (38.3 vs 48.4 mm, P < 0.001) and increased left ventricular diameter (12.0 vs 8.0 mm, P = 0.005). Sepsis alone was associated with higher diffusivity in the left frontal lobe (1.85 vs 1.68 × 10−3 mm2/s, P = 0.001) and right cingulum bundle (1.52 vs 1.45 × 10−3 mm2/s, P = 0.002). Neurobehavioral outcomes were worse in children exposed to maternal genitourinary infection (Cognitive Composite: β = −8.8, P = 0.001; Receptive Language Score: β = −2.7, P < 0.001; Language Composite: β = −14.9, P < 0.001) or histological chorioamnionitis (Language Composite β = −8.6, P = 0.006), but not neonatal infection.
Conclusion
Neonatal infection was associated with changes in brain structure but not with neurobehavioral outcomes whereas the opposite pattern was observed for maternal genitourinary tract infection. These findings emphasize the potential importance of infections during pregnancy on the neurodevelopmental outcomes of preterm infants.
Keywords: brain injury, metrics, diffusion, behavior
INTRODUCTION
Children born very preterm (<30 weeks’ gestation) score lower on intelligence scales1 and perform more poorly on tests of motor functioning,2 language,3 memory,4 attention, and executive functioning5, 6, 7 than children born full-term. The increasing survival rates of very preterm infants have thus led to greater research focus on the pathogenesis of these adverse neurobehavioral outcomes.
Neurodevelopmental outcomes in very preterm children may be influenced by many factors, two of which are prenatal and neonatal infection,8, 9, 10 thought to be related to a unique sensitivity of pre-oligodendrocytes in immature white matter to inflammatory-mediated injury.15, 16 For example, histological chorioamnionitis has been associated with increased rates of cerebral palsy.11 Maternal urinary tract infection12 and neonatal sepsis8 have also been associated with poor neurobehavioral outcomes. Interestingly, while neonatal sepsis is associated with increased extent of white matter injury,13, 14 an association was not observed between maternal infections ultrasonographic brain structural abnormalities.12 However, these studies did not assess both prenatal and neonatal infections in the same cohort. Furthermore, despite the association between chorioamnionitis and cerebral palsy, other studies have not observed additional neurobehavioral alterations.17, 18, 19 Given the high rates of prenatal17 and neonatal8 infections in very preterm infants, accurate identification of the risk of neurodevelopmental impairment associated with such infections may render an opportunity for targeted neuroprotection.
In this retrospective cohort study, we assessed the association between prenatal and neonatal infection and three magnetic resonance imaging (MRI) measures of brain structure: white matter injury, brain structure metrics (measures of growth), and diffusion (a measure of microstructure). We also assessed the association between infections and neurobehavioral outcome at two years of age. We hypothesized that the presence of prenatal or neonatal infection would correlate with adverse neurobehavioral outcomes and altered white matter structure.
MATERIALS AND METHODS
Study population
This investigation was a secondary retrospective analysis of patients recruited for a larger study prospectively investigating the factors influencing brain development in preterm infants. Patients in this cohort were recruited from the neonatal intensive care unit (NICU) at St. Louis Children’s Hospital between 2007 and 2012 in studies approved by the Washington University in St. Louis Institutional Review Board. All infants were enrolled within the first 48 hours of birth. Exclusion criteria included chromosomal abnormalities or proven congenital infections (e.g., cytomegalovirus, toxoplasma, rubella). Written, informed consent was obtained from parents.
Clinical methods
Chart review provided an extensive database of prenatal and neonatal data. Maternal report was employed to define infant race, which was dichotomized as African-American or non-African American. Chart abstraction of records and laboratory results at time of delivery and emergency room visits to Barnes-Jewish Hospital during pregnancy was used to determine the presence of genitourinary infections. These included urinary tract infections, gonorrhea/chlamydia, bacterial vaginosis, vaginitis, and trichomoniasis. Infants were categorized for neonatal infection as previously defined by Stoll et al.20 No analyses were conducted on meningitis because of the small number of affected infants (n=2).
Placental Evaluation
An experienced placental pathologist (P.H), blinded to infant and clinical outcomes, reviewed hematoxylin-and-eosin-stained slides for histological chorioamnionitis and fetal vasculitis.21 Histological chorioamnionitis included inflammation of the chorionic membrane, free membrane chorioamnionitis, subchorionitis, and chorionitis. Fetal vasculitis included vasculitis (in the umbilical cord or placenta), phlebitis, arteritis, or funisitis.
Imaging
Infants received serial magnetic resonance imaging (MRI) during their NICU stay as a part of the prospective study. Magnetic resonance images were collected at term-equivalent postmenstrual age (PMA) and assessed by a single investigator (H.K) blinded to infection status. A Siemens Magnetom Trio 3-T scanner with an infant head coil was used according to previously published acquisition methods.22 White matter injury was classified as periventricular leukomalacia (PVL), cerebellar hemorrhage, or intraventricular hemorrhage.23 In addition, intraventricular hemorrhage was recorded from independent evaluation of all cranial ultrasounds undertaken during the infants’ NICU stay, grading them on the basis of the Papile classification (grades 1–4). For brain structure metrics, six measurements were made on tissue and fluid spaces on MR images: bifrontal, biparietal, transverse cerebellar, right and left ventricular diameters, and interhemispheric distance.24 Diffusion MRI analysis was performed with laboratory-written software. Regions of interest were placed in the genu and splenium of the corpus callosum, cingulum bundle, posterior and anterior limbs of the internal capsule, and optic radiations. In addition, white matter regions of interest were placed in the superior frontal lobes and centrum semiovale. The following parameters were measured with Analyze (Mayo Clinic, Rochester, MN): mean diffusivity, fractional anisotropy, axial diffusivity, and radial diffusivity.
Neurobehavioral testing
At age two years, participants underwent developmental assessment with the Bayley III Scales of Infant Development and behavioral evaluation with the Infant Toddler Social and Emotional Assessment,25 which assesses social-emotional problems and competencies. Four domains are scored: social-emotional competence, externalizing, internalizing, and dysregulation.
Statistical analysis
SPSS 19 (IBM Corporation, 1989, 2010) was used for statistical analyses. For unadjusted comparisons, statistical significance was determined by Fisher’s Exact Test for dichotomous outcome variables, independent Student’s t-test for continuous and normally distributed outcome variables, and Mann-Whitney U test for continuous and nonparametric outcome variables (length of stay, days of ventilation, and days of total parenteral nutrition [TPN]). Levine’s test for equality of variance was used to test normality. Analysis of covariance was used to adjust the natural log of nonparametric outcomes for PMA. For continuous variables, including metrics and diffusion measures, adjusted models were analyzed with a general linear model. Missing data were eliminated listwise. Known predictors of outcomes were entered into models using forced entry. These included PMA at birth for impact on clinical factors and white matter injury; race, gender, and PMA at term-equivalent scan for brain metrics and diffusion; and social risk for two-year neurobehavioral outcomes. A social risk score, modified from another study,26 was calculated from family structure; primary caregiver education level, occupation, and employment status; language spoken at home; and maternal age at birth. Race was not used to control for two-year developmental outcomes because it was associated with social risk (data not shown).
Log conversion was undertaken to assess the influence of infection on highly skewed hospital factors such as days of TPN, days of ventilation, and length of stay. Additionally, because days of TPN and ventilation were associated with sepsis, they were dichotomized by upper quartile of exposure and used to control for outcome. Because of the number of variables examined, a P-value cutoff of 0.01 was considered significant to reduce the risk of Type I error. For our primary outcome measure of neurobehavioral outcome, we used a two-tailed t-test with alpha = 0.05 and possessed 80% power to detect true differences between means that were 7.75 in cognitive, 9.5 in language, and 9.8 in motor scores.
RESULTS
Study population
The study population consisted of 155 infants—102 singletons, 42 twins, and 11 triplets—with a median gestational age at birth of 26 weeks. This represented 43% of the 360 eligible infants admitted during the study period. Lack of parental access due to maternal illness or sedation was the most common reason for failure to enroll. Race and gender of the infants enrolled did not differ from those not enrolled (P > 0.05). We were unable to enroll one infant from each of four twin pairs and one triplet set because of very early death. Both maternal records and placental histology were available for 138 infants. Twelve records were not available because of birth at an outside hospital. An additional five placental records were missing because of obstetrical emergencies that limited placental examination. These consisted of emergent home delivery, severe maternal pre-eclampsia and bleeding, and death of the first twin.
Infections
Histological chorioamnionitis was the most frequent prenatal infectious/inflammatory exposure, affecting 60 infants (43.5%). Fetal vasculitis was noted in 36 infants (24.7%), all of whom were exposed to histological chorioamnionitis. Additionally, 43 infants (31.2%) were exposed to a maternal genitourinary tract infection. Although 53% of infants exposed to maternal genitounary tract infections were also exposed to histological chorioamnionitis, there was no association between infants exposed to histological chorioamnionitis or fetal vasculitis and those exposed to prenatal genitourinary tract infection. Three infants (5%) exposed to histological chorioamnionitis had early onset sepsis (sepsis within 48 hours of delivery). Fifty-eight infants (42.0%) were not exposed to any prenatal infection or inflammation.
Postnatally, four infants (2.6%) died within three days of birth, 35 (22.6%) had at least one episode of sepsis, 10 (6.5%) had necrotizing enterocolitis (NEC), seven (4.5%) had NEC with sepsis, two (1.3%) had meningitis, and 24 (15.5%) had clinically suspected infection. Forty-nine infants (31.6%) did not have any neonatal infection. Baseline characteristics between infected and control groups were not statistically different except for a higher rate of preeclampsia in the control group than in those with sepsis, histological chorioamnionitis, or fetal vasculitis (Table 1).
Table 1.
Maternal and infant characteristics in relation to prenatal and neonatal infection
| Prenatal Infection | Neonatal Infection | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| None | Maternal GU | Chorioamnionitis | Fetal vasculitis | None | Sepsis | NEC | Sepsis and NEC |
Clinically suspected infection |
|
| Number | 65 | 31 | 60 | 34 | 48 | 35 | 10 | 7 | 24 |
| Estimated gestational age, mean in wks (SD) | 26 (1) | 27 (2) | 26 (2) | 26 (2) | 26 (2) | 26 (2) | 26 (2) | 26 (2) | 26 (2) |
| Birthweight, median in gms (IQR) | 940 (785–1153) | 730 (700–960) | 765 (700–990) | 765 (700–1012) | 985 (840–1290) | 750 (700–930) | 750 (728–880) | 710 (660–740) | 745 (693–898) |
| Male, no. (%) | 31 (48) | 14 (45) | 32 (53) | 17 (50) | 15 (31) | 21 (60)* | 5 (50) | 4 (57) | 13 (54) |
| African-American, no. (%) | 30 (46) | 22 (71) | 36 (60) | 20 (59) | 23 (48) | 21 (60) | 4 (40) | 5 (71) | 13 (54) |
| Medicaid, no. (%) | 29 (64) | 16 (80) | 26 (68) | 12 (67) | 21 (55) | 13 (72) | 3 (75) | 3 (100) | 10 (71) |
| Maternal high school education, no (%) | 20 (53) | 9 (47) | 24 (67) | 10 (63) | 20 (56) | 10 (59) | 2 (40) | 1 (33) | 8 (67) |
| Preeclampsia, no. (%) | 28 (43) | 7 (23) | 7 (12)* | 1 (2.9)* | 20 (42) | 5 (14)* | 2 (20) | 3 (43) | 5 (21) |
| Prenatal Steroids, no. (%) | 55 (85) | 24 (77) | 49 (82) | 26 (77) | 42 (88) | 27 (77) | 8 (80) | 5 (71) | 19 (79) |
P < 0.01 compared to no infection
Abbreviations: GU – genitourinary; IQR – interquartile range; NEC – necrotizing enterocolitis; PMA – postmenstrual age; SD – standard deviation.
Associations between infection and hospital course
Prenatal genitourinary and placental infections were not associated with length of stay or days of ventilation or TPN (data not shown). For neonatal infection, clinically suspected infection and sepsis with NEC were associated with more days of ventilation and TPN than no infection. Sepsis and NEC without sepsis were also associated with more days of TPN. Clinically suspected infection and sepsis were associated with prolonged length of stay (Table 2). All associations were controlled for PMA at birth.
Table 2.
Impact of postnatal infection on hospital factors (controlled for PMA)
| Length of stay (95% CI) | Total days on TPN (95% CI) | Total days of ventilation (95% CI) | |
|---|---|---|---|
| P value* | P value* | P value* | |
| Sepsis | 16.2 (11.3, 23.4) | 30.5 (23.9, 38.8) | 87.7 (70.8, 108.5) |
| < 0.001 | < 0.001 | 0.018 | |
| Necrotizing enterocolitis | 53.1 (51.8, 74.4) | 28.1 (18.1, 43.5) | 5.7 (3.1, 10.3) |
| 0.479 | 0.003 | 0.016 | |
| Necrotizing enterocolitis with sepsis | 85.4 (56.6, 128.8) | 47.4 (29.0, 77.6) | 12.9 (6.4, 26.3) |
| 0.149 | < 0.001 | < 0.001 | |
| Clinically suspected infection | 19.3 (12.3, 30.3) | 47.4 (24.7, 44.1) | 96.8 (76.9, 122.1) |
| 0.001 | < 0.001 | 0.003 | |
| No infection | 2.5 (1.9, 3.4) | 13.4 (11.0, 16.4) | 62.4 (52.5–74.2) |
Compared to no infection
TPN - Total parenteral nutrition
Infection, death, and white matter injury
Eighteen infants died before discharge. After controlling for PMA, NEC without sepsis was the only prenatal or neonatal infection related to death (OR 14.4, 95% CI 2.1–99.5, P = 0.007), but not after additionally controlling for days of TPN and ventilation (P = 0.99). Prenatal infection was not associated with death.
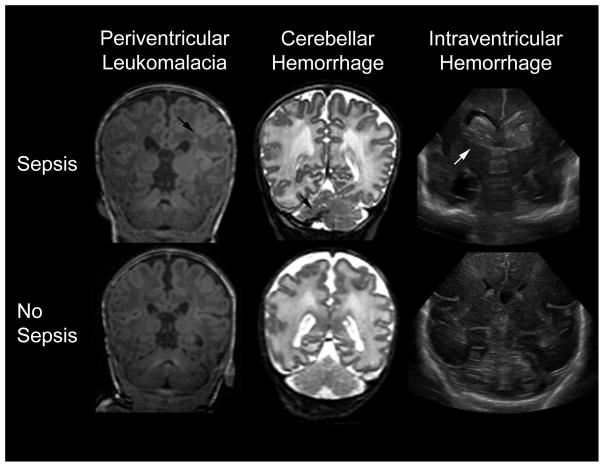
Clinically suspected infection was associated with cerebellar hemorrhage on univariate analysis, after controlling for PMA, and additionally after controlling for days on TPN and ventilation. Sepsis trended towards an association with intraventricular hemorrhage but did not meet our standard for statistical significance (Table 3). PVL was evaluated qualitatively and was not associated with any of the examined infections (data not shown). Representative images of PVL, cerebellar hemorrhage, and intraventricular hemorrhage are shown in Figure 1.
Table 3.
Impact of neonatal infection on white matter injury and death
| Unadjusted odds ratio (95% CI) | * Adjusted odds ratio (95% CI) | † Adjustment including TPN and vent (95% CI) | |
|---|---|---|---|
| P value | P value | P value | |
| Intraventricular hemorrhage | |||
| Sepsis | 5.2 (1.3, 20.9) | 5.8 (1.3, 24.8) | 4.9 (0.8, 29.3) |
| 0.02 | 0.02 | 0.08 | |
| Necrotizing enterocolitis | 6.4 (1.1–38.4) | 6.2 (1.0, 37.7) | 14.1 (1.4, 138.0) |
| 0.04 | 0.05 | 0.02 | |
| Clinically suspected infection | 3.0 (0.6, 14.7) | 3.1 (0.6, 15.6) | 4.1 (0.6, 26.1) |
| 0.175 | 0.165 | 0.133 | |
| Cerebellar hemorrhage | |||
| Sepsis | 3.6 (1.0, 12.5) | 3.5 (1.0, 12.4) | 1.8 (0.4, 8.6) |
| 0.05 | 0.05 | 0.5 | |
| Necrotizing enterocolitis | 3.8 (0.5, 26.4) | 3.9 (0.5, 27.9) | N/A |
| 0.177 | 0.182 | 0.999 | |
| Clinically suspected infection | 8.4 (2.3, 30.9) | 8.5 (2.3, 30.8) | 6.2 (1.5, 25.9) |
| 0.001 | 0.001 | 0.01 | |
| Any injury or death | |||
| Sepsis | 8.1 (2.5, 25.6) | 8.5 (2.6, 27.7) | 6.5 (1.6, 26.5) |
| < 0.001 | < 0.001 | 0.009 | |
| Necrotizing enterocolitis | 10.1 (1.7, 59.1) | 10.1 (1.7, 59.1) | 10.7 (1.6, 72.6) |
| 0.01 | 0.01 | 0.02 | |
| Clinically suspected infection | 13.3 (3.7, 47.6) | 14.0 (3.8, 51.6) | 10.7 (2.5, 44.8) |
| < 0.001 | 0.001 | 0.001 | |
adjustment model includes PMA
adjustment model includes PMA, days of TPN, and days of ventilation
Figure 1.
Representative T1-weighted image of periventricular leukomalacia (left), T2-weighted MR image of cerebellar hemorrhage (middle), and coronal ultrasound images of intraventricular hemorrhage (right). Images from infants exposed to sepsis (top) have arrows indicating the relevant pathologies. The bottom images are from a very preterm infant without any infectious exposure.
A combined outcome of intraventricular hemorrhage grades 3 and 4, PVL, cerebellar hemorrhage, or death before 44 weeks PMA was associated with neonatal infection (except for sepsis with NEC); this association persisted after controlling for PMA at scan (Table 3). After controlling for days of TPN and ventilation, only sepsis and clinically suspected infection were still associated with a higher rate of white matter injury or death. Prenatal infection was not associated with death, injury, or the combined outcome (data not shown).
Brain structure metrics at term equivalent
Univariate analysis between brain structure metrics and neonatal infection showed associations between sepsis with NEC and biparietal diameter, transcerebellar diameter, and left ventricular diameter (Table 4). These associations persisted after controlling for gender, PMA at scan, and race. Except for biparietal diameter, these associations persisted after additionally controlling for days on TPN and ventilation (Table 4). Prenatal infection was not associated with changes in brain metrics (data not shown).
Table 4.
Impact of proven neonatal sepsis on brain metrics and diffusion measures
| Unadjusted means | * Adjusted means | † Adjusted mean including TPN and vent | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (SE) | Sepsis (SE) | P value | Control (SE) | Sepsis (SE) | P value | Control (SE) | Sepsis (SE) | P value | |
|
Brain metrics (cm)
**
| |||||||||
| Bifrontal diameter | 59.8 (0.9) | 56.3 (2.7) | 0.224 | 60.7 (1.1) | 55.8 (3.0) | 0.141 | 60.0 (1.8) | 56.0 (3.5) | 0.367 |
| Biparietal diameter | 70.4 (0.9) | 63.5 (2.7) | 0.02 | 72.2 (1.0) | 62.6 (2.7) | 0.002 | 71.4 (1.6) | 63.3 (3.1) | 0.046 |
| Interhemispheric diameter | 3.7 (0.3) | 3.7 (1.0) | 0.959 | 3.9 (0.4) | 3.0 (1.1) | 0.455 | 3.7 (0.7) | 3.3 (1.3) | 0.822 |
| Transcerebellar diameter | 48.4 (0.7) | 38.3 (2.2) | < 0.001 | 49.5 (0.8) | 36.8 (2.3) | < 0.001 | 49.7 (1.4) | 37.0 (2.7) | 0.001 |
| Right ventricular diameter | 8.1 (0.6) | 11.9 (1.8) | 0.046 | 7.6 (0.7) | 12.5 (2.0) | 0.027 | 8.1 (1.0) | 13.6 (2.0) | 0.038 |
| Left ventricular diameter | 8.0 (0.4) | 12.0 (1.3) | 0.005 | 8.0 (0.5) | 12.3 (1.4) | 0.007 | 8.5 (0.7) | 13.0 (1.4) | 0.01 |
|
| |||||||||
|
Diffusion (× 10-3 mm2/s)
| |||||||||
| Right Frontal Lobe | |||||||||
| Mean diffusion | 1.71 (0.02) | 1.79 (0.04) | 0.17 | 1.69 (0.03) | 1.79 (0.05) | 0.05 | 1.70 (0.04) | 1.83 (0.05) | 0.02 |
| Axial diffusion | 1.93 (0.02) | 2.02 (0.05) | 0.12 | 1.90 (0.03) | 2.02 (0.05) | 0.04 | 1.90 (0.04) | 2.05 (0.05) | 0.01 |
| Radial diffusion | 1.61 (0.02) | 1.67 (0.05) | 0.22 | 1.58 (0.03) | 1.68 (0.05) | 0.07 | 1.60 (0.04) | 1.72 (0.05) | 0.04 |
| Left Frontal Lobe | |||||||||
| Mean diffusion | 1.71 (0.02) | 1.79 (0.04) | 0.07 | 1.68 (0.02) | 1.82 (0.04) | 0.004 | 1.68 (0.03) | 1.85 (0.04) | 0.001 |
| Axial diffusion | 1.93 (0.02) | 2.03 (0.04) | 0.04 | 1.90 (0.02) | 2.05 (0.04) | 0.003 | 1.88 (0.03) | 2.07 (0.04) | < 0.001 |
| Radial diffusion | 1.60 (0.02) | 1.67 (0.04) | 0.12 | 1.57 (0.02) | 1.70 (0.04) | 0.007 | 1.58 (0.03) | 1.74 (0.04) | 0.001 |
| Right Cingulum Bundle | |||||||||
| Mean diffusion | 1.46 (0.01) | 1.5 (0.02) | 0.08 | 1.46 (0.01) | 1.51 (0.02) | 0.04 | 1.45 (0.01) | 1.52 (0.02) | 0.002 |
| Axial diffusion | 2.01 (0.02) | 2.04 (0.04) | 0.42 | 2.00 (0.02) | 2.06 (0.04) | 0.12 | 1.99 (0.03) | 2.07 (0.04) | 0.09 |
| Radial diffusion | 1.19 (0.01) | 1.23 (0.02) | 0.18 | 1.19 (0.02) | 1.23 (0.03) | 0.21 | 1.18 (0.02) | 1.24 (0.03) | 0.03 |
adjustment model includes PMA at scan, gender, and race
adjustment model additionally includes upper quartiles of days of TPN and days of ventilation
Brain metrics compared infants with both sepsis and NEC
Diffusion measures
Univariate analyses revealed no significant associations between prenatal infections and measures of diffusion. After controlling for race, gender, and PMA at scan, histological chorioamnionitis was associated with higher fractional anisotropy in the body of the corpus callosum (0.52 vs. 0.46; P = 0.008). This resulted from lower radial diffusivity (1.06 vs. 1.2; P = 0.006). These associations remained significant after controlling for days on TPN and ventilation (0.44 vs 0.5, P = 0.003 and 1.1 vs 1.3, P = 0.004 respectively). Prenatal infections were not related to any other diffusion measures.
For neonatal infections, neonatal sepsis and alterations in mean diffusivity in the left frontal lobe and right cingulum. Additionally, higher values for axial and radial were observed in the left frontal lobe. For the right cingulum bundle, neonatal sepsis was not associated with increased axial or radial diffusivity (Table 4). All diffusion measures were controlled for gender, race, PMA at scan, and days of TPN and ventilation.
Neurobehavioral outcomes
Eighty-six of 104 eligible subjects (83%) returned for follow-up at two years of age. Infants with private insurance (P = 0.01) and who were not African American (p = 0.003) were more likely to return for follow up. The infant’s gender did not associate with return for follow-up. On univariate analysis, only maternal genitourinary infections were associated with changes in neurobehavioral outcomes (Table 5), which persisted after controlling for social risk. After controlling for social risk, histological chorioamnionitis was associated with a reduced Language Composite score (β = −8.6, 95% CI: −14.7 to −2.5, P = 0.006). Infection was not associated with Infant Toddler Social and Emotional Assessment scores.
Table 5.
Impact of maternal genitourinary tract infection on neurobehavioral outcomes
| Unadjusted beta [95% CI] | * Adjusted beta [95% CI] | |
|---|---|---|
| P value | P value | |
| Cognitive Composite | −7.0 [−12, −2.1] | −8.8 [−13.9, −3.7] |
| 0.006 | 0.001 | |
| Language Composite | −12.9 [−19.1, −6.6] | −14.9 [−21.6, −8.1] |
| < 0.001 | < 0.001 | |
| Receptive Language | −2.3 [−3.4, −1.3] | −2.7 [−3.8, −1.6] |
| < 0.001 | < 0.001 |
adjustment model includes social risk
DISCUSSION
This study complements recent literature on the adverse associations between prenatal and neonatal infection and white matter injury, brain structure metrics, and diffusion in very preterm infants. Importantly, maternal genitourinary tract infections were not associated with any of the measured neuroimaging structural domains, but were associated with adverse neurobehavioral outcomes. In contrast, neurobehavioral outcomes at two years of age did not differ significantly between those with and without neonatal infection, but did impact perinatal outcomes.
The association between histological chorioamnionitis and neurodevelopmental outcomes of infants has varied in the literature. In our study, histological chorioamnionitis was not associated with white matter injury or brain structure metrics. However, it was associated with higher anisotropy, reflected by lower radial diffusivity, in the corpus callosum. Reductions in radial diffusivity in the immature brain are often thought to reflect increasing myelination and maturation within the fiber tracts. However, one recent study showed a relationship between histological chorioamnionitis and a decreased mental developmental index score at 18 months;18 another reported an association between cerebral palsy and histological chorioamnionitis.11 Others have not observed associations between histological chorioamnionitis and neurobehavioral outcomes at two years.19, 40 Histological chorioamnionitis in our cohort was associated with a lower Bayley Composite Language score at two years of age. Unlike the previous studies, we uniquely controlled for social risk, which is associated with cognitive impairment at two years.40 The lack of association between fetal vasculitis and neurobehavioral outcomes in our study was not consistent with a prior report showing associations between funisitis and moderate to severe disability.19 However, we did not differentiate between fetal inflammation in different placental compartments.
Interestingly, the neurobehavioral consequences associated with infection were most notable in the domain of language and especially in association with maternal genitourinary tract infections during pregnancy. These associations were stronger after adjusting for sociodemographic variables that may be associated with prenatal infection. Prior studies have shown associations between maternal infection at delivery and the risk of cerebral palsy in preterm infants9 as well as between urinary tract infections and the risk for mental retardation. Although we were unable to disentangle the impact of antibiotic use on neurodevelopmental outcomes27 because of almost universal antibiotic use, our findings highlight the importance of maternal genitourinary tract infections as an arena that may be worthy of targeted fetal neuroprotection. Furthermore, the lack of MRI structural correlates complements prior studies examining ultrasonographic structural differences12, 28 and suggests the involvement of functional dysregulation in observed neurobehavioral changes.
Neonatal infection, in contrast, was not associated with two-year neurobehavioral outcomes, but was associated with several brain structural changes. We found associations between neonatal infections and smaller transcerebellar and left ventricular diameters, indicating that brain growth was impaired. Additionally, we observed associations between neonatal infections and increased diffusivity in the left frontal lobe, indicating that brain microstructure was abnormal. These findings are consistent with prior studies,29, 30, 31, 32, 33 and support the role of infection/inflammation in perturbing white matter microstructural integrity. The fact that neonatal infections were associated with brain structural changes but not neurobehavioral outcomes may be related to the timing, extent, or other unique characteristics of these infections. However, other studies, which used different outcome measures than we used, have observed an association between neonatal infection and neurobehavioral outcomes.8 It also is possible that our measurements of neurobehavioral outcomes at age two years may not accurately predict future clinical outcomes.34, 35, 36 Sophisticated testing of higher cognitive and executive skills at older ages may reveal an association between neonatal infections and neurobehavioral outcomes.
In addition to defects in brain structure metrics, neonatal infection was also associated with higher rates of white matter injury. We found an increased incidence of hemorrhagic brain injury, particularly cerebellar hemorrhage, and a combined outcome of death and injury among infants with sepsis or clinically suspected infection. Our study did not reveal an association between neonatal infection and PVL as was reported by Stoll et al20, which may be because of lower power in our study or improvements in hospital practices.37 The associations between neonatal infection and increased duration of hospitalization, TPN, and ventilator support were also similar to previously noted associations between neonatal infection and increased surfactant and postnatal steroid administration,8 and longer length of NICU stay.38 Interestingly, ventilation time has recently been shown to contribute to the inflammatory cascade39 thought to result in brain injury,8, 13, 14 but many of our associations persisted after controlling for days on ventilation. Although no differences in behavioral outcomes were observed, these associations may highlight the importance of neonatal sepsis as a marker for infants at increased risk for gross brain injury in the perinatal period. Furthermore, whether as a consequence of the infection itself or from complications of infection, ventilation and TPN are not benign interventions and may simultaneous increase the risk for further complications.
The strengths of the current study include the systematic review of placental pathology by an experienced placental pathologist and the evaluation of brain structure through multimodal MR techniques. The limitations include retrospective chart review, which precludes definitive conclusions about infectious contributions to worsened neurodevelopmental outcomes. For example, mothers who had emergency room records and were subsequently diagnosed with asymptomatic urinary tract infection may have had other factors associated with adverse neurodevelopmental outcomes, despite controlling for social risk. A further limitation of this study is the small sample size, which did not allow for subgroup analyses of specific types of maternal infection or organisms. Although infant death contributed to a smaller sample size for MRI measures and two-year follow-up testing, this is unlikely to bias our data because of the lack of association between infection and death in our cohort.
Despite these limitations, our data highlight the negative influence of infection/inflammation on neonatal brain health and development. Although neonatal infection was not associated with neurobehavioral changes, later neurobehavioral changes may emerge at older ages when more sophisticated psychometric testing is feasible. In addition, the lack of brain structural changes associated with maternal genitourinary tract infection during pregnancy suggests that the mechanism behind these neurobehavioral changes may be an area worthy of future study.
Acknowledgments
Funding Source: Funding support from National Institute of Health (NICHD) R01HD05805, P30HD062171 and the Doris Duke Foundation Distinguished Clinical Scientist Award to Dr. Inder. This work was also supported by a grant from the Doris Duke Charitable Foundation to Washington University in St. Louis for Clinical Research Fellow Iris Lee. Dr. Rogers is supported by Grant Number UL1 TR000448 and TL1 TR000449/KL2 TR000450 from the NIH-National Center for Research Resources (NCRR) and the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health. Dr. Smyser is supported by the Child Neurology Foundation, NIH K12 NS001690 and UL1 TR000448. Dr. Mysorekar is supported by the Preventing Prematurity Initiative grant from the Burroughs Wellcome Fund.
We wish to acknowledge those who assisted in acquiring, processing, or reading MRI and US scans at Washington University in St Louis, including Karen Lukas, Joe Ackerman, Tara Smyser, Jeanette Kenley, and Eilon Shany (visiting from Soroka University Medical Center, Israel). We are grateful for the advice on statistical processing from Mike Wallendorf and editing from Deborah Frank (both at Washington University in St Louis).
Footnotes
Conflict of interest: The authors declare no conflict of interest
References
- 1.Marlow N, Wolke D, Bracewell MA, Samara M. Neurologic and developmental disability at six years of age after extremely preterm birth. N Engl J Med. 2005;352(1):9–19. doi: 10.1056/NEJMoa041367. [DOI] [PubMed] [Google Scholar]
- 2.Williams J, Lee KJ, Anderson PJ. Prevalence of motor-skill impairment in preterm children who do not develop cerebral palsy: a systematic review. Dev Med Child Neurol. 2010;52(3):232–237. doi: 10.1111/j.1469-8749.2009.03544.x. [DOI] [PubMed] [Google Scholar]
- 3.Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. The Journal of pediatrics. 2011;158(5):766–774. e761. doi: 10.1016/j.jpeds.2010.10.032. [DOI] [PubMed] [Google Scholar]
- 4.Saavalainen P, Luoma L, Bowler D, Maatta S, Kiviniemi V, Laukkanen E, et al. Spatial span in very prematurely born adolescents. Dev Neuropsychol. 2007;32(3):769–785. doi: 10.1080/87565640701539535. [DOI] [PubMed] [Google Scholar]
- 5.Anderson P, Doyle LW. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. JAMA : the journal of the American Medical Association. 2003;289(24):3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 6.Anderson PJ, De Luca CR, Hutchinson E, Spencer-Smith MM, Roberts G, Doyle LW. Attention problems in a representative sample of extremely preterm/extremely low birth weight children. Dev Neuropsychol. 2011;36(1):57–73. doi: 10.1080/87565641.2011.540538. [DOI] [PubMed] [Google Scholar]
- 7.Mulder H, Pitchford NJ, Hagger MS, Marlow N. Development of executive function and attention in preterm children: a systematic review. Dev Neuropsychol. 2009;34(4):393–421. doi: 10.1080/87565640902964524. [DOI] [PubMed] [Google Scholar]
- 8.Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA : the journal of the American Medical Association. 2004;292(19):2357–2365. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 9.Neufeld MD, Frigon C, Graham AS, Mueller BA. Maternal infection and risk of cerebral palsy in term and preterm infants. J Perinatol. 2005;25(2):108–113. doi: 10.1038/sj.jp.7211219. [DOI] [PubMed] [Google Scholar]
- 10.Mann JR, McDermott S. Are maternal genitourinary infection and pre-eclampsia associated with ADHD in school-aged children? J Atten Disord. 2011;15(8):667–673. doi: 10.1177/1087054710370566. [DOI] [PubMed] [Google Scholar]
- 11.Shatrov JG, Birch SC, Lam LT, Quinlivan JA, McIntyre S, Mendz GL. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstetrics and gynecology. 2010;116(2 Pt 1):387–392. doi: 10.1097/AOG.0b013e3181e90046. [DOI] [PubMed] [Google Scholar]
- 12.Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Mental Retardation and Developmental Disabilities Research Reviews. 2002;8(1):46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 13.Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP. Tractography-based quantitation of corticospinal tract development in premature newborns. The Journal of pediatrics. 2010;156(6):882–888. 888 e881. doi: 10.1016/j.jpeds.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass HC, Bonifacio SL, Chau V, Glidden D, Poskitt K, Barkovich AJ, et al. Recurrent postnatal infections are associated with progressive white matter injury in premature infants. PEDIATRICS. 2008;122(2):299–305. doi: 10.1542/peds.2007-2184. [DOI] [PubMed] [Google Scholar]
- 15.Kaindl AM, Favrais G, Gressens P. Molecular mechanisms involved in injury to the preterm brain. Journal of Child Neurology. 2009;24(9):1112–1118. doi: 10.1177/0883073809337920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int J Dev Neurosci. 2011;29(4):423–440. doi: 10.1016/j.ijdevneu.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chau V, Poskitt KJ, McFadden DE, Bowen-Roberts T, Synnes A, Brant R, et al. Effect of chorioamnionitis on brain development and injury in premature newborns. Annals of Neurology. 2009;66(2):155–164. doi: 10.1002/ana.21713. [DOI] [PubMed] [Google Scholar]
- 18.Hendson L, Russell L, Robertson CM, Liang Y, Chen Y, Abdalla A, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. The Journal of pediatrics. 2011;158(3):397–402. doi: 10.1016/j.jpeds.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Rovira N, Alarcon A, Iriondo M, Ibanez M, Poo P, Cusi V, et al. Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants. Early Human Development. 2011;87(4):253–257. doi: 10.1016/j.earlhumdev.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 20.Stoll B, Hansen N, Adams-Chapman I, Fanaroff A, Hintz S, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA : the journal of the American Medical Association. 2004;292(19):2357–2422. doi: 10.1001/jama.292.19.2357. [DOI] [PubMed] [Google Scholar]
- 21.Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr Dev Pathol. 2003;6(5):435–448. doi: 10.1007/s10024-003-7070-y. [DOI] [PubMed] [Google Scholar]
- 22.Smith GC, Gutovich J, Smyser C, Pineda R, Newnham C, Tjoeng TH, et al. Neonatal intensive care unit stress is associated with brain development in preterm infants. Ann Neurol. 2011;70(4):541–549. doi: 10.1002/ana.22545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kidokoro H, Neil J, Inder T. A new MRI assessment tool to define brain abnormalities in very preterm infants at term. AJNR Am J Neuroradiol. 2013 Apr; doi: 10.3174/ajnr.A3521. Epub ahead of print. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen The Tich S, Anderson PJ, Shimony JS, Hunt RW, Doyle LW, Inder TE. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am J Neuroradiol. 2009;30(1):125–131. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter A, Briggs-Gowan M. Infant-Toddler Social and Emotional Assessment (ITSEA) (Unpublished Manual) University of Massachusetts - Boston, Department of Psychology, Yale University; Boston, MA, New Haven, CT: 2000. [Google Scholar]
- 26.Spittle AJ, Anderson PJ, Lee KJ, Ferretti C, Eeles A, Orton J, et al. Preventive care at home for very preterm infants improves infant and caregiver outcomes at 2 years. PEDIATRICS. 2010;126(1):e171–178. doi: 10.1542/peds.2009-3137. [DOI] [PubMed] [Google Scholar]
- 27.Kenyon S, Pike K, Jones DR, Brocklehurst P, Marlow N, Salt A, et al. Childhood outcomes after prescription of antibiotics to pregnant women with spontaneous preterm labour: 7-year follow-up of the ORACLE II trial. Lancet. 2008;372(9646):1319–1327. doi: 10.1016/S0140-6736(08)61203-9. [DOI] [PubMed] [Google Scholar]
- 28.Reiman M, Kujari H, Maunu J, Parkkola R, Rikalainen H, Lapinleimu H, et al. Does placental inflammation relate to brain lesions and volume in preterm infants? The Journal of pediatrics. 2008;152(5):642–647. 647 e641–642. doi: 10.1016/j.jpeds.2007.09.051. [DOI] [PubMed] [Google Scholar]
- 29.Chau V, Brant R, Poskitt KJ, Tam EW, Synnes A, Miller SP. Postnatal infection is associated with widespread abnormalities of brain development in premature newborns. Pediatric Research. 2012;71(3):274–279. doi: 10.1038/pr.2011.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. PEDIATRICS. 2006;117(4):1253–1261. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 31.Leviton A, Allred EN, Dammann O, Engelke S, Fichorova RN, Hirtz D, et al. Systemic Inflammation, Intraventricular Hemorrhage, and White Matter Injury. Journal of Child Neurology. 2012 doi: 10.1177/0883073812463068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stout MJ, Conlon B, Landeau M, Lee I, Bower C, Zhao Q, et al. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. American Journal of Obstetrics and Gynecology. 2013;208(3):226, e221–227. doi: 10.1016/j.ajog.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–694. doi: 10.1056/NEJMoa053792. [DOI] [PubMed] [Google Scholar]
- 34.Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. PEDIATRICS. 2005;116(2):333–341. doi: 10.1542/peds.2005-0173. [DOI] [PubMed] [Google Scholar]
- 35.Webster RI, Majnemer A, Platt RW, Shevell MI. The predictive value of a preschool diagnosis of developmental language impairment. Neurology. 2004;63(12):2327–2331. doi: 10.1212/01.wnl.0000147472.33670.b6. [DOI] [PubMed] [Google Scholar]
- 36.Dale PS, Price TS, Bishop DV, Plomin R. Outcomes of early language delay: I. Predicting persistent and transient language difficulties at 3 and 4 years. J Speech Lang Hear Res. 2003;46(3):544–560. doi: 10.1044/1092-4388(2003/044). [DOI] [PubMed] [Google Scholar]
- 37.Hamrick SE, Miller SP, Leonard C, Glidden DV, Goldstein R, Ramaswamy V, et al. Trends in severe brain injury and neurodevelopmental outcome in premature newborn infants: the role of cystic periventricular leukomalacia. The Journal of pediatrics. 2004;145(5):593–599. doi: 10.1016/j.jpeds.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 38.Apisarnthanarak A, Holzmann-Pazgal G, Hamvas A, Olsen MA, Fraser VJ. Ventilator-associated pneumonia in extremely preterm neonates in a neonatal intensive care unit: characteristics, risk factors, and outcomes. PEDIATRICS. 2003;112(6 Pt 1):1283–1289. doi: 10.1542/peds.112.6.1283. [DOI] [PubMed] [Google Scholar]
- 39.Bose CL, Laughon MM, Allred EN, Michael O’Shea T, Van Marter LJ, Ehrenkranz RA, et al. Systemic inflammation associated with mechanical ventilation among extremely preterm infants. Cytokine. 2013;61(1):315–322. doi: 10.1016/j.cyto.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helderman JB, O’Shea TM, Kuban KC, Allred EN, Hecht JL, Dammann O, et al. Antenatal antecedents of cognitive impairment at 24 months in extremely low gestational age newborns. PEDIATRICS. 2012;129(3):494–502. doi: 10.1542/peds.2011-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]