Abstract
Protons dissociated from organic acids in cells are partly buffered. If not, they are transported to the extracellular fluid through the plasma membrane and buffered in circulation or excreted in urine and expiration gas. Several transporters including monocarboxylate transporters and Na+/H+ exchanger play an important role in uptake and output of protons across plasma membranes in cells of metabolic tissues including skeletal muscle and the liver. They also contribute to maintenance of the physiological pH of body fluid. Therefore, impairment of these transporters causes dysfunction of cells, diseases, and a decrease in physical performance associated with abnormal pH. Additionally, it is known that fluid pH in the interstitial space of metabolic tissues is easily changed due to little pH buffering capacitance in interstitial fluids and a reduction in the interstitial fluid pH may mediate the onset of insulin resistance unlike blood containing pH buffers such as Hb (hemoglobin) and albumin. In contrast, habitual exercise and dietary intervention regulate expression/activity of transporters and maintain body fluid pH, which could partly explain the positive effect of healthy lifestyle on disease prognosis.
1. Introduction
Body fluid pH is determined by the content of protons (H+) generated from organic acids produced in living cells. Lactic acid (lactate−/H+) is a typical proton source and is involved in the regulation of physiological pH. In metabolic tissues such as skeletal muscle and adipose tissue, the glycolytic anaerobic metabolism mediates the conversion of glucose and glycogen into lactic acid. Because the pKa of lactic acid is 3.80, it is immediately dissociated into lactate (lactate−) and protons under physiological conditions, resulting in reduced intracellular pH. Pyruvic acid (pyruvate−/H+), an intermediate metabolite in the glycolytic system, is also a source of protons, although it generates much less protons compared to lactic acid. In addition, metabolites such as ketone bodies also act as proton sources. Beta-hydroxybutyric acid (beta-hydroxybutyrate−/H+), a typical ketone body, is generated as a result of fatty acid metabolism in the liver and is also dissociated into beta-hydroxybutyrate anions and protons, leading to the reduction of intracellular pH.
The intracellular pH in most living cells is alkaline compared to the pH generated by protons that are transported passively through the plasma membrane by electrochemical forces. In addition to buffering systems such as the bicarbonate-carbonate system, protein-proton binding, and phosphoric acid, several membrane transporters are responsible for proton removal from the cytosol and play important roles in maintaining the alkaline pH in cells (Figure 1). In most mammalian cells, H+-monocarboxylate cotransporters (MCTs) participate in the transport of monocarboxylic acids such as lactate, pyruvate, beta-hydroxybutyrate, and acetoacetate across the cellular membrane by cotransporting protons and monocarboxylate anions [1–3]. Other transporters such as the Na+/H+ exchanger (NHE) and bicarbonate-dependent exchanger also contribute to proton extrusion from the cytosol to the extracellular space [4, 5]. This review focuses on the critical role of the membrane transport system of protons in regulation of intracellular and extracellular fluid pH and its importance in maintaining physiological homeostasis and preventing diseases development.
Figure 1.
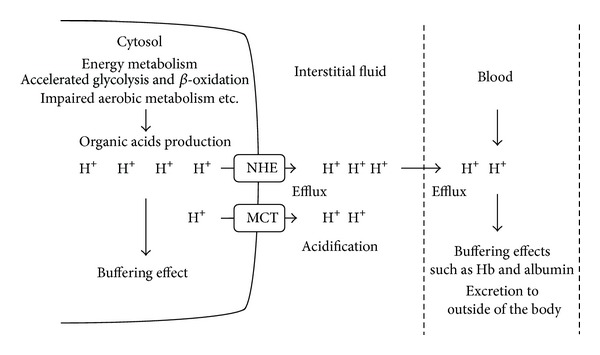
Proton production and its transporting kinetics in intracellular and extracellular fluid in metabolic tissues. The production of organic acids such as lactic acid and ketone bodies is accelerated by elevating glycolytic anaerobic metabolism and β-oxidation in metabolic cells. Body fluid pH is strictly maintained by buffering systems, efflux across plasma membrane, and acid excretion. Monocarboxylate transporter (MCT) and Na+/H+ exchanger (NHE) contribute to proton extrusion from the cytosol to the extracellular space. In contrast to intracellular fluid and blood containing pH buffers such as Hb (hemoglobin) and albumin, the interstitial fluid pH could be easily reduced by acid stress owing to the limited availability of the buffering factors such as proteins.
2. Proton Transport across the Plasma Membrane in pH Regulation
Regulation of body fluid pH is one of the most important physiological functions of homeostasis, because activity of most chemical reactions via enzyme proteins is dependent on fluid pH. To maintain homeostasis of body fluid pH, various buffering systems are utilized in addition to proton excretion from the cytosol to the extracellular space and ultimately outside of the body. However, if production of organic acid is elevated or the buffering and excretion systems are impaired, body fluid turns acidic, leading to abnormal conditions. A typical example is elevation of lactic acid production in skeletal muscle in response to strenuous exercise, which leads to body fluid acidosis, preventing muscle contraction [6, 7]. Proton transport across the plasma membrane of muscle cells is important for maintaining the appropriate intracellular pH. Skeletal muscle is a major metabolic organ that generates acids, in particular during contraction. Strenuous muscle contractions can cause a drastic reduction in intramuscular pH to −6.5 with accumulation of more than 40 mM lactate [6–8], regardless of cellular buffering capacity. Several studies have shown that intracellular pH is reduced during muscle contraction and has a delayed recovery to basal conditions during the recovery phase in the absence of proton transporters [9]. This delay suggests that proton transporters play a key role in maintaining pH homeostasis. Indeed, the function of proton transporters is involved in the capacity for pH maintenance [9, 10]. In particular, over 80% of intracellular proton is transported through lactate cotransport in contracting muscle, although remaining parts are transported through NHE and bicarbonate-depending transport [8, 11]. The liver, another organ that is closely associated with the metabolism of organic acids, generates ketone bodies (i.e., acetoacetic and β-hydroxybutyric acids), metabolizes lipids, and converts lactate to glucose via gluconeogenesis. Therefore, this organ generates acidic conditions [12–14] and intracellular pH should be maintained by proton extrusion along with buffering function.
MCTs, a part of the solute carrier (SLC) 16 that contains 14 members in total, play a crucial role in proton transport across the plasma membrane by cotransporting monocarboxylates. Each isoform has different transport kinetics and is specifically located on a particular subcellular site. It has been shown that MCT1–MCT4 transport aliphatic monocarboxylates such as lactate, pyruvate, and ketone bodies [2] and that the direction of transport across the plasma membrane in a 1 : 1 manner is determined by the concentration gradients of protons and monocarboxylate both inside and outside of the cell [15–17]. Thus, these isoforms play important roles in proton transport maintaining intracellular pH. In particular, the expression of two MCT isoforms (MCT1 and MCT4) is associated with lactate disposal in muscles. MCT1 is highly expressed and located in both the sarcolemmal and the mitochondrial membranes of oxidative muscles [18–20]; on the other hand, MCT4 is predominantly located on the plasma membrane of glycolytic muscle and is assumed to contribute to lactate efflux [19, 21]. In contrast, MCT2 is mainly located on the membranes of liver cells and contributes to the extrusion of ketone bodies [22]. Other members of the family have different substrate specificities. For example, MCT6 has been shown to transport bumetanide, a diuretic drug [23], MCT 8 acts as a thyroid hormone transporter [24], MCT9 is a potential carnitine extrusion transporter [25], and MCT10 is identified as a low-affinity transporter of aromatic amino acids along with iodothyronines [26]. In addition, NHE is known as another major proton transporter that plays an important role in intracellular pH homeostasis by exchanging intracellular proton with extracellular Na+ using the chemical gradient between intra- and extracellular Na+ concentrations [4, 27]. Currently, 10 isoforms are known to exist in mammals. NHE1–NHE5 are located on the plasma membrane of their specific tissues, while NHE6–NHE9 are located on the membrane of subcellular organelles [27–29]. In particular, NHE1 has been recognized as a ubiquitous isoform and plays an important role in maintaining homeostasis in metabolic organs.
Proton transport across the plasma membrane is important for maintenance of intracellular and extracellular fluid pH. In particular, proton excretion and bicarbonate reabsorption are recognized as important function of renal tubules. Proton excretion into urine is mainly mediated by both proton-ATPase and NHE3 located on the apical plasma membrane of the proximal convoluted tubule participating in approximately 80% of bicarbonate reabsorption occurring in the whole kidney, acting as the major buffering system in blood [30, 31], which has also pH buffers such as Hb (hemoglobin) and albumin. Bicarbonate reacts with protons via catalytic carbonic anhydrase on the apical membrane and generates CO2. Then, it is transported into the blood by sodium-bicarbonate cotransporters on the basolateral side [32].
3. pH Disturbance and Disease Development
The normal physiological pH of mammalian arterial blood is strictly maintained at 7.40; blood has pH buffers such as Hb (hemoglobin) and albumin. A decrease of more than 0.05 units from the normal pH results in acidosis. The body fluids of diabetic patients are chronically acidic and exhibit characteristic ketoacidosis caused by an increased level of ketone bodies in the blood [33, 34]. Insulin resistance in metabolic tissues such as skeletal muscle, adipose tissue, and the liver accelerates the utilization of lipids as an energy substrate instead of glucose. Excess lipolysis caused by impaired glucose metabolism leads to free fatty acids in circulation, which facilitate hepatic gluconeogenesis by the oxidation of fatty acids resulting in large quantities of ketone bodies. This further accelerates proton overloads, leading to the metabolic ketoacidosis found in diabetic patients. Such acidic conditions prevent the activity of metabolic enzymes such as phosphofructokinase and further accelerate the progression of pathological conditions [33–35]. Acidic conditions can also result in physical fatigue of diabetic patients. Therefore, maintaining normal pH is important for physiological homeostasis.
It has been suggested that loss of function of MCTs causes a change of body fluid pH. Several point mutations of the MCT gene have been shown to affect both specificity and transport activity. The spontaneously occurring mutation of arginine 306 to threonine in domain 8 of MCT1 resulted in reduced transport activity [36]. In addition, it has been shown that subjects who have mutations in MCT1 cDNA have drastically lower transport rates and a delayed decline of blood lactate after exercise [37, 38]. Healthy subjects feel severe chest pain and muscle cramping after strenuous exercise, along with a defect in lactate efflux from muscle. Furthermore, many amino acid differences that are not attributable to polymorphisms are found in MCT1 obtained from muscle tissues in these subjects [37, 39]; thus, mutations in MCT1 are related to physical fatigue and exercise performance. MCT dysfunction may lead to metabolic disorder. Indeed, lower level expression of MCT1 and MCT4 is found in the skeletal muscle of obese rats compared to normal rats [40]. In addition, the activity of lactate transport in muscle is also decreased by both denervation and aging [41, 42]. A significant negative correlation between the level of circulating lactate and degree of insulin sensitivity is found in humans [43], suggesting that lower lactic acid disposal caused by reduction of MCT function is associated with insulin resistance.
4. Interstitial Fluid pH and Disease Development
Body fluid acidosis could also contribute to the development of metabolic diseases. Our recent study indicates that before the development of diabetic symptoms the interstitial fluid pH in ascites and metabolic tissues of Otsuka Long-Evans Tokushima Fatty (OLETF) rats is lower than the normal pH (7.40) [44]. The buffering capacity is relatively high in the cytosol and blood but low in the interstitial fluid due to limited buffering factors such as proteins [45, 46]. Therefore, interstitial fluid pH in metabolic tissues easily changes (Figure 1) and may contribute to the onset of insulin resistance. We have shown the inhibitory effect of extracellular pH on the insulin signaling pathway in the L6 rat myotube. The phosphorylation level and binding affinity to insulin of insulin receptors were significantly diminished in media with low pH [47]. In addition, the levels of Akt phosphorylation, a downstream of the insulin receptor, are also decreased in low pH media, along with a reduction in glucose uptake. These in vitro observations support the hypothesis that lower extracellular pH may cause insulin resistance in skeletal muscle cells. Other studies [48–50] have suggested a close correlation between organic acid production and insulin sensitivity in both type 2 diabetes patients and healthy subjects. In a cross-sectional study of over 1,000 subjects [48], it has been demonstrated that body weight and waist circumference have a negative correlation with both insulin sensitivity and urine pH. Patients with metabolic syndrome have also reported a significantly lower pH of 24 h urine compared to the normal subjects and a negative correlation between the mean 24 h urine pH and the number of metabolic syndrome abnormalities [49, 50]. It has been suggested that lower levels of serum bicarbonate and higher levels of anion gap resulting from metabolic acidosis are associated with lower insulin sensitivity [51]. Hyperlactacidemia is found in patients with obesity and type 2 diabetes [43], which supports the strong relationship between acidic condition and insulin sensitivity. Even in healthy subjects, acids level could be an independent risk factor for the development of type 2 diabetes [52].
Insulin resistance is one of the major symptoms of metabolic disorders and is frequently associated with hypertension, high blood glucose levels, visceral obesity, and dyslipidemia. Insulin resistance also causes type 2 diabetes and plays a key role in developing cancer and cardiovascular disease. Thus, pH abnormalities can cause abnormal metabolic regulation in a predisease state. We recently found an observation that the interstitial pH around the hippocampus, an important region for memory [53], is lower in diabetic OLETF rats (26 weeks of age) than in normal Wistar rats [54]. It has been reported that diabetic patients have a high risk of developing dementia and Alzheimer's disease [55] and may experience defective memory functions. The insulin action is required for neuronal survival within the central nervous system [56]. Fluctuating glucose levels resulting from defective insulin have been suggested to lead to apoptosis, energy starvation, formation of neuritic plaques and neurofibrillary tangles, hallmark lesions of Alzheimer's disease, and altered acetylcholine levels in the hippocampus [57, 58]. Therefore, we indicate that maintenance of the interstitial fluid pH within the normal range or the recovery of the interstitial pH to the normal range could be one of the most important factors in developing molecular and cellular therapies for metabolic brain disorders.
5. pH Regulation by Diet and Exercise Intervention
The maintenance of pH in metabolic organs is achieved through various regulatory systems. Physical exercise and appropriate diet contribute to pH homeostasis. Habitual exercise adaptively accelerates the entry of fatty acids both from the plasma into the muscle cell and from the cytosol into the mitochondria, while also enhancing Krebs cycle function in the resting state. Their actions are caused by elevation of activity and expression of related enzymes in skeletal muscles [59–61]. Since the energy consumed in muscle during exercise is mainly supplied by carbohydrates and lipids, the exercise-induced lipid utilization may decrease the energy obtained from carbohydrates, further decreasing the lactate/proton production, or lactic acidosis. In addition, circulating and intramuscular buffering capacities are improved via habitual exercise increasing proteins, amino acids, and phosphate [62–64]. Peripheral circulation is also improved through vasodilation caused as a physiological adaptation to exercise [65], which further facilitates the proton washout. In particular there is evidence suggesting that excretion of protons from the cytosol to the extracellular space or into circulation via transporters located on the plasma membrane contributes to the prevention of intracellular acidosis. It has been reported that exercise training increases the MCT1 and MCT4 levels in the skeletal and cardiac muscle of humans and animals [66–68]. Although the regulation of MCT expression levels is not clearly understood, it has been suggested that protein kinases A and B are involved in the regulation of MCT expression [69] as an adaptation mechanism, which may be mediated by an increase in lactate movement across the membrane. In addition, our recent study has reported that MCT1 content in erythrocyte membranes is elevated by exercise training in rats [70, 71]. A proportion of the lactate released from skeletal muscles into the plasma is taken up by erythrocytes. The mature erythrocytes generate ATP only through the glycolytic pathway, since they have no mitochondrial machinery. Thus, erythrocytes cannot utilize lactate produced as a respiratory fuel and this necessitates the release of lactate into the plasma via MCT1 [72]. However, one of the most important roles of erythrocytes is to distribute released monocarboxylates by taking up monocarboxylates, since erythrocytes produce much less lactate than other tissues. Based on the results of our in vitro study, the skeletal muscle may be entirely dependent on MCT1-mediated lactate uptake by erythrocytes to maintain pH homeostasis [71]. In addition, there is a high correlation between the athletic performance of horses and their erythrocyte lactate concentrations after racing [73]. Therefore, efficient proton transport via MCTs induced by habitual exercise may contribute to the improvement of insulin sensitivity and muscle fatigue caused by lowered pH.
It is well known that adequate diet is important for controlling pathological conditions in patients with metabolic disorders. In addition, intervention studies in humans have reported that several bioactive factors included in foods such as antioxidants [74–77] and n-3 unsaturated fatty acids [78, 79] improve energy metabolism. Additional factors such as carotenoids, alpha lipoic acids, amino acids/peptides, and minerals may also offer preventive or therapeutic effects to combat hyperglycemia and several animal and culture studies have demonstrated their efficacy in improving insulin sensitivity [80–84]. The effects of these nutrients are only beneficial when administered in combination. In contrast to the successful application of dietary approaches or combined nutrients [85–87], various types of intervention studies using single nutrients have failed to clarify their beneficial action on cardiovascular risk and insulin resistance [88, 89]. Therefore, administration of multiple nutrients is considered more effective when compared to administration of a single bioactive factor. Propolis, a natural product derived from the plant resins collected by honeybees, contains various types of compounds including polyphenols, phenolic aldehydes, sesquiterpene quinones, coumarins, amino acids, steroids, and inorganic compounds [90] and has been reported to reduce the metabolic defects caused by abnormal blood glucose and insulin in young (18 weeks of age) OLETF rats [42] characterized by hyperphagia, obesity, decreased glucose infusion rate in a euglycemic clamp at 16–18 weeks of age, hyperinsulinemia around 25 weeks of age responding to an intravenous glucose infusion, and developing type 2 diabetes [91, 92]. Thus, our study indicates that propolis has a beneficial and preventive action on type 2 diabetes mellitus at early stages developing insulin resistance. Further, we have obtained evidence that intake of propolis elevates the pH of ascites and metabolic tissues compared with normal diet, indicating that dietary propolis diminishes production of organic acids or increases buffering capacity in those tissues. Therefore, propolis may be a useful compound to improve insulin sensitivity via prevention of metabolic acidosis. The molecular mechanism of how propolis improves interstitial pH is unclear, and we should strive to better understand the mechanism of this bioactive supplement.
6. Conclusion
Membrane transport of protons is required for preventing acidic states of body fluid, maintaining physical performance, and improving metabolic impairments. In contrast to the intracellular and blood pH, interstitial fluid pH can easily be reduced by acid stress. This can disturb homeostasis of the intracellular metabolism, leading to the development of metabolic diseases. However, detailed mechanisms including the involvement of membrane transport of protons responsible for the reduction of interstitial fluid pH are unknown. In addition, activity and expression of proton transporters such as MCT and NHE are easily altered by various changes in the cell environment. More studies are required to examine the detailed regulatory mechanisms of proton transporters, including gene expression, protein modification, and membrane trafficking, in addition to their contributions to metabolic homeostasis.
Acknowledgments
This work was supported by Grants-in-Aid from Japan Society of the Promotion of Science (25282199 and 25670111), Adaptable and Seamless Technology Transfer Program through Target-Driven R&D, Japan Science and Technology Agency (JST), Four-University Collaborative Research Grant, Salt Science Foundation (1235), and Cell Research Conference.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Garcia CK, Goldstein JL, Pathak RK, Anderson RGW, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76(5):865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 2.Halestrap AP. The monocarboxylate transporter family-structure and functional characterization. IUBMB Life. 2012;64(1):1–9. doi: 10.1002/iub.573. [DOI] [PubMed] [Google Scholar]
- 3.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. The American Journal of Physiology—Cell Physiology. 1993;264(4, part 1):C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 4.Burckhardt G, Di Sole F, Helmle-Kolb C. The Na+/H+ exchanger gene family. Journal of Nephrology. 2002;15, supplement 5:S3–S21. [PubMed] [Google Scholar]
- 5.Loh S-H, Chen W-H, Chiang C-H, et al. Intracellular pH regulatory mechanism in human atrial myocardium: Functional evidence for Na+/H+ exchanger and Na+/HCO3 − symporter. Journal of Biomedical Science. 2002;9(3):198–205. doi: 10.1007/BF02256066. [DOI] [PubMed] [Google Scholar]
- 6.Authier B, Albrand JP, Decorps M, Reutenauer H, Rossi A. Disruption of muscle energy metabolism due to intense ischaemic exercise: a 31P NMR study in rats. Physiological Chemistry & Physics & Medical NMR. 1987;19(2):83–93. [PubMed] [Google Scholar]
- 7.Fitts RH. Cellular mechanisms of muscle fatigue. Physiological Reviews. 1994;74(1):49–94. doi: 10.1152/physrev.1994.74.1.49. [DOI] [PubMed] [Google Scholar]
- 8.Juel C. Lactate/proton co-transport in skeletal muscle: regulation and importance for pH homeostasis. Acta Physiologica Scandinavica. 1996;156(3):369–374. doi: 10.1046/j.1365-201X.1996.206000.x. [DOI] [PubMed] [Google Scholar]
- 9.Juel C. Intracellular pH recovery and lactate efflux in mouse soleus muscles stimulated in vitro: the involvement of sodium/proton exchange and a lactate carrier. Acta Physiologica Scandinavica. 1988;132(3):363–371. doi: 10.1111/j.1748-1716.1988.tb08340.x. [DOI] [PubMed] [Google Scholar]
- 10.Bonen A, Baker SK, Hatta H. Lactate transport and lactate transporters in skeletal muscle. Canadian Journal of Applied Physiology. 1997;22(6):531–552. doi: 10.1139/h97-034. [DOI] [PubMed] [Google Scholar]
- 11.Juel C. Regulation of cellular pH in skeletal muscle fiber types, studied with sarcolemmal giant vesicles obtained from rat muscles. Biochimica et Biophysica Acta. 1995;1265(2-3):127–132. doi: 10.1016/0167-4889(94)00209-w. [DOI] [PubMed] [Google Scholar]
- 12.Eledrisi MS, Alshanti MS, Shah MF, Brolosy B, Jaha N. Overview of the diagnosis and management of diabetic ketoacidosis. The American Journal of the Medical Sciences. 2006;331(5):243–251. doi: 10.1097/00000441-200605000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Fafournoux P, Demigné C, Rémésy C. Mechanisms involved in ketone body release by rat liver cells: Influence of pH and bicarbonate. The American Journal of Physiology—Gastrointestinal and Liver Physiology. 1987;252(2, part 1):G200–G208. doi: 10.1152/ajpgi.1987.252.2.G200. [DOI] [PubMed] [Google Scholar]
- 14.Metcalfe HK, Monson JP, Welch SG, Cohen RD. Inhibition of lactate removal by ketone bodies in rat liver. Evidence for a quantitatively important role of the plasma membrane lactate transporter in lactate metabolism. Journal of Clinical Investigation. 1986;78(3):743–747. doi: 10.1172/JCI112635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth DA, Brooks GA. Lactate transport is mediated by a membrane-bound carrier in rat skeletal muscle sarcolemmal vesicles. Archives of Biochemistry and Biophysics. 1990;279(2):377–385. doi: 10.1016/0003-9861(90)90505-s. [DOI] [PubMed] [Google Scholar]
- 16.Juel C. Muscle lactate transport studied in sarcolemmal giant vesicles. Biochimica et Biophysica Acta—Biomembranes. 1991;1065(1):15–20. doi: 10.1016/0005-2736(91)90004-r. [DOI] [PubMed] [Google Scholar]
- 17.McDermott JC, Bonen A. Lactate transport by skeletal muscle sarcolemmal vesicles. Molecular and Cellular Biochemistry. 1993;122(2):113–121. doi: 10.1007/BF01076095. [DOI] [PubMed] [Google Scholar]
- 18.Poole RC, Halestrap AP. Transport of lactate and other monocarboxylates across mammalian plasma membranes. The American Journal of Physiology. 1993;264(4) part 1:C761–C782. doi: 10.1152/ajpcell.1993.264.4.C761. [DOI] [PubMed] [Google Scholar]
- 19.Bonen A, Tonouchi M, Miskovic D, Heddle C, Heikkila JJ, Halestrap AP. Isoform-specific regulation of the lactate transporters MCT1 and MCT4 by contractile activity. The American Journal of Physiology—Endocrinology and Metabolism. 2000;279(5):E1131–E1138. doi: 10.1152/ajpendo.2000.279.5.E1131. [DOI] [PubMed] [Google Scholar]
- 20.Brooks GA, Dubouchaud H, Brown M, Sicurello JP, Butz CE. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(3):1129–1134. doi: 10.1073/pnas.96.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilegaard H, Terzis G, Halestrap A, Juel G. Distribution of the lactate/H+ transporter isoforms MCT1 and MCT4 in human skeletal muscle. The American Journal of Physiology—Endocrinology and Metabolism. 1999;276(5):E843–E848. doi: 10.1152/ajpendo.1999.276.5.E843. [DOI] [PubMed] [Google Scholar]
- 22.Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. Journal of Biological Chemistry. 1995;270(4):1843–1849. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 23.Murakami Y, Kohyama N, Kobayashi Y, et al. Functional characterization of human monocarboxylate transporter 6 (SLC16A5) Drug Metabolism and Disposition. 2005;33(12):1845–1851. doi: 10.1124/dmd.105.005264. [DOI] [PubMed] [Google Scholar]
- 24.Friesema ECH, Ganguly S, Abdalla A, Manning Fox JE, Halestrap AP, Visser TJ. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. Journal of Biological Chemistry. 2003;278(41):40128–40135. doi: 10.1074/jbc.M300909200. [DOI] [PubMed] [Google Scholar]
- 25.Suhre K, Shin S-Y, Petersen A-K, et al. Human metabolic individuality in biomedical and pharmaceutical research. Nature. 2011;477(7362):54–62. doi: 10.1038/nature10354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Visser WE, Friesema ECH, Visser TJ. Minireview: thyroid hormone transporters: the knowns and the unknowns. Molecular Endocrinology. 2011;25(1):1–14. doi: 10.1210/me.2010-0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiological Reviews. 1997;77(1):51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- 28.Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Archiv European Journal of Physiology. 2004;447(5):549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- 29.Donowitz M, Tse CM. Molecular physiology of mammalian epithelial Na+/H+ exchangers NHE2 and NHE3. Current Topics in Membranes. 2001;50:437–498. [Google Scholar]
- 30.Bobulescu IA, Moe OW. Na+/H+ exchangers in renal regulation of acid-base balance. Seminars in Nephrology. 2006;26(5):334–344. doi: 10.1016/j.semnephrol.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner CA, Devuyst O, Bourgeois S, Mohebbi N. Regulated acid-base transport in the collecting duct. Pflügers Archiv. 2009;458(1):137–156. doi: 10.1007/s00424-009-0657-z. [DOI] [PubMed] [Google Scholar]
- 32.Harvey BJ, Ehrenfeld J. Epithelial pH and ion transport regulation by proton pumps and exchangers. Ciba Foundation Symposium. 1988;139:139–164. doi: 10.1002/9780470513699.ch9. [DOI] [PubMed] [Google Scholar]
- 33.Sumi S, Mineo I, Kono N, Shimizu T, Nonaka K, Tarui S. Decreases in hepatic fructose-2,6-bisphosphate level and fructose-6-phosphate,2-kinase activity in diabetic mice: a close relationship to the development of ketosis. Biochemical and Biophysical Research Communications. 1984;120(1):103–108. doi: 10.1016/0006-291x(84)91419-0. [DOI] [PubMed] [Google Scholar]
- 34.Lemieux G, Aranda MR, Fournel P, Lemieux C. Renal enzymes during experimental diabetes mellitus in the rat. Role of insulin, carbohydrate metabolism, and ketoacidosis. Canadian Journal of Physiology and Pharmacology. 1984;62(1):70–75. doi: 10.1139/y84-010. [DOI] [PubMed] [Google Scholar]
- 35.Gil J, Carreras J, Bartrons R. Effects of diabetes on fructose 2,6-P2, glucose 1,6-P2 and 6-phosphofructo 2-kinase in rat liver. Biochemical and Biophysical Research Communications. 1986;136(2):498–503. doi: 10.1016/0006-291x(86)90468-7. [DOI] [PubMed] [Google Scholar]
- 36.Rahman B, Schneider HP, Bröer A, Deitmer JW, Bröer S. Helix 8 and helix 10 are involved in substrate recognition in the rat monocarboxylate transporter MCT1. Biochemistry. 1999;38(35):11577–11584. doi: 10.1021/bi990973f. [DOI] [PubMed] [Google Scholar]
- 37.Merezhinskaya N, Fishbein WN, Davis JI, et al. Mutations in MCT1 cDNA in patients with symptomatic deficiency in lactate transport. Muscle & Nerve. 2000;23(1):90–97. doi: 10.1002/(sici)1097-4598(200001)23:1<90::aid-mus12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Fishbein WN. Lactate transporter defect: a new disease of muscle. Science. 1986;234(4781):1254–1256. doi: 10.1126/science.3775384. [DOI] [PubMed] [Google Scholar]
- 39.Merezhinskava N, Fishbein WN. Muscle monocarboxylate transporter (MCT1) mutations in 5 patients with red cell irbc lactate transport deficiency (LTD) FASEB Journal. 1997;11(9, article 656) [Google Scholar]
- 40.Py G, Lambert K, Perez-Martin A, Raynaud E, Prefaut C, Mercier J. Impaired sarcolemmal vesicle lactate uptake and skeletal muscle MCT1 and MCT4 expression in obese Zucker rats. American Journal of Physiology: Endocrinology and Metabolism. 2001;281(6):E1308–E1315. doi: 10.1152/ajpendo.2001.281.6.E1308. [DOI] [PubMed] [Google Scholar]
- 41.Juel C, Honig A, Pilegaard H. Muscle lactate transport studied in sarcolemmal giant vesicles: dependence on fibre type and age. Acta Physiologica Scandinavica. 1991;143(4):361–365. doi: 10.1111/j.1748-1716.1991.tb09246.x. [DOI] [PubMed] [Google Scholar]
- 42.McCullagh KJA, Bonen A. Reduced lactate transport in denervated rat skeletal muscle. The American Journal of Physiology—Regulatory Integrative and Comparative Physiology. 1995;268(4, part 2):R884–R888. doi: 10.1152/ajpregu.1995.268.4.R884. [DOI] [PubMed] [Google Scholar]
- 43.Reaven GM, Hollenbeck C, Jeng C-Y, Wu MS, Chen Y-DI. Measurement of plasma glucose, free fatty acid, lactate, and insulin for 24 h in patients with NIDDM. Diabetes. 1988;37(8):1020–1024. doi: 10.2337/diab.37.8.1020. [DOI] [PubMed] [Google Scholar]
- 44.Aoi W, Hosogi S, Niisato N, et al. Improvement of insulin resistance, blood pressure and interstitial pH in early developmental stage of insulin resistance in OLETF rats by intake of propolis extracts. Biochemical and Biophysical Research Communications. 2013;432(4):650–653. doi: 10.1016/j.bbrc.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 45.Fogh-Andersen N, Altura BM, Altura BT, Siggaard-Andersen O. Composition of interstitial fluid. Clinical Chemistry. 1995;41(10):1522–1525. [PubMed] [Google Scholar]
- 46.Aukland K, Fadnes HO. Protein concentration of interstitial fluid collected from rat skin by a wick method. Acta Physiologica Scandinavica. 1973;88(3):350–358. doi: 10.1111/j.1748-1716.1973.tb05459.x. [DOI] [PubMed] [Google Scholar]
- 47.Hayata H, Miyazaki H, Niisato N, Yokoyama N, Marunaka Y. Lowered extracellular pH is involved in the pathogenesis of skeletal muscle insulin resistance. Biochemical and Biophysical Research Communications. 2014;445(1):170–174. doi: 10.1016/j.bbrc.2014.01.162. [DOI] [PubMed] [Google Scholar]
- 48.Otsuki M, Kitamura T, Goya K, et al. Association of urine acidification with visceral obesity and the metabolic syndrome. Endocrine Journal. 2011;58(5):363–367. doi: 10.1507/endocrj.k10e-319. [DOI] [PubMed] [Google Scholar]
- 49.Maalouf NM, Cameron MA, Moe OW, Adams-Huet B, Sakhaee K. Low urine pH: a novel feature of the metabolic syndrome. Clinical Journal of the American Society of Nephrology. 2007;2(5):883–888. doi: 10.2215/CJN.00670207. [DOI] [PubMed] [Google Scholar]
- 50.Maalouf NM, Cameron MA, Moe OW, Sakhaee K. Metabolic basis for low urine pH in type 2 diabetes. Clinical Journal of the American Society of Nephrology. 2010;5(7):1277–1281. doi: 10.2215/CJN.08331109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Farwell WR, Taylor EN. Serum bicarbonate , anion gap and insulin resistance in the National Health and Nutrition Examination Survey. Diabetic Medicine. 2008;25(7):798–804. doi: 10.1111/j.1464-5491.2008.02471.x. [DOI] [PubMed] [Google Scholar]
- 52.Ohlson L-O, Larsson B, Bjorntorp P, et al. Risk factors for type 2 (non-insulin-dependent) diabetes mellitus: thirteen and one-half years of follow-up of the participants in a study of Swedish men born in 1913. Diabetologia. 1988;31(11):798–805. doi: 10.1007/BF00277480. [DOI] [PubMed] [Google Scholar]
- 53.Packard MG, Goodman J. Factors that influence the relative use of multiple memory systems. Hippocampus. 2013;23(11):1044–1052. doi: 10.1002/hipo.22178. [DOI] [PubMed] [Google Scholar]
- 54.Marunaka Y, Yoshimoto K, Aoi W, Hosogi S, Ikegaya H. Low pH of interstitial fluid around hippocampus of the brain in diabetic OLETF rats. Molecular and Cellular Therapies. 2014;2, article 6 doi: 10.1186/2052-8426-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mirza Z, Kamal MA, Abuzenadah AM, et al. Establishing genomic/transcriptomic links between alzheimer’s disease and type II diabetes mellitus by meta-analysis approach. CNS & Neurological Disorders-Drug Targets. 2013;13(3):501–516. doi: 10.2174/18715273113126660154. [DOI] [PubMed] [Google Scholar]
- 56.Dudek H, Datta SR, Franke TF, et al. Regulation of neuronal survival by the serine-threonine protein kinase Akt. Science. 1997;275(5300):661–665. doi: 10.1126/science.275.5300.661. [DOI] [PubMed] [Google Scholar]
- 57.Rasgon N, Jarvik L. Insulin resistance, affective disorders, and alzheimer’s disease: review and hypothesis. The Journals of Gerontology A: Biological Sciences and Medical Sciences. 2004;59(2):178–183. doi: 10.1093/gerona/59.2.m178. [DOI] [PubMed] [Google Scholar]
- 58.Steen E, Terry BM, Rivera EJ, et al. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease: is this type 3 diabetes? Journal of Alzheimer’s Disease. 2005;7(1):63–80. doi: 10.3233/jad-2005-7107. [DOI] [PubMed] [Google Scholar]
- 59.Holloway GP, Bezaire V, Heigenhauser GJF, et al. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/ CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. Journal of Physiology. 2006;571(1):201–210. doi: 10.1113/jphysiol.2005.102178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wibom R, Hultman E, Johansson M, Matherei K, Constantin-Teodosiu D, Schantz PG. Adaptation of mitochondrial ATP production in human skeletal muscle to endurance training and detraining. Journal of Applied Physiology. 1992;73(5):2004–2010. doi: 10.1152/jappl.1992.73.5.2004. [DOI] [PubMed] [Google Scholar]
- 61.Bradley NS, Snook LA, Jain SS, Heigenhauser GJF, Bonen A, Spriet LL. Acute endurance exercise increases plasma membrane fatty acid transport proteins in rat and human skeletal muscle. The American Journal of Physiology—Endocrinology and Metabolism. 2012;302(2):E183–E189. doi: 10.1152/ajpendo.00254.2011. [DOI] [PubMed] [Google Scholar]
- 62.Susuki Y, Ito O, Takahashi H, Takamatsu K. The effect of sprint training on skeletal muscle carnosine in humans. International Journal of Sport and Health Science. 2004;2:105–110. [Google Scholar]
- 63.Parkhouse WS, McKenzie DC, Hochachka PW, Ovalle WK. Buffering capacity of deproteinized human vastus lateralis muscle. Journal of Applied Physiology. 1985;58(1):14–17. doi: 10.1152/jappl.1985.58.1.14. [DOI] [PubMed] [Google Scholar]
- 64.Arthur PG, Hogan MC, Bebout DE, Wagner PD, Hochachka PW. Modeling the effects of hypoxia on ATP turnover in exercising muscle. Journal of Applied Physiology. 1992;73(2):737–742. doi: 10.1152/jappl.1992.73.2.737. [DOI] [PubMed] [Google Scholar]
- 65.DeSouza CA, Shapiro LF, Clevenger CM, et al. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102(12):1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 66.Dubouchaud H, Butterfield GE, Wolfel EE, Bergman BC, Brooks GA. Endurance training, expression, and physiology of LDH, MCT1, and MCT4 in human skeletal muscle. American Journal of Physiology: Endocrinology and Metabolism. 2000;278(4):E571–E579. doi: 10.1152/ajpendo.2000.278.4.E571. [DOI] [PubMed] [Google Scholar]
- 67.Baker SK, Mccullagh KJA, Bonen A. Training intensity-dependent and tissue-specific increases in lactate uptake and MCT-1 in heart and muscle. Journal of Applied Physiology. 1998;84(3):987–994. doi: 10.1152/jappl.1998.84.3.987. [DOI] [PubMed] [Google Scholar]
- 68.Bonen A, McCullagh KJA, Putman CT, Hultman E, Jones NL, Heigenhauser GJF. Short-term training increases human muscle MCT1 and femoral venous lactate in relation to muscle lactate. American Journal of Physiology: Endocrinology and Metabolism. 1998;274(1, part 1):E102–E107. doi: 10.1152/ajpendo.1998.274.1.E102. [DOI] [PubMed] [Google Scholar]
- 69.Narumi K, Furugen A, Kobayashi M, Otake S, Itagaki S, Iseki K. Regulation of monocarboxylate transporter 1 in skeletal muscle cells by intracellular signaling pathways. Biological and Pharmaceutical Bulletin. 2010;33(9):1568–1573. doi: 10.1248/bpb.33.1568. [DOI] [PubMed] [Google Scholar]
- 70.Aoi W, Tsuzuki M, Fujie M, Iwashita S, Suzuki M. Sustained voluntary climbing exercise increases erythrocyte monocarboxylate transporter 1 in rats. Journal of Clinical Biochemistry and Nutrition. 2002;32:23–29. [Google Scholar]
- 71.Aoi W, Iwashita S, Fujie M, Suzuki M. Sustained swimming increases erythrocyte MCT1 during erythropoiesis and ability to regulate pH homeostasis in rat. International Journal of Sports Medicine. 2004;25(5):339–344. doi: 10.1055/s-2004-815846. [DOI] [PubMed] [Google Scholar]
- 72.Skelton MS, Kremer DE, Smith EW, Gladden LB. Lactate influx into red blood cells from trained and untrained human subjects. Medicine and Science in Sports and Exercise. 1998;30(4):536–542. doi: 10.1097/00005768-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 73.Rassanan LA, Lampinen KJ, Poso AR. Responses of blood and plasma lactate and plasma purine concentrations to maximal exercise and their relation to performance in standardbred trotters. The American Journal of Veterinary Research. 1995;56(12):1651–1656. [PubMed] [Google Scholar]
- 74.Nagao T, Meguro S, Hase T, et al. A catechin-rich beverage improves obesity and blood glucose control in patients with type 2 diabetes. Obesity. 2009;17(2):310–317. doi: 10.1038/oby.2008.505. [DOI] [PubMed] [Google Scholar]
- 75.Squadrito F, Marini H, Bitto A, et al. Genistein in the metabolic syndrome: results of a randomized clinical trial. The Journal of Clinical Endocrinology & Metabolism. 2013;98(8):3366–3374. doi: 10.1210/jc.2013-1180. [DOI] [PubMed] [Google Scholar]
- 76.Yoshida M, Jacques PF, Meigs JB, et al. Effect of vitamin K supplementation on insulin resistance in older men and women. Diabetes Care. 2008;31(11):2092–2096. doi: 10.2337/dc08-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Asemi Z, Samimi M, Tabassi Z, Shakeri H, Esmaillzadeh A. Vitamin D supplementation affects serum high-sensitivity C-reactive protein, insulin resistance, and biomarkers of oxidative stress in pregnant women. Journal of Nutrition. 2013;143(9):1432–1438. doi: 10.3945/jn.113.177550. [DOI] [PubMed] [Google Scholar]
- 78.Ramel A, Martinéz A, Kiely M, Morais G, Bandarra NM, Thorsdottir I. Beneficial effects of long-chain n-3 fatty acids included in an energy-restricted diet on insulin resistance in overweight and obese European young adults. Diabetologia. 2008;51(7):1261–1268. doi: 10.1007/s00125-008-1035-7. [DOI] [PubMed] [Google Scholar]
- 79.Vessby B, Uusitupa M, Hermansen K, et al. Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU study. Diabetologia. 2001;44(3):312–319. doi: 10.1007/s001250051620. [DOI] [PubMed] [Google Scholar]
- 80.Bhuvaneswari S, Anuradha CV. Astaxanthin prevents loss of insulin signaling and improves glucose metabolism in liver of insulin resistant mice. Canadian Journal of Physiology and Pharmacology. 2012;90(11):1544–1552. doi: 10.1139/y2012-119. [DOI] [PubMed] [Google Scholar]
- 81.Takikawa M, Inoue S, Horio F, Tsuda T. Dietary anthocyanin-rich bilberry extract ameliorates hyperglycemia and insulin sensitivity via activation of amp-activated protein kinase in diabetic mice. Journal of Nutrition. 2010;140(3):527–533. doi: 10.3945/jn.109.118216. [DOI] [PubMed] [Google Scholar]
- 82.Greene EL, Nelson BA, Robinson KA, Buse MG. α-Lipoic acid prevents the development of glucose-induced insulin resistance in 3T3-L1 adipocytes and accelerates the decline in immunoreactive insulin during cell incubation. Metabolism: Clinical and Experimental. 2001;50(9):1063–1069. doi: 10.1053/meta.2001.25601. [DOI] [PubMed] [Google Scholar]
- 83.Lee H-S, Lee HJ, Suh HJ. Silk protein hydrolysate increases glucose uptake through up-regulation of GLUT 4 and reduces the expression of leptin in 3T3-L1 fibroblast. Nutrition Research. 2011;31(12):937–943. doi: 10.1016/j.nutres.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 84.Wang Y-Q, Yao M-H. Effects of chromium picolinate on glucose uptake in insulin-resistant 3T3-L1 adipocytes involve activation of p38 MAPK. Journal of Nutritional Biochemistry. 2009;20(12):982–991. doi: 10.1016/j.jnutbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 85.Lindström J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. The Lancet. 2006;368(9548):1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 86.Plantinga Y, Ghiadoni L, Magagna A, et al. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. American Journal of Hypertension. 2007;20(4):392–397. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 87.Zureik M, Galan P, Bertrais S, et al. Effects of long-term daily low-dose supplementation with antioxidant vitamins and minerals on structure and function of large arteries. Arteriosclerosis, Thrombosis, and Vascular Biology. 2004;24(8):1485–1491. doi: 10.1161/01.ATV.0000136648.62973.c8. [DOI] [PubMed] [Google Scholar]
- 88.Eskurza I, Monahan KD, Robinson JA, Seals DR. Ascorbic acid does not affect large elastic artery compliance or central blood pressure in young and older men. The American Journal of Physiology—Heart and Circulatory Physiology. 2004;286(4):H1528–H1534. doi: 10.1152/ajpheart.00879.2003. [DOI] [PubMed] [Google Scholar]
- 89.Woods MN, Wanke CA, Ling P-R, et al. Effect of a dietary intervention and n-3 fatty acid supplementation on measures of serum lipid and insulin sensitivity in persons with HIV. The American Journal of Clinical Nutrition. 2009;90(6):1566–1578. doi: 10.3945/ajcn.2009.28137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Khalil ML. Biological activity of bee propolis in health and disease. sian Pacific Journal of Cancer Prevention. 2006;7(1):22–31. [PubMed] [Google Scholar]
- 91.Yagi K, Kim S, Wanibuchi H, Yamashita T, Yamamura Y, Iwao H. Characteristics of diabetes, blood pressure, and cardiac and renal complications in Otsuka Long-Evans Tokushima Fatty rats. Hypertension. 1997;29(3):728–735. doi: 10.1161/01.hyp.29.3.728. [DOI] [PubMed] [Google Scholar]
- 92.Kawano K, Hirashima T, Mori S, Natori T. OLETF (Otsuka Long-Evans Tokushima fatty) rat: a new NIDDM rat strain. Diabetes Research and Clinical Practice. 1994;24:S317–S320. doi: 10.1016/0168-8227(94)90269-0. [DOI] [PubMed] [Google Scholar]


