Abstract
At high concentrations, the glutamine synthetase inhibitor L-methionine-S,R-sulfoximine is a convulsant, especially in dogs. Nevertheless, sub-convulsive doses of MSO are neuroprotective in rodent models of hyperammonemia, acute liver disease, and amyotrophic lateral sclerosis and suggest MSO may be clinically useful. Previous work has also shown that much lower doses of MSO are required to produce convulsions in dogs than in primates. Evidence from the mid-20th century suggests that humans are also less sensitive. In the present work, the inhibition of recombinant human glutamine synthetase with MSO is shown to be biphasic – an initial reversible competitive inhibition (Ki 1.19 mM) is followed by rapid irreversible inactivation. This Ki value for the human enzyme accounts, in part, for relative insensitivity of primates to MSO and suggests that this inhibitor could be used to safely inhibit glutamine synthetase activity in humans.
Keywords: Amyotrophic lateral sclerosis, glutamine synthetase, hyperammonemia, L-methionine-S, R-sulfoximine
Introduction
Glutamine synthetase catalyzes the ATP-dependent condensation of ammonia 1 and glutamate to glutamine:
| [1] |
This enzyme plays unique roles in the biology of prokaryotes and eukaryotes. For example prokaryotes utilize glutamine generated by glutamine synthetase to synthesize the cell walls that surround these organisms (Harth and Horwitz 1999, 2003, Nilsson et al. 2009). The prokaryote glutamine synthetases are sufficiently different from the mammalian enzymes to have warranted significant research effort to produce antibacterial agents based on this difference (Krajewski et al. 2005, Krajewski et al. 2008, Nilsson et al. 2009). In the human brain, glutamine synthetase is found primarily in the astrocytes and acts to regulate ammonia concentrations and supply neurons with glutamine (Pamiljans et al. 1962, Martinez-Hernandez et al. 1977, Norenberg and Martinez-Hernandez 1979, Pow and Robinson 1994, Laake et al. 1995, Eisenberg et al. 2000, Kruchkova et al. 2001). A portion of this pool of glutamine is subsequently converted to glutamate and used in part as a neurotransmitter. Thus, certain pathological states involving elevations in either ammonia or glutamate might benefit from regulation of glutamine synthetase. Elevations in cerebral ammonia result in increased levels of glutamine within astrocytes (Brusilow et al. 2010, Cooper 2012a, 2012b). Glutamine is a significant osmolyte, and therefore, the increase in its concentrations adds to the brain swelling characteristic of hyperammonemia, particularly in the acute form of this condition (Desjardins et al. 1999, Tok et al. 2009, Brusilow et al. 2010, Mardini et al. 2011, Cudalbu et al. 2012). Astrocytic glutamine contributes to excitotoxicity by acting as a precursor of the excitatory neurotransmitter glutamate (Ghoddoussi et al. 2010, Bame et al. 2012). These observations suggest that limiting the production of glutamine in astrocytes could mitigate the toxicity due to either hyperammonemia or glutamate excitotoxicity. Despite these possibilities, little information exists concerning the kinetic behavior of human glutamine synthetase or it regulation by specific inhibitors.
One possible inhibitor that might be used in humans to regulate either the endogenous or prokaryote forms of this enzyme is L-methionine-S,R-sulfoximine (MSO). A number of studies indicate that MSO is therapeutic in the treatment of hyperammonemia and glutamate excitotoxicity. For example, in an acute murine model of liver disease, the animals were protected by prior administration of MSO (Warren and Schenker 1964, Jambekar et al. 2011). Similarly, the chronic administration of MSO in a murine model of amyotrophic lateral sclerosis (ALS) increased the survival time of the affected animals (Ghoddoussi et al. 2010, Bame et al. 2012). The finding that MSO has therapeutic benefit in a model of ALS is particularly exciting because at present only one drug has been approved to treat this disease (Hardiman et al. 2011). The drug - Riluzole - increases average life expectancy in ALS patients by a modest 3 to 6 months and comes with a risk of hepatic complications. Nevertheless, enthusiasm for the use of MSO in humans is tempered by the observation that, at high concentrations, this compound causes convulsions and eventually death in experimental animals (Mellanby 1946, Pollock 1949, Gershoff and Elvehjem 1951). This ability of MSO to induce convulsions is exploited in some murine models of epilepsy (Boissonnet et al. 2012, Boissonnet et al. 2013). In contrast, low doses of MSO may mitigate the onset of epileptic seizures (Sun et al. 2013). Given the potential use of this inhibitor in humans, we sought to characterize the inhibition of human glutamine synthetase by MSO.
Materials
The materials for protein purification: CHT ceramic hydroxyapatite (Type II, 40 μm particle size); Bio-Rad protein assay; Amicon Ultra-4 Centrifugal Filter Units (50 KDa); and UltrogelACA44 were purchased from Bio-Rad, Millipore and the Pall Corporation, respectively. Sigma-Aldrich supplied all other reagents used in the studies described below.
Methods
Recombinant human glutamine synthetase was overexpressed in Escherichia coli cells and purified as described by Listrom et al. (1997). The purified protein migrated as a single band on SDS-PAGE with an estimated monomer Mr of ~45,000 (not shown). Mammalian glutamine synthetase exists as a decamer of two concentric pentameric rings (Krajewski et al. 2008). The amounts of glutamine synthetase cited in the text refer to the decamer rather the monomer. Protein concentrations were determined using the Bio-Rad dye-binding assay and bovine serum albumin was used as a protein standard. A unit of enzyme activity (U) is defined as the amount of enzyme that catalyzes the formation of 1 μmol of glutamine per minute under standard assay conditions.
Glutamine synthetase activity was assayed using a modification of the method of (Kingdon et al. 1968), which couples the formation of product ADP to the oxidation of NADH. The standard reaction mixture contained 10 mM imidazole-HCl, 100 mM KCl, 40 mM MgCl2, 0.3 mM EDTA, 12 mM phosphoenolpyruvate, 10 mM ATP, 20 mM L-glutamate, 0.25 mM NADH, 10 mU pyruvate kinase, 13.3 mU lactate dehydrogenase, and 10 mM NH4Cl at a final pH of 7.5 and a volume of 1 mL. This mixture was warmed to 37°C and the reaction was then initiated by the addition of enzyme. The oxidation of NADH to NAD+ was continuously monitored as the loss of absorbance at 340 nm and quantified using the extinction coefficient 6.23 × 103 M−1cm−1.
Results
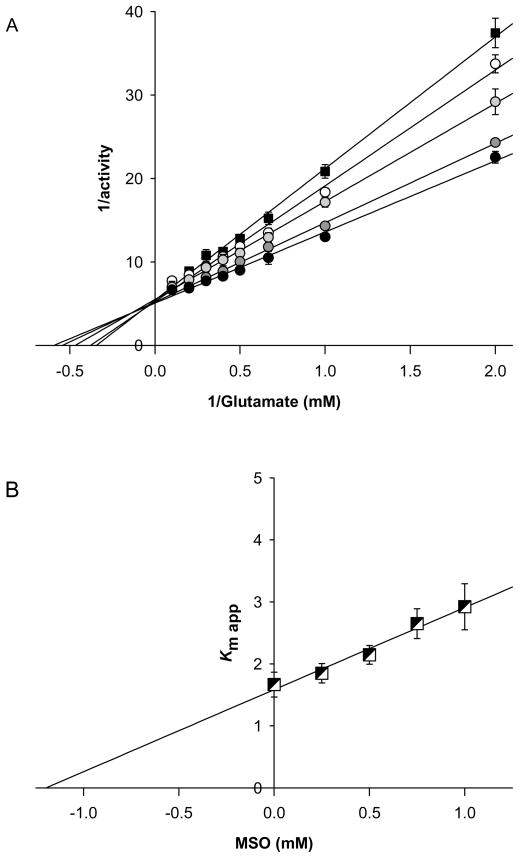
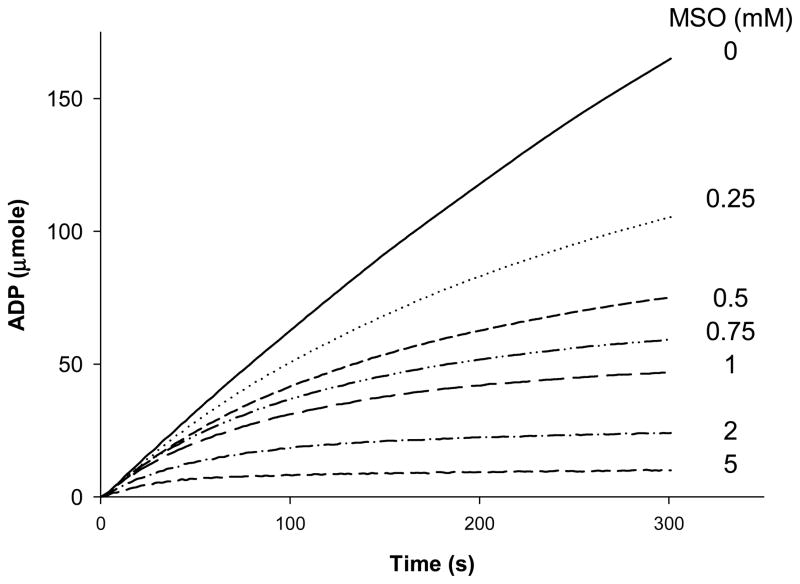
Recombinant human glutamine synthetase was purified as described by described by Listrom et al. (1997) and exhibited a specific activity of 17.9 U/mg with a 4.5% recovery and 11.1-fold increase in specific activity. The purified protein migrated as a single band on SDS-PAGE with an estimated monomer Mr of ~45,000 (not shown). Mammalian glutamine synthetase exists as a decamer of two concentric pentameric rings (Krajewski et al. 2008). The amounts of glutamine synthetase cited in the text refer to the decamer rather the monomer. A Lineweaver-Burk plot constructed from the reciprocal of initial rates versus the reciprocal of substrate concentration (Fig. 1, solid squares) indicated a Km value for L-glutamate of 1.67 mM, a Vmax of 16.0 ± 1.26 μmole/min/mg and maximal turnover number of 11.1/s for this enzyme(Table 1), and consistent with the earlier measurements of Listrom et al. (1997) The addition of MSO to the reaction mixtures caused an apparent increase in the Km of the enzyme for glutamate, but no change in Vmax, as shown in Figs. 1a (circles) in a manner consistent with competitive inhibition. A Dixon plot of these apparent Km values yielded a Ki of 1.19 mM for the competitive inhibition of glutamine synthetase by MSO (Fig. 1b). Thus, MSO initially binds competitively to human glutamine synthetase with an affinity similar to that of the natural substrate glutamate (c.f. Ki MSO ~ 1.19 mM & Km Glu ~ 1.67 mM: Table 1). Studies with other mammalian forms of this enzyme indicate that glutamine synthetase becomes rapidly inactivated in the presence of MSO (Sellinger 1967, Tate et al. 1972, Griffith and Meister 1978, Meister 1980). This is also the case for the recombinant human glutamine synthetase as shown in Fig 2. Even though the inactivation is not instantaneous, it is rapid. For example, the t1/2 for inactivation of human glutamine synthetase due to 5 mM MSO, in the presence of 20 mM L-glutamate, is ~ 25 sec (Fig. 2).
Figure 1. Competitive inhibition of human glutamine synthetase by MSO.
Panel A depicts the Lineweaver-Burke plot for the inhibition of 300 nM human glutamine synthetase by 0, 0.25, 0.5, 0.75, or 1.0 mM MSO. Reaction rates were determined as quickly as possible after addition of glutamine synthetase to avoid errors in estimation of initial reversible, competitive reversible inhibition resulting from slower but irreversible inhibition. Panel B represents the replotted data (Dixon plot) derived from Panel A. The values shown are the means SD for three independent measurements. Where error bars are not shown the SD is less than the width of the symbol.
Table 1.
Kinetic properties of the human glutamine synthetase
| Parameter | Mean ± SD (n) units |
|---|---|
| Vmax | 15.9 ± 1.26 (5) μmol/min/mg |
| km glutamate | 1.67 ± 0.20 (5) mM |
| Kcat | 11.1/s |
| Ki MSO | 1.19 ± 0.20 (3) mM |
Figure 2. Inactivation of human glutamine synthetase by MSO.
Shown are the progress curves of the glutamine synthetase reaction in the presence of 20 mM glutamate and 0, 0.25, 0.5, 0.75, or 1.0 mM MSO. The curves represent the mean values of three separate determinations. For the sake of clarity, the SD values are not shown but were less than 5% of the mean in all cases.
Discussion
MSO inhibits reaction 1 by two distinct mechanisms: competitive inhibition followed by irreversible inactivation. Competitive inhibition occurs subsequent to the binding of ATP to glutamine synthetase. Nucleotide binding increases the affinity of the enzyme for glutamate, which binds to the active site by way of its α carboxyl and amino groups (Eisenberg et al. 2000). MSO has similarly placed carboxyl and amino groups and competes with glutamate for binding to the active site of glutamine synthetase (2). The competitive nature of this inhibition has been confirmed for all of the forms of the enzyme studied thus far, including the human enzyme (Table 2).
Table 2.
Reported values for the inhibition of glutamine synthetases by MSO
| Source | Ki MSO (mM) | Km Glu (mM) | Km ATP (mM) | References |
|---|---|---|---|---|
| Human | 1.19a | 1.67a, 3.00b & 3.50b | 1.80b & 2.8b | a This study & bListrom et al. (1997) |
| Ovine brain | 0.210 | 2.70c & 2.50d | 2.3d | cLogusch et al. (1989) & dPamiljans et al. (1962) |
| Canine long form | 0.124 | 1.30 | 1.90 | Shin et al. (2004) |
| Canine short form | 0.067 | 1.10 | 1.30 | Shin et al. (2004) |
| Various Plants | n.d. | ~5 – 10 | ~0.3 – 1.3 | Acaster et al. (1985) |
| Pea seed | 0.200c | n.d. | 2.00d | cWedler et al. (1976) & dKnight et al. (1988) |
| Pea leaf | 0.161 | n.d. | n.d. | Leason et al. (1982) |
| Spinach | 0.100 | n.d. | n.d. | Lea et al. (1989) |
| Yeast | n.d. | 5.40 | 0.300 | (Kim and Rhee 1987) Kim et al. (1987) |
| S. Typhimurium | n.d. | 1.10e | 0.580f | eLiaw et al. (1993) & fLiaw et al. (1994) |
| E. Coli | 0.001g,h | 3.00g,h, 5.50i | 0.400i | gVillafranca et al. (1976), hWedler et al. (1976) & iAlibhai et al. (1994) |
MSO and glutamate are phosphorylated after binding to the enzyme (Tsuda et al. 1971). Phosphorylation of the γ-carboxyl of glutamate renders the resulting intermediate susceptible to nucleophilic attack by NH3 (Eisenberg et al. 2000). An attack on this intermediate by water is prevented by prior closure of the channel to the active site for amino acids by a flap consisting of Gly302-Phe303-His304-Glu305-Thr306 (Eisenberg et al. 2000, Krajewski et al. 2008). These residues refer to human glutamine synthetase. Homologs of this flap are also present in the prokaryote and other eukaryote forms of this enzyme (Eisenberg et al. 2000, van Rooyen et al. 2011). In addition to occluding the amino acid channel, the flap forms a binding pocket for ammonium by the juxtaposition of flap residue Glu305 with Asp 63 in the active site (Gill and Eisenberg 2001). This binding pocket for ammonium, however, is not formed when MSO is bound to glutamine synthetase. Instead, the protonated sulfoximine N atom of MSO hydrogen bonds with a carboxylate oxygen of Glu305 and prevents the interaction with Asp63 (Gill and Eisenberg 2001). These actions prevent the binding of ammonium and leads to the essentially irreversible inhibition of glutamine synthetase by MSO2.
The inhibition of human glutamine synthetase by MSO is remarkable in that the Ki value for the reversible inhibition is largest thus far reported among the enzymes investigated (Table 2). Moreover, this value is three orders of magnitude greater than the Ki values reported for the bacterial forms of the enzyme and supports the contention that MSO or its variants may be useful in treatment of tuberculosis. Indeed, Hart and Horowitz (1999) reported that the Mycobacterium tuberculosis glutamine synthetase is a 100 times more sensitive to inhibition by MSO than a mammalian glutamine synthetase. Unfortunately, M. tuberculosis rapidly develops resistance to MSO by upregulating three alternative forms of glutamine synthetase (Carroll et al. 2011) and later efforts to inhibit this group of enzymes have focused on the nucleotide binding sites (Krajewski et al. 2005, Nilsson et al. 2009). At present, it is not possible to account for the differences in competitive inhibition of the various eukaryotic glutamine synthetases by MSO based on the available structural information. The published structures are of the enzymes complexed with MSO or a comparable inhibitor (Eisenberg et al. 2000, Krajewski et al. 2005, Unno et al. 2006, Krajewski et al. 2008, van Rooyen et al. 2011). In these studies, the position of MSO in these enzymes is apparently invariant and suggests conservation in the catalytic fold regardless of any sequence divergence in other parts of the enzyme (Eisenberg et al. 2000, Krajewski et al. 2008, van Rooyen et al. 2011). The MSO in these studies, however, was phophorylated. Thus, the reported structures reflect the conformation of the enzymes in an irreversibly-inhibited state rather than the competitively-inhibited state.
One of the motivations for conducting the present studies was to examine whether differences in the catalytic activity of the human and canine glutamine synthetase could account for sensitivity of dogs to the induction of convulsions by MSO. Given the potential use of MSO in humans it is important to understand why some mammals are more sensitive to the effect of this compound than others (Pollock 1949, Proler and Kellaway 1965, Rowe and Meister 1970, Griffith and Meister 1978). Dogs are especially sensitive to MSO (Gershoff and Elvehjem 1951) and also produce two forms of glutamine synthetase distinguished by size (Shin and Park 2004). The reported Ki values for the inhibition of the short and long forms of the canine glutamine synthetase are 0.067 and 0.124 mM, respectively (Shin and Park 2004). These values though are likely to be an underestimate of the actual Ki for initial competitive inhibition of these enzymes by MSO (i.e., they over-estimate the affinity for MSO), because they were derived using an end-point assay, stopped 15 minutes after the initiation of the reaction. As can be seen from Fig. 2, the human glutamine synthetase is substantially inactivated within five minutes. Inactivation by MSO is a common feature of eukaryote and prokaryote glutamine synthetases (Sellinger 1967, Tate et al. 1972, Griffith and Meister 1978, Meister 1980, Rhee et al. 1981, Maurizi and Ginsburg 1982, Kim and Rhee 1987) and indicates that end-point assays cannot be used to determine Ki values for MSO. For this reason, the Ki MSO reported for the P. Laninosum glutamine synthetase (Blanco et al. 1989) is also liable to be in error. Crystallographic studies suggest that the active sites of the human and canine enzymes are identical and imply that the actual Ki value for the competitive inhibition of the short canine glutamine synthetase is comparable to value obtained for the human enzyme. As discussed above, the reported structures for the canine and human glutamine synthetase represent these enzymes in an irreversibly-inhibited conformation and therefore cannot be used to infer information about the competitively-inhibited glutamine synthetases. Thus, the possibility that the sensitivity of dogs to MSO-induced convulsion is due a tighter binding of MSO to glutamine synthetase cannot be assessed with the currently available data.
MSO inhibits γ-glutamylcysteine synthetase as well glutamine synthetase in in vitro studies (Richman et al. 1973, Griffith and Meister 1978). γ-Glutamylcysteine synthetase catalyzes the rate-limiting step of glutathione biosynthesis (Franklin et al. 2009). Administration of MSO to rodents, however, did not alter the content of glutathione in the brains of these animals (Ghittoni et al. 1970, Palekar et al. 1975, Griffith and Meister 1978). This lack of an effect of MSO on in the activity of γ-glutamylcysteine synthetase in vivo presumably reflects the slow turnover of glutathione in the brain (Chang et al. 1997) and the fact that glutathione synthesis is driven by the availability of cysteine rather than that of glutamine (Jeitner and Lawrence 2001). Taken together, these observations indicate that MSO preferentially depletes the brain of glutamine synthetase activity.
ALS is a particularly devastating neurodegenerative disorder (Hardiman et al. 2011). The patients almost always die within two to five years of onset due to the pulmonary complications of this disease. As noted in the Introduction, Riluzole is the only drug approved for the treatment of ALS and its therapeutic benefits are relatively modest (Hardiman et al. 2011). Riluzole slows the course of ALS, in part, by limiting neuronal glutamate release (Hardiman et al. 2011). MSO also stems the release of glutamate by neurons (Somers and Beckstead 1990, Zou et al. 2010). The recent reports of MSO improving both survival rates and locomotor activity in a murine model of ALS suggest that this agent could be used to treat ALS (Ghoddoussi et al. 2010, Bame et al. 2012). MSO is tolerated well by humans (Newell et al. 1949, Pollock 1949) and as discussed below, unlikely to cause the hepatic damage reported in ~10% of patients taking Riluzole (Bensimon and Doble 2004).
Another condition for which the administration of MSO may have therapeutic value is hyperammonemia. Elevated levels of brain ammonia can arise in a variety of disease states, including acute and chronic liver disease and inborn errors of the urea cycle (Brusilow et al. 2010, Ghoddoussi et al. 2010, Cooper 2012a, 2012b). Accumulation of ammonia in turn leads to encephalopathy and in some cases death. The neurotoxicity due to hyperammonemia is likely to be due at least in part to the excess production of glutamine and MSO decreases the amounts of this amino acid in vivo (Ghoddoussi et al. 2010). Indeed, MSO protects against acute ammonia toxicity in rodents (Warren and Schenker 1964) and acute liver failure in rodents (Jambekar et al. 2011). Thus, MSO may by extension be of use in the treatment of hyperammonemia in human patients.
One objection to the use of MSO in human is the fact at relatively high concentrations it can cause seizures in a variety of experimental animals (Mellanby 1946, Pollock 1949, Gershoff and Elvehjem 1951, Proler and Kellaway 1965, Boissonnet et al. 2012, Boissonnet et al. 2013, Sun et al. 2013). It was the occurrence of these seizures that led to the discovery of MSO. Early in 20th century many hunting dogs in the US and the UK exhibited running fits. These convulsions were eventually linked to the ingestion of biscuits made with agenized flour3 (Mellanby 1946). The actual convulsant was later identified as the L,S-diastereoisomer of MSO (Manning et al. 1969, Rowe and Meister 1970). Humans had, however, consumed products made from agenized flour for several decades with no apparent ill effects (Newell et al. 1949). The tolerance of humans to MSO has been confirmed by several experimental studies (Pollock 1949, Krakoff 1961). In addition, Rhesus monkeys acutely treated with MSO exhibited a ~60% reduction in cerebral glutamine synthetase activity with no discernible neurological deficits (Brusilow et al. 2010). Mice treated chronically with MSO that produced a sustained ~85% decrement in glutamine synthetase activity, were also not adversely affected by this treatment (Blin et al. 2002). These observations suggest that MSO is not harmful to primates and rodents at levels that reduce glutamine synthetase activity substantially and may be safe to use in humans.
Acknowledgments
Mark Horswill and Michael Haywood (Department of Pediatrics, Medical College of Wisconsin) provided technical assistance and Dr. Chad D. Listrom (University of Maryland) supplied the glutamine synthetase expression system. Drs. Owen Griffith and William Antholine of the Departments of Biochemistry and Biophysics at the Medical College of Wisconsin are thanked for their insights into these studies and their review of the manuscript. Funding was generously supplied by National Institutes of Health grants RO3-NS074286 (TMJ) and RO1 ES 008421 (AJLC) and the Theresa Pantnode Santmann Foundation Award (TMJ).
Abbreviations used
- ALS
amyotrophic lateral sclerosis
- MSO
L-methionine-S,R-sulfoximine
Footnotes
Ammonia free base (NH3) has a pKa of ~9.2. Thus, under normal intracellular physiological conditions (pH 7.2 – 7.4) ammonia exists predominantly (~99%) as the conjugate acid, ammonium (NH4+). For convenience, unless otherwise stated, the term ammonia is used throughout the text to indicate the sum of NH3 plus NH4+.
Glutamine synthetase inactivated by MSO can be reactivated by certain non-physiological manipulations (Maurizi and Ginsburg 1982).
Flour bleached with NCl3, which converts some protein methionine residues to MSO.
Bibliography
- Bame M, Pentiak PA, Needleman R, Brusilow WS. Effect of Sex on Lifespan, Disease Progression, and the Response to Methionine Sulfoximine in the SOD1 G93A Mouse Model for ALS. Gend Med. 2012;9:524–535. doi: 10.1016/j.genm.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Bensimon G, Doble A. The tolerability of riluzole in the treatment of patients with amyotrophic lateral sclerosis. Expert Opin Drug Saf. 2004;3:525–534. doi: 10.1517/14740338.3.6.525. [DOI] [PubMed] [Google Scholar]
- Blanco F, Alana A, Llama MJ, Serra JL. Purification and properties of glutamine synthetase from the non-N2-fixing cyanobacterium Phormidium laminosum. J Bacteriol. 1989;171:1158–1165. doi: 10.1128/jb.171.2.1158-1165.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin M, Crusio WE, Hevor T, Cloix JF. Chronic inhibition of glutamine synthetase is not associated with impairment of learning and memory in mice. Brain Res Bull. 2002;57:11–15. doi: 10.1016/s0361-9230(01)00631-1. [DOI] [PubMed] [Google Scholar]
- Boissonnet A, Hevor T, Cloix JF. Phenotypic differences between fast and slow methionine sulfoximine-inbred mice: seizures, anxiety, and glutamine synthetase. Epilepsy Res. 2012;98:25–34. doi: 10.1016/j.eplepsyres.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Boissonnet A, Hevor T, Landemarre L, Cloix JF. Monoamines and glycogen levels in cerebral cortices of fast and slow methionine sulfoximine-inbred mice. Epilepsy Res. 2013;104:217–225. doi: 10.1016/j.eplepsyres.2012.11.007. [DOI] [PubMed] [Google Scholar]
- Brusilow SW, Koehler RC, Traystman RJ, Cooper AJ. Astrocyte glutamine synthetase: importance in hyperammonemic syndromes and potential target for therapy. Neurotherapeutics. 2010;7:452–470. doi: 10.1016/j.nurt.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll P, Waddell SJ, Butcher PD, Parish T. Methionine sulfoximine resistance in Mycobacterium tuberculosis is due to a single nucleotide deletion resulting in increased expression of the major glutamine synthetase, GlnA1. Microb Drug Resist. 2011;17:351–355. doi: 10.1089/mdr.2010.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang ML, Klaidman LK, Adams JD., Jr The effects of oxidative stress on in vivo brain GSH turnover in young and mature mice. Mol Chem Neuropathol. 1997;30:187–197. doi: 10.1007/BF02815097. [DOI] [PubMed] [Google Scholar]
- Cooper AJ. Possible treatment of end-stage hyperammonemic encephalopathy by inhibition of glutamine synthetase. Metab Brain Dis. 2012a;28:19–25. doi: 10.1007/s11011-012-9338-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper AJ. The role of glutamine synthetase and glutamate dehydrogenase in cerebral ammonia homeostasis. Neurochem Res. 2012b;37:2439–2455. doi: 10.1007/s11064-012-0803-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudalbu C, Lanz B, Duarte JM, Morgenthaler FD, Pilloud Y, Mlynarik V, Gruetter R. Cerebral glutamine metabolism under hyperammonemia determined in vivo by localized (1)H and (15)N NMR spectroscopy. J Cereb Blood Flow Metab. 2012;32:696–708. doi: 10.1038/jcbfm.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins P, Rao KV, Michalak A, Rose C, Butterworth RF. Effect of portacaval anastomosis on glutamine synthetase protein and gene expression in brain, liver and skeletal muscle. Metab Brain Dis. 1999;14:273–280. doi: 10.1023/a:1020741226752. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Gill HS, Pfluegl GM, Rotstein SH. Structure-function relationships of glutamine synthetases. Biochim Biophys Acta. 2000;1477:122–145. doi: 10.1016/s0167-4838(99)00270-8. [DOI] [PubMed] [Google Scholar]
- Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoff SN, Elvehjem CA. The relative effect of methionine sulfoximine on different animal species. J Nutr. 1951;45:451–458. doi: 10.1093/jn/45.3.451. [DOI] [PubMed] [Google Scholar]
- Ghittoni NE, Ohlsson WG, Sellinger OZ. The effect of methionine on the regional and intracellular disposition of [3H]-methionine sulphoximine in rat brain. J Neurochem. 1970;17:1057–1068. doi: 10.1111/j.1471-4159.1970.tb02259.x. [DOI] [PubMed] [Google Scholar]
- Ghoddoussi F, Galloway MP, Jambekar A, Bame M, Needleman R, Brusilow WS. Methionine sulfoximine, an inhibitor of glutamine synthetase, lowers brain glutamine and glutamate in a mouse model of ALS. J Neurol Sci. 2010;290:41–47. doi: 10.1016/j.jns.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Gill HS, Eisenberg D. The crystal structure of phosphinothricin in the active site of glutamine synthetase illuminates the mechanism of enzymatic inhibition. Biochemistry. 2001;40:1903–1912. doi: 10.1021/bi002438h. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Meister A. Differential inhibition of glutamine and gamma-glutamylcysteine synthetases by alpha-alkyl analogs of methionine sulfoximine that induce convulsions. J Biol Chem. 1978;253:2333–2338. [PubMed] [Google Scholar]
- Hardiman O, van den Berg LH, Kiernan MC. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol. 2011;7:639–649. doi: 10.1038/nrneurol.2011.153. [DOI] [PubMed] [Google Scholar]
- Harth G, Horwitz MA. An inhibitor of exported Mycobacterium tuberculosis glutamine synthetase selectively blocks the growth of pathogenic mycobacteria in axenic culture and in human monocytes: extracellular proteins as potential novel drug targets. J Exp Med. 1999;189:1425–1436. doi: 10.1084/jem.189.9.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harth G, Horwitz MA. Inhibition of Mycobacterium tuberculosis glutamine synthetase as a novel antibiotic strategy against tuberculosis: demonstration of efficacy in vivo. Infect Immun. 2003;71:456–464. doi: 10.1128/IAI.71.1.456-464.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jambekar AA, Palma E, Nicolosi L, Rasola A, Petronilli V, Chiara F, Bernardi P, Needleman R, Brusilow WS. A glutamine synthetase inhibitor increases survival and decreases cytokine response in a mouse model of acute liver failure. Liver Int. 2011;31:1209–1221. doi: 10.1111/j.1478-3231.2011.02553.x. [DOI] [PubMed] [Google Scholar]
- Jeitner TM, Lawrence DA. Mechanisms for the cytotoxicity of cysteamine. Toxicol Sci. 2001;63:57–64. doi: 10.1093/toxsci/63.1.57. [DOI] [PubMed] [Google Scholar]
- Kim KH, Rhee SG. Subunit interaction elicited by partial inactivation with L-methionine sulfoximine and ATP differently affects the biosynthetic and gamma-glutamyltransferase reactions catalyzed by yeast glutamine synthetase. J Biol Chem. 1987;262:13050–13054. [PubMed] [Google Scholar]
- Kingdon HS, Hubbard JS, Stadtman ER. Regulation of glutamine synthetase. XI. The nature and implications of a lag phase in the Escherichia coli glutamine synthetase reaction. Biochemistry. 1968;7:2136–2142. doi: 10.1021/bi00846a016. [DOI] [PubMed] [Google Scholar]
- Krajewski WW, Jones TA, Mowbray SL. Structure of Mycobacterium tuberculosis glutamine synthetase in complex with a transition-state mimic provides functional insights. Proc Natl Acad Sci U S A. 2005;102:10499–10504. doi: 10.1073/pnas.0502248102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewski WW, Collins R, Holmberg-Schiavone L, Jones TA, Karlberg T, Mowbray SL. Crystal structures of mammalian glutamine synthetases illustrate substrate-induced conformational changes and provide opportunities for drug and herbicide design. J Mol Biol. 2008;375:217–228. doi: 10.1016/j.jmb.2007.10.029. [DOI] [PubMed] [Google Scholar]
- Krakoff IH. Effect of methionine sulfoximine in man. Clin Pharmacol Ther. 1961;2:599–604. doi: 10.1002/cpt196125599. [DOI] [PubMed] [Google Scholar]
- Kruchkova Y, Ben-Dror I, Herschkovitz A, David M, Yayon A, Vardimon L. Basic fibroblast growth factor: a potential inhibitor of glutamine synthetase expression in injured neural tissue. J Neurochem. 2001;77:1641–1649. doi: 10.1046/j.1471-4159.2001.00390.x. [DOI] [PubMed] [Google Scholar]
- Laake JH, Slyngstad TA, Haug FM, Ottersen OP. Glutamine from glial cells is essential for the maintenance of the nerve terminal pool of glutamate: immunogold evidence from hippocampal slice cultures. J Neurochem. 1995;65:871–881. doi: 10.1046/j.1471-4159.1995.65020871.x. [DOI] [PubMed] [Google Scholar]
- Manning JM, Moore S, Rowe WB, Meister A. Identification of L-methionine S-sulfoximine as the diastereoisomer of L-methionine SR-sulfoximine that inhibits glutamine synthetase. Biochemistry. 1969;8:2681–2685. doi: 10.1021/bi00834a066. [DOI] [PubMed] [Google Scholar]
- Mardini H, Smith FE, Record CO, Blamire AM. Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J Hepatol. 2011;54:1154–1160. doi: 10.1016/j.jhep.2010.09.030. [DOI] [PubMed] [Google Scholar]
- Martinez-Hernandez A, Bell KP, Norenberg MD. Glutamine synthetase: glial localization in brain. Science. 1977;195:1356–1358. doi: 10.1126/science.14400. [DOI] [PubMed] [Google Scholar]
- Maurizi MR, Ginsburg A. Reactivation of glutamine synthetase from Escherichia coli after auto-inactivation with L-methionine-S-sulfoximine, ATP, and Mn2+ J Biol Chem. 1982;257:4271–4278. [PubMed] [Google Scholar]
- Meister A. Catalytic mechanism of glutamine synthetase: overview of glutamine metabolism. Glutamine Metabolism, Enzymology, Regulation. 1980:1–40. [Google Scholar]
- Mellanby E. Diet and canine hysteria; experimental production by treated flour. Br Med J. 1946;2:885–887. doi: 10.1136/bmj.2.4484.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell GW, Erickson TC, et al. Studies on human subjects receiving highly agenized food materials. J Lab Clin Med. 1949;34:239–245. [PubMed] [Google Scholar]
- Nilsson MT, Krajewski WW, Yellagunda S, Prabhumurthy S, Chamarahally GN, Siddamadappa C, Srinivasa BR, Yahiaoui S, Larhed M, Karlen A, Jones TA, Mowbray SL. Structural basis for the inhibition of Mycobacterium tuberculosis glutamine synthetase by novel ATP-competitive inhibitors. J Mol Biol. 2009;393:504–513. doi: 10.1016/j.jmb.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Norenberg MD, Martinez-Hernandez A. Fine structural localization of glutamine synthetase in astrocytes of rat brain. Brain Res. 1979;161:303–310. doi: 10.1016/0006-8993(79)90071-4. [DOI] [PubMed] [Google Scholar]
- Palekar AG, Tate SS, Meister A. Decrease in glutathione levels of kidney and liver after injection of methionine sulfoximine into rats. Biochem Biophys Res Commun. 1975;62:651–657. doi: 10.1016/0006-291x(75)90448-9. [DOI] [PubMed] [Google Scholar]
- Pamiljans V, Krishnaswamy PR, Dumville G, Meister A. Studies on the mechanism of glutamine synthesis; isolation and properties of the enzyme from sheep brain. Biochemistry. 1962;1:153–158. doi: 10.1021/bi00907a023. [DOI] [PubMed] [Google Scholar]
- Pollock GH. Species specificity of agene toxicity. J Appl Physiol. 1949;1:802–806. doi: 10.1152/jappl.1949.1.11.802. [DOI] [PubMed] [Google Scholar]
- Pow DV, Robinson SR. Glutamate in some retinal neurons is derived solely from glia. Neuroscience. 1994;60:355–366. doi: 10.1016/0306-4522(94)90249-6. [DOI] [PubMed] [Google Scholar]
- Proler ML, Kellaway P. Behavioral and convulsive phenomena induced in the cat by methionine sulfoximine. Ablation and electrographic studies. Neurology. 1965;15:931–940. doi: 10.1212/wnl.15.10.931. [DOI] [PubMed] [Google Scholar]
- Rhee SG, Chock PB, Wedler FC, Sugiyama Y. Subunit interaction in unadenylylated glutamine synthetase from Escherichia coli. Evidence from methionine sulfoximine inhibition studies. J Biol Chem. 1981;256:644–648. [PubMed] [Google Scholar]
- Richman PG, Orlowski M, Meister A. Inhibition of gamma-glutamylcysteine synthetase by L-methionine-S-sulfoximine. J Biol Chem. 1973;248:6684–6690. [PubMed] [Google Scholar]
- Rowe WB, Meister A. Identification of L-methionine-S-sulfoximine as the convulsant isomer of methionine sulfoximine. Proc Natl Acad Sci U S A. 1970;66:500–506. doi: 10.1073/pnas.66.2.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellinger OZ. Inactivation of cerebral glutamine synthetase by DL-methionine-DL-sulfoximine. Biochim Biophys Acta. 1967;132:514–516. doi: 10.1016/0005-2744(67)90172-6. [DOI] [PubMed] [Google Scholar]
- Shin D, Park C. N-terminal extension of canine glutamine synthetase created by splicing alters its enzymatic property. J Biol Chem. 2004;279:1184–1190. doi: 10.1074/jbc.M309940200. [DOI] [PubMed] [Google Scholar]
- Somers DL, Beckstead RM. Chronic methionine sulfoximine administration reduces synaptosomal aspartate and glutamate in rat striatum. Neurosci Lett. 1990;115:335–340. doi: 10.1016/0304-3940(90)90478-r. [DOI] [PubMed] [Google Scholar]
- Sun HL, Zhang SH, Zhong K, Xu ZH, Feng B, Yu J, Fang Q, Wang S, Wu DC, Zhang JM, Chen Z. A Transient Upregulation of Glutamine Synthetase in the Dentate Gyrus Is Involved in Epileptogenesis Induced by Amygdala Kindling in the Rat. PLoS One. 2013;8:e66885. doi: 10.1371/journal.pone.0066885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate SS, Leu FY, Meister A. Rat liver glutamine synthetase. Preparation, properties, and mechanism of inhibition by carbamyl phosphate. J Biol Chem. 1972;247:5312–5321. [PubMed] [Google Scholar]
- Tok CY, Chew SF, Peh WY, Loong AM, Wong WP, Ip YK. Glutamine accumulation and up-regulation of glutamine synthetase activity in the swamp eel, Monopterus albus (Zuiew), exposed to brackish water. J Exp Biol. 2009;212:1248–1258. doi: 10.1242/jeb.025395. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Stephani RA, Meister A. Direct evidence for the formation of an acyl phosphate by glutamine synthetase. Biochemistry. 1971;10:3186–3189. doi: 10.1021/bi00793a004. [DOI] [PubMed] [Google Scholar]
- Unno H, Uchida T, Sugawara H, Kurisu G, Sugiyama T, Yamaya T, Sakakibara H, Hase T, Kusunoki M. Atomic structure of plant glutamine synthetase: a key enzyme for plant productivity. J Biol Chem. 2006;281:29287–29296. doi: 10.1074/jbc.M601497200. [DOI] [PubMed] [Google Scholar]
- van Rooyen JM, Abratt VR, Belrhali H, Sewell T. Crystal structure of Type III glutamine synthetase: surprising reversal of the inter-ring interface. Structure. 2011;19:471–483. doi: 10.1016/j.str.2011.02.001. [DOI] [PubMed] [Google Scholar]
- Warren KS, Schenker S. Effect of an Inhibitor of Glutamine Synthesis (Methionine Sulfoximine) on Ammonia Toxicity and Metabolism. J Lab Clin Med. 1964;64:442–449. [PubMed] [Google Scholar]
- Zou J, Wang YX, Dou FF, Lu HZ, Ma ZW, Lu PH, Xu XM. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochem Int. 2010;56:577–584. doi: 10.1016/j.neuint.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]