The incidence of constrictive pericarditis in HIV un-infected patients with pericardial tuberculosis is very high (31.65 cases per 1000 person-years) despite modern rifampicin-based antituberculosis treatment.1 The cellular mediators and molecular mechanisms of post tuberculous pericardial fibrosis are unknown. N-acetyl-seryl-aspartyl-lysl-proline (Ac-SDKP) is a ubiquitous tetrapeptide with important antifibrotic properties and galectin-3 is an activator of myofibroblasts, promoter of collagen and extracellular matrix deposition and is associated with organ fibrosis.2,3 AcSDKP, which is inactivated by angiotensin converting enzyme (ACE), exerts part of its anti-fibrotic effect by inhibiting galectin-3 (figure 1).4 Currently, it is not known whether endogenous Ac-SDKP and galectin-3 are present in normal pericardial effusion, and whether there are any changes in the context of pericarditis.
METHODS
We conducted a pilot study of adults (≥18 years) with a normal pericardium and cases with tuberculous pericarditis to define the levels of endogenous Ac-SDKP and galectin-3 in normal pericardial fluid, and to assess the effect of pericardial infection with tuberculosis on levels of these factors. All participants gave written informed consent and the study was approved by the University of Cape Town Human Research Ethics Committee (HREC REF: 402/2008). Eligible participants had echocardiographic evidence of a moderate or large effusion. The pericardial fluid was considered tuberculous if it was an inflammatory exudate with one of the following criteria: Mycobacterium tuberculosis was identified by microscopy, culture or by polymerase chain reaction; levels of interferon- gamma (IFN-γ) was >50pg/ml or adenosine deaminase >40IU/L.
Patients undergoing elective coronary bypass surgery were used as normal controls. Participants with either a history of pericardial disease or evidence of prior myocardial infarction (by history or electrocardiogram) were excluded. The SPIBIO-A05881 was used to measure Ac-SDKP while the BenderMed BMS 279/2CE ELISA kit was used for measurement of galectin-3.
The Shapiro-Wilk test was used to test if the measured levels were normally distributed. Differences between the two groups (cases with tuberculosis and controls without pericardial disease) were tested using the Student t test, or Mann-Whitney test, where appropriate for continuous variables, and the chi square test for categorical variables. All tests were two sided and a p-value <0.05 was considered significant.
RESULTS
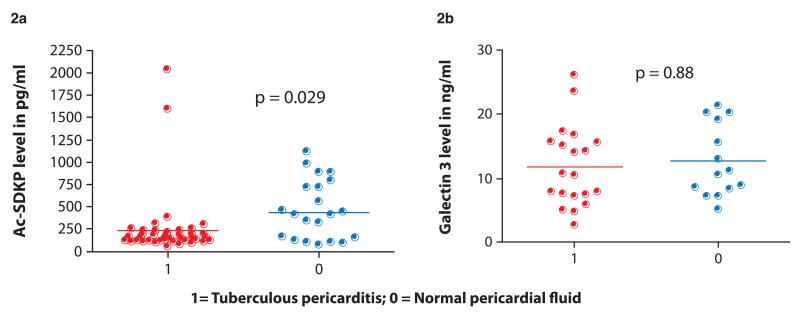
AcSDKP and galectin-3 levels were assayed in 49 and 52 patients with tuberculous pericarditis, respectively. The levels were compared with those of 20 control participants with no pericardial disease. The median level of AcSDKP in the participants with tuberculous pericarditis (156 pg/ml [interquartile range {IQR} 126.8-187.4]) was significantly lower than normal controls (412 pg/ml [IQR 146.7-717.9]), p=0.029 (figure 2A). The median level of galectin-3 measured in the cell free pericardial fluid of patients with tuberculous pericarditis was 11ng/ml (IQR 7.55-15.6). This was similar to the 12 ng/ml (IQR 7.49-19.62) found in the pericardial fluid of normal controls (p=0.191) (figure 2B).
DISCUSSION
In this pilot study we have shown, for the first time, that AcSDKP and galectin-3 are detectable in normal pericardial fluid, and that tuberculous pericarditis is associated with low levels of pericardial AcSDKP and normal galectin-3 levels. These observations pertaining to the levels of AcSDKP and galectin-3 in normal pericardial fluid and tuberculous pericardial effusion are important for a number of reasons. The findings suggest that AcSDKP and galectin-3 play a housekeeping function within the normal pericardium similar to that in ventricles and kidneys.5 Furthermore, our results raise the possibility that both molecules play an important role maintaining the health of the pericardium during times of physiological or pathological stress. The, depressed levels of AcSDKP in conjunction with normal or low levels of galectin-3 within the pericardium, may provide a novel explanation for the high incidence of constrictive pericarditis associated with tuberculous pericarditis.1
Thus, further elucidation of the physiological and pathological role of Ac-SDKP and galectin-3 could enhance the limited understanding of the constitution and function of normal pericardial fluid, the pathogenesis of pericardial inflammation and fibrosis, and provide important information on the prospect of using these molecules as novel pharmacological targets for the prevention of constriction.
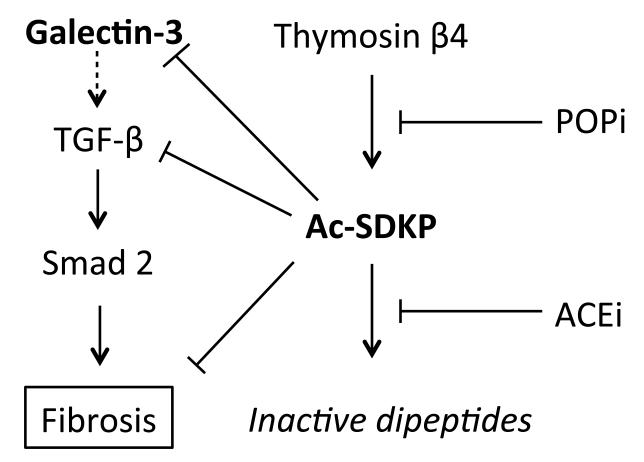
Figure 1.
Hypothesised mechanism by which the tetrapeptide N-acetyl-seryl-aspartyly-lysyl-proline (Ac-SDKP) inhibits fibrosis
Ac-SDKP is generated from the G actin binding peptide, thymosin β4, by prolyl oligopeptidase (POP) through one-step enzymatic cleavge. Angiotensin-1 converting enzyme (ACE) hydrolyses Ac-SDKP into inactive dipeptides. ACE inhibitors (ACEi) increase the concentration of Ac-SDKP whereas POP inhibitors (POPi) decrease AcSDKP production. Ac-SDKP prevents the galectin-3 induced effect on fibrosis by inhibiting the TGF-β/Smad2 signaling pathway. Ac-SDKP may also inhibit fibrosis directly by blocking collagen synthesis.
Figure 2.
Scatter plots of pericardial AcSDKP levels in pg/m (2a) and pericardial galectin-3 levels in ng/ml (2b) grouped by cases with tuberculous pericarditis (1) and controls undergoing cardiac surgery without pericarditis (0)
ACKNOWLEDGEMENTS
We are grateful to the patients who consented to be enrolled in this study, and to the physicians who contributed to the IMPI Africa Registry. Veronica Francis, Unita September and Simphiwe Nkepu are thanked for assistance with recruitment and follow up of patients.
FUNDING This work was supported by the Wellcome Trust of Great Britain (references 084323, 088316, 083226). Additional support was provided by the Medical Research Councils of the UK and South Africa and the Lily and Hausmann Research Trust.
Footnotes
COMPETING INTERESTS None.
REFERENCES
- 1.Imazio M, Brucato A, Maestroni S, et al. Risk of Constrictive Pericarditis After Acute Pericarditis. Circulation. 2011;124:1270–5. doi: 10.1161/CIRCULATIONAHA.111.018580. [DOI] [PubMed] [Google Scholar]
- 2.Castoldi G, di Gioia CR, Bombardi C, et al. Prevention of myocardial fibrosis by N-acetyl-seryl-aspartyl-lysyl-proline in diabetic rats. Clin Sci (Lond) 2009;118:211–20. doi: 10.1042/cs20090234. [DOI] [PubMed] [Google Scholar]
- 3.Henderson NC, Mackinnon AC, Farnworth SL, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–98. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu YH, D’Ambrosio M, Liao TD, et al. N-acetyl-seryl-aspartyl-lysyl-proline prevents cardiac remodeling and dysfunction induced by galectin-3, a mammalian adhesion/growth-regulatory lectin. Am J Physiol Heart Circ Physiol. 2009;296:H404–12. doi: 10.1152/ajpheart.00747.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cavasin MA, Liao TD, Yang XP, et al. Decreased endogenous levels of Ac-SDKP promote organ fibrosis. Hypertension. 2007;50:130–6. doi: 10.1161/HYPERTENSIONAHA.106.084103. [DOI] [PubMed] [Google Scholar]