Abstract
In the 30 years since the rabbit antithymocyte globulin (rATG) Thymoglobulin® was first licensed, its use in solid organ transplantation and hematology has expanded progressively. Although the evidence base is incomplete, specific roles for rATG in organ transplant recipients using contemporary dosing strategies are now relatively well-identified. The addition of rATG induction to a standard triple or dual regimen reduces acute cellular rejection, and possibly humoral rejection. It is an appropriate first choice in patients with moderate or high immunological risk, and may be used in low-risk patients receiving a calcineurin inhibitor (CNI)-sparing regimen from time of transplant, or if early steroid withdrawal is planned. Kidney transplant patients at risk of delayed graft function may also benefit from the use of rATG to facilitate delayed CNI introduction. In hematopoietic stem cell transplantation, rATG has become an important component of conventional myeloablative conditioning regimens, following demonstration of reduced acute and chronic graft-versus-host disease. More recently, a role for rATG has also been established in reduced-intensity conditioning regimens. In autoimmunity, rATG contributes to the treatment of severe aplastic anemia, and has been incorporated in autograft projects for the management of conditions such as multiple sclerosis, Crohn’s disease, and systemic sclerosis. Finally, research is underway for the induction of tolerance exploiting the ability of rATG to induce immunosuppresive cells such as regulatory T-cells. Despite its long history, rATG remains a key component of the immunosuppressive armamentarium, and its complex immunological properties indicate that its use will expand to a wider range of disease conditions in the future.
Key Points
| In the 30 years since the rabbit antithymocyte globulin (rATG) Thymoglobulin® was first licensed, there have been profound advances in its use, including a widening of its role in optimizing immunosuppression for solid organ transplant recipients and in hematology indications. |
| rATG dosing has decreased over time, refining the risk:benefit ratio and reducing early safety concerns. |
| A growing understanding of the complex immunological properties has prompted new research into other therapeutic fields. |
Introduction
It has been 30 years since the rabbit antithymocyte globulin (rATG) Thymoglobulin® was first licensed for clinical use in April 1984. Of the three polyclonal agents currently available—Thymoglobulin®, the rabbit preparation manufactured by Fresenius (ATG-Fresenius), and, in certain markets, the equine antithymocyte globulin (eATG, ATGAM®)—rATG is the most widely used. Over time, its role in the management of solid organ transplant patients and other therapeutic areas has expanded progressively. It is now the most widely prescribed induction agent in the US [1], administered to approximately half of all de novo kidney transplant recipients [2]. From the early evidence focusing on kidney transplantation, rATG administration has widened to all types of solid organ transplantation, including liver, heart, lung, pancreas, and intestinal transplantation, as well as to hematopoietic stem cell transplantation (HSCT) and aplastic anemia [3]. It has also been included in immunosuppressive regimens following pioneering composite tissue transplant procedures (hands, face, etc.), and as part of experimental protocols attempting to achieve operational tolerance. Part of this expansion has arisen from increasing awareness of additional clinical applications for the drug. As well as providing rejection prophylaxis, and treatment for steroid-resistant rejection episodes, it is used to help avoid delayed graft function (DGF) in at-risk individuals and, more recently, to facilitate steroid-sparing and calcineurin inhibitor (CNI)-sparing regimens in the quest for reduced long-term morbidity after organ transplantation.
Over the intervening years there has also been a steady shift towards lower rATG dosing as experience has grown, refining the risk:benefit balance. Moreover, understanding of the immunological impact of rATG has improved, with recent studies confirming a significant depletion of CD3+, CD4+, CD8+ and natural killer (NK) cell depletion, followed by preferential reconstitution of central memory CD4+ T-cells at the expense of naïve CD4+ cells [4–6]. Long-term follow-up data have shown that CD4+ T-cell reconstitution remains impaired for up to 21 years in kidney transplant patients treated with rATG compared with controls receiving interleukin (IL)-2 receptor antagonist (IL-2RA) induction [7].
There is now a substantial evidence base relating to the use of rATG in different therapeutic settings from the last three decades [3, 8–10]. At this milestone in its development, it is timely to focus on the most recent clinical data regarding the use of rATG based on contemporary treatment protocols.
Methodology
A literature search on the PubMed database was undertaken with no time limits. Single search terms included ‘ATG’, ‘rATG’, ‘antithymocyte globulin’, and ‘Thymoglobulin’, across all therapy areas. Only articles with an English abstract were reviewed. Abstracts from relevant congresses during 2013 and 2014 were also searched using the same search terms. While all relevant publications were reviewed, emphasis is placed on randomized trials where possible.
The Basis for Rabbit Antithymocyte Globulin (rATG) use in Solid Organ Transplantation
rATG exerts its immunomodulatory and immunosuppressive effects via a wide range of immune and non-immune targets, adhesion molecules, and chemokine receptors [11]. rATG administration induces a significant depletion of CD3+, CD4+ and CD8+ T-cells and NK cells, as well as other T-cell subsets [11], while leaving the B-cell count unaffected [4]. CD3+ cell count diminishes rapidly, with most patients having almost no CD3+ cells within 24 h after the start of treatment [12]. The proportion of naïve CD4+ cells decreases, while central memory CD4+ T-cells increase. Using a typical dosing strategy of 1.5 mg/kg on days 0–3 (cumulative dose 6 mg/kg), rATG remains at therapeutic levels for approximately 19 days [4]. The half-life of rATG is about 3 weeks, with complete elimination from the serum within 1 year [12].
Randomized trials and non-randomized trials of rATG during the 1990s and early 2000s established the immunosuppressive potency of rATG for both the treatment and prevention of solid organ graft rejection, but also highlighted safety concerns. The first randomized trials of rATG were comparative studies versus other lymphocyte-depleting agents for the treatment of allograft rejection following kidney transplantation [13, 14]. In one trial, a non-significant trend to improved rates of response and prevention of recurrent rejection was observed versus muromonab-CD3 (OKT3), with a more favorable safety profile [14]. Another early randomized study showed that compared with eATG, rATG achieved a higher rate of rejection reversal and a lower incidence of recurrent rejection, with a similar safety profile [13]. Shortly afterwards, a randomized, double-blind trial of rATG versus eATG for prevention of rejection in de novo kidney transplant patients was published [15]. In this trial, 72 patients received rATG (1.5 mg/kg/day) or eATG (15 mg/kg/day) for 7 days, with maintenance immunosuppression comprising cyclosporine, azathioprine and steroids in both groups. At 1 year, the incidence of acute rejection was significantly lower in the rATG arm (4 % vs. 25 %; p = 0.014) and a composite endpoint of death, graft loss or rejection was less frequent (6 % vs. 27 %; p = 0.0005), with a lower incidence of cytomegalovirus (CMV) infection [15]. Mourad et al. [16] also demonstrated the efficacy of rATG in preventing rejection after kidney transplantation in a multicenter study of rATG (1.25 mg/kg/day for 10 days) with tacrolimus initiated on day 9 post-transplant versus immediate tacrolimus with no induction. Biopsy-proven acute rejection (BPAR) was less frequent in the rATG group (15.2 % vs. 30.4 %; p = 0.001), but adverse events such as CMV infection, herpes simplex infection, fever, and thrombocytopenia were increased in rATG-treated patients. A large multicenter trial of 555 de novo kidney transplant patients randomized to rATG with tacrolimus, rATG with cyclosporine, or tacrolimus with no induction, all with azathioprine and steroids, showed the lowest rate of acute rejection in the rATG-tacrolimus group (p = 0.004 vs. tacrolimus and no induction) [17]. Again, with an rATG dose of 1.25 mg/kg/day for 10 days (adjusted as necessary based on clinical findings), higher rates of adverse events, including hematological disturbances and infections, were observed in patients receiving rATG. In a randomized trial of 50 heart transplant patients published in 2002, rATG and ATG-Fresenius were associated with a similar incidence and number of rejections when combined with a maintenance regimen of cyclosporine, azathioprine, and steroids [18]. However, the study used relatively high doses of both rATG (five doses of 2.5 mg/kg/day) and ATG-Fresenius (five doses of approximately 3.2 mg/kg/day), and the overall rate of infections was high (rATG 58 %, ATG-Fresenius 75 %) [18].
Contemporary rATG Dosing Regimens in Solid Organ Transplantation
As experience with rATG grew during the late 1990s and early 2000s, it became apparent that the high doses of rATG (typically 1.25–1.5 mg/kg/day for 7–10 days) administered in early clinical studies [13, 15–18] were not necessary to achieve sustained lymphocyte depletion. Protocol-specified doses have since declined (Fig. 1a–c). In a non-randomized trial, Agha et al. [19] demonstrated that a total dose of 6 mg/kg achieved similar efficacy to a dose of 10.5 mg/kg (1.5 mg/kg/day over 7 days) in a cohort of historical controls. Indeed, with an intraoperative dose of 3 mg/kg and two subsequent doses of 1.5 mg/kg, lymphocyte depletion appeared to be more sustained than with the high-dose regimen [19]. Even lower doses (<6 mg/kg in total) have been explored [20, 57–61] (Table 1), but T-cell depletion appears to be diminished [21]. Limited retrospective data have suggested that early acute rejection may be more frequent if the total rATG dose is less than 6 mg/kg [66], although good outcomes with lower doses have also been reported [61]. Cumulative doses in the range of 3–6 mg/kg may be adequate in elderly recipients, in whom a retrospective analysis has reported excellent outcomes with an average total rATG dose of 5.4 mg/kg [67], and in lower-risk individuals such as living-donor recipients [68]. However, very low doses of rATG are likely to be inadequate, even in low-risk groups. A retrospective analysis of 100 living-donor kidney transplant patients given a single rATG dose of 1.5 mg/kg found the rate of acute rejection at 1 year to be 17 % and 35 % in recipients of related and unrelated grafts, respectively [62]. In a controlled study of 40 kidney transplants given a total dose of 1.5 mg/kg, 3.0 mg/kg, or 6.0 mg/kg of rATG as induction with tacrolimus, mycophenolate mofetil (MMF) and steroids, the T-cell count returned to normal by month 3 in the 1.5 mg/kg group, and during the first year in the 3.0 mg/kg group, but remained lower than in controls in the patients given a dose of 6.0 mg/kg [69]. Experimental evidence indicates that a total dose of approximately 6.4 mg/kg achieves lymphocyte depletion in peripheral blood and the spleen and lymph nodes [70]. Overall, it appears that a cumulative rATG dose of 6 mg/kg is generally appropriate for induction therapy [4, 69].
Fig. 1.
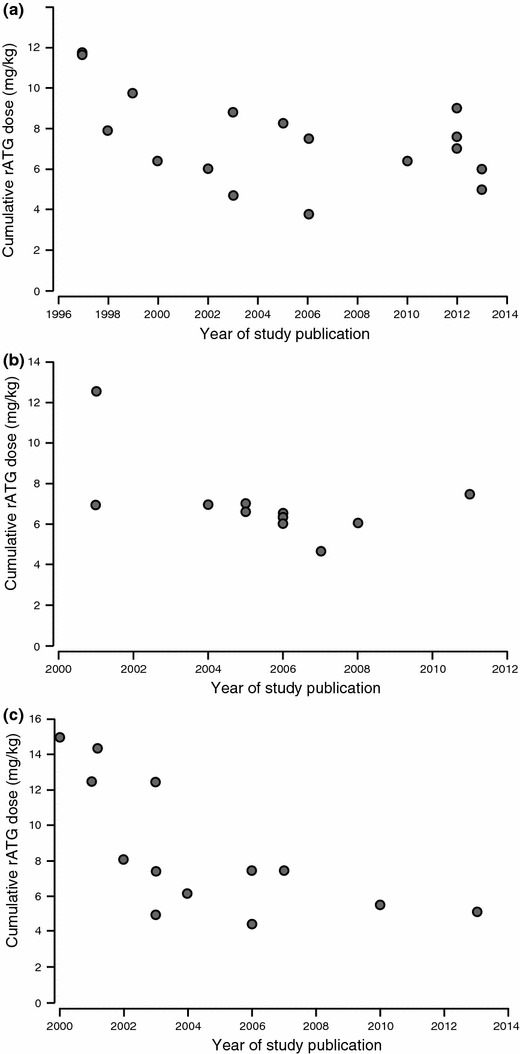
rATG (Thymoglobulin®) dose in clinical studies according to year of publication with (a) standard triple maintenance regimen [4, 15, 19–34]; (b) steroid-sparing regimen [35–46] and (c) CNI-sparing regimen [16, 17, 47–56]. Doses shown are protocol specified or, if unavailable, mean dose administered. CNI calcineurin inhibitor, rATG rabbit antithymocyte globulin
Table 1.
Alternative dosing strategies for rATG (Thymoglobulin®) induction therapy in solid organ transplant patients
| Study (year) | Organ | N | Study design | rATG regimen | Comparator group/s | Maintenance immunosuppression | Follow-up | Treatment group |
Acute rejection (%) | Other outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Low-dose and/or short-course regimen | ||||||||||
| Grafals et al. (2013) [59] | Kidney | 68 |
Prospective Randomized Single-center |
Total dose 2.25 mg/kg (0.75 mg/kg days 0–2) | Total dose 3.75 mg/kg (1.25 mg/kg days 0–2) | Not stated | Mean 9.9 and 11.8 months | 2.25 mg/kg | 10.0 | DGF more frequent in 2.25 mg/kg group (40 % vs. 14.3 %; p = 0.041) |
| 3.75 mg/kg | 9.5 | |||||||||
| Popat et al. (2013) [60] | Kidney | 45 |
Prospective Non-randomized Single-center |
Total dose 3.75 mg/kg (2.5 mg/kg on day 0, 1.25 mg/kg on day 4) | IL-2RA induction |
CNI MMF ± Steroids |
3 years | rATG | 0 | Significantly lower rates of DGF and hospitalization for infection in the rATG group |
| IL-2RA |
13 (p = 0.001) |
|||||||||
| Laftavi et al. (2011) [57] | Kidney | 90 |
Retrospective Single-arm Single-center |
Mean (SD) total dose 3.0 (1.3) mg/kg in older patients (>65 years) or 3.2 (2.1) mg/kg in younger patients (<65 years) |
CNI MMF Low-dose steroids |
6 months | Older patients | 0 | 4.4 % and 6.7 % viral infections in the older and younger patients, respectively | |
| Younger patients | 2.5 | |||||||||
| Wong et al. (2006) [21] | Kidney | 16 |
Prospective Single-arm Single-center |
Total 3.0 mg/kg (first dose intraoperative, days 0–2) | 4.5 mg/kg (first dose intraoperative, days 0–2) |
Tacrolimus MMF Steroids |
2 years | 3 mg/kg | 5 | T-cell count lower at month 6 with 4.5 mg/kg total dose (p = 0.016) |
| Goggins et al. (2003) [20] | Kidney | 58 |
Prospective Randomized Single-center |
Total 3–6 mg/kg (first dose intraoperative) | 3–6 mg/kg in week 1 (no intraoperative) |
CNI MMF Steroids |
Mean 15.1 months | Intraoperative | 3.6 | Less DGF (p < 0.05) and lower mean serum creatinine (p < 0.005) with intraoperative first dose |
| Postoperative |
16.0 (NS) |
|||||||||
| Agha et al. (2002) [19] | Kidney | 88 |
Prospective Historical controls Single-center |
Total 6 mg/kg (days 0–2) | Total 10.5 mg/kg (1.5 mg days 0–6) | Not stated | 1 year | 6 mg/kg | 5 |
Lower lymphocyte count at month 1 with 6 mg/kg regimen (p < 0.05) Similar safety profile |
| Eason et al. (2003) [58] | Liver | 119 |
Prospective Randomized Single-center |
Total 3.0 mg/kg (days 0–1) |
Steroids to month 3 (no steroids in rATG group) |
Tacrolimus MMF (withdrawn after month 3) |
2 years | rATG (steroid-free) | 25 | Similar patient survival at 1 and 2 years. Greater requirement for steroid treatment of rejection in the steroid group (p = 0.03) |
| Steroids | 31 (NS) | |||||||||
| Single-dose regimen | ||||||||||
| Schenker et al. (2011) [62] | Kidney | 100 |
Retrospective Single-arm Single-center |
Total dose 1.5 mg/kg | – |
Tacrolimus MMF Low-dose steroids |
Mean 52.6 months | Living related | 17 | Low rates of infection |
| Living unrelated | 35 | |||||||||
| Stevens et al. (2008) [22] | Kidney | 142 |
Prospective Randomized Single-center |
Total dose 6 mg/kg (single dose over 24 hours) | Total dose 6 mg/kg (1.5 mg/kg × 4 doses) |
CNI to month 6 MMF or sirolimus |
6 months | Single dose | 8 | Change in eGFR to month 6 was better in the single-dose group (p = 0.02) |
| 4 doses |
12 (NS) |
|||||||||
| De Ruvo et al. (2005) [63] | Liver | 52 |
Retrospective Historical controls Multicenter |
Total dose 5 mg/kg (single dose over 4 h) | No rATG |
Tacrolimus Steroids to month 3 in non-rATG group |
1 year | rATG single dose | 36.4 | Lower tacrolimus dose in rATG group (p < 0.05) |
| No rATG | 40 (NS) | |||||||||
| Dosing based on immune monitoring | ||||||||||
| Peddi et al. (2002) [64] | Kidney | 41 |
Prospective Single-arm Single-center |
1.5 mg/kg daily until CD3 + ≤30 cells/mm3 (mean total dose 4.1 mg/kg) |
– |
CNI MMF Steroids |
Mean 340 days | T-cell adapted rATG | 12.2 |
Low rate of rejection in a high-risk population CNI delayed for mean of 6 days post-transplant |
| Djamali et al. (2000) [23] | Kidney | 39 |
Prospective Non-randomized Single-center |
50 mg/day × 3 days then only if CD3+ T-cells were >10 cells/mm3 Mean (SD) total dose 6.6 (2.9) mg/kg (mean 7.3 doses) |
50 mg/day daily Mean (SD) total dose 9.1 (2.2) mg/kg (mean 11.5 doses) |
CsA AZA Steroids |
1 year | T-cell adapted rATG | 19 episodes |
Similar depletion of T-cells and peripheral blood lymphocytes Infections and hematological complications were similar |
| Standard rATG | 13 episodes (NS) | |||||||||
| Koch et al. (2005) [65] | Heart | 62 |
Retrospective Single-center Historical controls |
1.5 mg/kg daily until total lymphocytes <100 cells/mm3, CD4+ T-cells <50 cells/mm3 and CD8+ <50 cells/mm3 | Total dose 12 mg/kg (1.5 mg/kg × 8 days) (equine ATG Merieux) |
CsA AZA Steroids |
1 year | T-cell adapted rATG | Mean 0.4 (0.7) episodes |
Significantly lower ATG dose (p < 0.05) with T-cell adapted dosing Similar patient survival CMV seroconversion 23 % vs. 13 % with standard dosing Deep sternal infection 1.6 % vs. 3.2 % with standard dosing |
| Standard equine ATG | Mean 1.1 (1.7) episodes | |||||||||
AZA azathioprine, CMV cytomegalovirus, CNI calcineurin inhibitor, CsA cyclosporine, DGF delayed graft function, eGFR estimated glomerular filtration rate, IL-2RA interleukin-2 receptor antagonist, MMF mycophenolate mofetil, NS not significant, rATG rabbit antithymocyte globulin, SD standard deviation
A further imperative to reduce rATG dosing came from the realization that early high-dose regimens increased the risk of infectious disease, particularly CMV infection [15]. In one study, a dose of 1.5 mg/kg/day for 9 days (13.5 mg/kg in total) was associated with a significantly higher rate of CMV disease versus no induction in kidney transplant patients [16], while in liver transplantation a very high total dose of 25 mg/kg over 10 days resulted in a higher rate of fatal infections than a 3-day course with a total dose of 7.5 mg/kg [71].
Following a gradual decline over time, the typical dose of rATG now used in kidney transplant patients receiving a standard triple-dose maintenance regimen is 6.0–7.5 mg/kg (Fig. 1a). Trial results indicate that this dose level achieves high efficacy with a good safety profile in kidney transplant patients, even in high-risk individuals [4, 24, 26, 72]. In recent trials of liver transplantation, total doses have generally been similar to those in kidney transplantation (6–7.5 mg/kg) [73]. Data are more limited in heart transplantation [74–77]. There is evidence suggesting that 1.5 mg/kg/day for 5 days may offer less effective rejection prophylaxis than a 7-day course following heart transplantation [78].
Various different innovative dosing strategies for rATG have also been explored, notably single-dose regimens or dosing based on immune monitoring (Table 1). In one study, a single infusion of 6 mg/kg instead of four divided doses of 1.5 mg/kg/day was found to offer improved renal function at month 6 after kidney transplantation [22], an intriguing finding that merits further investigation since two infusions of 3 mg/kg instead of four divided doses of 1.5 mg/kg/day induce a similar pattern of T-cell and NK-cell depletion and reconstitution [4]. However, perhaps of more interest is adaptation of dosing based on T-cell count, which has been explored in kidney and heart transplantation (Table 1). Prospective [23, 64] and retrospective [64] studies indicate that the total dose of rATG can be reduced markedly [23, 79] without compromising lymphocyte suppression or immunosuppressive potency. In a prospective, non-randomized trial, Djamali et al. [23] observed that withholding rATG until CD3+ T-cell count exceeded 10 cells/mm3 led to an approximately 25 % reduction in rATG dose but provided similar T-cell depletion to a standard daily dosing (mean total dose 6.6 mg/kg vs. 9.1 mg/kg). Lymphocyte count has been proposed by some investigators in the field of solid organ transplantation as the preferred tool to adjust rATG dosage, but randomized trials are lacking. This approach is not widely accepted, and overall dosage based on milligrams per kilogram remains the most common mode of administration. Using weight-adjusted dosing, caution should be exercised in patients with body weight below 40 kg or greater than 80 kg, to avoid under- or over-immunosuppression. Pharmacokinetic monitoring techniques are emerging but are not yet routinely used.
Lastly, intraoperative administration of the first dose may help to minimize the risk of DGF in at-risk kidney transplant recipients [20, 80], possibly by ameliorating ischemia-reperfusion injury through suppression of inflammatory cells and mediators as a result of leukocyte and T-lymphocyte depletion [81–83], but this remains unconfirmed.
The duration and dose of rATG therapy, of course, impacts on drug purchase costs. There is also evidence that a shorter course—for example, 3 days at 2 mg/kg/day versus 4 days at 1.5 mg/kg/day—may result in indirect cost savings, largely due to reduced hospital stay [84, 85]. Dosing based on CD3+ monitoring may also lower drug costs versus a fixed-dose regimen [86].
rATG in Kidney Transplantation
Risk Stratification
rATG induction is typically used in high-risk or medium-risk kidney transplant patients, specifically those who are sensitized, or in patients at risk of DGF [87]. The decision to administer rATG or other induction therapy after kidney transplantation is based on demographic characteristics such as age and race, taking into account other clinical factors such as previous transplantation and the type of donor. These variables are coupled with immunological markers of rejection risk: the degree of human leukocyte antigen (HLA) matching, the presence of anti-HLA prior to transplantation [88]. More recently, growing awareness of the predictive role of pre-transplant donor-specific antibodies (DSA) has received intense attention. The presence of pre-transplant DSA (class I or II) [89] and the DSA titer [90] show a close correlation with the risk of acute antibody-mediated rejection after kidney transplantation. However, DSA measurement by the Luminex technique is not always reproducible and suitable cutoff points using other detection techniques, such as complement-dependent cytotoxicity (CDC) cross-match, have not been established. Testing for DSA prior to transplantation is not yet standard. Nevertheless, there is a growing movement towards inclusion of DSA during risk stratification of transplant recipients in order to target rATG induction and other interventions appropriately.
Rejection Prophylaxis and Graft Survival
The efficacy of rATG in preventing acute rejection in kidney transplant patients is well-established [3, 8, 9]. Randomized trials have shown a lower rate of rejection for rATG versus the antilymphocyte preparation ATGAM [14], or versus IL-2RA induction in patients with moderate to high immunological risk [24, 91] with similar rejection rates to IL-2RA induction in low-risk patients [47, 72, 92, 93] (Table 2). Meta-analyses have confirmed that rATG induction confers a lower rejection risk compared with no induction [95], with similar rates of rejection to alemtuzumab induction [96]. A large-scale analysis of data from the Organ Procurement and Transplant Network (OPTN) showed rejection to be less likely under rATG than IL-2RA induction [87].
Table 2.
Randomized trials of rATG (Thymoglobulin®) induction therapy in kidney transplant patients using contemporary dosing regimens (cumulative dose (≤7.5 mg/kg)
| Study (year) | N | Immunological risk status | rATG regimen | Comparator group/s | Maintenance immunosuppression | Follow-up | Treatment group |
Patient survival (%) |
Graft survival (%) |
Acute rejection (%)a |
|---|---|---|---|---|---|---|---|---|---|---|
| rATG vs. IL-2RA induction | ||||||||||
| Brennan et al. (2006) [24] | 278 | High |
Total dose 7.5 mg/kg/day (days 0–4) |
Basiliximab |
CsA by day 4 MMF Steroids |
12 months | rATG | 95.7 | 90.8 | 15.6 |
| IL-2RA | 95.6 | 89.8 | 25.5 | |||||||
| p-Value | 0.90 | 0.68 | 0.02 b | |||||||
| Ciancio et al. (2005) [92] | 60 | Low |
Total dose 7.0 mg/kg/day (days 0–7) |
Daclizumab |
Tacrolimus MMF Steroids |
Median 15 months |
rATG | 92 | 88 | 16.6 |
| IL-2RA | 88 | 88 | 16.6 | |||||||
| p-Value | NS | NS | 0.99 | |||||||
| Abou-Ayache et al. (2008) [93] | 109 | Low |
1.0–1.5 mg/kg 4–9 infusions |
Daclizumab |
Delayed CsA MMF Steroids |
12 months | rATG | 98 | 95 | 14.5 |
| IL-2RA | 98 | 94 | 16.7 | |||||||
| p-Value | NS | NS | NS | |||||||
| Mourad et al. (2004) [72] | 105 | Low |
1.0 mg/kg Mean 5.4 infusions |
Basiliximab |
Delayed CsA MMF Steroids |
12 months | rATG | 98.1 | 96.2 | 9.4 |
| IL-2RA | 98.1 | 94.2 | 9.6 | |||||||
| p-Value | NS | NS | NS | |||||||
| Steroid-sparing vs. standard steroid regimen | Steroid regimen | |||||||||
| Woodle et al. (2009) [94] | 151 | Low | Total dose 5–6 mg/kg | Steroid withdrawal by day 8 |
Tacrolimus MMF |
12 months | Withdrawal | 98.9 | 98.9 | 13.9 |
| Standard | 100 | 100 | 19.4 | |||||||
| p-Value | NS | NS | NS | |||||||
| CNI-free vs. standard CNI regimens | CNI regimen | |||||||||
| Glotz et al. (2010) [48] | 141 | Standard |
1.25–1.5 mg/kg 4 dosesc |
CNI free |
Sirolimus MMF Steroids |
12 months | CNI free | 95.8 | 85.9 | 16.9 |
| Tacrolimus |
MMF Steroids |
CNI | 97.1 | 95.7 | 12.9 | |||||
| p-Value | NS | 0.044 | NS | |||||||
| Büchler et al. (2007) [49] | 145 | Standard |
Total dose 7.5 mg/kg/day (days 0–4) |
CNI free |
MMF Steroids (to month 6) |
12 months | CNI free | 97 | 90 | 14.3 |
| CsA |
MMF Steroids (to month 6) |
CNI | 97 | 93 | 8.6 | |||||
| p-Value | NS | NS | 0.30 | |||||||
| Larson et al. (2006) [50] | 165 | Standard |
Total dose 7.5 mg/kg/day (days 0–4) |
Sirolimus |
Sirolimus MMF Steroids |
12 months | CNI free | 98 | 94 | 19 |
| Tacrolimus |
MMF Steroids |
CNI | 96 | 92 | 14 | |||||
| p-Value | NS | NS | NS | |||||||
| Lo et al. (2004) [51] | 70 | High | Total dose 4.5–10.5 mg/kg | CNI free |
Sirolimus MMF Steroids |
12 months | CNI free | 100 | 89 | 7 |
| Low CNI |
Sirolimus Reduced tacrolimus Steroids |
Low CNI | 98 | 80 | 10 | |||||
| p-Value | NS | NS | NS | |||||||
Significant p-values are shown in bold
CNI calcineurin inhibitor, CsA cyclosporine, DGF delayed graft function, IL-2RA interleukin-2 receptor antagonist, MMF mycophenolate mofetil, NS not significant, rATG rabbit antithymocyte globulin
aOr biopsy-proven acute rejection
bSteroid-resistant acute rejection: 1.4 % with Thymoglobulin®, 8.0 % with IL-2RA induction (p = 0.005)
cOnly given to tacrolimus-treated patients in the event of DGF
In a prospective, randomized study of kidney transplantation comparing rATG versus alemtuzumab induction, in combination with tacrolimus plus MMF and a 5-day course of corticosteroids, alemtuzumab was associated with a lower rate of early BPAR in low-risk individuals but there was no difference in high-risk patients [97]. A meta-analysis of randomized trials in kidney transplantation found no significant difference in the risk of BPAR when rATG induction was compared with alemtuzumab [96].
A recent retrospective case-control series also indicated that in patients undergoing a second kidney transplant, rATG induction achieves similar lymphocyte depletion to that observed after a first course, and is as well tolerated [98]. In pediatric kidney transplant recipients, good outcomes have been reported with rATG at a dose of 1.5–2.5 mg/kg/day for 5–10 days [99–103], with promising rates of graft survival in high-risk patients, but dose-finding studies and robust comparisons with other regimens are lacking.
Determining whether an effect on rejection rates translates to higher graft survival rates is more difficult to determine. The number of patients and duration required means that it is impractical for a controlled trial to be adequately powered. The available evidence is largely restricted to retrospective single-center studies or registry analyses, in which patient characteristics are not always fully captured. There are reports in the literature that rATG induction is associated with improved graft survival rates [35, 61, 104–106] compared with no induction or IL-2RA induction therapy, but data from a recent meta-analysis (n = 892) [95] and large-scale registry analyses [87, 107] are conflicting. One recent analysis of graft survival rates, based on data from the OPTN for kidney transplants during 2001–2005, reported a lower 6-month incidence of a combined endpoint of rejection, graft failure or death with rATG versus IL-2RA induction or no induction [108]. Moreover, any effect of rATG on graft survival appears to be influenced by the type of patient population; no effect was observed in one analysis of zero-mismatched deceased-donor recipients [109], whereas an analysis of 12,100 deceased-donor recipients with DGF demonstrated a significant improvement in death-censored graft failure (p = 0.04) and death with a functioning graft (p = 0.0005) versus IL-2RA induction therapy after adjustment for confounding factors [110]. In a large retrospective analysis of 475 kidney transplants undertaken during 2001–2009 at a single center, a low rATG dose (mean 3.2 mg/kg) was associated with higher graft survival than IL-2RA induction, a difference that was more pronounced among obese recipients (90.3 % vs. 63.6 % at an average of 47 months’ follow-up; p < 0.04) but was still significant in non-obese individuals (88.7 % vs. 68.2 %; p < 0.01) [61]. No firm conclusions on an effect of rATG on kidney allograft survival can be drawn, although there are some encouraging indications.
Based on the available data, rATG induction is appropriate in patients with moderate or high immunological risk as rejection prophylaxis, but in low-risk patients is required only if early CNI-sparing or steroid withdrawal is planned. Use of rATG induction in low-risk kidney transplant patients receiving a standard triple-therapy maintenance regimen is unlikely to be cost effective and unnecessarily exposes the patients to risk of over-immunosuppression.
Ischemia-Reperfusion Injury and Delayed Graft Function
Beyond its traditional use in rejection prophylaxis and treatment, it is possible that rATG may ameliorate ischemia-reperfusion injury by inhibiting expression of adhesion molecules and cytokines [82]. Coupled with T-cell depletion, such an effect could help to prevent DGF. In a randomized trial in liver transplant patients, rATG resulted in less clinical evidence of ischemia-reperfusion injury [111], but a similar histological study in kidney transplant patients is lacking. Clinical data are not adequate to assume a benefit for rATG in terms of diminishing injury.
In 2003, Goggins et al. [20] reported a lower incidence of DGF using intraoperative rATG compared with postoperative administration (14.8 % vs. 35.5 %; p < 0.05). More recently, Harrison and colleagues observed no difference in renal function during the first year post-transplant in patients given the first rATG dose before reperfusion or postoperatively [112]. In two randomized trials in which the first dose of rATG was given before graft reperfusion, the rate of DGF was significantly lower versus IL-2RA induction in one study of 227 immunologically high-risk, HLA-sensitized patients [91], but no difference was observed in the second trial of 278 patients at high risk for acute rejection or DGF [23]. Other studies have not shown a reduction in DGF rates [34, 47, 74] in patients receiving rATG induction, although the risk and/or severity of rejection is generally lower in patients with DGF who receive rATG [15, 34]. The multifactorial etiology of DGF means it is difficult to confirm a contribution of rATG induction conclusively, but it appears that rATG may offer some benefit in decreasing the risk of DGF [113]; further data are awaited. A recently proposed scale to classify patients according to their DGF risk [114] may help identify high-risk individuals.
Reducing Maintenance Immunosuppression
In recent years, clinical investigation of rATG in kidney transplantation has centered on its ability to support either early steroid withdrawal or CNI-sparing regimens. The most robust data relating to early steroid withdrawal comes from a multicenter trial in which 151 living-donor kidney transplant patients were randomized to rATG (total dose 5–6 mg/kg) with steroids to day 7, or to an induction-free standard-steroids regimen, both with tacrolimus and MMF [94] (Table 2). At 12 months post-transplant, rates of BPAR and all efficacy endpoints were similar between the two groups, but total cholesterol was lower in the steroid-withdrawal group with trends towards a lower triglyceride level and less weight gain, although leukopenia was more frequent in the rATG cohort. A large, randomized trial by Kandaswamy et al. [36], in which standard-risk patients received rATG at a total dose of 5.0–7.5 mg/kg, demonstrated a low rate of rejection when steroids were withdrawn after day 5 with a variety of maintenance regimens. Steroid withdrawal at month 3 from a maintenance regimen of cyclosporine and MMF also appears feasible based on data from a randomized, double-blind trial reported by Lebranchu et al. [115] in which 104 patients were given rATG induction according to local practice with cyclosporine and MMF maintenance therapy, but rATG dosing information was not provided. Other prospective or retrospective non-comparative trials have also shown a low rate of rejection following early steroid withdrawal supported by rATG induction [37–39]. In low-risk children, non-randomized trials have suggested that rATG induction can support early (<1 week) steroid discontinuation [116] or steroid-free immunosuppression [117] with a maintenance regimen of CNI and MMF, with no increase in rejection risk. One retrospective study has compared rATG induction (6.0 mg/kg) versus IL-2RA induction with basiliximab in 99 kidney transplant patients for whom early steroid discontinuation on day 6 was planned [118]. By 1-year post-transplant, the incidence of BPAR was lower (7 % vs. 26 %), and mean time to first BPAR (151.4 vs. 53.6 days) was longer, with rATG versus IL-2RA induction. As with standard maintenance immunosuppression, the dose of rATG administered in patients receiving a steroid-sparing regimen has declined over time (Fig. 1b).
The use of rATG induction to facilitate delayed introduction of CNI therapy by up to 9 days—often in an attempt to avoid DGF—has been shown to maintain immunosuppressive efficacy following kidney transplantation [16, 24, 47, 93] and is a recognized therapeutic strategy in patients at risk of DGF. More controversial, single-arm studies [119, 120] and randomized, controlled trials [48–51] have administered rATG induction with an entirely CNI-free, sirolimus-based maintenance immunosuppressive regimen (Table 2). In each of the comparative studies, the rate of acute rejection was low and similar to a standard CNI regimen [48–51], although graft survival was lower with the CNI-free group in one study [48]. In all but one randomized trial [50], renal function was superior at 1 year in the CNI-free arm, either overall [48, 51] or among the subpopulation who remained on a CNI-free regimen [49]. Five-year follow-up data from the Spiesser study [49] confirmed that estimated glomerular filtration rate (GFR) remained higher in the CNI-free treatment group, with no difference in acute rejection rates [121]. In a small, prospective study of seven patients receiving a kidney from an HLA-identical living donor, rATG with MMF or sirolimus and no CNI therapy achieved 100 % graft and patient survival over a median of 26 months’ follow-up; rATG induction was continued for 10 days at an unspecified dose [122]. In contrast, de novo immunosuppression with sirolimus and no CNI therapy in patients receiving IL-2RA induction is associated with a high rate of acute rejection (>30 % at 1 year) [123, 124]. It appears that adequate immunosuppression in the early period after kidney transplantation may require rATG induction if CNI avoidance is attempted.
One novel strategy to reduce both CNI and steroid exposure is to use low-dose rATG in combination with IL-2RA induction. This approach has been assessed in a prospective, single-center, non-randomized study which compared rATG at a total dose of 200 mg combined with basiliximab (total dose 40 mg) versus basiliximab alone [125]. In the rATG group, tacrolimus exposure was reduced and steroids were selectively withdrawn at 3–6 months. At 1 year, the incidence of acute rejection was similar in both groups despite lower exposure to both CNI and steroids. Alternatively, rATG induction (up to 6 mg/kg in total) coupled with maintenance therapy using the co-stimulation blocker belatacept and a mammalian target of rapamycin inhibitor or MMF may enable avoidance of both CNIs and steroids with an acceptable rate of rejection [126]; further data are awaited.
rATG and Donor-Specific Antibodies
Awareness that the presence of de novo DSA nearly doubles the risk for antibody-mediated rejection after kidney transplantation [89] has focused attention on preventative strategies. There are early data to suggest that rATG preferentially inhibits the proliferation of donor antigen-activated T-cells in kidney transplant patients by inducing expression of donor-specific helios−FOXP3+ regulatory T-cells, an effect not seen with IL-2RA induction agents [127]. B-cell expression and phenotype remained unchanged after administration of low-dose rATG [128], or even after high doses (3 mg/kg/day) [4]. Clinically, a retrospective study of rATG induction with intravenous immunoglobulin (IVIG) in kidney transplant patients with preformed DSA receiving tacrolimus-based triple therapy has demonstrated that sensitized patients with positive flow cytometry cross-match can achieve excellent graft survival rates with acceptable levels of antibody-mediated rejection [129]. In liver transplantation, rATG with rituximab induction has also been shown to result in low rates of antibody-mediated rejection [130].
Data regarding a possible effect of rATG induction therapy on the risk of post-transplant de novo DSA are starting to emerge. In a series of 114 moderately sensitized DSA-positive patients, occurrence of de novo DSA (defined as absence of pre-transplant DSA) increasing at least threefold was monitored for a mean of 12.4 months [32]. rATG was given to 85 of the patients (mean total dose 4.98 mg/kg), and the IL-2RA induction agent basiliximab was given to 29 patients. Multivariate analysis showed that rATG induction was associated with a significant reduction in risk of both de novo DSA and acute antibody-mediated humoral rejection [32] (Table 3). Of all factors assessed, rATG induction was the single most important variable associated with both de novo DSA and humoral rejection. In a single-center, matched-cohort study of 16 kidney transplant patients and 32 controls [128], there was a lower incidence of de novo DSA at 1-year post-transplant in patients treated with rATG or basiliximab versus alemtuzumab (p = 0.011). The difference was maintained at 2 years (p = 0.010) because alemtuzumab induces B-cell depletion and regeneration. If these data are confirmed, use of rATG in patients at risk of developing de novo DSA would be of considerable clinical interest. However, other single-center, retrospective analyses have observed no difference in DSA production in kidney transplant patients with or without rATG induction [131, 132]. Further prospective trials are required, including examination of the effect of rATG therapy on the capacity of DSA to bind complement fraction C1q, a variable that shows a strong correlation with risk of antibody-mediated rejection in the first year after kidney transplantation [133].
Table 3.
Univariate and multivariate analysis of association between rATG induction and risk of dnDSA and acute AMR in 114 moderately sensitized DSA-positive kidney transplant patients receiving rATG (mean total dose 4.98 mg/kg) or IL-2RA induction [32]
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| HR (95 % CI) | p-Value | HR (95 % CI) | p-Value | |
| dnDSA | 0.1 (0.02–0.48) | 0.003 | 0.16 (0.04–0.5) | 0.003 |
| Acute AMR | 0.01 (0.001–0.15) | 0.0007 | 0.16 (0.05–0.6) | 0.006 |
Reference IL-2RA induction
AMR antibody-mediated rejection, CI confidence interval, dnDSA de novo donor-specific antibody, DSA donor-specific antibody, HR hazard ratio, IL-2RA interleukin-2 receptor antagonist, rATG rabbit antithymocyte globulin
rATG in Liver Transplantation
Rejection Prophylaxis
Induction therapy is used far less frequently in liver transplant patients compared with kidney transplant recipients [1], although recent evidence that induction therapy is associated with improved long-term graft and patient survival following liver transplantation [134, 135] may contribute to greater use in the future. At present, data relating to the use of rATG after liver transplantation are relatively limited [3, 73, 136].
Induction with rATG in liver transplant patients receiving a standard triple or dual regimen has been compared with no induction in two single-center, randomized trials [111, 137] (Table 4). With follow-up to 5 years [137] or 3 months [111], there were no significant differences in acute rejection, graft or patient survival in either trial, although one trial noted a longer mean time to rejection [137] and the other reported a shorter hospital stay [111] in the rATG cohorts. Relatively large retrospective studies, in which patients have received a variety of maintenance regimens, have suggested that rATG induction may reduce the risk of acute rejection versus no induction, but poor study design limits the validity of these findings [138, 139]. Interestingly, a recent retrospective review of 112 positive cross-match liver transplant patients receiving combined induction therapy with rATG and rituximab reported low rates of acute cellular rejection (9 %) and chronic rejection (4 %), and graft survival was only slightly lower than in negative cross-match patients (85 % vs. 89 %; p = 0.26) [130]. Such an approach in this very high-risk patient group may merit further investigation. Elsewhere, another retrospective, single-center experience has reported good outcomes in liver transplant patients receiving delayed rATG in combination with a single dose of rituximab in standard-risk recipients [136]. There is also preliminary evidence (published in abstract form only) from a single-center, retrospective analysis of 89 patients that rATG may significantly reduce the rate of ischemic cholangiopathy (12.5 % vs. 35.2 % with IL-2RA induction; p = 0.017) after liver transplantation from a donor after circulatory death [140]. Ischemic cholangiopathy is a major cause of liver graft loss in patients undergoing a liver transplantation from donors after cardiac death and this potentially important finding requires examination in further studies.
Table 4.
Randomized trials of rATG (Thymoglobulin®) induction therapy in liver transplant patients
| Study (year) | N | Immunological risk status | rATG regimen | Comparator group/s | Maintenance immunosuppression | Follow-up | Treatment group |
Patient survival (%) |
Graft survival (%) |
Acute rejection (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| Boillot et al. (2009) [137] | 93 | Standard | Mean total dose 8.78 mg/kg | No induction |
Tacrolimus MMF Steroids (withdrawn after month 3) |
5 years | rATG | 77.3 | 77.3 | 11.4 |
| No induction | 87.8 | 87.8 | 14.3 | |||||||
| p-Value | NS | NS | NS | |||||||
| Bogetti et al. (2005) [111] | 22 | Standard | Total dose 4.5 mg/kg (days 0–4) | No induction |
CNI Steroids |
3 months | rATG | 100 | 100 | 25 |
| No induction | 100 | 100 | 30 | |||||||
| p-Value | NS | NS | NS | |||||||
| Eason et al. (2003) [58] | 119 | Standard | Total dose 3.0 mg/kg (days 0–1) | Steroids to month 3 (no steroids in rATG group) |
Tacrolimus MMF (withdrawn after month 3) |
2 years | rATG (steroid-free) | 85 | 82 | 25 |
| Steroids | 85 | 80 | 31.0 | |||||||
| p-Value | NS | NS | NS |
CNI calcineurin inhibitor, MMF mycophenolate mofetil, NS not significant, rATG rabbit antithymocyte globulin
Comparative trials versus IL-2RA agents or other induction therapies are lacking in liver transplant patients.
Reducing Maintenance Immunosuppression
Randomized trials of CNI reduction or avoidance in liver transplant patients using rATG induction are lacking, but retrospective, single-center data from a series of 391 recipients suggest that rATG induction with CNI initiation delayed to day 3 may reduce the risk of rejection versus a standard CNI therapy (14.5 % vs. 31.8 %; p = 0.0008) [71]. Other retrospective analyses have also indicated that rATG induction with reduced tacrolimus exposure and no steroids [63], or delayed tacrolimus [141], effectively maintains immunosuppressive efficacy. Such approaches may be particularly helpful in liver transplant patients with renal impairment at the time of transplantation, to minimize CNI-related nephrotoxicity and avoid acute renal failure to help preserve long-term kidney function [142].
In terms of steroid avoidance, a randomized trial in 119 liver transplant recipients showed that a low-dose rATG regimen (total dose 3 mg/kg) demonstrated a similar rate of acute rejection in patients receiving a steroid-free regimen versus a standard steroids protocol [58]. These promising data await confirmation in other controlled trials.
Hepatitis C Virus Recurrence
With modern maintenance regimens, and the decrease in rATG dose over time, early concerns that lymphocyte depletion with OKT3 induction could lead to a permissive environment for hepatitis C virus (HCV) replication following liver transplantation [143] have not been borne out by recent results. Registry analyses of patients receiving induction with an ATG preparation or an IL-2RA agent [135, 144], as well as in subpopulation analyses of HCV-positive patients in randomized trials [135, 145] and retrospective studies [63, 138, 141, 146], have not indicated any increase in HCV recurrence in patients receiving rATG induction.
rATG in Heart Transplantation
Rejection Prophylaxis
There is a scarcity of randomized trials relating to rATG induction, or other forms of induction therapy, in heart transplantation [77]. Comparative trials of rATG versus no induction, either prospective or retrospective, are lacking. Only two randomized trials of rATG have been published which used a contemporary dosing regimen [147, 148] (Table 5).
Table 5.
Randomized trials of rATG (Thymoglobulin®) induction therapy in heart transplant patients
| Study (year) | N | Immunological risk status | rATG regimen | Comparator group | Maintenance immunosuppression | Follow-up | Treatment group |
Patient survival (%) |
Acute rejection (%) |
|---|---|---|---|---|---|---|---|---|---|
| Yamani et al. (2008) [147] | 32 | Low | Total dose 6 mg/kg (steroid-free) | Single dose of rATG (1.5 mg/kg) |
Tacrolimus MMF Steroids only in induction-free group |
12 months | rATG | 93.8 | 50a |
| Standard steroids | 93.8 | 69a | |||||||
| p-Value | NS | NS | |||||||
| Carrier et al. (2007) [148] | 35 | Standard | Total dose 5.2 mg/kg | Basiliximab |
CsA MMF Steroids |
6 months | rATG | 78 | 17a |
| Basiliximab | 77 | 35a | |||||||
| p-Value | 0.9955 | * | |||||||
| Mattei et al. (2007) [74] | 80 | Standard | Total dose 7.5–12.5 mg/kg (mean ~8.6 mg/kg) | Basiliximab |
CsA MMF Steroids |
6 months | rATG | 78.6 | 45.2b |
| Basiliximab | 86.8 | 50.0b | |||||||
| p-Value | 0.388 | NS | |||||||
| Schnetzler et al. (2002) [18] | 50 | Standard | Mean total ~12.5 mg/kg | ATG-Fresenius |
CsA AZA Steroids |
12 months | rATG | 84.6 | 91.7c |
| ATG- Fresenius | 87.5 | 84.6c | |||||||
| p-Value | NS | NS |
aGrade ≥3
bGrade ≥1B
cAny rejection
* Non-inferiority for basiliximab was not shown
AZA azathioprine, CsA cyclosporine, MMF mycophenolate mofetil, NS not significant, rATG rabbit antithymocyte globulin
In a randomized trial of 35 standard-risk patients, the addition of rATG, at a mean total dose of 5.2 mg/kg, to a CNI-based triple regimen has been shown to achieve a lower rate of grade ≥3A acute rejection than induction with basiliximab (17 % vs. 35 %; the non-inferiority criterion for basiliximab versus rATG was not met) [148]. However, earlier high-dose, randomized [74] or retrospective trials [74, 76] and small, retrospective trials using a total rATG dose of ≤7.5 mg/kg [75, 149], have not reported a lower rejection rate compared with IL-2RA induction. A trial of 721 de novo heart transplant recipients randomized to everolimus at a dose of 1.5 mg/day or 3.0 mg/day with reduced-dose cyclosporine, or to MMF with standard-dose cyclosporine, permitted centers to chose between basiliximab or rATG induction (dosed according to local practice) [150]. A numerically higher rate of mortality was observed in the everolimus 1.5 mg group compared with the MMF cohort at month 12. The difference was largely attributed to a higher incidence of infection-related death during the first 3 months post-transplant in patients receiving everolimus with rATG, particularly if a left ventricular assist device had been used. By month 24, mortality rates were similar in the two groups. The intensity of immunosuppression in the everolimus arm (rATG, everolimus, CNI, and steroids) appears to have over-immunosuppressed patients. A lower level of CNI exposure than in this study is targeted by most centers that use rATG induction in heart transplant patients, and may be a safer approach.
Reducing Maintenance Immunosuppression
The International Society of Heart and Lung Transplantation (ISHLT) guidelines for the care of heart transplant recipients comment that rATG can allow for CNI delay due to renal insufficiency [151]. Delgado et al. [152] delayed CNI introduction after a mean of 3.2 days in seven patients receiving rATG induction, and found that renal function was preserved to last follow-up at month 6 with a lower rate of acute rejection than in patients given IL-2RA induction and there were no episodes of rejection with hemodynamic compromise. Delaying CNI initiation until serum creatinine was <150 μM with rATG induction (total dose 7.5 mg/kg) in a small series of 15 patients with postoperative renal dysfunction was associated with a similar rate of rejection as control patients, and renal function improved in the delayed-CNI cohort compared with matched controls [153]. One retrospective, single-center analysis, currently published in abstract form only, has suggested that rATG induction (total dose 4.5 mg/kg) with reduced CNI exposure and everolimus therapy in patients with moderate to severe chronic kidney disease achieves effective rejection prophylaxis with a low rate of infection, and preserves renal function [154], a regimen that merits further evaluation.
Evidence relating to a steroid-sparing role for rATG in adult heart transplant recipients is currently limited to a single, randomized trial [138]. Yamani et al. [147] undertook a single-center randomized trial in which 32 patients at low immunological risk were randomized to rATG induction (total dose 6 mg/kg) without steroids, or to a standard triple-therapy regimen with no induction (Table 5). At 1 year, the rate of acute rejection grade ≥3A was similar between groups (mean number of episodes 0.81 with rATG vs. 1.07 in the control arm). The steroid-avoidance patients showed significantly greater muscle strength and less bone loss. Preliminary evidence from a series of 70 children (median age 7.1 years) undergoing heart transplantation at a single center who were treated with rATG then a steroid-free tacrolimus-based dual therapy regimen indicated that 87 % of patients were free of rejection at 1 year, with 88 % survival, among the 50 patients who survived to the point of hospital discharge [155]. Further data are awaited.
Cardiac Allograft Vasculopathy
Experimental evidence [81–83] and preliminary evidence from liver transplantation [111] that ATG preparations may reduce ischemia-reperfusion injury, which, by limiting immunologic damage [156], could potentially attenuate cardiac allograft vasculopathy (CAV) [77]. At present, data are limited to three single-center, retrospective analyses [157–159]. In the largest of these (n = 662), 16.9 % of patients showed signs of CAV during a follow-up period of 1–5 years [157]. On multivariate analysis, rATG induction therapy was significantly predictive of freedom from CAV (risk ratio 0.634; p < 0.001) or severe CAV (risk ratio 0.277; p < 0.001). The dose of rATG was not specified. Two other single-center series have reported 10-year follow-up data [158, 159]. In one analysis by Carrier et al. [159], in which 163 patients were given rATG at a mean total dose of approximately 5.2 mg/kg, the 10-year incidence of CAV was 50 % compared with 70 % in 48 induction-free controls. In another single-center analysis, 40 patients were given rATG or an IL-2RA induction agent with a triple-therapy maintenance regimen. The rATG cohort had a lower rate of CAV at 10 years post-transplant (20 % vs. 40 %; p = 0.031) but the total rATG dose was exceptionally high (20 mg/kg) [158]. Data from patients receiving ATG-Fresenius are consistent with these findings [160, 161]. There are limited data from patients treated with ATG-Fresenius to suggest that an effect on CAV may be dose-dependent [160], but an effect using modern rATG dose levels appears possible [159] and further investigation is merited.
rATG in Lung Transplantation
Immunosuppressive protocols following lung transplantation must be modified to take into account certain organ-specific features. Maintaining adequate immunosuppression appears to be particularly important, especially during the immediate post-transplant period, due to various factors such as T-cell activation caused by donor dendritic cell activity and ongoing exposure of the lungs to endogenous antigens [162]. Acute rejection contributes to the risk of bronchiolitis obliterans syndrome (BOS) [163], the principal factor limiting long-term graft survival after lung transplantation [164]. CMV pneumonitis is also believed to contribute to the development of BOS [163, 165], heightening the potential impact of over-immunosuppression.
Retrospective analyses of data from the ISHLT registry have shown that induction therapy generally is associated with a reduced risk of BOS, presumably due to reduced rejection [162, 166, 167]. One analysis of ISHLT data [158] and one based on OPTN data [124] have indicated higher lung allograft and patient survival with induction versus no induction.
Early published studies of rATG dosing indicate that lower doses were used in lung transplant recipients than in other types of solid organ transplantation [168, 169]. In 2002, Krasinskas et al. [79] demonstrated that by using CD3+ T-cell monitoring in lung transplant patients, the total dose could be reduced by 48 % without affecting the rate of acute rejection. The first robustly designed trial to assess rATG in lung transplantation was a single-center, randomized trial of 44 single or bilateral lung transplant patients given rATG (total dose 4.5 mg/kg) or no induction, both with standard triple therapy comprising cyclosporine, azathioprine, and steroids [168]. The incidence of acute rejection was significantly reduced in the presence of rATG (23 % vs. 55 %; p = 0.03), with a non-significant reduction in the incidence of BOS (20 % with rATG vs. 38 % vs. no induction), findings that were maintained long-term [170]. The rate of post-transplant infections was similar in both arms. In a comparative trial of cyclosporine versus tacrolimus with MMF and steroids, both groups received rATG at a total dose of 7.5 mg/kg, with no marked safety concerns [169].
Few studies have compared induction regimens following lung transplantation. A randomized trial of rATG (total dose 10 mg/kg) versus daclizumab in 50 lung transplant recipients showed similar rates of rejection and BOS in both arms but a significantly higher rate of CMV infection with daclizumab, possibly due to greater CMV serology mismatching [171]. Studies assessing the feasibility of CNI- or steroid-sparing regimens using rATG induction in lung transplant patients are lacking.
rATG in Pancreas and Islet Transplantation
Randomized trials of rATG in pancreas or simultaneous pancreas kidney (SPK) transplantation are sparse. A retrospective, single-center analysis of 128 SPK procedures performed during 2001–2008 indicated a lower rate of both acute rejection overall and steroid-resistant rejection, with similar graft and patient survival rates and safety profiles, but rATG dosing and maintenance immunosuppression evolved over the study period and the findings are not necessarily applicable to current regimens [172].
The use of rATG to delay the introduction of CNI therapy was investigated in a randomized trial of 50 patients undergoing SPK transplantation [173]. Patients received either rATG (1.5 mg/kg/day for 10 days, adjusted as necessary) with delayed cyclosporine, or no induction with standard cyclosporine, both in conjunction with azathioprine and steroids. After a mean follow-up of 36 months, the incidence of acute kidney rejection episodes was lower with rATG (36 % vs. 76 %; p < 0.01), but at this dose level adverse events were more frequent with rATG (80 % vs. 40 %). No other study has assessed delayed CNI with a more contemporary rATG dose, but a recent randomized trial (published only in abstract form to date) assessed both CNI- and steroid-sparing immunosuppression in a population of 100 SPK transplant patients [174]. Induction comprised a 10-day course of rATG, at an unspecified dose. After an initial regimen of tacrolimus, low-dose steroids, and MMF, tacrolimus was replaced by sirolimus and steroids were withdrawn during days 60–90 post-transplant. At 1-year post-transplant, outcomes (rejection rates, switch of regimen, renal histology, and de novo DSA) favored the control group in whom tacrolimus and steroids were continued.
While CNI withdrawal may be inadvisable after SPK transplantation, steroid reduction in patients receiving rATG induction appears feasible. In an early prospective study of 40 SPK patients given rATG (total dose 8 mg/kg over days 1–14), steroids were withdrawn after day 6 [175]. Maintenance therapy comprised tacrolimus and MMF, or tacrolimus and sirolimus. Compared with historical controls who did not receive rATG and who continued standard steroid therapy, the rejection rate was lower in the rATG/steroid elimination group (2.5 % vs. 19.8 %; p = 0.034). Since then, retrospective studies from the mid-2000s using a variety of rATG protocols [176–178], and a more recent retrospective study in which patients received a total rATG dose of 6 mg/kg [179], have also indicated that withdrawal of steroids from a regimen of tacrolimus and MMF with or without sirolimus by the end of the first week after SPK (or pancreas after kidney transplantation) maintains immunosuppressive efficacy. There are also limited data to support rATG induction in an entirely steroid-free protocol [54] but these have not been confirmed.
In the relatively new field of islet transplantation, there is some initial positive experience with an immunosuppression regimen of rATG induction, tacrolimus, and MMF [180], and studies are ongoing [181].
rATG in Other Types of Transplantation
Intestinal Transplantation
No standard immunosuppressive regimen for intestinal or multivisceral transplant recipients has been established, but for the last 15 years rATG has been the most frequently used antibody agent in this setting [182]. The high risk of rejection following such procedures, and the high rate of graft-versus-host disease (GvHD) [183] means that more than one induction agent may be used. In one series of 27 patients undergoing intestinal transplantation at a single center, induction comprised rATG, rituximab, and steroids, with tacrolimus and tapered steroids as maintenance therapy [184]. There were eight episodes of severe infection, and two cases of steroid-responsive skin GvHD with a 1-year graft survival rate of 76 %, outcomes that would be considered good in such an immunogenic setting. An analysis of data from 211 intestinal and multivisceral procedures performed at major centers during 2006–2010 compared acute rejection and infection rates between patients receiving IL-2RA induction (daclizumab) with tacrolimus and steroids, alemtuzumab induction with tacrolimus, or rATG induction with rituximab and tacrolimus [185]. The incidence of moderate acute rejection was 0, 26.3 and 11.7 % in the three groups, respectively, but the infection rates were 62.5, 52.0 and 7.4 %, respectively, with the highest graft and patient survival rates in the rATG/rituximab/tacrolimus group due to the low rate of infection. The authors concluded that the latter regimen was optimal for balancing the risks of rejection and infection [185].
Separately, there is also intriguing preliminary evidence that pre-treatment with a single dose of rATG (5 mg/kg) followed by minimal-dose tacrolimus monotherapy may achieve partial immune tolerance after intestinal transplantation [186, 187].
Composite Tissues
Transplantation of vascularized composite tissue—for example, face and upper extremities—is still in its infancy but offers a reconstructive approach impossible by other means. More than 150 such procedures have now been performed [188], the majority of which have included rATG in the immunosuppressive protocol [189]. The first ever face allograft recipient was treated with rATG induction, and maintenance immunosuppression comprising tacrolimus, MMF, and steroids [190]. Immunosuppression was well tolerated, and despite two episodes of acute rejection during the first year, after 5 years the patient was doing well and reported normal social interactions [191]. More recent reports of near-total or full-face transplants, including rATG induction therapy, have reported similarly successful outcomes [192, 193]. rATG has also contributed to uneventful postoperative courses following human hand allografting [194], and management of steroid-resistant atypical rejection following hand transplantation [195]. Interestingly, despite high levels of immunosuppression related to the combination of rATG, tacrolimus, and MMF, the incidence of skin rejection in face or hand transplantation is much higher than for internal organ transplants. However, the incidence of chronic rejection has so far been low. The reasons for this discrepancy remain elusive [196].
Safety Profile of rATG in Solid Organ Transplantation
Historically, the two key safety concerns related to rATG therapy have been infectious complications and risk of malignancy or post-transplant lymphoproliferative disease (PTLD). Adverse events, notably fevers and chills, and hematological abnormalities such as lymphopenia, neutropenia, and thrombocytopenia, can occur but are usually managed successfully by dose adjustments. As a result of progressive decreases in the cumulative rATG dose and duration of exposure, the incidence of serum sickness is now estimated to have declined to 0.25 % in patients receiving a cumulative dose of 6 mg/kg over no more than 7 days [171], and can be managed by a combination of plasmapheresis and steroids.
Malignancy and Post-Transplant Lymphoproliferative Disease
The risk of cancer is estimated to be twice as high in solid organ transplant recipients as in the general population, with the difference most marked for infection-related malignancy diagnosis such as non-Hodgkin lymphoma [197]. Nevertheless, the incidence of PTLD following kidney transplantation is no more than 1 % at 5 years [198, 199], with lower rates for non-Hodgkin lymphoma and other common cancers [197, 200]. Assessment of the risk associated with specific immunosuppressive agents therefore requires the statistical power provided by large-scale transplant registries. Many such analyses have not specifically evaluated rATG induction therapy (and even fewer have specifically assessed Thymoglobulin®) as opposed to lymphocyte-depleting agents as a class. An analysis from 1995 to 2004, which was based on the Collaborative Transplant Study database, observed a marked increase in non-Hodgkin lymphoma with rATG induction versus non-induction [200]. During that period, rATG doses were higher than at present, and this finding is consistent with other registry analyses based solely on data from the 1990s or early 2000s (Table 6). A somewhat more recent analysis of 2,151 adult heart transplant recipients (1995–2008) receiving ATG in the UK (the preparation of ATG was not specified) found no evidence of increased death from lymphoma [201]. An analysis of patients registered with the Australian and New Zealand Dialysis and Transplantation Registry (ANZDATA) registry during 1997 to 2009 indicated that acute rejection treated with lymphocyte-depleting therapy (rATG was not specifically assessed) appears to be associated with an increased risk of cancer [206]. The increase was due to more genitourinary tract cancers but not other site-specific malignancies or PTLD [206]. The authors point out that acute rejection may share a common causal pathway with malignancy, making it difficult to distinguish the contribution of rATG to such an effect, and results of other long-term analyses are awaited [207].
Table 6.
Safety data reported in registry analyses of transplant patients receiving ATG as induction therapy
| Study (year) | Organ | Registry (years of transplant) | Induction | N | Follow-up | Safety outcome |
|---|---|---|---|---|---|---|
| Neoplasms | ||||||
| Emin et al. (2011) [201] | Heart | UK Cardiothoracic Transplant Audit | ATG (unspecified formulation) | 2,086 | 10 years | Similar rate of death from lymphoid malignancy (1.0 % vs. 1.4 %; p = 0.38) or non-lymphoid malignancy (3.9 % vs. 2.8 %; p = 0.40) with ATG vs. no induction |
| No induction | ||||||
| Gajarski et al. (2011) [202] | Heart (pediatric) | Pediatric Heart Transplant Study | rATG (Thymoglobulin®) | 2,374 | 5 years | No significant association between rATG and risk of lymphoma on multivariate analysis |
| No induction | ||||||
| Kirk et al. (2007) [198] | Kidney | Organ Procurement and Transplant Network (2000–2004) | rATG (Thymoglobulin®) | 13,110 | ≤730 days | Relative risk of PTLD for rATG vs. no induction 1.63 (95 % CI 1.19–2.24; p = 0.003) |
| Opelz et al. (2006) [107] | Kidney |
Collaborative Transplant Study (1995–2004) |
rATG (Thymoglobulin®) | 1,875 | 3 years |
Relative risk of non-Hodgkin lymphoma vs. no inductiona: rATG 21.1 ATG-Fresenius 3.0 ATGAM 22.1 OKT3 31.3 IL-2RA 7.4 |
| ATG-Fresenius | 856 | |||||
| ATGAM | 440 | |||||
| OKT3 | 1,760 | |||||
| IL-2RA | 6,209 | |||||
| No induction | 23,066 | |||||
| Bustami et al. (2004) [203] | Kidney | Scientific Registry of Transplant Recipients (1995–2002) | rATG (unspecified) | Not stated | 0–4 years |
Relative risk of de novo solid tumor 1.53 (93 % CI 0.92–2.56; p = 0.10) Relative risk of PLTD for rATG vs. no induction 3.00 (95 % CI 1.53–5.89; p = 0.001) |
| No induction | Not stated | |||||
| Infections | ||||||
| Emin et al. (2011) [201] | Heart | UK Cardiothoracic Transplant Audit | ATG (unspecified formulation) | 2,086 | 1 year | Adjusted risk of infection for ATG vs. no induction 1.21 (95 % CI 1.02–1.44; p = 0.027) |
| No induction | ||||||
| Gajarski et al. (2011) [202] | Heart (pediatric) | Pediatric Heart Transplant Study | rATG (Thymoglobulin®) | 2,374 | 5 years | No significant association between rATG and risk of viral, fungal, or bacterial infection |
| No induction | ||||||
| Dharnidharka et al. (2009) [204] | Kidney | Organ Procurement and Transplant Network (2003–2006) | rATG (Thymoglobulin®) | 16,746 | 2 years | Adjusted HR for treated BKV infection with rATG vs. no induction 1.42 (95 % CI 1.24–1.63; p < 0.001) |
| No induction | 13,050 | |||||
| Schold et al. (2009) [205] | Kidney | Scientific Registry of Transplant Recipients (2004–2006) | rATG (Thymoglobulin®) | Not stated | 1 year | Adjusted OR for treated BKV infection with rATG vs. IL-2RA induction 1.23 (95 % CI 1.03–1.45) |
| IL-2RA | Not stated | |||||
| Non-comparative analyses | ||||||
| Gaber et al. (2012) [68] | Kidney | United Network for Organ Sharing (2003–2008) | rATG (Thymoglobulin®) | 2,322 | Hospital discharge | 0.005 % serious adverse events possibly or probably related to rATG |
| 1 year | Incidence of CMV infection 4.2 % | |||||
| 5 years | Incidence of PTLD 0.9 % | |||||
ATG antithymocyte globulin, ATGAM equine ATG, BKV BK polyomavirus, CI confidence interval, CMV cytomegalovirus, HR hazard ratio, IL-2RA interleukin-2 receptor antagonist, OKT3 muromonab-CD3, OR odds ratio, PTLD post-transplant lymphoproliferative disorder, rATG rabbit antithymocyte globulin
aStandardized incidence risk compared to a non-transplant control population
A systematic review of trials of rATG (Thymoglobulin®), published during 1999–2009, in kidney and heart transplant recipients (n = 2,246) with at least 3 years’ follow-up recently concluded that the cumulative dose showed no association with risk of PTLD [208]. The overall incidence of PTLD was 0.98 % and 1.05 % in kidney and heart transplant patients, respectively. Evidence from registry studies regarding the risk of PTLD in patients receiving rATG is difficult to interpret since only one has specifically assessed Thymoglobulin®, reporting an increased risk versus no induction for patients transplanted during 2000–2004 [198]. Other registry analyses have described mixed findings, with some showing an increased risk for PTLD with rATG or ATG preparations of any type [199, 203, 209, 210] and others reporting no effect of rATG or polyclonal lymphocyte-depleting agents [211–213]. In one of the most recent large-scale datasets, derived from the TAILOR registry, a non-comparative evaluation of PTLD in 2,322 patients receiving rATG (Thymoglobulin®) after living-donor kidney transplantation during 2003–2008 showed the 5-year incidence of PTLD to be 0.9 % [68]. This is comparable with published incidence rates for the kidney transplant population as a whole [198, 199]. Unlike most registries, TAILOR captures the rATG dose, and in this series the mean cumulative dose was ~5.3 mg/kg. It appears that the risk of PTLD and malignancy associated with modern rATG induction regimens may be less of a concern than during the high-dose era, but confirmatory data are required. However, close monitoring remains mandatory and includes both short-term evaluation of blood T-cell depletion and longer-term assessment of immune reconstitution [9].
Infections
Concerns about infectious complications associated with rATG therapy focus on viral infections, most notably CMV infection. Randomized trials of rATG induction versus no induction [16, 17], published in the early 2000s, reported a higher rate of CMV infection in kidney transplant patients receiving rATG [16, 17]. In these studies, the dose of rATG was relatively high by today’s standards (12.5 mg/kg), and CMV prophylaxis was not specified. Randomized comparative trials of rATG versus IL-2RA induction have shown mixed results [24, 92, 93, 121]. The differences are largely due to the fact that some trials included systematic CMV prophylaxis while others did not, and because of variations in the incidence of rejection and the consequent requirement for increased immunosuppression. Higher rATG doses appear to increase CMV infection rates compared with IL-2RA induction [47, 91]. In a United Network for Organ Sharing (UNOS) analysis of 2,322 patients undergoing kidney transplantation with rATG induction during 2003–2008, a period when rATG dose was declining, the CMV infection rate was reported to be only 4.2 % [68]. It would appear that with contemporary rATG dosing regimens, and wider use of CMV prophylaxis therapy, the risk of increased CMV infections in rATG-treated kidney transplant patients has diminished but cannot be discounted.
Data in liver transplantation, while limited, do not indicate a higher rate of CMV infections using contemporary rATG regimens [73]. Randomized trials [74, 147] and retrospective analyses [75, 76] in heart transplant recipients have shown no difference in rates of infection overall, or CMV infection specifically, when rATG was compared with IL-2RA induction. A large observational study in pediatric heart transplant recipients found that rATG induction appeared to be associated with a lower rate of infection [202] (Table 6), presumably due to a reduced requirement for rejection treatment or high-dose maintenance therapy. rATG-treated patients should receive prophylactic antiviral therapy, an approach that is typically considered mandatory in donor-positive, recipient-negative transplants.
A more recent potential safety issue to emerge is whether rATG therapy could impact on the risk of BK virus infection after kidney transplantation. Two registry analyses of patients in the US who received a kidney transplant during 2003–2006 [203] or 2004–2006 [205] have suggested that rATG induction is associated with a higher rate of treatment for BK virus infection. These retrospective data are consistent with results from a single-center, prospective study during 2001–2003 in which rATG dose was 7.5 mg/kg, which reported a higher risk of BK virus infection with rATG induction versus IL-2RA induction [214]. A more recent retrospective, single-center analysis, which did not specify rATG dose, found no relationship between rATG administration and risk of BK virus infection on multivariate analysis [215]. Generally, BK virus infection is considered to be associated with any intensive immunosuppressive regimen rather than a specific agent [216], and no direct link to rATG therapy has been established [217].
Monitoring for CMV infection for at least 3 months, with at least 6 months’ screening for BK virus infection and 1 year for Epstein-Barr virus (EBV) infection, with risk-adjusted prophylaxis for CMV and pneumocystis infection has been recommended in patients receiving rATG, especially when used to treat steroid-resistant rejection [218].
rATG in Hematopoietic Stem Cell Transplantation
Since the early days of HSCT, rATG was thought to be an effective agent for treating and preventing GvHD. Following evidence of improved GvHD prophylaxis with rATG versus other antibody therapies [219], it has since been widely adopted within conditioning regimens before allogeneic HSCT from an unrelated donor. rATG has been included in the conventional myeloablative conditioning regimens, administered prior to HSCT to facilitate engraftment. In more recent years a role for rATG has also been established in less aggressive non-myeloablative and reduced-intensity conditioning regimens [3].
rATG in Myeloablative Conditioning Regimens
An early randomized trial in patients with hematological malignancy undergoing unrelated HSCT reported significant protection against extensive chronic GvHD in rATG-treated patients versus controls (Fig. 2) [220, 221]. In particular, the study demonstrated a highly significant reduction in chronic lung dysfunction, a severe and often fatal complication of HSCT, in patients receiving rATG compared with controls (19 % vs. 51 %; p = 0.005). However, reduction of acute GvHD appeared to require high rATG dose (15 mg/kg) [220]. Subsequently, further studies confirmed significantly better outcomes with rATG versus controls following unrelated [222–225] or matched-related [224, 226, 227] HSCT, bone marrow or cord blood cell transplantation in terms of chronic GvHD, relapse, and mortality. A retrospective analysis of 110 patients undergoing HSCT for the treatment of β-thalassemia major (all but six of whom received a graft from a related donor) transplanted over the period 1985–2007 at 21 centers in France showed a significant association between rATG as part of the conditioning regimen versus no rATG in terms of thalassemia-free survival (55.1 % vs. 82.5 %; p = 0.002) [228]. In a recent prospective study, 47 patients undergoing unrelated HSCT received rATG at a dose of 4.5 mg/kg (1.5 mg/kg/day on days −3, −2 and −1) with tacrolimus and sirolimus for the prevention of acute GvHD [229]. At 2 years, the incidences of acute graft vascular disease (GVD) and chronic GvHD were 23.4 and 33.0 %, respectively, and the regimen was well tolerated. Typically, rATG is now given at a total dose ranging between 4.5 and 7.5 mg/kg within myeloablative conditioning regimens [224, 226, 230].
Fig. 2.
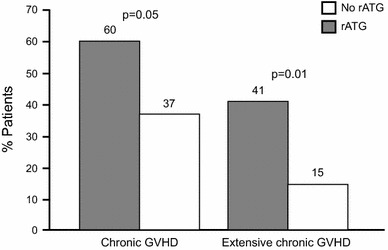
Chronic GvHD in patients with hematological malignancy undergoing unrelated HSCT randomized to rATG or no rATG [221]. GvHD graft-versus-host disease, HSCT hematopoietic stem cell transplantation, rATG rabbit antithymocyte globulin
Initial experience in umbilical cord transplantation, an alternative to conventional allogeneic stem cell transplantation, suggests that the use of rATG as part of the pre-transplant conditioning regimen may improve outcomes and ameliorate GvHD [231].
rATG in Reduced-Intensity Conditioning
The optimal dose and administration schedule for rATG within reduced-intensity conditioning to prevent GvHD in patients undergoing HSCT is less well defined. Trials in the early 2000s used a total rATG dose of 7.5–10.0 mg/kg [232, 233] but lower doses later proved to be more relevant. A randomized trial compared two doses of rATG (total dose 4.5 mg/kg and 7.5 mg/kg) within a reduced-intensity regimen in 20 recipients of HSCT from an unrelated donor and found no difference in the incidence of acute or chronic GvHD, or any other efficacy endpoint, at 100 days [234]. The authors concluded that an rATG dose of 4.5 mg/kg was preferable, and a high rATG dose may be unfavorable. A retrospective analysis of reduced-intensity conditioning in 110 consecutive recipients of HSCT from matched unrelated donors found that an rATG total dose of 6 mg/kg was associated with improved results compared with 8 mg/kg; the lower dose predicted improved relapse-free survival on multivariate analysis [235]. Depending on the adjunctive conditioning regimen, relatively low doses have proved effective. A randomized, multicenter study has compared two reduced-intensity conditioning regimens, one with and one without rATG [236]. One hundred and thirty-nine patients with hematological malignancies received HSCT from an HLA-identical sibling. All patients received conditioning with fludarabine, and were randomized to either receive rATG at a low total dose (2.5 mg/kg) with busulfan, or no rATG with total body irradiation (TBI), with a median follow-up of 54 months. The cohort randomized to rATG and busulfan had a higher rate of acute GvHD (47 % vs. 27 %; p = 0.01) and non-relapse mortality (38 % vs. 22 %; p = 0.027) but no difference in chronic GvHD and a lower relapse rate (27 % vs. 54 %; p < 0.01). At 5 years’ follow-up, all outcomes were similar between groups (Fig. 3) [236]. The authors commented that rATG with busulfan offers greater disease control than low-dose TBI, although this did not translate to improved survival. In a small series of 19 children undergoing transplantation for non-malignant indications (12 mismatched unrelated donors, 7 unrelated donors), a regimen of rATG, busulfan, and fludarabine was associated with excellent survival but with a high graft failure rate (21 %) accounted for by the unrelated donor cohort [237].
Fig. 3.
Five-year outcomes from a randomized trial of patients with hematologic malignancies receiving reduced-intensity conditioning with fludarabine and either rATG (2.5 mg/kg) with busulfan (n = 69) or total body irradiation (n = 70) prior to HSCT from an HLA-identical sibling [236]. HLA human leukocyte antigen, HSCT hematopoietic stem cell transplantation, rATG rabbit antithymocyte globulin
rATG as Pre-Emptive Treatment of Acute Graft-versus-Host Disease
Patients at high risk of GvHD and death can be identified on day 7 after HSCT based on laboratory data [238]. In a prospective, randomized trial, all patients grafted from unrelated donors received rATG 7.5 mg/kg pre-transplant [238]. On day +7, they were assigned to receive an additional rATG dose of 1.25 mg/kg/day on days 7 and 9 post-transplant, or enter a control arm [239]. One hundred and seventy patients were randomized and were evaluable for non-relapse-related mortality (the primary endpoint) or GvHD (a secondary endpoint). Non-relapse-related mortality was 29 % in the rATG-treated patients versus 35 % in the control arm (p = 0.3). GvHD was significantly less frequent under rATG therapy; the incidence of acute GvHD III–IV was 5 % versus 15 % in controls (p = 0.02), and the incidence of extensive chronic GvHD was 11 % versus 27 % (p = 0.03). Rates of relapse and survival were comparable. These results indicate that rATG can be given to high-risk patients before and after an alternative donor stem cell transplant, and this approach further mitigates acute and chronic GvHD without interfering with the graft versus leukemia (GvL) effect. This strategy can be referred to as pre-emptive therapy of GvHD.
Although the drug is approved in this setting, treatment of established severe acute GvHD has been less satisfactory. This is especially true in steroid-refractory cases, where no agent has been shown to be superior to steroids alone. In a prospective, randomized trial, the combination of rATG and steroids did not prove superior to steroids alone for the therapy of steroid refractory acute GvHD [240]. Nevertheless rATG is commonly used in the management of acute GvHD [241], although an early use (pre-emptive) has been shown to be more effective than later on in the course of the disease.
Epstein-Barr Virus Reactivation and Lymphoproliferative Diseases
The risk of EBV reactivation is increased in patients receiving high doses of ATG in the conditioning regimen [242], and may lead to potentially life-threatening lymphoproliferative disorders [242]. Currently, this complication can be prevented through close and regular monitoring of EBV reactivation and use of pre-emptive therapy with rituximab. A low dose of anti-CD20 antibody (rituximab) administered on day +5 after HSCT may also prevent this complication [243]. The risk of EBV reactivation after HSCT should be appreciated, and prevented or treated appropriately.
rATG in Autoimmunity
Severe Aplastic Anemia
The role of ATG in conditioning regimens for HSCT in severe aplastic anemia has been the topic of several studies. In a prospective, randomized study performed in the US, patients receiving ATG had comparable survival to controls [244]. In contrast, a recent report from the European Group of Blood and Marrow Transplantation (EBMT) observed that patients with severe aplastic anemia receiving ATG had significantly superior survival compared with controls [245]. In another EBMT study, 100 patients with severe aplastic anemia all received rATG as part of the conditioning regimen, together with fludarabine and cyclophosphamide (FCA), with or without low-dose TBI [246]. Actuarial survival was 73 % for rATG with FCA and 79 % with FCA-TBI. For patients grafted within 2 years of diagnosis, the overall survival rate was 87 %.
While HSCT is the treatment of choice for acquired aplastic anemia, ATG with cyclosporine may be an effective option for patients with severe disease who do not have a matched sibling donor or whose age (>50 years) makes transplantation an unsuitable option [247–249]. The majority of data in this setting relates to eATG, with rATG reserved for second-line therapy in the event of non-response to eATG or relapse [249]. eATG is now available only in some markets. Data for rATG as first-line immunosuppressive therapy remain relatively limited [250–254]. A prospective pilot study comparing rATG with cyclosporine as first-line treatment for 35 patients with aplastic anemia versus 105 matching controls treated with eATG and cyclosporine observed higher 2-year overall survival rates with eATG (86 % vs. 68 %; p = 0.009) and higher transplant-free survival (76 % vs. 52 %; p = 0.002). Another smaller, prospective, non-randomized study observed a non-significant difference in response rates that was numerically in favor of eATG [250]. A recent published report from a single-center study in Japan found similar response rates with either rATG (75 %) or eATG (67 %), based on an rATG dose of 12.5 mg/kg. A prospective, randomized trial performed at the National Institute of Health in the US indicated that rATG is inferior in terms of response and survival compared with eATG [255]. The trial enrolled 120 patients (60 in each group) and the response rate at 6 months was 68 % with eATG versus 37 % with rATG (p < 0.001), associated with a borderline advantage in survival (p = 0.04).
Other Autoimmune Disorders
The EBMT has developed several trials of autografts in patients with autoimmune disorders, including multiple sclerosis, Crohn’s disease, systemic sclerosis, and systemic lupus erythematosus [256]. These trials are based on the hypothesis that high-dose therapy combined with rATG can ‘reset’ the immune system and induce long-term remission. rATG is typically given at the time autologous stem cells are re-infused, in combination with high-dose cyclophosphamide (200 mg/kg) or high-dose combination chemotherapy (BEAM). This hypothesis has been proven in several phase II trials, and very recently in three prospective, randomized trials which compared this procedure with best available treatment—the ASTIM study in multiple sclerosis [257], ASTIC for Crohn’s Disease, and ASTIS for systemic sclerosis. The EBMT registry of autoimmune disorders now has data on 1,700 patients, and the field is moving fast, with increasing numbers of patients being treated following the positive results of these randomized trials.
Pre-Transplant rATG
Pre-transplant rATG is now considered the standard of care for patients undergoing an unrelated donor–donor transplant. It has been proven to reduce the incidence of acute and especially chronic GvHD, without interfering significantly with the so-called GvL effect. Survival of patients receiving rATG prior to transplant is comparable to patients not given rATG, but with the highly important difference of less chronic GvHD for rATG-treated patients, which has a major impact on quality of life, the duration of immunosuppressive therapy, number of hospital admissions, and cost of treatment. The use of rATG post-transplant in a subgroup of high-risk patients can further reduce the risk of GvHD.
Induction of Tolerance
Patients undergoing solid organ transplantation require life-long immunosuppressive therapy, with its associated side effects and complications, including reduced quality of life and chronic graft rejection. rATG induces regulatory NK T-cells (CD161+, CD3+), particularly when combined with total lymphoid irradiation (TLI) [258]. The Stanford group has developed a conditioning regimen of TLI 800 rads in 10 fractions, combined with rATG 7.5 mg/kg (1.5 mg/kg/day for five doses), and followed by allogeneic hematopoietic stem cells with cyclosporine and MMF [233]. This regimen results in a high rate of complete donor chimerism, with little or no GvHD. The same group has since developed a program of combined HSCT and renal transplantation from HLA-identical family donors, the endpoint being discontinuation of immunosuppression. Initial results are encouraging [259]. More patients have been transplanted since 2008 and results remain promising, but work remains in its early stages.
Conclusions
Specific roles for rATG in modern immunosuppressive regimens are now relatively well-identified. In solid organ transplantation, the addition of rATG induction to a standard triple or dual regimen is an effective strategy to reduce acute cellular rejection, and possibly humoral rejection. It is an appropriate first choice in patients with moderate or high immunological risk. In patients at low risk, rATG induction may be used if a CNI-sparing regimen is administered from time of transplant, or if early steroid withdrawal is planned. Kidney transplant patients at risk of DGF may also benefit from the use of rATG to facilitate delayed CNI introduction.
rATG also has an established position in HSCT, as an agent which can reduce the risk of both acute and chronic GvHD. In addition to its current applications in solid organ transplantation, there is growing interest in the potential to harness the biological effects of rATG beyond T-cell depletion. The use of rATG combined with stem cell transplantation with the ultimate goal of achieving operational tolerance after allografting shows promise, although clinical data to date is highly preliminary [3, 260–263]. The broad spectrum of rATG activity means a possible role for rATG in new therapeutic areas is being assessed. These include rATG induction with autologous HSCT in patients with type 1 diabetes, which may improve β-cell function and improve glycemic control [264–266], myelodysplastic syndrome in patients with aplastic anemia [267, 268] and B-cell-mediated autoimmune diseases such as lupus erythematosus [269, 270].
Despite the long history of its use, rATG remains a key component of the immunosuppressive armamentarium, and its intriguing properties indicate that its use is to expand to a wider range of immunological conditions in the future.
Acknowledgments
The authors would like to thank Caroline Dunstall for editorial assistance supported by a grant from Sanofi. No honoraria were provided to the authors for their participation. The authors would like to apologize for those colleagues whose work could not be cited for reasons of space.
Conflicts of interest
Mohamad Mohty has received research support and speaker’s honoraria from Sanofi, whose product is discussed in this manuscript. Andrea Bacigalupo is a member of the speakers’ bureau for Sanofi-Genzyme. Faouzi Saliba has received speaker’s fees from Sanofi-Genzyme. Andreas Zuckermann has received research funding and is a member of an advisory board for Sanofi-Genzyme. Emmanuel Morelon has received speaker’s honoraria and research funding from Sanofi-Genzyme. Yvon Lebranchu has received speaker’s honoraria and research funding from Sanofi-Genzyme.
References
- 1.Organ Procurement and Transplant Network (OPTN) Annual Report 2011. http://srtr.transplant.hrsa.gov/annual_reports/2011/default.aspx. Accessed 14 Jan 2014.
- 2.Cai J, Terasaki P. The current trend of induction and maintenance treatment in patients of different PRA levels: a report on OPTN/UNOS Kidney Transplant Registry data. Clin Transplant 2010;45–52. [PubMed]
- 3.Gaber AO, Monaco AP, Russell JA, Lebranchu Y, Mohty M. Rabbit antithymocyte globulin (thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs. 2010;70:691–732. doi: 10.2165/11315940-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Büchler M, Longuet H, Lemoine R, Herr F, Gatault P, Thibault G, et al. Pharmacokinetic and pharmacodynamic studies of two different rabbit antithymocyte globulin dosing regimens: results of a randomized trial. Transpl Immunol. 2013;28:120–126. doi: 10.1016/j.trim.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Lindemans CA, Chiesa R, Amrolia PJ, Rao K, Nikolejava O, de Wildt A, et al. Impact of thymoglobulin prior to pediatric unrelated umbilical cord blood transplantation on immune reconstitution and clinical outcome. Blood. 2014;123:126–132. doi: 10.1182/blood-2013-05-502385. [DOI] [PubMed] [Google Scholar]
- 6.Morelon E, Lefrançois N, Besson C, Prévautel J, Brunet M, Touraine JL, et al. Preferential increase in memory and regulatory subsets during T-lymphocyte immune reconstitution after Thymoglobulin induction therapy with maintenance sirolimus vs cyclosporine. Transpl Immunol. 2010;23:53–58. doi: 10.1016/j.trim.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Longuet H, Sautenet B, Gatault P, Thibault G, Barbet C, Marliere JF, et al. Risk factors for impaired CD4+ T-cell reconstitution following rabbit antithymocyte globulin treatment in kidney transplantation. Transpl Int. 2014;27(3):271–9. doi:10.1111/tri.12249. [DOI] [PMC free article] [PubMed]
- 8.Thiyagarajan UM, Ponnuswamy A, Bagul A. Thymoglobulin and its use in renal transplantation: a review. Am J Nephrol. 2013;37:586–601. doi: 10.1159/000351643. [DOI] [PubMed] [Google Scholar]
- 9.Mourad G, Morelon E, Noël C, Glotz D, Lebranchu Y. The role of Thymoglobulin induction in kidney transplantation: an update. Clin Transplant. 2012;26:E450–E464. doi: 10.1111/ctr.12021. [DOI] [PubMed] [Google Scholar]
- 10.Deeks ED, Keating GM. Rabbit antithymocyte globulin (thymoglobulin): a review of its use in the prevention and treatment of acute renal allograft rejection. Drugs. 2009;69:1483–1512. doi: 10.2165/00003495-200969110-00007. [DOI] [PubMed] [Google Scholar]
- 11.Popow I, Leitner J, Grabmeier-Pfistershammer K, Majdic O, Zlabinger GJ, Kundi M, et al. A comprehensive and quantitative analysis of the major specificities in rabbit antithymocyte globulin preparations. Am J Transplant. 2013;13:3103–3113. doi: 10.1111/ajt.12514. [DOI] [PubMed] [Google Scholar]
- 12.Ternant D, Büchler M, Thibault G, Ohresser M, Watier H, Lebranchu Y, et al. Influence of FcγRIIIA genetic polymorphism on T-lymphocyte depletion induced by rabbit antithymocyte globulins in kidney transplant patients. Pharmacogenet Genomics. 2014;24:26–34. doi: 10.1097/FPC.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 13.Gaber AO, First MR, Tesi RJ, Gaston RS, Mendez R, Mulloy LL, et al. Results of the double-blind, randomized, multicenter, phase III clinical trial of Thymoglobulin versus Atgam in the treatment of acute graft rejection episodes after renal transplantation. Transplantation. 1998;66:29–37. doi: 10.1097/00007890-199807150-00005. [DOI] [PubMed] [Google Scholar]
- 14.Mariat C, Alamartine E, Diab N, de Filippis JP, Laurent B, Berthoux F. A randomized prospective study comparing low-dose OKT3 to low-dose ATG for the treatment of acute steroid-resistant rejection episodes in kidney transplant recipients. Transpl Int. 1998;11:231–236. doi: 10.1007/s001470050133. [DOI] [PubMed] [Google Scholar]
- 15.Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. A randomized, double-blinded comparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67:1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 16.Mourad G, Garrigue V, Squifflet JP, Besse T, Berthoux F, Alamartine E, et al. Induction versus noninduction in renal transplant recipients with tacrolimus-based immunosuppression. Transplantation. 2001;72:1050–1055. doi: 10.1097/00007890-200109270-00012. [DOI] [PubMed] [Google Scholar]
- 17.Charpentier B, Rostaing L, Berthoux F, Lang P, Civati G, Touraine JL, et al. A three-arm study comparing immediate tacrolimus therapy with antithymocyte globulin induction therapy followed by tacrolimus or cyclosporine A in adult renal transplant recipients. Transplantation. 2003;75:844–851. doi: 10.1097/01.TP.0000056635.59888.EF. [DOI] [PubMed] [Google Scholar]
- 18.Schnetzler B, Leger P, Völp A, Dorent R, Pavie A, Gandjbakhch I. A prospective randomized controlled study on the efficacy and tolerance of two antilymphocytic globulins in the prevention of rejection in first-heart transplant recipients. Transpl Int. 2002;15:317–325. doi: 10.1007/s00147-002-0418-9. [DOI] [PubMed] [Google Scholar]
- 19.Agha IA, Rueda J, Alvarez A, Singer GG, Miller BW, Flavin K, et al. Short course induction immunosuppression with thymoglobulin for renal transplant recipients. Transplantation. 2002;73:473–475. doi: 10.1097/00007890-200202150-00025. [DOI] [PubMed] [Google Scholar]
- 20.Goggins WC, Pascual MA, Powelson JA, Magee C, Tolkoff-Rubin N, Farrell ML, et al. A prospective, randomized, clinical trial of intraoperative versus postoperative Thymoglobulin in adult cadaveric renal transplant recipients. Transplantation. 2003;76:798–802. doi: 10.1097/01.TP.0000081042.67285.91. [DOI] [PubMed] [Google Scholar]
- 21.Wong W, Agrawal N, Pascual M, Anderson DC, Hirsch HH, Fujimoto K, et al. Comparison of two dosages of thymoglobulin used as a short-course for induction in kidney transplantation. Transpl Int. 2006;19:629–635. doi: 10.1111/j.1432-2277.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 22.Stevens RB, Mercer DF, Grant WJ, Freifeld AG, Lane JT, Groggel GC, et al. Randomized trial of single-dose versus divided-dose rabbit anti-thymocyte globulin induction in renal transplantation: an interim report. Transplantation. 2008;82:1391–1399. doi: 10.1097/TP.0b013e3181722fad. [DOI] [PubMed] [Google Scholar]
- 23.Djamali A, Turc-Baron C, Portales P, Leverson G, Chong G, Clot J, et al. Low dose antithymocyte globulins in renal transplantation: daily versus intermittent administration based on T-cell monitoring. Transplantation. 2000;69:799–805. doi: 10.1097/00007890-200003150-00021. [DOI] [PubMed] [Google Scholar]
- 24.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D, Thymoglobulin Induction Study Group Thymoglobulin Induction Study Group. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 25.Zaltzman JS, Paul LC. Single center experience with thymoglobulin in renal transplantation. Transplant Proc. 1997;29:27S–28S. [PubMed] [Google Scholar]
- 26.Büchler M, Hurault de Ligny B, Madec C, Lebranchu Y, French Thymoglobuline Pharmacovigilance Study Group Induction therapy by anti-thymocyte globulin (rabbit) in renal transplantation: a 1-yr follow-up of safety and efficacy. Clin Transplant. 2003;17:539–545. doi: 10.1046/j.1399-0012.2003.00102.x. [DOI] [PubMed] [Google Scholar]
- 27.Guttmann RD, Flemming C. Sequential biological immunosuppression. Induction therapy with rabbit antithymocyte globulin. Clin Transplant. 1997;11:185–192. [PubMed] [Google Scholar]
- 28.Haririan A, Morawski K, Sillix DH, El-Amm JM, Garnick J, West MS, et al. Induction therapy with basiliximab versus Thymoglobulin in African-American kidney transplant recipients. Transplantation. 2005;79:716–721. doi: 10.1097/01.tp.0000153506.07816.f0. [DOI] [PubMed] [Google Scholar]
- 29.Thibaudin D, Alamartine E, de Filippis JP, Diab N, Laurent B, Berthoux F. Advantage of antithymocyte globulin induction in sensitized kidney recipients: a randomized prospective study comparing induction with and without antithymocyte globulin. Nephrol Dial Transplant. 1998;13:711–715. doi: 10.1093/ndt/13.3.711. [DOI] [PubMed] [Google Scholar]
- 30.Rostaing L, Lavayssière L, Kamar N. Hematologic adverse effects of 2 different polyclonal antilymphocyte preparations in de novo kidney transplant patients. Exp Clin Transplant. 2010;8:178–180. [PubMed] [Google Scholar]
- 31.Kim JM, Jang HR, Ko JS, Kwon CH, Kwak MS, Hur WS, et al. Comparison between thymoglobulin and ATGAM as an induction agent in adult kidney transplantation: a single-center experience. Transplant Proc. 2012;44:171–174. doi: 10.1016/j.transproceed.2011.11.061. [DOI] [PubMed] [Google Scholar]
- 32.Brokhof MM, Sollinger HW, Hager DR, Muth BL, Pirsch JD, Fernandez LA, et al. Antithymocyte globulin is associated with a lower incidence of de novo donor-specific antibodies in moderately sensitized renal transplant recipients. Transplantation. 2014;97:612–617. doi: 10.1097/TP.0000000000000031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaMattina JC, Mezrich JD, Hofmann RM, Foley DP, D’Alessandro AM, Sollinger HW, et al. Alemtuzumab as compared to alternative contemporary induction regimens. Transpl Int. 2012;25:518–526. doi: 10.1111/j.1432-2277.2012.01448.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Requião-Moura LR, Ferraz E, Matos AC, Tonato EJ, Ozaki KS, Durão MS, et al. Comparison of long-term effect of thymoglobulin treatment in patients with a high risk of delayed graft function. Transplant Proc. 2012;44:2428–2433. doi: 10.1016/j.transproceed.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 35.Libório AB, Mendoza TR, Esmeraldo RM, Oliveira ML, Paes FJ, Silva Junior GB, et al. Induction antibody therapy in renal transplantation using early steroid withdrawal: long-term results comparing anti-IL2 receptor and anti-thymocyte globulin. Int Immunopharmacol. 2011;11:1832–1836. doi: 10.1016/j.intimp.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Kandaswamy R, Melancon JK, Dunn T, Tan M, Casingal V, Humar A, et al. A prospective randomized trial of steroid-free maintenance regimens in kidney transplant recipients–an interim analysis. Am J Transplant. 2005;5:1529–1536. doi: 10.1111/j.1600-6143.2005.00885.x. [DOI] [PubMed] [Google Scholar]
- 37.Birkeland SA. Steroid-free immunosuppression in renal transplantation: a long-term follow-up of 100 consecutive patients. Transplantation. 2001;71:1089–1090. doi: 10.1097/00007890-200104270-00013. [DOI] [PubMed] [Google Scholar]
- 38.Khwaja K, Asolati M, Harmon JV, Melancon JK, Dunn TB, Gillingham KJ, et al. Rapid discontinuation of prednisone in higher-risk kidney transplant recipients. Transplantation. 2004;78:1397–1399. doi: 10.1097/01.tp.0000136964.59494.ff. [DOI] [PubMed] [Google Scholar]
- 39.Matas AJ, Kandaswamy R, Gillingham KJ, McHugh L, Ibrahim H, Kasiske B, et al. Prednisone-free maintenance immunosuppression: a 5-year experience. Am J Transplant. 2005;5:2473–2478. doi: 10.1111/j.1600-6143.2005.01051.x. [DOI] [PubMed] [Google Scholar]
- 40.Heilman RL, Reddy KS, Mazur MJ, Moss AA, Post DJ, Petrides S, et al. Acute rejection risk in kidney transplant recipients on steroid-avoidance immunosuppression receiving induction with either antithymocyte globulin or basiliximab. Transplant Proc. 2006;38:1307–1313. doi: 10.1016/j.transproceed.2006.02.116. [DOI] [PubMed] [Google Scholar]
- 41.Woodle ES, First MR, Pirsch J, Shihab F, Gaber AO, Van Veldhuisen P. Astellas Corticosteroid Withdrawal Study Group. A prospective, randomized, double-blind, placebo-controlled multicenter trial comparing early (7 day) corticosteroid cessation versus long-term, low-dose corticosteroid therapy. Ann Surg. 2008;248:564–577. doi: 10.1097/SLA.0b013e318187d1da. [DOI] [PubMed] [Google Scholar]
- 42.Gruber SA, West MS, Sillix DH, El-Amm JM, Garnick J, Morawski K, et al. Preliminary results with early corticosteroid withdrawal in African American renal allograft recipients. Surgery. 2005;138:772–778. doi: 10.1016/j.surg.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 43.Haririan A, Sillix DH, Morawski K, El-Amm JM, Garnick J, Doshi MD, et al. Short-term experience with early steroid withdrawal in African-American renal transplant recipients. Am J Transplant. 2006;6:2396–2402. doi: 10.1111/j.1600-6143.2006.01477.x. [DOI] [PubMed] [Google Scholar]
- 44.Jaber JJ, Feustel PJ, Elbahloul O, Conti AD, Gallichio MH, Conti DJ. Early steroid withdrawal therapy in renal transplant recipients: a steroid-free sirolimus and Cell Cept-based calcineurin inhibitor-minimization protocol. Clin Transplant. 2007;21:101–109. doi: 10.1111/j.1399-0012.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 45.Matas AJ, Ramcharan T, Paraskevas S, Gillingham KJ, Dunn DL, Gruessner RW, et al. Rapid discontinuation of steroids in living donor kidney transplantation: a pilot study. Am J Transplant. 2001;1:278–283. doi: 10.1034/j.1600-6143.2001.001003278.x. [DOI] [PubMed] [Google Scholar]
- 46.Rajab A, Pelletier RP, Henry ML, Ferguson RM. Excellent clinical outcomes in primary kidney transplant recipients treated with steroid-free maintenance immunosuppression. Clin Transplant. 2006;20:537–546. doi: 10.1111/j.1399-0012.2006.00521.x. [DOI] [PubMed] [Google Scholar]
- 47.Lebranchu Y, Bridoux F, Büchler M, Le Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant. 2002;2:48–56. doi: 10.1034/j.1600-6143.2002.020109.x. [DOI] [PubMed] [Google Scholar]
- 48.Glotz D, Charpentier B, Abramovicz D, Lang P, Rostaing L, Rifle G, et al. Thymoglobulin induction and sirolimus versus tacrolimus in kidney transplant recipients receiving mycophenolate mofetil and steroids. Transplantation. 2010;89:1511–1517. doi: 10.1097/TP.0b013e3181db09e4. [DOI] [PubMed] [Google Scholar]
- 49.Büchler M, Caillard S, Barbier S, Thervet E, Toupance O, Mazouz H, et al. SPIESSER Group. Sirolimus versus cyclosporine in kidney recipients receiving thymoglobulin, mycophenolate mofetil and a 6-month course of steroids. Am J Transplant. 2007;7:2522–2531. doi: 10.1111/j.1600-6143.2007.01976.x. [DOI] [PubMed] [Google Scholar]
- 50.Larson TS, Dean PG, Stegall MD, Griffin MD, Textor SC, Schwab TR, et al. Complete avoidance of calcineurin inhibitors in renal transplantation: a randomized trial comparing sirolimus and tacrolimus. Am J Transplant. 2006;6:514–522. doi: 10.1111/j.1600-6143.2005.01177.x. [DOI] [PubMed] [Google Scholar]
- 51.Lo A, Egidi MF, Gaber LW, Amiri HS, Vera S, Nezakatgoo N, et al. Comparison of sirolimus-based calcineurin inhibitor-sparing and calcineurin inhibitor-free regimens in cadaveric renal transplantation. Transplantation. 2004;77:1228–1235. doi: 10.1097/01.tp.0000121504.69676.5e. [DOI] [PubMed] [Google Scholar]
- 52.Grinyó JM, Gil-Vernet S, Seron D, Hueso M, Fulladosa X, Cruzado JM, et al. Primary immunosuppression with mycophenolate mofetil and antithymocyte globulin for kidney transplant recipients of a suboptimal graft. Nephrol Dial Transplant. 1998;13:2061–2064. doi: 10.1093/ndt/13.10.2601. [DOI] [PubMed] [Google Scholar]
- 53.Alexander JW, Goodman HR, Cardi M, Austin J, Goel S, Safdar S, et al. Simultaneous corticosteroid avoidance and calcineurin inhibitor minimization in renal transplantation. Transpl Int. 2006;19:295–302. doi: 10.1111/j.1432-2277.2006.00280.x. [DOI] [PubMed] [Google Scholar]
- 54.Cantarovich D, Giral-Classe M, Hourmant M, Dantal J, Blancho G, Lerat L, et al. Prevention of acute rejection with antithymocyte globulin, avoiding corticosteroids, and delaying cyclosporin after renal transplantation. Nephrol Dial Transplant. 2000;15:1673–1676. doi: 10.1093/ndt/15.10.1673. [DOI] [PubMed] [Google Scholar]
- 55.Shaffer D, Langone A, Nylander WA, Goral S, Kizilisik AT, Helderman JH. A pilot protocol of a calcineurin-inhibitor free regimen for kidney transplant recipients of marginal donor kidneys or with delayed graft function. Clin Transplant. 2003;17(Suppl 9):31–34. doi: 10.1034/j.1399-0012.17.s9.5.x. [DOI] [PubMed] [Google Scholar]
- 56.Wang CJ, Tuffaha A, Zhang D, Diederich DA, Wetmore JB. A CD3+ count-based thymoglobulin induction regimen permits delayed introduction of calcineurin inhibitors in kidney transplantation. Clin Transplant. 2012;26:900–909. doi: 10.1111/j.1399-0012.2012.01656.x. [DOI] [PubMed] [Google Scholar]
- 57.Laftavi MR, Patel S, Soliman MR, Alnimri M, Kohli R, Said M, et al. Low-dose thymoglobulin use in elderly renal transplant recipients is safe and effective induction therapy. Transplant Proc. 2011;43:466–468. doi: 10.1016/j.transproceed.2011.01.039. [DOI] [PubMed] [Google Scholar]
- 58.Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE., Jr Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75:1396–1399. doi: 10.1097/01.TP.0000062834.30922.FE. [DOI] [PubMed] [Google Scholar]
- 59.Grafals M, Simpson M, Gilligan H, Pomposelli J, Akoad M, Kwaja K, et al. Prospective randomized study of low dose antithymocyte globulin as induction in non sensitized adult renal transplant recipients [abstract no. 1351]. Am J Transplant. 2013;13 Suppl 5.
- 60.Popat R, Syed A, Puliatti C, Cacciola R. Outcome and cost analysis of induction immunosuppression with IL2Mab or ATG in DCD kidney transplants. Transplantation. 2014;97:1161–1165. doi: 10.1097/01.tp.0000442505.10490.20. [DOI] [PubMed] [Google Scholar]
- 61.Patel S, Pankewycz O, Kohli R, Said M, Alnimri M, Feng L, et al. Obesity in renal transplantation: the role of induction therapy on long-term outcomes. Transplant Proc. 2011;43:469–471. doi: 10.1016/j.transproceed.2011.01.040. [DOI] [PubMed] [Google Scholar]
- 62.Schenker P, Ozturk A, Vonend O, Krüger B, Jazra M, Wunsch A, et al. Single-dose thymoglobulin induction in living-donor renal transplantation. Ann Transplant. 2011;16:50–58. doi: 10.12659/aot.881865. [DOI] [PubMed] [Google Scholar]
- 63.De Ruvo N, Cucchetti A, Lauro A, Masetti M, Cautero N, Di Benedetto F, et al. Preliminary results of a “prope” tolerogenic regimen with thymoglobulin pre-treatment and hepatitis C virus recurrence in liver transplantation. Transplantation. 2005;80:8–12. doi: 10.1097/01.tp.0000164349.54297.95. [DOI] [PubMed] [Google Scholar]
- 64.Peddi VR, Bryant M, Roy-Chaudhury P, Woodle ES, First MR. Safety, efficacy, and cost analysis of thymoglobulin induction therapy with intermittent dosing based on CD3 + lymphocyte counts in kidney and kidney-pancreas transplant recipients. Transplantation. 2002;73:1514–1518. doi: 10.1097/00007890-200205150-00025. [DOI] [PubMed] [Google Scholar]
- 65.Koch A, Daniel V, Dengler TJ, Schnabel PA, Hagl S, Sack FU. Effectivity of a T-cell-adapted induction therapy with anti-thymocyte globulin (Sangstat) J Heart Lung Transplant. 2005;24:708–713. doi: 10.1016/j.healun.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 66.Tsapepas D, Mohan S, Crew RJ, Cohen D, Ratner LE. Small thymoglobulin dose adjustments have profound impact on early rejections in renal transplantation [abstract no. 389]. Am J Transplant 2011;11 Suppl 2.
- 67.Khanmoradi K, Knorr JP, Feyssa EL, Parsikia A, Jawa P, Dinh H-B, et al. Evaluating safety and efficacy of rabbit antithymocyte globulin induction in elderly kidney transplant recipients. Exp Clin Transplant. 2013;11:222–228. doi: 10.6002/ect.2012.0211. [DOI] [PubMed] [Google Scholar]
- 68.Gaber AO, Matas AJ, Henry ML, Brennan DC, Stevens RB, Kapur S, et al. Thymoglobulin Antibody Immunosuppression in Living Donor Recipients Investigators. Antithymocyte globulin induction in living donor renal transplant recipients: final report of the TAILOR registry. Transplantation. 2012;94:331–337. doi: 10.1097/TP.0b013e31825a7d1f. [DOI] [PubMed] [Google Scholar]
- 69.Kho MM, Bouvy AP, Cadogan M, Kraaijeveld R, Baan CC, Weimar W. The effect of low and ultra-low dosages Thymoglobulin on peripheral T, B and NK cells in kidney transplant recipients. Transpl Immunol. 2012;26:186–190. doi: 10.1016/j.trim.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 70.Préville X, Flacher M, LeMauff B, Beauchard S, Davelu P, Tiollier J, et al. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460–468. doi: 10.1097/00007890-200102150-00021. [DOI] [PubMed] [Google Scholar]
- 71.Soliman T, Hetz H, Burghuber C, Gyöi G, Silberhumer G, Steininger R, et al. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transplantation. 2007;13:1039–1044. doi: 10.1002/lt.21185. [DOI] [PubMed] [Google Scholar]
- 72.Mourad G, Rostaing L, Legendre C, Garrigue V, Thervet E, Durand D. Sequential protocols using basiliximab versus antithymocyte globulins in renal-transplant patients receiving mycophenolate mofetil and steroids. Transplantation. 2004;78:584–590. doi: 10.1097/01.tp.0000129812.68794.cc. [DOI] [PubMed] [Google Scholar]
- 73.Rostaing L, Saliba F, Calmus Y, Dharancy S, Boillot O. Review article: use of induction therapy in liver transplantation. Transplant Rev (Orlando) 2012;26:246–260. doi: 10.1016/j.trre.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 74.Mattei M, Redonnet M, Gandjbakhch I, Bandini AM, Billes A, Epailly E, et al. Lower risk of infectious deaths in cardiac transplant patients receiving basiliximab versus anti-thymocyte globulin as induction therapy. J Heart Lung Transplant. 2007;26:693–699. doi: 10.1016/j.healun.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 75.Flaman F, Zieroth S, Rao V, Ross H, Delgado DH. Basiliximab versus rabbit anti-thymocyte globulin for induction therapy in patients after heart transplantation. J Heart Lung Transplant. 2006;25:1358–1362. doi: 10.1016/j.healun.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 76.Carlsen J, Johansen M, Boesgaard S, Andersen CB, Arendrup H, Aldershvilet J, et al. Induction therapy after cardiac transplantation: a comparison of anti-thymocyte globulin and daclizumab in the prevention of acute rejection. J Heart Lung Transplant. 2005;24:296–302. doi: 10.1016/j.healun.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Aliabadi A, Grömmer M, Cochrane A, Salameh O, Zuckermann A. Induction therapy in heart transplantation: where are we now? Transpl Int. 2013;26:684–695. doi: 10.1111/tri.12107. [DOI] [PubMed] [Google Scholar]
- 78.Goland S, Lawrence S, Czer C, De Robertis MA, Mirocha J, Zivari K, et al. Induction therapy with thymoglobulin after heart transplantation: impact of therapy duration on lymphocyte depletion and recovery, rejection, and cytomegalovirus infection rates. J Heart Lung Transplant. 2008;27:1115–1121. doi: 10.1016/j.healun.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Krasinskas AM, Kreisel D, Acker MA, Bavaria JE, Pochettino A, Kotloff RM, et al. CD3 monitoring of antithymocyte globulin therapy in thoracic organ transplantation. Transplantation. 2002;73:1339–1341. doi: 10.1097/00007890-200204270-00026. [DOI] [PubMed] [Google Scholar]
- 80.Hardinger KL, Bohl DL, Schnitzler MA, Lockwood M, Storch GA, Brennan DC. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation. 2005;80:41–46. doi: 10.1097/01.tp.0000162980.68628.5a. [DOI] [PubMed] [Google Scholar]
- 81.Walther S, Beiras-Fernandez A, Csapo C, Münzing S, Stief CG, Hammer C, et al. Influence of polyclonal antithymocyte globulins on the expression of adhesion molecules of isolated human umbilical vein endothelial cells. Transplantation Proc. 2010;42:1931–1934. doi: 10.1016/j.transproceed.2009.11.038. [DOI] [PubMed] [Google Scholar]
- 82.Beiras-Fernandez A, Chappell D, Hammer C, Beiras A, Reichart B, Thein E. Impact of polyclonal anti-thymocyte globulins on the expression of adhesion and inflammation molecules after ischemia-reperfusion injury. Transpl Immunol. 2009;20:224–228. doi: 10.1016/j.trim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 83.Beiras-Fernandez A, Thein E, Chappell D, Gallego R, Fernandez-Roel D, Kemming G, et al. Polyclonal anti-thymocyte globulins influence apoptosis in reperfused tissues after ischemia in a non-human primate model. Transpl Int. 2004;17:453–457. doi: 10.1007/s00147-004-0736-1. [DOI] [PubMed] [Google Scholar]
- 84.Hardinger KL, Rasu RS, Skelton R, Miller BW, Brennan DC. Thymoglobulin induction dosing strategies in a low-risk kidney transplant population: three or four days? J Transplant. 2010;2010:957549. doi: 10.1155/2010/957549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Marfo K, Akalin E, Wang C, Lu A. Clinical and economic analysis of short-course versus standard-course antithymocyte globulin (rabbit) induction therapy in deceased-donor renal transplant recipients. Am J Health Syst Pharm. 2011;68:2276–2282. doi: 10.2146/ajhp110120. [DOI] [PubMed] [Google Scholar]
- 86.Uber WE, Uber LA, VanBakel AB, Crumbley AJ, 3rd, Pereira NL, Ikonomidis JS, et al. CD3 monitoring and thymoglobulin therapy in cardiac transplantation: clinical outcomes and pharmacoeconomic implications. Transplant Proc. 2004;36:3245–3249. doi: 10.1016/j.transproceed.2004.11.099. [DOI] [PubMed] [Google Scholar]
- 87.Bunnapradist S, Takemoto SK. Multivariate analysis of antibody induction therapy and their associated outcomes in deceased donor transplants. Transplant Proc. 2005;37:889–891. doi: 10.1016/j.transproceed.2004.12.080. [DOI] [PubMed] [Google Scholar]
- 88.Lebranchu Y, Baan C, Biancone L, Legendre C, Morales JM, Naesens M, et al. Pretransplant identification of acute rejection risk following kidney transplantation. Transpl Int. 2014;27:129–138. doi: 10.1111/tri.12205. [DOI] [PubMed] [Google Scholar]
- 89.Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, et al. Donor-specific antibodies adversely affect kidney allograft outcomes. J Am Soc Nephrol. 2012;23:2061–2071. doi: 10.1681/ASN.2012070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burns JM, Cornell LD, Perry DK, Pollinger HS, Gloor JM, Kremers WK, et al. Alloantibody levels and acute humoral rejection early after positive crossmatch kidney transplantation. Am J Transplant. 2008;8:2684–2694. doi: 10.1111/j.1600-6143.2008.02441.x. [DOI] [PubMed] [Google Scholar]
- 91.Noël C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, et al. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20:1385–1392. doi: 10.1681/ASN.2008101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005;80:457–465. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- 93.Abou-Ayache R, Büchler M, Lepogamp P, Westeel PF, Le Meur Y, Etienne I, et al. CMV infections after two doses of daclizumab versus thymoglobulin in renal transplant patients receiving mycophenolate mofetil, steroids and delayed cyclosporine A. Nephrol Dial Transplant. 2008;23:2024–2032. doi: 10.1093/ndt/gfm873. [DOI] [PubMed] [Google Scholar]
- 94.Woodle ES, Peddi VR, Tomlanovich S, Mulgaonkar S, Kuo PC. TRIMS Study Investigators. A prospective, randomized, multicenter study evaluating early corticosteroid withdrawal with Thymoglobulin in living-donor kidney transplantation. Clin Transplant. 2009;24:73–83. doi: 10.1111/j.1399-0012.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- 95.Tian JH, Wang X, Yang KH, Liu AP, Luo XF, Zhang J. Induction with and without antithymocyte globulin combined with cyclosporine/tacrolimus-based immunosuppression in renal transplantation: a meta-analysis of randomized controlled trials. Transplant Proc. 2009;41:3671–3676. doi: 10.1016/j.transproceed.2009.06.184. [DOI] [PubMed] [Google Scholar]
- 96.Morgan RD, O’Callaghan JM, Knight SR, Morris PJ. Alemtuzumab induction therapy in kidney transplantation: a systematic review and meta-analysis. Transplantation. 2012;93:1179–1188. doi: 10.1097/TP.0b013e318257ad41. [DOI] [PubMed] [Google Scholar]
- 97.Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, et al. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364:1909–1919. doi: 10.1056/NEJMoa1009546. [DOI] [PubMed] [Google Scholar]
- 98.Rodríguz-Reimundes E, Buron F, Chauvet C, Daoud S, Thaunat O, Brunet M, et al. Retreatment by antithymocyte globulin for second kidney transplantation:efficacy, tolerance and safety. Transpl Immunol. 2013;28:6–8. doi: 10.1016/j.trim.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 99.Khositseth S, Matas A, Cook ME, Gillingham KJ, Chavers BM. Thymoglobulin versus ATGAM induction therapy in pediatric kidney transplant recipients: a single-center report. Transplantation. 2005;79:958–963. doi: 10.1097/01.tp.0000158325.12837.a2. [DOI] [PubMed] [Google Scholar]
- 100.Colleen Hastings M, Wyatt RJ, Lau KK, Jones DP, Powell SL, Hays DW, et al. Five years’ experience with thymoglobulin induction in a pediatric renal transplant population. Pediatr Transplant. 2006;10:805–810. doi: 10.1111/j.1399-3046.2006.00509.x. [DOI] [PubMed] [Google Scholar]
- 101.Schwartz JJ, Ishitani MB, Weckwerth J, Morgenstern B, Milliner D, Stegall MD. Decreased incidence of acute rejection in adolescent kidney transplant recipients using antithymocyte induction and triple immunosuppression. Transplantation. 2007;84:715–721. doi: 10.1097/01.tp.0000281907.54832.cb. [DOI] [PubMed] [Google Scholar]
- 102.Brophy PD, Thomas SE, McBryde KD, Bunchman TE. Comparison of polyclonal induction agents in pediatric renal transplantation. Pediatr Transplant. 2001;5:174–178. doi: 10.1034/j.1399-3046.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- 103.Kamel MH, Mohan P, Little DM, Awan A, Hickey DP. Rabbit antithymocyte globulin as induction immunotherapy for pediatric deceased donor kidney transplantation. J Urol. 2005;174:703–707. doi: 10.1097/01.ju.0000164752.37118.9c. [DOI] [PubMed] [Google Scholar]
- 104.Hardinger KL, Schnitzler MA, Koch MJ, Labile E, Stirnemann PM, Miller B, et al. Thymoglobulin induction is safe and effective in live-donor renal transplantation: a single center experience. Transplantation. 2006;81:1285–1289. doi: 10.1097/01.tp.0000209825.91632.ea. [DOI] [PubMed] [Google Scholar]
- 105.Martins L, Fonseca I, Almeida M, Henriques AC, Dias L, Sarmento AM, et al. Immunosuppression with antithymocyte globulin in renal transplantation: better long-term graft survival. Transplant Proc. 2005;37:2755–2758. doi: 10.1016/j.transproceed.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 106.Abouna GM, Kumar MS, Stephan R, Prior JE, Lyons P, Bulova SI, et al. Induction therapy with antithymocyte globulin reduces the incidence of allograft rejection and improves graft survival in cadaver renal transplantation. Transplant Proc. 1993;25:2241–2242. [PubMed] [Google Scholar]
- 107.Opelz G, Naujokat C, Daniel V, Terness P, Döhler B. Disassociation between risk of graft loss and risk of non-Hodgkin lymphoma with induction agents in renal transplant recipients. Transplantation. 2006;81:1227–1233. doi: 10.1097/01.tp.0000219817.18049.36. [DOI] [PubMed] [Google Scholar]
- 108.Willoughby LM, Schnitzler MA, Brennan DC, Pinsky BW, Dzebisashvili N, Buchanan PM, et al. Early outcomes of thymoglobulin and basiliximab induction in kidney transplantation: application of statistical approaches to reduce bias in observational comparisons. Transplantation. 2009;87:1520–1529. doi: 10.1097/TP.0b013e3181a484d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kuo HT, Huang E, Emami S, Pham PT, Wilkinson AH, Danovitch GM, et al. Effects of antibody induction on transplant outcomes in human leukocyte antigen zero-mismatch deceased donor kidney recipients. Transplantation. 2012;93:493–502. doi: 10.1097/TP.0b013e3182427fc3. [DOI] [PubMed] [Google Scholar]
- 110.Oweis A, Zaltzman J, Kim J. Comparative effectiveness of rabbit anti-thymocyte globulin vs. interleukin-2 receptor blockers in deceased donor kidney transplants with delayed graft function. Am J Transplant 2013;13(Suppl 5):Abstract 173.
- 111.Bogetti D, Sankary HN, Jarzembowski TM, Manzelli A, Knight PS, Thielke J, et al. Thymoglobulin induction protects liver allografts from ischemia/reperfusion injury. Clin Transplant. 2005;19:507–511. doi: 10.1111/j.1399-0012.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 112.Harrison JJ, Hamandi B, Li Y, Famure O, Kim SJ. Timing of rabbit antithymocyte globulin induction therapy in kidney transplantation: an observational cohort study. Transplant Res. 2014;3:1. doi: 10.1186/2047-1440-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mehrabi A, Mood ZhA, Sadeghi M, Schmied BM, Müller SA, Welsch T, et al. Thymoglobulin and ischemia reperfusion injury in kidney and liver transplantation. Nephrol Dial Transplant. 2007;22(Suppl 8):viii54–viii60. doi: 10.1093/ndt/gfm651. [DOI] [PubMed] [Google Scholar]
- 114.Chapal M, Le Borgne F, Legendre C, Kreis H, Mourad G, Garrigue V, et al. The DGFS: a useful scoring system for the prediction and management of delayed graft function following kidney transplantation from cadaveric donors. Kidney Int 2014. doi:10.1038/ki.2014.188. [DOI] [PubMed]
- 115.Lebranchu Y, Aubert P, Bayle F, Bedrossian J, Berthoux F, Bourbigot B, et al. Could steroids be withdrawn in renal transplant patients sequentially treated with ATG, cyclosporine, and cellcept? One-year results of a double-blind, randomized, multicenter study comparing normal dose versus low-dose and withdrawal of steroids. M 55002 French Study Group. Transplant Proc. 2000;32:396–397. doi: 10.1016/s0041-1345(99)00992-6. [DOI] [PubMed] [Google Scholar]
- 116.Chavers BM, Chang YC, Gillingham KJ, Matas A. Pediatric kidney transplantation using a novel protocol of rapid (6-day) discontinuation of prednisone: 2-year results. Transplantation. 2009;88:237–241. doi: 10.1097/TP.0b013e3181ac6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li L, Chaudhuri A, Chen A, Zhao X, Bezchinsky M, Concepcion W, et al. Efficacy and safety of thymoglobulin induction as an alternative approach for steroid-free maintenance immunosuppression in pediatric renal transplantation. Transplantation. 2010;90:1516–1520. doi: 10.1097/TP.0b013e3181fc8937. [DOI] [PubMed] [Google Scholar]
- 118.Martin ST, Roberts KL, Malek SK, Tullius SG, Vadivel N, De Serres S, et al. Induction treatment with rabbit antithymocyte globulin versus basiliximab in renal transplant recipients with planned early steroid withdrawal. Pharmacotherapy. 2011;31:566–573. doi: 10.1592/phco.31.6.566. [DOI] [PubMed] [Google Scholar]
- 119.Swanson SJ, Hale DA, Mannon RB, Kleiner DE, Cendales LC, Chamberlain CE, et al. Kidney transplantation with rabbit antithymocyte globulin induction and sirolimus monotherapy. Lancet. 2002;360:1662–1664. doi: 10.1016/S0140-6736(02)11606-0. [DOI] [PubMed] [Google Scholar]
- 120.Grinyó JM, Gil-Vernet S, Cruzado JM, Caldés A, Riera L, Serón D, et al. Calcineurin inhibitor-free immunosuppression based on antithymocyte globulin and mycophenolate mofetil in cadaveric kidney transplantation: results after 5 years. Transpl Int. 2003;16:820–827. doi: 10.1007/s00147-003-0638-7. [DOI] [PubMed] [Google Scholar]
- 121.Lebranchu Y, Snanoudj R, Toupance O, Weestel PF, Hurault de Ligny B, Buchler M, et al. Five-year results of a randomized trial comparing de novo sirolimus and cyclosporine in renal transplantation: the SPIESSER study. Am J Transplant. 2012;12:1801–1810. doi: 10.1111/j.1600-6143.2012.04036.x. [DOI] [PubMed] [Google Scholar]
- 122.Venot M, Abboud I, Duboust A, Michel C, Suberbielle C, Vérine J, et al. Calcineurin inhibitor-free monotherapy in human leukocyte antigen-identical live donor renal transplantation. Transplantation. 2011;91:330–333. doi: 10.1097/tp.0b013e3182033ef0. [DOI] [PubMed] [Google Scholar]
- 123.Ekberg H, Tedesco-Silva H, Demirbas A, Vítko S, Nashan B, Gürkan A, et al; ELITE-Symphony Study. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med. 2007;357:2562-75. [DOI] [PubMed]
- 124.Flechner SM, Glyda M, Cockfield S, Grinyó J, Legendre Ch, Russ G, et al. The ORION Study: comparison of two sirolimus-based regimens versus tacrolimus and mycophenolate mofetil in renal allograft recipients. Am J Transplant. 2011;11:1633–1644. doi: 10.1111/j.1600-6143.2011.03573.x. [DOI] [PubMed] [Google Scholar]
- 125.Favi E, Spagnoletti G, Silvestrini N, Salerno MP, Pedroso JA, Romagnoli J, et al. Thymoglobulin plus basiliximab versus basiliximab induction in deceased donor kidney transplant recipients treated with tacrolimus and MMF: 1-year results of a prospective clinical trial. Transpl Int. 2013;26 Suppl s2:83.
- 126.Ferguson R, Grinyó J, Vincenti F, Kaufman DB, Woodle ES, Marder BA, et al. Immunosuppression with belatacept-based, corticosteroid-avoiding regimens in de novo kidney transplant recipients. Am J Transplant. 2011;11:66–76. doi: 10.1111/j.1600-6143.2010.03338.x. [DOI] [PubMed] [Google Scholar]
- 127.Bouvy A, Klepper M, Kho M, Ijzermans J, Litjens N, Betjes M, et al. Rabbit antithymocyte globulin induction therapy induces donor-specific helios−FOXP3+ regulatory T cells in kidney transplant patients [abstract no. 237]. Am J Transplant. 2013;13 Suppl.
- 128.Todeschini M, Cortinovis M, Perico N, Poli F, Innocente A, Cavinato RA, et al. In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor-specific anti-HLA antibody development. J Immunol. 2013;191:2818–2828. doi: 10.4049/jimmunol.1203261. [DOI] [PubMed] [Google Scholar]
- 129.Mai ML, Ahsan N, Wadei HM, Genco PV, Geiger XJ, Willingham DL, et al. Excellent renal allograft survival in donor-specific antibody positive transplant patients-role of intravenous immunoglobulin and rabbit antithymocyte globulin. Transplantation. 2009;87:227–232. doi: 10.1097/TP.0b013e31818c962b. [DOI] [PubMed] [Google Scholar]
- 130.Kubal CA, Mangus RS, Saxena R, Lobashevsky A, Higgins N, Agarwal A, et al. Crossmatch-positive liver transplantation in patients receiving thymoglobulin-rituximab induction. Transplantation. 2014;97:56–63. doi: 10.1097/TP.0b013e3182a688c0. [DOI] [PubMed] [Google Scholar]
- 131.Cooper JE, Gralla J, Cagle L, Goldberg R, Chan L, Wiseman AC. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation. 2011;91:1103–1109. doi: 10.1097/TP.0b013e3182139da1. [DOI] [PubMed] [Google Scholar]
- 132.Huang Y, Ramon D, Luan FL, Sung R, Samaniego M. Incidences of preformed and de novo donor-specific HLA antibodies and their clinicohistological correlates in the early course of kidney transplantation. Clin Transplant. 2012;247–56. [PubMed]
- 133.Loupy AD, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med. 2013;369:1215–1226. doi: 10.1056/NEJMoa1302506. [DOI] [PubMed] [Google Scholar]
- 134.Cai J, Terasaki PI. Induction immunosuppression improves long-term graft and patient outcome in organ transplantation: an analysis of United Network for Organ Sharing registry data. Transplantation. 2010;90:1511–1515. doi: 10.1097/TP.0b013e3181fecfcb. [DOI] [PubMed] [Google Scholar]
- 135.Moonka DK, Kim D, Kapke A, Brown KA, Yoshida A. The influence of induction therapy on graft and patient survival in patients with and without hepatitis C after liver transplantation. Am J Transplant. 2010;10:590–601. doi: 10.1111/j.1600-6143.2009.02880.x. [DOI] [PubMed] [Google Scholar]
- 136.Mangus R, Fridell AJ, Vianna RM, Kwo PY, Chen J, Tector AJ. Immunosuppression induction with rabbit anti-thymocyte globulin with or without rituximab in 1000 liver transplant patients with long-term follow-up. Liver Transpl. 2012;18:786–795. doi: 10.1002/lt.23381. [DOI] [PubMed] [Google Scholar]
- 137.Boillot O, Seket B, Dumortier J, Pittau G, Boucaud C, Bouffard Y, et al. Thymoglobulin induction in liver transplant recipients with a tacrolimus, mycophenolate mofetil, and steroid immunosuppressive regimen: a five-year randomized prospective study. Liver Transpl. 2009;15:1426–1434. doi: 10.1002/lt.21905. [DOI] [PubMed] [Google Scholar]
- 138.Horton PJ, Tchervenkov J, Barkun JS, Rochon C, Chaudhury PK, Znajda TL, et al. Antithymocyte globulin induction therapy in hepatitis C-positive liver transplant recipients. J Gastrointest Surg. 2005;9:896–902. doi: 10.1016/j.gassur.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 139.Tchervenkov J, Flemming C, Guttmann RD, Des Gachons G. Use of thymoglobulin induction therapy in the prevention of acute graft rejection episodes following liver transplantation. Transplant Proc. 1997;29(Suppl 7A):13S–15S. [PubMed] [Google Scholar]
- 140.Halldorson J, Bakthavatsalam B, Dick A, Rayhill S, Perkins J, Reyes J. Antithymocyte globulin induction is associated with improved graft survival and reduced ischemic cholangiopathy after DCD liver transplantation as compared to basiliximab [abstract no. 265]. Am J Transplant. 2013;13 Suppl 5.
- 141.Tector AJ, Fridell JA, Mangus RS, Shah A, Milgrom M, Kwo P, et al. Promising early results with immunosuppression using rabbit anti-thymocyte globulin and steroids with delayed introduction of tacrolimus in adult liver transplant recipients. Liver Transpl. 2004;10:404–407. doi: 10.1002/lt.20085. [DOI] [PubMed] [Google Scholar]
- 142.Varo E, López A, Rivero C. Initial immunosuppression in liver transplant recipients with impaired renal function. Transplant Proc. 2005;37:3909–3912. doi: 10.1016/j.transproceed.2005.09.115. [DOI] [PubMed] [Google Scholar]
- 143.Rosen HR, Shackleton CR, Higa L, Gralnek IM, Farmer DA, McDiarmid SV, et al. Use of OKT3 is associated with early and severe recurrence of hepatitis C after liver transplantation. Am J Gastroenterol. 1997;92:1453–1457. [PubMed] [Google Scholar]
- 144.Uemura T, Schaefer E, Hollenbeak CS, Khan A, Kadry Z. Outcome of induction immunosuppression for liver transplantation comparing anti-thymocyte globulin, daclizumab, and corticosteroid. Transpl Int. 2011;24:640–650. doi: 10.1111/j.1432-2277.2011.01250.x. [DOI] [PubMed] [Google Scholar]
- 145.Nair S, Lipscomb J, Eason J. Efficacy of interferon based antiviral therapy for recurrent hepatitis C in patients who received steroid free immunosuppression for liver transplantation. Transplantation. 2008;86:418–422. doi: 10.1097/TP.0b013e31817c1543. [DOI] [PubMed] [Google Scholar]
- 146.Kamar N, Ribes D, Sandres-Saune K, Suc B, Barange K, Cointault O, et al. Efficacy and safety of induction therapy with rabbit antithymocyte globulins in liver transplantation for hepatitis C. Transplant Proc. 2004;36:2757–2761. doi: 10.1016/j.transproceed.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 147.Yamani MH, Taylor DO, Czerr J, Haire C, Kring R, Zhou L, et al. Thymoglobulin induction and steroid avoidance in cardiac transplantation: results of a prospective, randomized, controlled study. Clin Transplant. 2008;22:76–81. [PubMed] [Google Scholar]
- 148.Carrier M, Leblanc MH, Perrault LP, White M, Doyle D, Beaudoin D, et al. Basiliximab and rabbit anti-thymocyte globulin for prophylaxis of acute rejection after heart transplantation: a non-inferiority trial. Heart Lung Transplant. 2007;26:258–263. doi: 10.1016/j.healun.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 149.Chou NK, Wang SS, Chen YS, Yu HY, Chi NH, Wang CH, et al. Induction immunosuppression with basiliximab in heart transplantation. Transplant Proc. 2008;40:2623–2625. doi: 10.1016/j.transproceed.2008.07.113. [DOI] [PubMed] [Google Scholar]
- 150.Eisen HJ, Kobashigawa J, Starling RC, Pauly DR, Kfoury A, Ross H, et al. Everolimus versus mycophenolate mofetil in heart transplantation: a randomized, multicenter trial. Am J Transplant. 2013;13:1203–1216. doi: 10.1111/ajt.12181. [DOI] [PubMed] [Google Scholar]
- 151.Baran DA, Carboni M, Dengler T, Feldman D, Frigerio M, Kfoury A, et al. The International Society of Heart and Lung Transplantation guidelines for the care of heart transplant recipients. Task Force 2: immunosuppression and rejection. ISHLT; 2010: pp. 1–41.
- 152.Delgado DH, Miriuka SG, Cusimano RJ, Feindel C, Rao V, Ross HJ. Use of basiliximab and cyclosporine in heart transplant patients with pre-operative renal dysfunction. J Heart Lung Transplant. 2005;24:166–169. doi: 10.1016/j.healun.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 153.Cantarovich M, Giannetti N, Barkun J, Cecere R. Antithymocyte globulin induction allows a prolonged delay in the initiation of cyclosporine in heart transplant patients with postoperative renal dysfunction. Transplantation. 2004;78:779–781. doi: 10.1097/01.tp.0000130179.18176.3d. [DOI] [PubMed] [Google Scholar]
- 154.Fuchs U, Zittermann A, Amini A, Ensminger SM, Gummert JF, Schulz U. Clinical outcome in heart transplant recipients with chronic kidney disease receiving Thymoglobulin for induction therapy. German Transplant Society (DTG) Congress 2013.
- 155.Singh TP, Faber C, Blume ED, Worley S, Almond CS, Smoot LB, et al. Safety and early outcomes using a corticosteroid-avoidance immunosuppression protocol in pediatric heart transplant recipients. J Heart Lung Transplant. 2010;29:517–522. doi: 10.1016/j.healun.2009.11.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131–2141. doi: 10.1161/CIRCULATIONAHA.107.711911. [DOI] [PubMed] [Google Scholar]
- 157.Zuckermann A, Ploner M, Czerny M, Keziban U, Birsan T, Laufer G, et al. Low incidence of graft arteriosclerosis after cardiac transplantation: risk factor analysis for patients with induction therapy. Transplant Proc. 2002;34:1869–1871. doi: 10.1016/s0041-1345(02)03103-2. [DOI] [PubMed] [Google Scholar]
- 158.Bonaros N, Dunkler D, Kocher A, Imhof M, Grimm M, Zuckermann A, et al. Ten-year follow-up of a prospective, randomized trial of BT563/bb10 versus anti-thymocyte globulin as induction therapy after heart transplantation. J Heart Lung Transplant. 2006;25:1154–1163. doi: 10.1016/j.healun.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 159.Carrier M, White M, Perrault LP, Pelletier GB, Pellerin M, Robitaille D, et al. A 10-year experience with intravenous thymoglobuline in induction of immunosuppression following heart transplantation. J Heart Lung Transplant. 1999;18:1218–1223. doi: 10.1016/s1053-2498(99)00100-x. [DOI] [PubMed] [Google Scholar]
- 160.Faggian G, Forni A, Milano AD, Chiominto B, Walpoth BH, Scarabelli T, et al. Antithymocyte globulin induction therapy in heart transplantation: prospective randomized study of high vs standard dosage. Transplant Proc. 2010;42:3679–3687. doi: 10.1016/j.transproceed.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 161.Zhang R, Haverich A, Strüber M, Simon A, Bara C. Delayed onset of cardiac allograft vasculopathy by induction therapy using anti-thymocyte globulin. J Heart Lung Transplant. 2008;27:603–609. doi: 10.1016/j.healun.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 162.Sweet SC. Induction therapy in lung transplantation. Transplant Int. 2013;26:696–703. doi: 10.1111/tri.12115. [DOI] [PubMed] [Google Scholar]
- 163.Hayes D., Jr A review of bronchiolitis obliterans syndrome and therapeutic strategies. J Cardiothorac Surg. 2011;6:92. doi: 10.1186/1749-8090-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Todd JL, Palmer SM. Bronchiolitis obliterans syndrome: the final frontier for lung transplantation. Chest. 2011;140:502–508. doi: 10.1378/chest.10-2838. [DOI] [PubMed] [Google Scholar]
- 165.Lease ED, Zaas DW. Update on infectious complications following lung transplantation. Curr Opin Pulm Med. 2011;17:206–209. doi: 10.1097/MCP.0b013e328344dba5. [DOI] [PubMed] [Google Scholar]
- 166.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dipchand AI, Dobbels F, et al. The Registry of the International Society for Heart and Lung Transplantation: 29th adult lung and heart-lung transplant report-2012. J Heart Lung Transplant. 2012;31:1073–1086. doi: 10.1016/j.healun.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 167.Hachem RR, Edwards LB, Yusen RD, Chakinala MM, Alexander Patterson G, Trulock EP. The impact of induction on survival after lung transplantation: an analysis of the International Society for Heart and Lung Transplantation Registry. Clin Transplant. 2008;22:603–608. doi: 10.1111/j.1399-0012.2008.00831.x. [DOI] [PubMed] [Google Scholar]
- 168.Palmer SM, Miralles AP, Lawrence CM, Gaynor JW, Davis RD, Tapson VF. Rabbit antithymocyte globulin decreases acute rejection after lung transplantation: results of a randomized, prospective study. Chest. 1999;116:127–133. doi: 10.1378/chest.116.1.127. [DOI] [PubMed] [Google Scholar]
- 169.Zuckermann A, Reichenspurner H, Birsan T, Treede H, Deviatko E, Reichart B, et al. Cyclosporine A versus tacrolimus in combination with mycophenolate mofetil and steroids as primary immunosuppression after lung transplantation: one-year results of a 2-center prospective randomized trial. J Thorac Cardiovasc Surg. 2003;125:891–900. doi: 10.1067/mtc.2003.71. [DOI] [PubMed] [Google Scholar]
- 170.Hartwig MG, Snyder LD, Appel JZ, 3rd, Cantu E, 3rd, Lin SS, Palmer SM, et al. Rabbit anti-thymocyte globulin induction therapy does not prolong survival after lung transplantation. J Heart Lung Transplant. 2008;27:547–553. doi: 10.1016/j.healun.2008.01.022. [DOI] [PubMed] [Google Scholar]
- 171.Mullen JC, Oreopoulos A, Lien DC, Bentley MJ, Modry DL, Stewart K, et al. A randomized, controlled trial of daclizumab vs anti-thymocyte globulin induction for lung transplantation. J Heart Lung Transplant. 2007;26:504–510. doi: 10.1016/j.healun.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 172.Bazerbachi F, Selzner M, Boehnert MU, Marquez MA, Norgate A, McGilvray ID, et al. Thymoglobulin versus basiliximab induction therapy for simultaneous kidney-pancreas transplantation: impact on rejection, graft function, and long-term outcome. Transplantation. 2011;92:1039–1043. doi: 10.1097/TP.0b013e3182313e4f. [DOI] [PubMed] [Google Scholar]
- 173.Cantarovich D, Karam G, Giral-Classe M, Hourmant M, Dantal J, Blancho G, et al. Randomized comparison of triple therapy and antithymocyte globulin induction treatment after simultaneous pancreas-kidney transplantation. Kidney Int. 1998;54:1351–1356. doi: 10.1046/j.1523-1755.1998.00094.x. [DOI] [PubMed] [Google Scholar]
- 174.Cantarovich D, Papuchon E, Guillot-Guéguen C, Blancho G, Dantal J, Giral-Classe M, et al. CNI- and steroid-free immunosuppression after simultaneous pancreas-kidney transplantation: one-year results of a prospective and randomized study. Transpl Int. 2013;26 Suppl s2:172.
- 175.Kaufman DB, Leventhal JR, Koffron AJ, Gallon LG, Parker MA, Fryer JP, et al. A prospective study of rapid corticosteroid elimination in simultaneous pancreas-kidney transplantation: comparison of two maintenance immunosuppression protocols: tacrolimus/mycophenolate mofetil versus tacrolimus/sirolimus. Transplantation. 2002;73:169–177. doi: 10.1097/00007890-200201270-00004. [DOI] [PubMed] [Google Scholar]
- 176.Fridell JA, Agarwal A, Powelson JA, Goggins WC, Milgrom M, Pescovitz MD, et al. Steroid withdrawal for pancreas after kidney transplantation in recipients on maintenance prednisone immunosuppression. Transplantation. 2006;82:389–392. doi: 10.1097/01.tp.0000228904.01482.88. [DOI] [PubMed] [Google Scholar]
- 177.Aoun M, Eschewege P, Hamoudi Y, Beaudreuil S, Duranteau J, Cheisson G, et al. Very early steroid withdrawal in simultaneous pancreas-kidney transplants. Nephrol Dial Transplant. 2007;22:899–905. doi: 10.1093/ndt/gfl660. [DOI] [PubMed] [Google Scholar]
- 178.Freise CE, Kang SM, Feng S, Posselt A, Hirose K, Hirose R, et al. Experience with steroid-free maintenance immunosuppression in simultaneous pancreas-kidney transplantation. Transplant Proc. 2004;36:1067–1068. doi: 10.1016/j.transproceed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 179.Reddy KS, Devarapalli Y, Mazur M, Hamawi K, Chakkera H, Moss A, et al. Alemtuzumab with rapid steroid taper in simultaneous kidney and pancreas transplantation: comparison to induction with antithymocyte globulin. Transplant Proc. 2010;42:2006–2008. doi: 10.1016/j.transproceed.2010.05.090. [DOI] [PubMed] [Google Scholar]
- 180.O’Connell PJ, Holmes-Walker DJ, Goodman D, Hawthorne WJ, Loudovaris T, Gunton JE, et al. Multicenter Australian trial of islet transplantation: improving accessibility and outcomes. Am J Transplant. 2013;13:1850–1858. doi: 10.1111/ajt.12250. [DOI] [PubMed] [Google Scholar]
- 181.Jamiolkowski RM, Guo LY, Li YR, Shaffer SM, Naji A. Islet transplantation in type I diabetes mellitus. Yale J Biol Med. 2012;85:37–43. [PMC free article] [PubMed] [Google Scholar]
- 182.Cai J. Intestine and multivisceral transplantation in the United States: a report of 20-year national registry data (1990–2009). Clin Transplant. 2009;83–101. [PubMed]
- 183.Wu G, Selvaggi G, Nishida S, Moon J, Island E, Ruiz P, et al. Graft-versus-host disease after intestinal and multivisceral transplantation. Transplantation. 2011;91:219–224. doi: 10.1097/TP.0b013e3181ff86ec. [DOI] [PubMed] [Google Scholar]
- 184.Vianna RM, Mangus RS, Fridell JA, Weigman S, Kazimi M, Tector J. Induction immunosuppression with thymoglobulin and rituximab in intestinal and multivisceral transplantation. Transplantation. 2008;85:1290–1293. doi: 10.1097/TP.0b013e31816dd450. [DOI] [PubMed] [Google Scholar]
- 185.Trevizol AP, David AI, Dias ER, Mantovani D, Pécora R, D’Albuquerque LA. Intestinal and multivisceral transplantation immunosuppression protocols: literature review. Transplant Proc. 2012;44:2445–2448. doi: 10.1016/j.transproceed.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 186.Abu-Elmagd KM, Costa G, Bond GJ, Wu T, Murase N, Zeevi A, et al. Evolution of the immunosuppressive strategies for the intestinal and multivisceral recipients with special reference to allograft immunity and achievement of partial tolerance. Transpl Int. 2009;22:96–109. doi: 10.1111/j.1432-2277.2008.00785.x. [DOI] [PubMed] [Google Scholar]
- 187.Abu-Elmagd KM, Costa G, Bond GJ, Soltys K, Martin L, Koritsky DA, et al. A decade of experience with a single dose of rabbit antithymocyte globulin or alemtuzumab pretreatment for intestinal and multivisceral transplantation. Clin Transplant. 2012:155–66. [PubMed]
- 188.Diaz-Siso JR, Bueno EM, Sisk GC, Marty FM, Pomahac B, Tullius SG. Vascularized composite tissue allotransplantation: state of the art. Clin Transplant. 2013;27:330–337. doi: 10.1111/ctr.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Murphy BD, Zuker RM, Borschel GH. Vascularized composite allotransplantation: an update on medical and surgical progress and remaining challenges. J Plast Reconstr Aesthet Surg. 2013;66:1449–1455. doi: 10.1016/j.bjps.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 190.Devauchelle B, Badet L, Lengelé B, Morelon E, Testelin S, Michallet M, et al. First human face allograft: early report. Lancet. 2006;368:203–209. doi: 10.1016/S0140-6736(06)68935-6. [DOI] [PubMed] [Google Scholar]
- 191.Petruzzo P, Testelin S, Kanitakis J, Badet L, Lengelé B, Girbon JP, et al. First human face transplantation: 5 years outcomes. Transplantation. 2012;93:236–240. doi: 10.1097/TP.0b013e31823d4af6. [DOI] [PubMed] [Google Scholar]
- 192.Siemionow MZ, Papay F, Djohan R, Bernard S, Gordon CR, Alam D, et al. First U.S. near-total human face transplantation: a paradigm shift for massive complex injuries. Plast Reconstr Surg. 2010;125:111–122. doi: 10.1097/PRS.0b013e3181c15c4c. [DOI] [PubMed] [Google Scholar]
- 193.Barret JP, Gavaldà J, Bueno J, Nuvials X, Pont T, Masnou N, et al. Full face transplant: the first case report. Ann Surg. 2011;254:252–256. doi: 10.1097/SLA.0b013e318226a607. [DOI] [PubMed] [Google Scholar]
- 194.Dubernard JM, Owen E, Herzberg G, Lanzetta M, Martin X, Kapila H, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353:1315–1320. doi: 10.1016/S0140-6736(99)02062-0. [DOI] [PubMed] [Google Scholar]
- 195.Schneeberger S, Gorantla VS, van Riet RP, Lanzetta M, Vereecken P, van Holder C, et al. Atypical acute rejection after hand transplantation. Am J Transplant. 2008;8:688–696. doi: 10.1111/j.1600-6143.2007.02105.x. [DOI] [PubMed] [Google Scholar]
- 196.Morelon E, Kanitakis J, Petruzzo P. Immunological issues in clinical composite tissue allotransplantation: where do we stand today? Transplantation. 2012;93:855–859. doi: 10.1097/TP.0b013e31824728b8. [DOI] [PubMed] [Google Scholar]
- 197.Engels EA, Pfeiffer RM, Fraumeni JF, Jr, Kasiske BL, Israni AK, Snyder JJ, et al. Spectrum of cancer risk among US solid organ transplant recipients. JAMA. 2011;306:1891–1901. doi: 10.1001/jama.2011.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Kirk AD, Cherikh WS, Ring M, Burke G, Kaufman D, Knechtle SJ, et al. Dissociation of depletional induction and posttransplant lymphoproliferative disease in kidney recipients treated with alemtuzumab. Am J Transplant. 2007;7:2619–2625. doi: 10.1111/j.1600-6143.2007.01972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Caillard S, Lamy FX, Quelen C, Dantal J, Lebranchu Y, Lang P, et al. French Transplant Centers. Epidemiology of posttransplant lymphoproliferative disorders in adult kidney and kidney pancreas recipients: report of the French registry and analysis of subgroups of lymphomas. Am J Transplant. 2012;12:682–693. doi: 10.1111/j.1600-6143.2011.03896.x. [DOI] [PubMed] [Google Scholar]
- 200.Opelz G, Döhler B. Impact of HLA mismatching on incidence of posttransplant non-hodgkin lymphoma after kidney transplantation. Transplantation. 2010;89:567–572. doi: 10.1097/TP.0b013e3181c69855. [DOI] [PubMed] [Google Scholar]
- 201.Emin A, Rogers C, Thekkudan J, Bonser RS, Banner NR. Steering Group, UK Cardiothoracic Transplant Audit. Antithymocyte globulin therapy for adult heart transplantation: a UK national study. J Heart Lung Transplant. 2011;30:770–777. doi: 10.1016/j.healun.2011.01.716. [DOI] [PubMed] [Google Scholar]
- 202.Gajarski R, Blume E, Urschel S, Schechtman K, Zheng J, West LJ, et al. Pediatric Heart Transplant Study Investigators. Infection and malignancy after pediatric transplantation: the role of induction therapy. J Heart Lung Transplant. 2011;30:299–308. doi: 10.1016/j.healun.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 203.Bustami RT, Ojo AO, Wolfe RA, Merion RM, Bennett WM, McDiarmid SV, et al. Immunosuppression and the risk of post-transplant malignancy among cadaveric first kidney transplant recipients. Am J Transplant. 2004;4:87–93. doi: 10.1046/j.1600-6135.2003.00274.x. [DOI] [PubMed] [Google Scholar]
- 204.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019–1026. doi: 10.1097/TP.0b013e31819cc383. [DOI] [PubMed] [Google Scholar]
- 205.Schold JD, Rehman S, Kayle LK, Magliocca J, Srinivas TR, Meier-Kriesche HU. Treatment for BK virus: incidence, risk factors, and outcomes for kidney transplant recipients in the United States. Transpl Int. 2009;22:626–634. doi: 10.1111/j.1432-2277.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 206.Lim WH, Turner RM, Chapman JR, Ma MK, Webster AC, Craig JC, et al. Acute rejection, T-cell-depleting antibodies, and cancer after transplantation. Transplantation. 2014;97:817–825. doi: 10.1097/01.TP.0000442773.38510.32. [DOI] [PubMed] [Google Scholar]
- 207.Snanoudj R, Legendre C. T-cell-depleting antibodies and risk of cancer after transplantation. Transplantation. 2014;97:808–809. doi: 10.1097/01.TP.0000442780.88287.e7. [DOI] [PubMed] [Google Scholar]
- 208.Marks WH, Ilsley JN, Dharnidharka VR. Posttransplantation lymphoproliferative disorder in kidney and heart transplant recipients receiving thymoglobulin: a systematic review. Transplant Proc. 2011;43:1395–1404. doi: 10.1016/j.transproceed.2011.03.036. [DOI] [PubMed] [Google Scholar]
- 209.Opelz G, Döhler B. Lymphomas after solid organ transplantation: a collaborative transplant study report. Am J Transplant. 2004;4:222–230. doi: 10.1046/j.1600-6143.2003.00325.x. [DOI] [PubMed] [Google Scholar]
- 210.Caillard S, Dharnidharka V, Agodoa L, Bohen E, Abbott K. Posttransplant lymphoproliferative disorders after renal transplantation in the United States in era of modern immunosuppression. Transplantation. 2005;80:1233–1243. doi: 10.1097/01.tp.0000179639.98338.39. [DOI] [PubMed] [Google Scholar]
- 211.Cherikh WS, Kauffman HM, McBride MA, Maghirang J, Swinnen LJ, Hanto DW. Association of the type of and induction immunosuppression with posttransplant lymphoproliferative disorder, graft survival, and patient survival after primary kidney transplantation. Transplantation. 2003;76:1289–1293. doi: 10.1097/01.TP.0000100826.58738.2B. [DOI] [PubMed] [Google Scholar]
- 212.Dharnidharka VR, Stevens G. Risk for post-transplant lymphoproliferative disorder after polyclonal antibody induction in kidney transplantation. Pediatr Transplant. 2005;9:622–626. doi: 10.1111/j.1399-3046.2005.00361.x. [DOI] [PubMed] [Google Scholar]
- 213.Faull RJ, Hollett P, McDonald SP. Lymphoproliferative disease after renal transplantation in Australia and New Zealand. Transplantation. 2005;80:193–197. doi: 10.1097/01.tp.0000165098.49658.f3. [DOI] [PubMed] [Google Scholar]
- 214.Dadhania D, Snopkowski C, Ding R, Muthukumar T, Chang C, Aull M, et al. Epidemiology of BK virus in renal allograft recipients: independent risk factors for BK virus replication. Transplantation. 2008;86:521–528. doi: 10.1097/TP.0b013e31817c6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Borni-Duval C, Caillard S, Olagne J, Perrin P, Braun-Parvez L, Heibel F, et al. Risk factors for BK virus infection in the era of therapeutic drug monitoring. Transplantation. 2013;95:1498–1505. doi: 10.1097/TP.0b013e3182921995. [DOI] [PubMed] [Google Scholar]
- 216.Ramos E, Drachenberg CB, Wali R, Hirsch HH. The decade of polyomavirus BK-associated nephropathy: state of affairs. Transplantation. 2009;87:621–630. doi: 10.1097/TP.0b013e318197c17d. [DOI] [PubMed] [Google Scholar]
- 217.Acott P, Babel N. BK virus replication following kidney transplant: does the choice of immunosuppressive regimen influence outcomes? Ann Transplant. 2012;17:86–99. doi: 10.12659/aot.882640. [DOI] [PubMed] [Google Scholar]
- 218.Issa NC, Fishman JA. Infectious complications of antilymphocyte therapies in solid organ transplantation. Clin Infect Dis. 2009;48:772–786. doi: 10.1086/597089. [DOI] [PubMed] [Google Scholar]
- 219.Remberger M, Svahn BM, Hentschke P, Löfgren C, Ringdén O. Effect on cytokine release and graft-versus-host disease of different anti-T cell antibodies during conditioning for unrelated haematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:823–830. doi: 10.1038/sj.bmt.1701991. [DOI] [PubMed] [Google Scholar]
- 220.Bacigalupo A, Lamparelli T, Bruzzi P, Guidi S, Alessandrino PE, di Bartolomeo P, et al. Antithymocyte globulin for graft-versus-host disease prophylaxis in transplants from unrelated donors: 2 randomized studies from Gruppo Italiano Trapianti Midollo Osseo (GITMO) Blood. 2001;98:2942–2947. doi: 10.1182/blood.v98.10.2942. [DOI] [PubMed] [Google Scholar]
- 221.Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biol Blood Marrow Transplant. 2006;12:560–565. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 222.Duggan P, Booth K, Chaudhry A, Stewart D, Ruether JD, Glück S, et al. Unrelated donor BMT recipients given pretransplant low dose antithymocyte globulin have outcomes equivalent to matched sibling BMT: a matched pair analysis. Bone Marrow Transplant. 2002;30:681–686. doi: 10.1038/sj.bmt.1703674. [DOI] [PubMed] [Google Scholar]
- 223.Remberger M, Storer B, Ringdén O, Anasetti C. Association between pretransplant Thymoglobulin and reduced non-relapse mortality rate after marrow transplantation from unrelated donors. Bone Marrow Transplant. 2002;29:391–397. doi: 10.1038/sj.bmt.1703374. [DOI] [PubMed] [Google Scholar]
- 224.Deeg HJ, Storer BE, Boeckh M, Martin PJ, McCune JS, Myerson D, et al. Reduced incidence of acute and chronic graft-versus-host disease with the addition of thymoglobulin to a targeted busulfan/cyclophosphamide regimen. Biol Blood Marrow Transplant. 2006;12:573–584. doi: 10.1016/j.bbmt.2005.12.036. [DOI] [PubMed] [Google Scholar]
- 225.Call SK, Kasow KA, Barfield R, Madden R, Leung W, Horwitz E, et al. Total and active rabbit antithymocyte globulin (rATG; Thymoglobulin) pharmacokinetics in pediatric patients undergoing unrelated donor bone marrow transplantation. Biol Blood Marrow Transplant. 2009;15:274–278. doi: 10.1016/j.bbmt.2008.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Russell JA, Turner AR, Larratt L, Chaudhry A, Morris D, Brown C, et al. Adult recipients of matched related donor blood cell transplants given myeloablative regimens including pretransplant antithymocyte globulin have lower mortality related to graft-versus-host disease: a matched pair analysis. Biol Blood Marrow Transplant. 2007;13:299–306. doi: 10.1016/j.bbmt.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 227.Russell JA, Duan Q, Chaudhry MA, Savoie ML, Balogh A, Turner AR, et al. Transplantation from matched siblings using once-daily intravenous busulfan/fludarabine with thymoglobulin: a myeloablative regimen with low nonrelapse mortality in all but older patients with high-risk disease. Biol Blood Marrow Transplant. 2008;14:888–895. doi: 10.1016/j.bbmt.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 228.Galambrun C, Pondarré C, Bertrand Y, Loundou A, Bordigoni P, Frange P, et al. French multicenter 22-year experience in stem cell transplantation for beta-thalassemia major: lessons and future directions. Biol Blood Marrow Transpl. 2013;9:62–68. doi: 10.1016/j.bbmt.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 229.Al-Kadhimi Z, Gul Z, Rodriguez R, Chen W, Smith D, Mitchell A, et al. Anti-thymocyte globulin (thymoglobulin), tacrolimus, and sirolimus as acute graft-versus-host disease prophylaxis for unrelated hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1734–1744. doi: 10.1016/j.bbmt.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Nath CE, Shaw PJ. Busulphan in blood and marrow transplantation: dose, route, frequency and role of therapeutic drug monitoring. Curr Clin Pharmacol. 2007;2:75–91. doi: 10.2174/157488407779422249. [DOI] [PubMed] [Google Scholar]
- 231.Mohty M, Gaugler B. Advances in umbilical cord transplantation: the role of thymoglobulin/ATG in cord blood transplantation. Best Pract Res Clin Haematol. 2010;23:275–82. [DOI] [PubMed]
- 232.Mohty M, Bay JO, Faucher C, Choufi B, Bilger K, Tournilhac O, et al. Graft-versus- host disease following allogeneic transplantation from HLA-identical sibling with antithymocyte globulin-based reduced-intensity preparative regimen. Blood. 2003;102:470–476. doi: 10.1182/blood-2002-12-3629. [DOI] [PubMed] [Google Scholar]
- 233.Lowsky R, Takahashi T, Liu YP, Dejbakhsh-Jones S, Grumet FC, Shizuru JA, et al. Protective conditioning for acute graft-versus-host disease. N Engl J Med. 2005;353:1321–1331. doi: 10.1056/NEJMoa050642. [DOI] [PubMed] [Google Scholar]
- 234.Bashir Q, Munsell MF, Giralt S, de Padua Silva L, Sharma M, Couriel D, et al. Randomized phase II trial comparing two dose levels of thymoglobulin in patients undergoing unrelated donor hematopoietic cell transplant. Leuk Lymphoma. 2012;53:915–919. doi: 10.3109/10428194.2011.634039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Remberger M, Ringdén O, Hägglund H, Svahn BM, Ljungman P, Uhlin M, et al. A high antithymocyte globulin dose increases the risk of relapse after reduced intensity conditioning HSCT with unrelated donors. Clin Transplant. 2013;27:E368–E374. doi: 10.1111/ctr.12131. [DOI] [PubMed] [Google Scholar]
- 236.Blaise D, Tabrizi R, Boher JM, Le Corroller-Soriano AG, Bay JO, Fegueux N, et al. Randomized study of 2 reduced-intensity conditioning strategies for human leukocyte antigen-matched, related allogeneic peripheral blood stem cell transplantation: prospective clinical and socioeconomic evaluation. Cancer. 2013;119:602–611. doi: 10.1002/cncr.27786. [DOI] [PubMed] [Google Scholar]
- 237.Horn B, Baxter-Lower LA, Englert L, McMillan A, Quinn M, Desantes K, et al. Reduced intensity conditioning using intravenous busulfan, fludarabine and rabbit ATG for children with nonmalignant disorders and CML. Bone Marrow Transplant. 2006;37:263–269. doi: 10.1038/sj.bmt.1705240. [DOI] [PubMed] [Google Scholar]
- 238.Sormani MP, Oneto R, Bruno B, Fiorone M, Lamparelli T, Gualandi F, et al. A revised day +7 predictive score for transplant-related mortality: serum cholinesterase, total protein, blood urea nitrogen, gamma glutamil transferase, donor type and cell dose. Bone Marrow Transplant. 2003;32:205–211. doi: 10.1038/sj.bmt.1704085. [DOI] [PubMed] [Google Scholar]
- 239.Bacigalupo A, Lamparelli T, Milone G, Sormani MP, Ciceri F, Peccatori J, et al. Gruppo Italiano Trapianto Midollo Osseo (GITMO). Pre-emptive treatment of acute GVHD: a randomized multicenter trial of rabbit anti-thymocyte globulin, given on day + 7 after alternative donor transplants. Bone Marrow Transplant. 2010;45:385–391. doi: 10.1038/bmt.2009.151. [DOI] [PubMed] [Google Scholar]
- 240.Van Lint MT, Milone G, Leotta S, Uderzo C, Scime R, Dallorso S, et al. Treatment of acute graft-versus-host disease with prednisolone: significant survival advantage for day +5 responders and no advantage for nonresponders receiving anti-thymocyte globulin. Blood. 2006;107:4177–4181. doi: 10.1182/blood-2005-12-4851. [DOI] [PubMed] [Google Scholar]
- 241.Antin JH, Chen AR, Couriel DR, Ho VT, Nash RA, Weisdorf D. Novel approaches to the therapy of steroid-resistant acute graft-versus-host disease. Biol Blood Marrow Transplant. 2004;10:655–668. doi: 10.1016/j.bbmt.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 242.Van Esser JW, van der Holt B, Meijer E, Niesters HG, Trenschel R, Thijsen SF, et al. Epstein-Barr virus (EBV) reactivation is a frequent event after allogeneic stem cell transplantation (SCT) and qualitatively predicts EBV-lymphoproliferative disease following T-cell-depleted SCT. Blood. 2001;98:972–978. doi: 10.1182/blood.v98.4.972. [DOI] [PubMed] [Google Scholar]
- 243.Dominietto A, Tedone E, Soracco M, Bruno B, Raiola AM, Van Lint MT, et al. In vivo B-cell depletion with rituximab for alternative donor hemopoietic SCT. Bone Marrow Transplant. 2012;47:101–106. doi: 10.1038/bmt.2011.28. [DOI] [PubMed] [Google Scholar]
- 244.Champlin RE, Perez WS, Passweg JR, Klein JP, Camitta BM, Gluckman E, et al. Bone marrow transplantation for severe aplastic anemia: a randomized controlled study of conditioning regimens. Blood. 2007;109:4582–4585. doi: 10.1182/blood-2006-10-052308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Bacigalupo A, Socié G, Schrezenmeier H, Tichelli A, Locasciulli A, Fuehrer M, et al. Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation (WPSAA-EBMT). Bone marrow versus peripheral blood as the stem cell source for sibling transplants in acquired aplastic anemia: survival advantage for bone marrow in all age groups. Haematologica. 2012;97:1142–1148. doi: 10.3324/haematol.2011.054841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Bacigalupo A, Socie’ G, Lanino E, Prete A, Locatelli F, Locasciulli A, et al. Severe Aplastic Anemia Working Party of the European Group for Blood and Marrow Transplantation. Fludarabine, cyclophosphamide, antithymocyte globulin, with or without low dose total body irradiation, for alternative donor transplants, in acquired severe aplastic anemia: a retrospective study from the EBMT-SAA working party. Haematologica. 2010;95:976–982. doi: 10.3324/haematol.2009.018267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kang HJ, Shin HY, Park JE, Chung NG, Cho B, Kim HK, et al. Successful engraftment with fludarabine, cyclophosphamide, and thymoglobulin conditioning regimen in unrelated transplantation for severe aplastic anemia: a phase II prospective multicenter study. Biol Blood Marrow Transplant. 2010;16:1582–1588. doi: 10.1016/j.bbmt.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 248.Korthof ET, Békássy AN, Hussein AA. Management of acquired aplastic anemia in children. Bone Marrow Transplant. 2013;48:191–195. doi: 10.1038/bmt.2012.235. [DOI] [PubMed] [Google Scholar]
- 249.European Blood and Marrow Transplant Group Severe Aplastic Anaemia Working Party. Rabbit ATG for aplastic anaemia treatment: a backward step? Lancet. 2011;378:1831–1833. doi: 10.1016/S0140-6736(11)60817-9. [DOI] [PubMed] [Google Scholar]
- 250.Afable MG, 2nd, Shaik M, Sugimoto Y, Elson P, Clemente M, Makishima H, et al. Efficacy of rabbit antithymocyte globulin in severe aplastic anemia. Haematologica. 2011;96:1269–1275. doi: 10.3324/haematol.2011.042622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Atta EA, Dias DS, Marra VLN, de Azevedo AM. Comparison between horse and rabbit antithymocyte globulin as first line treatment for patients with severe aplastic anemia: a single-center retrospective study. Ann Hematol. 2010;89:851–859. doi: 10.1007/s00277-010-0944-y. [DOI] [PubMed] [Google Scholar]
- 252.Halkes CJM, Brand A, von dem Borne PA, Marijt EW, Willemze R, Veelken J, et al. Increasing the dose of rabbit-ATG does not lead to a higher response rate in the first-line treatment of severe aplastic anaemia. Bone Marrow Transplant. 2011;46(1 Suppl):S373. [Google Scholar]
- 253.Marsh JC, Bacigalupo A, Schrezenmeier H, Tichelli A, Risitano AM, Passweg JR, et al. Prospective study of rabbit antithymocyte globulin and cyclosporine for aplastic anemia from the EBMT Severe Aplastic Anaemia Working Party. Blood. 2012;119:5391–5396. doi: 10.1182/blood-2012-02-407684. [DOI] [PubMed] [Google Scholar]
- 254.Sakamoto T, Obara N, Kurita N, Sakata-Yanagimoto M, Nishikii H, Yokoyama Y, et al. Effectiveness and safety of rabbit anti-thymocyte globulin in Japanese patients with aplastic anemia. Int J Hematol. 2013;98:319–322. doi: 10.1007/s12185-013-1418-5. [DOI] [PubMed] [Google Scholar]
- 255.Scheinberg P, Nunez O, Weinstein B, Scheinberg P, Biancotto A, Wu CO, et al. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365:430–438. doi: 10.1056/NEJMoa1103975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Snowden JA, Saccardi R, Allez M, Ardizzone S, Arnold R, Cervera R, et al. EBMT Autoimmune Disease Working Party (ADWP); Paediatric Diseases Working Party (PDWP). Haematopoietic SCT in severe autoimmune diseases: updated guidelines of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2012;47:770–790. doi: 10.1038/bmt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Saccardi R, Freedman MS, Sormani MP, Atkins H, Farge D, Griffith LM, et al. European Blood and Marrow Transplantation Group; Center for International Blood and Marrow Research; HSCT in MS International Study Group. A prospective, randomized, controlled trial of autologous haematopoietic stem cell transplantation for aggressive multiple sclerosis: a position paper. Mult Scler. 2012;18:825–834. doi: 10.1177/1352458512438454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Strober S. Protective conditioning against GVHD and graft rejection after combined organ and hematopoietic cell transplantation. Blood Cells Mol Dis. 2008;40:48–54. doi: 10.1016/j.bcmd.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 259.Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362–368. doi: 10.1056/NEJMoa074191. [DOI] [PubMed] [Google Scholar]
- 260.Scandling JD, Busqueb S, Dejbakhsh-Jones S, Beniked C, Sarwale M, Millanb MT, et al. Tolerance and withdrawal of immunosuppressive drugs in patients given kidney and hematopoietic cell transplants. Am J Transplant. 2012;12:1133–1145. doi: 10.1111/j.1600-6143.2012.03992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Libetta A, Canevari M, Margiotta E, Martinelli C, Borettaz I, Esposito P, et al. Preliminary data of controlled randomised study (EVER TWIST) on tolerance induction. Transpl Int. 2011;26(Suppl 2):20. [Google Scholar]
- 262.Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, et al. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502–1510. doi: 10.1016/s0140-6736(03)13175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353–361. doi: 10.1056/NEJMoa071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Couri CE, Oliveira MC, Stracieri AB, Moraes DA, Pieroni F, Barros GM, et al. C-peptide levels and insulin independence following autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2009;301:1573–1579. doi: 10.1001/jama.2009.470. [DOI] [PubMed] [Google Scholar]
- 265.Voltarelli JC, Couri CE, Stracieri AB, Oliveira MC, Moraes DA, Pieroni F, et al. Autologous nonmyeloablative hematopoietic stem cell transplantation in newly diagnosed type 1 diabetes mellitus. JAMA. 2007;297:1568–1576. doi: 10.1001/jama.297.14.1568. [DOI] [PubMed] [Google Scholar]
- 266.Thomas HR, Gitelman SE. Altering the course of type 1 diabetes: an update on prevention and new-onset clinical trials. Pediatr Diab. 2013;14:311–321. doi: 10.1111/pedi.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Kadia TM, Borthakur G, Garcia-Manero G, Faderl S, Jabbour E, Estrov Z, et al. Final results of the phase II study of rabbit anti-thymocyte globulin, ciclosporin, methylprednisone, and granulocyte colony-stimulating factor in patients with aplastic anaemia and myelodysplastic syndrome. Br J Haematol. 2012;157:312–320. doi: 10.1111/j.1365-2141.2012.09064.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Garg R, Faderl S, Garcia-Manero G, Cortes J, Koller C, Huang X, et al. Phase II study of rabbit anti-thymocyte globulin, cyclosporine and granulocyte colony-stimulating factor in patients with aplastic anemia and myelodysplastic syndrome. Leukemia. 2009;23:1297–1302. doi: 10.1038/leu.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Burt RK, Marmont A, Oyama Y, Slavin S, Arnold R, Hiepe F, et al. Randomized controlled trials of autologous hematopoietic stem cell transplantation for autoimmune diseases: the evolution from myeloablative to lymphoablative transplant regimens. Arthritis Rheum. 2006;54:3750–3760. doi: 10.1002/art.22256. [DOI] [PubMed] [Google Scholar]
- 270.Lytton SD, Denton CP, Nutzenberger AM. Treatment of autoimmune disease with rabbit anti-T lymphocyte globulin: clinical efficacy and potential mechanisms of action. Ann N Y Acad Sci. 2007;1110:285–296. doi: 10.1196/annals.1423.030. [DOI] [PubMed] [Google Scholar]


