Abstract
Many synthetic polycationic vectors for non-viral gene delivery show high efficiency in vitro, but their usually excessive charge density makes them toxic for in vivo applications. Here we describe the synthesis of a series of high molecular weight terpolymers with low charge density, and show that they exhibit efficient gene delivery, some surpassing the efficiency of the commercial transfection reagents Polyethylenimine and Lipofectamine 2000. The terpolymers were synthesized via enzyme-catalyzed copolymerization of lactone with dialkyl diester and amino diol, and their hydrophobicity adjusted by varying the lactone content and by selecting a lactone comonomer of specific ring size. Targeted delivery of the pro-apoptotic TRAIL gene to tumour xenografts by one of the terpolymers results in significant inhibition of tumour growth, with minimal toxicity both in vitro and in vivo. Our findings suggest that the gene delivery ability of the terpolymers stems from their high molecular weight and increased hydrophobicity, which compensates for their low charge density.
Non-viral vectors for gene delivery have attracted much attention in the past few decades because of their potential for limited immunogenicity, their ability to accommodate and deliver large-size genetic materials, and the potential for modification of their surface structures. The major categories of non-viral vectors include cationic lipids and cationic polymers. Cationic lipid-derived vectors, which were pioneered by Feigner and colleagues1, represent the most extensively investigated systems for non-viral gene delivery2,3. Cationic polymer non-viral vectors have gained increasing attention because of flexibility in their synthesis and structural modifications for specific biomedical applications. Both cationic lipid and cationic polymer systems deliver genes by forming condensed complexes with negatively charged deoxyribonucleic acid (DNA) through electrostatic interactions: complex formation protects DNA from degradation and facilitates its cellular uptake and intracellular traffic into the nucleus. Polyplexes formed between cationic polymers and DNA are relatively more stable than lipoplexes formed between cationic lipids and DNA (ref. 4), but both are often unstable in physiological fluids, which contain serum components and salts, and in which the complexes tend to break apart or aggregate5. Furthermore, although some work suggests that anionic polymers6,7 or even naked DNA (refs 8,9) can provide some level of transfection under certain conditions, transfection by both lipids and polymers usually requires materials with excess charge, resulting in polyplexes or lipoplexes with net positive charges on the surface. When injected into the circulatory system in vivo, the positive surface charge initiates rapid formation of complex aggregates with negatively charged serum molecules or membranes of cellular components, which are then cleared by the reticuloendothelial system (RES). More importantly, many cationic vectors developed so far exhibit substantial toxicity5,10–13, which has limited their clinical applicability. This too appears to depend on charge: excess positive charges on the surface of the complexes can interact with cellular components, such as cell membranes11, and inhibit normal cellular processes, such as clathrin-mediated endocytosis10, activity of ion channels, of membrane receptors and enzymes11, and cell-survival signalling12. As a result, cationic lipids often cause acute inflammatory responses in animals and humans5,13, whereas cationic polymers, such as polyethylenimine (PEI), destabilize the plasma membrane of red blood cells and induce cell necrosis, apoptosis and autophagy5,10,13. Because of these undesirable effects, there is a need for highly efficient non-viral vectors that have lower charge densities.
Recently, we synthesized a family of biodegradable poly(amine-co-esters) formed through enzymatic copolymerization of diesters with amino-substituted diols14,15. Diesters with various chain lengths (for example, from succinate to dodecanedioate) were successfully copolymerized with diethanolamines with either an alkyl (methyl, ethyl, n-butyl, t-butyl) or an aryl (phenyl) substituent on the nitrogen. The high tolerance of the lipase catalyst allowed the copolymerization reactions to complete in one step without protection and deprotection of the amino functional groups. Following protonation in slightly acidic conditions, these poly(amine-co-esters) readily condense DNA and form nanosized polyplexes. Screening studies revealed that one of these materials, poly(N-methyldiethyleneamine sebacate) (PMSC), transfected a variety of cells, including Human Embryonic Kidney (HEK) 293, U87-MG, and 9L, with efficiencies comparable to that of leading commercial products, such as Lipofectamine 2000 and PEI (ref. 14). PMSC had been previously used for gene delivery16,17, but the delivery efficiency of the enzymatically synthesized materials was approximately five orders of magnitude higher than any previously reported. We hypothesize that this greater efficiency is due to the higher molecular weight obtained by our enzymatic synthesis: for example, PMSC synthesized via polycondensation of sebacoyl chloride with N-methyldiethanolamine has a weight-average molecular weight (Mw) less than 18.5kDa (refs 16,17), whereas the PMSC prepared using the lipase catalyst has a Mw of over 30kDa.
We hypothesize that molecular weight and hydrophobicity are important factors for gene delivery, particularly for polycationic polymers with a low charge density. Cationic polymers with a high charge density, such as PEI, are able to condense DNA predominantly through ionic interactions. In contrast, ionic interactions alone might not be sufficient for formation of stable polyplexes with DNA using polymers with a low charge density. For those polycations, a further chain entanglement effect, which depends on polymer molecular weight, might be required18. Furthermore, DNA condensation is an entropically driven process, which relies on the release of counter ions of the DNA phosphate groups19. Compared with low molecular weight polycation polymers, high molecular weight polycations can afford more entropy loss, and thus are theoretically more efficient in condensing DNA (ref. 20). As well as the favourable molecular weight of the enzymatically synthesized PMSC, the hydrophobicity of the sebacate units in the polymer should also contribute to its gene delivery efficiency. Compared with most cationic polymers, PMSC has low nitrogen content (4.9 wt% versus 33 wt% in PEI); thus, relatively weak ionic interactions between PMSC and DNA are anticipated. In aqueous medium, this factor can potentially be compensated by strong interactions between the sebacate units of PMSC, which might form hydrophobic domains that non-covalently crosslink PMSC-DNA polyplexes and enhance their stability. Others have provided evidence that hydrophobicity is an important influencing factor for gene delivery21, which functions to: (1) enhance DNA condensation through cooperative binding22,23; (2) promote polyplex charge inversion and allow interaction with cell membranes22; (3) strengthen interactions with phospholipid membranes, thus promoting endocytosis24,25; (4) alleviate serum inhibition26,27; and (5) facilitate DNA release from polycation carriers28. Unfortunately, the role of hydrophobicity of cationic polymers in gene delivery is underappreciated.
Synthesis and characterization of terpolymers
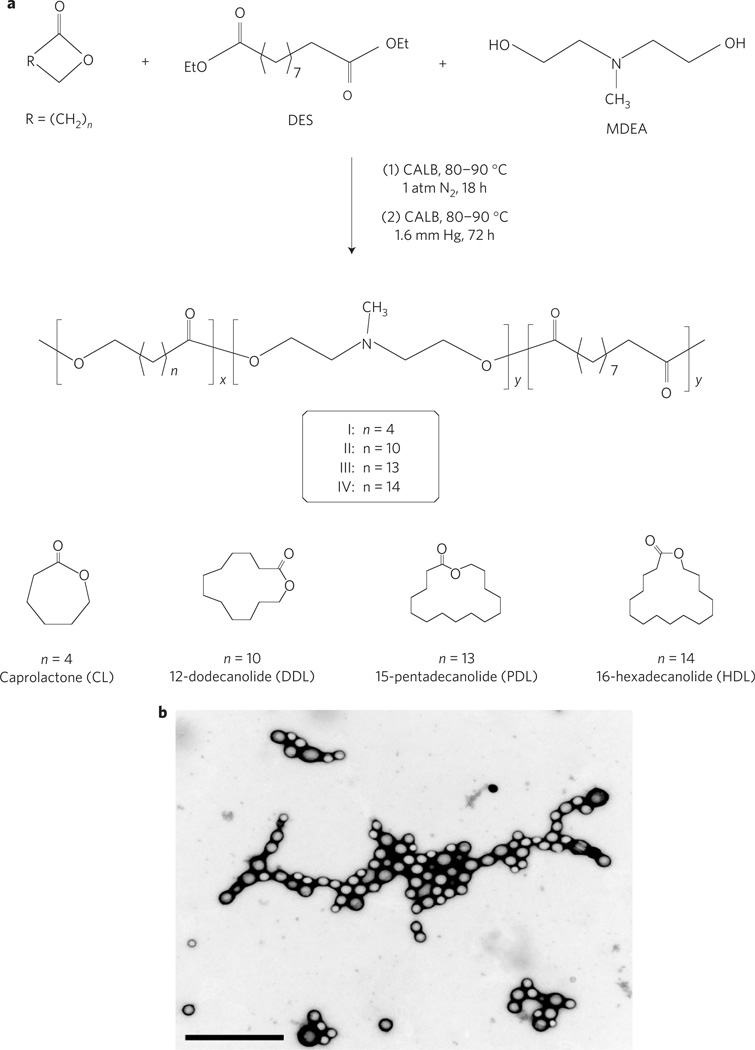
In prior work, we found that PMSC was an efficient vector for in vitro transfection of cell lines and for direct intratumoral gene delivery in vivo14, but was not able to deliver genes after systemic administration (unpublished data). We hypothesized that modification of PMSC chain structures with additional hydrophobic repeat units might lead to more efficient gene vectors. For this purpose, we have developed a novel method for the synthesis of terpolymers from lactone, diethyl sebacate (DES) and N-methyldiethanolamine (MDEA) using Candida antarctica lipase B (CALB) as catalyst. As reported previously, CALB is an efficient catalyst for combined ring-opening and condensation copolymerization of lactone with dialkyl diester and conventional diol monomers29,30. The terpolymerization of DES and MDEA with lactones of various ring sizes was performed in two stages: oligomerization under 1 atmospheric pressure of nitrogen, followed by polymerization at 1.6 mmHg vacuum (Fig. 1a). During the initial oligomerization, the monomers were converted to non-volatile oligomers. The subsequent use of high vacuum facilitates removal of the by-product ethanol, thus accelerating polymer chain growth. This method allowed for the synthesis of novel poly(amine-co-esters) with diverse chain structures and tunable hydrophobicity; the method is unique in that lactones in a wide range of ring sizes (C6 to C16) can serve as comonomers, and in that the copolymerization reactions were accomplished in one step without protection and deprotection of the amino group of MDEA. Such amino-bearing copolyesters would be extremely difficult to synthesize using conventional organometallic catalysts, as metal catalysts are often sensitive to (or deactivated by) organic amines31 and are known to be inefficient for polymerizing large ring lactone monomers32. Furthermore, enzymatic polymerization catalysis has distinct advantages for producing biomedical polymers owing to the high activity and extraordinary selectivity of enzyme catalysts and the resultant high purity of products that are also metal-free19. In the design of the current polycationic gene carriers, lactone was chosen as one of the comonomers, because the hydrophobicity of lactone-DES-MDEA terpolymers could be effectively altered by choosing a lactone with a specific ring size and/or by adjusting lactone unit content in the terpolymers. Furthermore, readily available lactones of various ring sizes are known to possess low toxicity: for example, polyesters derived from small lactones, such as poly(ε-caprolactone) and poly(p-dioxanone), are commercial biomaterials and have been used in clinical applications. Large (for example, C16–C24) lactones and their polyester derivatives are natural products that were found to be present in several different species of bee33–35.
Figure 1. Synthesis and characteristics of polymers and terpolymer/DNA complexes.
a, Two-stage process for terpolymerization of lactone with DES and MDEA. b, Visualization of |||-20% PDL/DNA polyplexes at 100:1 weight ratio using TEM. Scale bar represents 1 µm.
Table 1 shows the yield, composition, molecular weight, poly-dispersity, and other characterization data of selected lactone-DES-MDEA terpolymers that were prepared as described above. Complete data on all terpolymers, including those samples that have low solubility in polar organic solvents (for example, dimethyl Sulphoxide (DMSO)) and thus were not able to form polyplexes with DNA, are summarized in Supplementary Table S1. To simplify nomenclature in this report, CL-DES-MDEA, DDL-DES-MDEA, PDL-DES-MDEA, and HDL-DES-MDEA terpolymers are designated as polymer I, II, III, and IV, respectively (Table 1a; CL is ε-caprolactone, DDL is 12-dodecanolide, PDL is 15-pentadecanolide and HDL is 16-hexadecanolide). The composition of each individual terpolymer is further denoted as x% lactone, indicating the lactone unit content (mol% versus (lactone+sebacate) units) in the polymer. For example, II-40%DDL and III-20%PDL represent DDL-DES-MDEA copolymer with 40% DDL and PDL-DES-MDEA copolymer with 20% PDL, respectively. The lactone-DES-MDEA terpolymers were obtained in good yields (80–86%) and the compositions of the terpolymers were readily controlled by adjusting the corresponding monomer feed ratio (Table 1). The molecular weight (Mw) of the polymers ranged from 18,000 to 39,000, with polydispersity (Mw/Mn) between 1.8 and 2.3. Compared with PEI, which contains 32.6 wt% nitrogen, the lactone-DES-MDEA terpolymers had low nitrogen contents (1.9–4.7 wt%). In general, the solubility of lactone-DES-MDEA terpolymer in DMSO decreases with increasing lactone ring size at a given lactone content. Among terpolymers synthesized from the same lactone, solubility in DMSO is lower at a higher lactone content.
Table 1.
Characterization of selected lactone-DES-MDEA terpolymers.
| Name* | Lactone/DES/MDEA (feed molar ratio) |
Lactone/Sebacate/MDEA (unit molar ratio)† |
Isolated yield (%) |
Mw† | Mw/Mn‡ | Nitrogen content (wt%) |
Solubility in DMSO mg ml−1 |
|---|---|---|---|---|---|---|---|
| PMSC | 0:50:50 | 0:50:50 | - | 31,800 | 2.3 | 4.9 | >25 |
| I-10%CL | 10:90:90 | 10:90:90 | 85 | 18,400 | 1.9 | 4.7 | >25 |
| I-20%CL | 20:80:80 | 20:80:80 | 80 | 19,100 | 1.9 | 4.5 | >25 |
| I-40%CL | 40:60:60 | 40:60:60 | 83 | 18,400 | 1.8 | 3.9 | >25 |
| I-60%CL | 60:40:40 | 60:40:40 | 81 | 17,800 | 1.8 | 3.1 | >25 |
| I-80%CL | 80:20:20 | 80:20:20 | 86 | 20,300 | 2.0 | 1.9 | >25 |
| II-10%DDL | 10:90:90 | 10:90:90 | 82 | 24,900 | 1.9 | 4.6 | >25 |
| II-20%DDL | 20:80:80 | 20:80:80 | 80 | 29,300 | 2.0 | 4.2 | >25 |
| II-40%DDL | 40:60:60 | 40:60:60 | 81 | 25,800 | 1.8 | 3.4 | >25 |
| III-10%PDL | 10:90:90 | 10:90:90 | 81 | 30,700 | 2.1 | 4.5 | >25 |
| III-20%PDL | 20:80:80 | 20:80:80 | 83 | 38,700 | 2.3 | 4.1 | ≈25 |
| IV-10%HDL | 10:90:90 | 10:90:90 | 80 | 25,700 | 1.8 | 4.5 | <25 |
The polymer names are abbreviated or simplified as described in the main text. PMSC: poly(N-methyldiethyleneamine sebacate). The structures of terpolymers I–IV are shown in Fig. 1a. Each terpolymer is denoted with x% lactone indicating the lactone unit content (mol% versus (lactone+sebacate) units) in the polymer.
Measured by 1H NMR spectroscopy.
Measured by gel permeation chromatography (GPC) using narrow polydispersity polystyrene standards.
The lactone-DES-MDEA terpolymers were characterized by 1H and 13C NMR spectroscopy. As expected, the polymer chains consist of three different types of repeat unit: lactone, MDEA, and sebacate (Fig. 1a). Proton NMR spectra were used to measure the composition (repeat unit ratio) of the terpolymers. The repeat unit sequence distributions (diad distributions) in the polymers were analysed by 13C NMR spectroscopy and the experimental results were compared against the values calculated for statistically random terpolymers at same compositions. Consistent with the microstructures of PDL-diethyl succinate-1,4-butanediol terpolymers that were prepared previously using the same catalyst29,30, the unit arrangements in lactone-DES-MDEA copolymers were also random. Thus, these polymers can also be described as poly(lactone-co-diethyleneamine-co-sebacate). Further analyses on the structure, composition, and unit sequence distribution of lactone-DES-MDEA terpolymers are presented in the Supplementary Information.
Because of the branching nature of the tertiary amino groups in polymer chains, PMSC was a viscous liquidatambient temperature. Incorporation of lactone into the poly(amine-co-ester) resulted in lactone-DES-MDEA terpolymers in which the physical properties vary substantially depending on the ring size of the lactone and its content in the polymers. In general, the terpolymers with a small-ring lactone and low lactone content are liquids, and those with a large lactone and a high lactone content are waxy or solid materials. Thus, I-(10–80)% CL, II-(10–40)% DDL, III-(10–20)% PDL, and IV-10% HDL were viscous liquids at room temperature whereas II-(60–80)% DDL, III-(40–80)% PDL, and IV-(20–80)% HDL were either semisolidor solid polymers (Supplementary Table S1).
Gene delivery with terpolymers
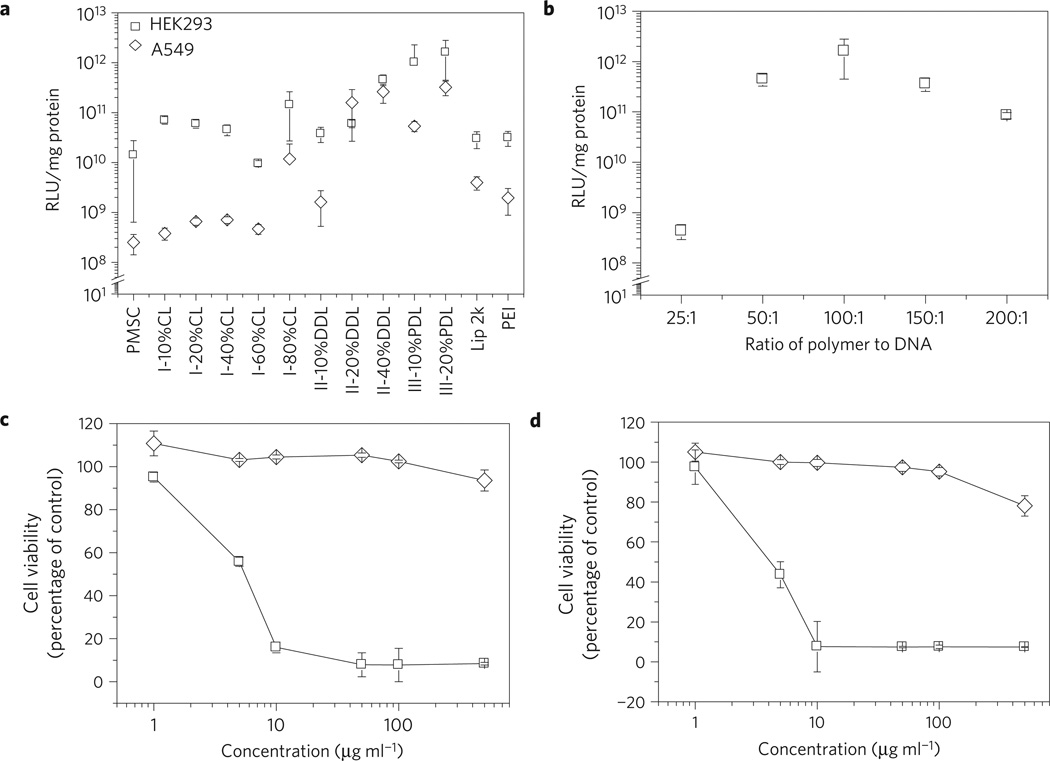
All liquid terpolymers were evaluated for luciferase gene transfection on HEK293 and A549 cells. Gene transfection efficiency increased with increasing lactone content for both terpolymer II and III (Fig. 2a). Thus, II-40% DDL transfected A549 cells with an efficiency that is 2, 162, and 1,047 times higher than that of II-20% DDL, II-10% DDL, and PMSC, respectively. On the other hand, III-20% PDL was 6 and 1,259 times more efficient than III-10% PDL and PMSC, respectively, in transfecting A549 cells (Fig. 2a). A similar trend was observed for transfecting HEK293 cells (Fig. 2a). Although group I terpolymers in general were more effective gene carriers than PMSC, the correlation between their transfection efficiency and lactone content does not follow a simple, consistent trend as observed for terpolymers II and III. Lactone ring size also significantly affects the gene delivery performance of the terpolymers. At a given lactone content, terpolymers with long chain lactone units deliver genes with higher efficiency than those with short chain lactone units. For example, the efficiency of HEK293 cell transfection was 27 times higher for III-10% PDL versus II-10% DDL, and was 27 times higher for III-20% PDL versus II-20% DDL (Fig. 2a). Similar lactone size effects were observed for the transfection of A549 cells (Fig. 2a). These results demonstrate that gene transfection efficiency of lactone-DES-MDEA terpolymers can be improved by using a large lactone and by adjusting lactone content in the polymers. The remarkable effects of lactone ring size and lactone unit content on gene transfection performance of lactone-DES-MDEA terpolymers support our hypothesis that hydrophobicity plays an important role in influencing transfection efficiency of cationic polymers.
Figure 2. Gene delivery efficiency and toxicity of terpolymer.
a, Gene delivery efficiency of terpolymers on HEK293 cells (open square) and A549 cells (open diamond). Polyplexes of DNA and terpolymer were prepared at a weight ratio of 1:100. Transfections by Lipofectamine 2000 and PEI were performed according to the manufacturer’s standard protocols. The same amount of DNA was used for all transfection experiments. b, The effect of III-20% PDL to DNA ratio on transfection efficiency on HEK293 cells. The same amount of DNA but various amount of polymer, as indicated, was used for forming polyplexes. c, d, Toxicity of PEI (open square) and III-20% PDL (open diamond) on HEK293 cells (c) and A549 cells (d). Toxicity is given as the percentage of viable cells remained after treatment for three days, compared against the control vehicle treated cells. Cell number was determined by the standard MTT assay. All experiments were carried out in triplicate and the standard deviation is shown by the error bars. Luciferase signal was detected 48 h after transfection. Luciferase signal is normalized by the amount of protein for comparison.
Among all terpolymers evaluated, III-20% PDL showed the best gene delivery ability. Terpolymer III with a higher PDL content (for example, III-40% PDL and III-60% PDL) and lactone-DES-MDEA terpolymers with a larger lactone (for example, group IV terpolymers) had low solubility in polar organic solvents (for example, DMSO), and were not able to form polyplexes in aqueous solution. Despite the fact that III-20% PDL has lower nitrogen density than PMSC (Table 1), the optimal polyplex composition for gene delivery was the same (at 100:1 weight ratio of polymer to DNA) for both polymers (Fig. 2b and ref. 14). There was a dramatic increase in transfection efficiency between particles with 25:1 and 50:1 polymer to DNA ratio, which potentially can be explained by the difference in size of these complexes (see Supplementary Fig. S4). A polymer to DNA ratio of 100:1 was selected for all subsequent experiments: at this ratio, nanoparticle complexes are spherical in shape, as determined by both transmission electron microsopy (TEM) and scanning elctron microscopy (SEM) (Fig. 1b and Supplementary Fig. S5). III-20% PDL/DNA complexes are more stable than traditional polyplexes, such as PEI/DNA complexes: incubation of complexes in 2% heparin released 65% of DNA from III-20% PDL/DNA complexes, compared with 97% for PEI/DNA complexes (see Supplementary Fig. S6). In a typical III-20% PDL/DNA suspension, 80% of III-20% PDL is associated with DNA (see Supplementary Fig. S7). Because of the low charge density and predominantly hydrophobic composition of III-20% PDL, the terpolymer that is not associated with DNA is water insoluble, so the remaining 20% of terpolymer is probably present as particulates (Supplementary Fig. S7). Further experiments demonstrated that III-20% PDL with 100:1 polymer to DNA ratio was sufficient to condense DNA (Supplementary Fig. S8) and protect DNA from enzymatic degradation (Supplementary Fig. S9).
At the 100:1 weight ratio, III-20% PDL transfected A549 cells with 81 and 166 times higher efficiency than Lipofectamine 2000 and PEI, respectively. For transfection of HEK293 cells, III-20% PDL was ∼50 times more efficient than Lipofectamine 2000 and PEI (Fig. 2a). However, under conditions that allow optimal transfection efficiency, Lipofectamine 2000 and PEI are toxic. Incubation of complexes of DNA with Lipofectamine 2000 at the optimal transfection conditions in this study inhibited cell proliferation by over 50%. PEI has similar toxicity. For this reason, Lipofectamine 2000 and PEI are normally used to transfect cells only at high confluence, where sufficient numbers of cells can survive. III-20% PDL is much less toxic than Lipofectamine 2000 and PEI (Fig. 2c,d). PEI killed all cells three days after treatment at 10µgml−1. In contrast, III-20% PDL was non-toxic even at concentrations as high as 500 µgml−1 (Fig. 2c,d).
Effectiveness of gene delivery in vivo
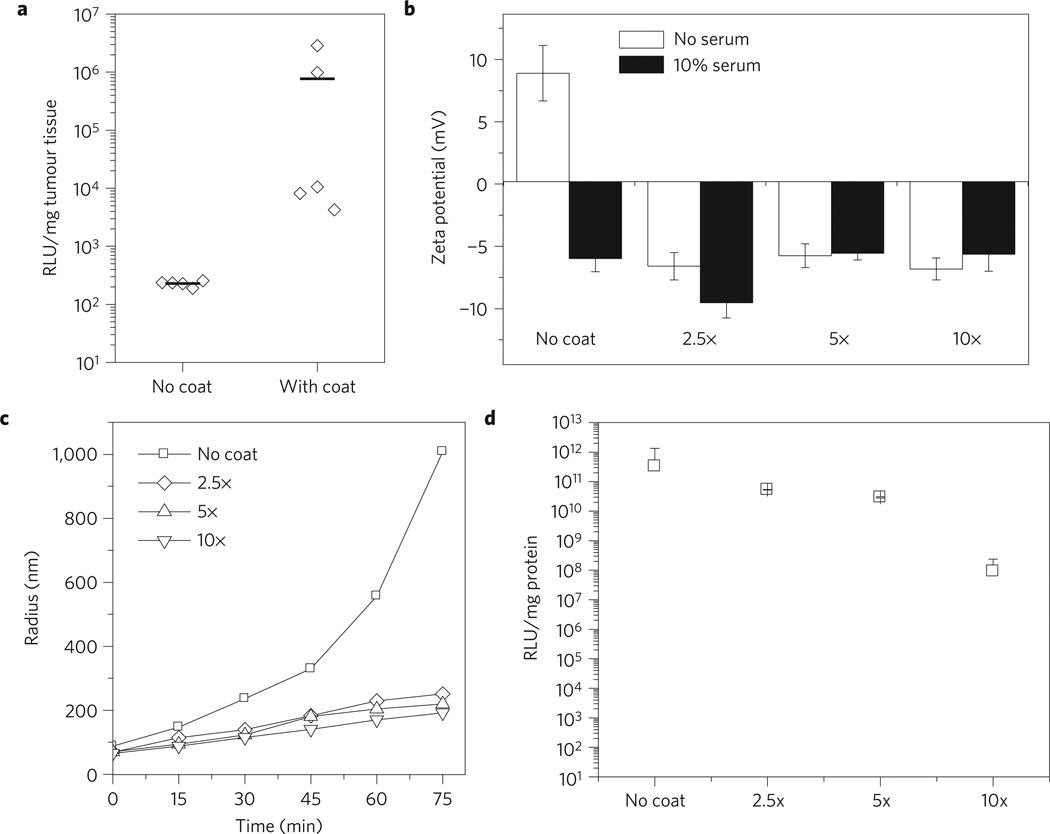
Because of its excellent transfection capability and low toxicity, III-20% PDL was tested for in vivo gene therapy by injection into mice bearing A549-derived tumour xenografts. First, polyplexes of III-20% PDL terpolymer with luciferase plasmid (pLucDNA) was administered through the tail vein: this treatment resulted in limited expression of luciferase in the tumours (no coat, Fig. 3a). We hypothesized that this low gene delivery efficiency was caused by (1) the positive charges on the polyplex surface (zeta potential of the polyplex: +8.9 mV) (Table 2), which attracts and binds with negatively charged plasma proteins in the blood during circulation, leading to its rapid clearance by the RES and (2) the instability of the polyplex nanoparticles. As evidence of instability, polyplex particles incubated in NaAc buffer solution containing 10% serum nearly doubled in size within 15 min and increased by over ten fold after 75min (Fig. 3c). As the result of this increase in size, polyplexes might be cleared from the circulation by uptake in the liver. Based on this hypothesis, to improve in vivo gene delivery we modified the surface of III-20% PDL/pLucDNA polyplexes by coating the particles with polyE-mRGD, a synthetic peptide containing three distinct segments. The first segment is a 16-(amino acid) polyglutamic acid (polyE), which is negatively charged at physiological pH and, therefore, capable of electrostatic binding to the positively charged surface of the polyplexes. The second segment is a 6-unit neutral polyglycine, which serves as a neutral linker. The third segment is the amino acid sequence arginine–glycine–aspartic acid-lysine (RGDK, mRGD), which includes the RGD sequence that binds the tumour endothelium through the interaction of RGD with αvβ3 and αvβ5. Furthermore, R/KxxR/K allows binding to neuropilin-1 (refs 36,37). Bindings with integrins and neuropilin-1 are thought to be critical for tumour-targeted and tissue-penetrating delivery to tumours in vivo36,37. Similar approaches have been reported to facilitate ligand-specific gene delivery in vitro38 and targeted gene delivery to liver, spleen, and bone marrow in vivo39. Coating with polyE–mRGD reversed the surface charge of III-20% PDL/pLucDNA polyplex (Fig. 3b): when polyE–mRGD was added at 5:1 peptide/DNA weight ratio, the zeta potential of the polyplex changed from +8.9 mV to −5.8mV. Peptide coated polyplexes were stable on incubation in NaAc buffer containing 10% serum (Fig. 3b), and were resistant to aggregation (Fig. 3c), suggesting that the modified polyplexes can escape clearance by the RES during circulation in vivo. Resistance to aggregation is particularly important, because small particle size limits clearance by the liver and maintains transfection ability of polyplex particles at the tumour site. We observed, however, that overcoating of III-20% PDL/pLucDNA polyplex significantly decreased transfection (Fig. 3d), despite our observation that uptake efficiency for overcoated (10×) polyplexes was not reduced in comparison to optimally coated (5×) particles (Supplementary Fig. S10). On the basis of these results, the ratio of peptide to DNA at 5:1 was selected for the subsequent in vivo studies.
Figure 3. Coating III-20% PDL/DNA polyplexes with peptide polyE–mRGD for improved stability in vitro and gene delivery in vivo.
a, Comparison between coated and uncoated polyplexes for in vivo luciferase transfection. Polyplexes were administrated through tail vein injection. Luciferase expression was determined 48 h after the last treatment of three consecutive daily treatments. b, Coating with polyE–mRGD prevented change of surface charge in serum. Polyplexes was prepared by mixing polymer and DNA and incubated at room temperature for 10 min. Then, polyE–mRGD was added at the indicated concentrations and coating allowed for 5 min. The zeta potential of the coated polyplexes was determined 5 min after their incubation in NaAc buffer containing 10% FBS. c, Change of coated and non-coated polyplex size in NaAc buffer containing 10% FBS. Coated polyplexes were prepared as described in b. Sizes of polyplexes were determined by dynamic light scattering at various time intervals. d, The effect of coating on transfection efficiency in vitro. When polyE–mRGD was added at 2.5:1 and 5:1 peptide/DNA weight ratios, a slight decrease in transfection efficiency of III-20% PDL/pLucDNA polyplex was observed. In contrast, when the peptide to DNA ratio increased to 10:1, the transfection efficiency of the polyplex decreased by over 3,000 times. All experiments were carried out in triplicate and the standard error are shown by the error bars.
Table 2.
Physical properties of polyplex nanoparticles formed from DNA (pGL4.13) and lactone-DES-MDEA terpolymer.
| Polymer name* | N/P† (molar ratio) |
Mean particle radius (nm) |
Zeta potential (mV) |
|---|---|---|---|
| PMSC‡ | 116 | 70 | 15.7 |
| I-10%CL | 111 | 73 | 11.3 |
| I-20%CL | 105 | 107 | 10.8 |
| I-40%CL | 91 | 45 | 15.3 |
| I-60%CL | 72 | 43 | 16.7 |
| I-80%CL | 45 | 117 | 7.3 |
| II-10%DDL | 107 | 69 | 10.4 |
| II-20%DDL | 99 | 93 | 11.6 |
| II-40%DDL | 79 | 90 | 8.9 |
| III-10%PDL | 106 | 86 | 13.2 |
| III-20%PDL | 96 | 75 | 8.9 |
| IV-10%HDL | 105 | - | - |
See Table 1 for polymer nomenclature.
All polyplex nanoparticles were formed at 100:1 weight ratio (polymer/DNA).
PMSC is included here as a reference polymer.
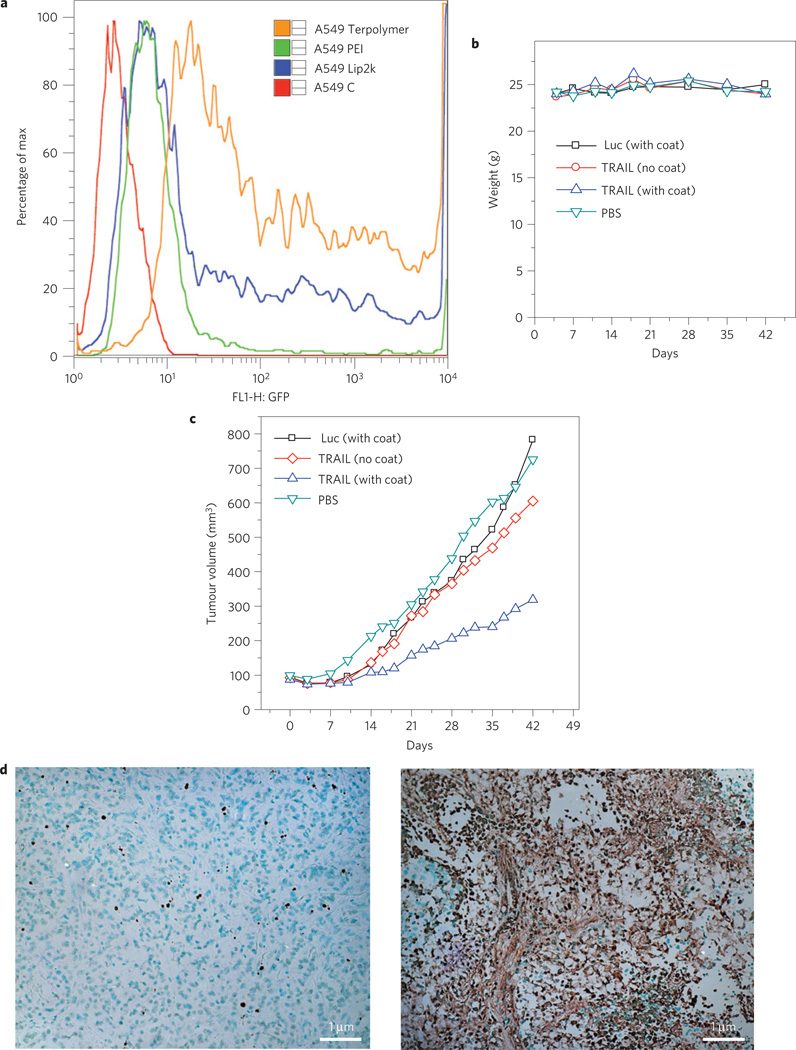
As polyE–mRGD contains tumour-targeting and tissue-penetrating functionality, we anticipated that intravenous administration of coated III-20% PDL/pLucDNA polyplexes would allow delivery of genes, and expression of luciferase, in the tumour. Indeed, compared with the uncoated polyplex particles, the coated polyplexes exhibited ∼14,000 times higher gene expression in tumour (Fig. 3a). Subsequently, we tested to see whether polyE–mRGD coated polyplex particles of III-20% PDL with therapeutic DNA could deliver genes to inhibit tumour growth. It is well known that the tumour-necrosis–factor-related apoptosis-inducing ligand (TRAIL) can preferentially kill malignant tumour cells40,41, inhibit tumour-related angiogenesis42, but not harm normal cells40,41. As well as direct tumour cell killing via transfection, TRAIL is also known to induce apoptosis in adjacent tumour cells owing to a bystander effect40. For this reason, a construct containing a fused TRAIL-green fluorescent protein (GFP) segment, pEGFP-TRAIL, was chosen as a therapeutic agent. III-20% PDL/pEGFP-TRAIL polyplexes were prepared, coated with polyE-mRGD, and then injected via tail vein in animals bearing tumours40. In preliminary in vitro studies, we observed that III-20% PDL complexed with pEGFP delivered the gene with an efficiency significantly higher than that of Lipofectamine 2000 or PEI (Fig. 4a). Cytotoxicity due to TRAIL was efficiently achieved in vitro by III-20% PDL/pEGFP-TRAIL polyplexes: five days after transfection, proliferation of A549 cells was inhibited by 58%, compared with cells treated with a vector control. In contrast, treatments with Lipofectamine/TRAIL and PEI/TRAIL complexes inhibited cell proliferation by only 21% and 9%, respectively (Supplementary Fig. S11). Furthermore, 5× coating, which was selected as the optimal for our in vivo study, did not coat Lipofectamine/DNA complexes and was insufficient to coat PEI/TRAIL complexes (Supplementary Fig. S12). For in vivo tumour treatment, polyE–mRGD coated III-20% PDL/pEGFP-TRAIL polyplexes were administrated three times a week for six weeks at a dose of 1.7 mg per mouse. During the entire course of treatment, no toxicity—as measured by weight loss, for example—was observed (Fig. 4b). Treatment of polyE–mRGD coated III-20% PDL/pEGFP-TRAIL polyplexes significantly inhibited tumour growth. By the end of the experiment, the average tumour size in the mouse group treated with the coated TRAIL polyplex was ∼300mm3, which was significantly smaller than the average tumour size for the groups treated with control polyplexes (polyE–mRGD coated III-20% PDL/pLuc) or phosphate buffered saline (PBS), which were ∼700 mm3 (p < 0.05, one-way analysis of variance (ANOVA); Fig. 4c). Histochemical analysis by terminal deoxynucleotidyl transferase (TUNEL) staining revealed a significant increase in the number of apoptotic cells after treatment with TRAIL (Fig. 4d). The tumour inhibition activity of coated III-20% PDL/pEGFP-TRAIL polyplexes was not due to either naked DNA or polyE-mRGD, because, independently, both of them exhibited limited toxicity (see Supplementary Fig. S13).
Figure 4. Evaluation of coated III-20%PDL/TRAIL DNA polyplexes in vivo.
a, Flow cytometry analysis of A549 cells transfected with pEGFP, using III-20% PDL, Lipofectamine 2000 and PEI. A549C: control untransfected A549 cells. Cell transfection was performed in a 6-well plate. DNA complexes with polymer or lipofectamine were prepared as described in the main text. 4 µg of plasmid was used for all vectors. GFP analysis was performed two days after transfection. b, Change of mouse weight during systemic administration of III-20% PDL/TRAIL polyplexes. Polyplexes were administrated through tail vein injection three days a week, at the dose of 1.7 mg per mouse, for 6 weeks. The dose was chosen based on the maximum amount of the polymer that can be used in 200 µl buffer solution for injection. Luc: luciferase. c, Antitumour effects of III-20% PDL/TRAIL polyplexes. Treatment started when the tumour reached a size of ∼50 mm3. Data are given as mean (n = 5). d, TUNEL staining demonstrated a marked increase in the number of apoptotic cells following treatment with TRAIL, as indicated by the significantly increased number of TUNEL-positive cells in the coated III-20% PDL/TRAIL group (right), as compared with the control group (left); ×20 magnification.
Methods
Synthesis and purification of lactone-DES-MDEA terpolymers
The copolymerization of lactone with diethyl sebacate (DES) and MDEA was performed in diphenyl ether solution using a parallel synthesizer connected to a vacuum line with the vacuum (±0.2 mmHg) controlled by a digital vacuum regulator. In a typical experiment, reaction mixtures were prepared, which contained three monomers (lactone, DES, and MDEA), Novozym 435 catalyst (10 wt% versus total monomer), and diphenyl ether solvent (200 wt% versus total monomer). The copolymerization reactions were carried out at a constant temperature in two stages: first-stage oligomerization, followed by second-stage polymerization. The reaction temperature was 80°C for the reactions of CL with DES and MDEA, and was set at 90°C for the copolymerizations of all other lactones (DDL, PDL, HDL) with DES and MDEA. During the first-stage reaction, the reaction mixtures were stirred under 1 atm of nitrogen gas, after which the reaction pressure was reduced to 1.6 mmHg and the reactions were continued for a further 72 h. The terpolymer products were isolated and purified according to the following procedures.
Because the solubility and physical properties of the terpolymers vary substantially depending on the ring size of the lactones and the lactone unit content in the polymers, two different purification methods were developed to isolate the polymer products. For purification of those polymers that are viscous liquids or waxy solids (for example, CL-DES-MDEA terpolymers with ≤80% CL, DDL-DES-MDEA terpolymers with ≤40% DDL, PDL-DES-MDEA terpolymers with ≤20% PDL, and HDL-DES-MDEA terpolymers with ≤20% HDL), the crude product mixtures were first mixed with hexane to cause the precipitation of the polymers. The precipitated polymers were then washed several times with fresh hexane to extract and remove the residual diphenyl ether solvent from the polymers. Subsequently, the terpolymers were dissolved in dichloromethane and filtered to remove catalyst particles. Evaporation and complete removal of the CH2Cl2 solvent from the filtrates at 40°C under high vacuum (1.0 mm Hg) yielded the purified terpolymers. On the other hand, for purification of the solid lactone-DES-MDEA terpolymers (for example, DDL-DES-MDEA terpolymers with > 40% DDL, PDL-DES-MDEA terpolymers with > 20% PDL, and HDL-DES-MDEA terpolymers with >20% HDL), the crude product mixtures were first dissolved in chloroform. The resultant polymer solutions were then filtered to remove the enzyme catalyst. After being concentrated under vacuum, the filtrates were added dropwise to stirring methanol to cause precipitation of the terpolymers. The obtained white solid polymers were subsequently washed with methanol three times and dried at 40°C under high vacuum (1.0 mm Hg) for 16 h. The isolated yield, composition, molecular weight (Mw), and polydispersity (Mw/Mn) of selected terpolymers are reported in Table 1. The complete data for all synthesized lactone-DES-MDEA copolymers are summarized in Supplementary Table S1. Analyses on the composition and molecular structures of the terpolymers by 1H and 13C NMR spectroscopy are also discussed in the Supplementary Information.
Evaluation of polyplexes
In vitro transfection and characterization of polyplexes are described in Supplementary Information. For in vivo evaluation, female athymic (NCR-nu/nu) nude mice were purchased from the National Cancer Institute and maintained in a sterile environment. All procedures were approved by the Institutional Animal Care and Utilization Committee (IACUC) of Yale University. To establish tumours, mice were injected with 1 × 106 A549 tumour cells subcutaneously. For luciferase transfection assay, experiments started when tumour volumes reached 200–300 mm3. 200 µl of the coated polyplexes, which were prepared as described in Supplementary Information, were then injected through tail vein. Transfection was conducted for three consecutive days. Two days after the last transfection, mice were killed. Tumours were excised, homogenized in lysis buffer and subjected to luciferase assay. For treatment evaluation, experiments were started when tumour volumes reached ∼50 mm3. Mice were randomly divided into four groups of eight mice per treatment group as follows: group 1, PBS control; group 2, coated luciferase polyplexes; group 3, non-coated TRAIL polyplexes; group 4, coated TRAIL polyplexes. Injections were performed through tail vein three days a week (Monday, Wednesday and Friday) until the end of the experiments. Tumour size was measured two times a week using traceable digital vernier calipers (Fisher). The tumour volumes were determined by measuring the length (l) and the width (w) and calculating the volume (V = 1/2 × lw2). The growth curve was plotted with respect to tumour volumes. One-way ANOVA analysis was performed to determine the statistical significance of treatment-related changes in tumour volume in athymic nude mice. p value smaller than 0.05 was considered to be significant. The animals were killed two days after the last treatment and the tumours were excised and formalin-fixed for immunohistochemistry. Slides of serial sections were stained with TUNEL for analysis of therapeutic effects.
Supplementary Material
Acknowledgements
We thank M. Graham in the EM Core Facility at the Yale School of Medicine for technical assistance and Nha Duong for editorial assistance. This work was supported by US National Institutes of Health (grant EB000487), Chicago Institute of Neurosurgery and Neuroresearch Foundation, the Voices Against Brain Cancer Foundation, and a pilot grant from the Yale Institute for Nanoscience and Quantum Engineering (YINQE).
Footnotes
Author contributions
J.Z., J.L., Z.J. and W.M.S. designed the experiments. J.Z., J.L., C.J.C., T.R.P., C.E.W. and Z.J. performed the experiments. All the authors were involved in the analyses and interpretation of data. J.Z., Z.J. and W.M.S. wrote the paper, with the help of the co-authors.
The authors declare no competing financial interests.
Supplementary information accompanies this paper on www.nature.com/naturematerials.
References
- 1.Felgner PL, et al. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl Acad. Sci. USA. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Templeton NS, et al. Improved DNA: Liposome complexes for increased systemic delivery and gene expression. Nature Biotechnol. 1997;15:647–652. doi: 10.1038/nbt0797-647. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Bathula SR, Yang Q, Huang L. Targeted nanoparticles deliver siRNA to melanoma. J. Invest. Dermatol. 2010;130:2790–2798. doi: 10.1038/jid.2010.222. [DOI] [PubMed] [Google Scholar]
- 4.Al-Dosari MS, Gao X. Nonviral gene delivery: Principle, limitations, and recent progress. Am. Assoc. Pharm. Sci. J. 2009;11:671–681. doi: 10.1208/s12248-009-9143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tros de Ilarduya C, Sun Y, Duzgunes N. Gene delivery by lipoplexes and polyplexes. Eur. J. Pharm. Sci. 2010;40:159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 6.Nicol F, et al. Poly-L-glutamate, an anionic polymer, enhances transgene expression for plasmids delivered by intramuscular injection with in vivo electroporation. Gene Ther. 2002;9:1351–1358. doi: 10.1038/sj.gt.3301806. [DOI] [PubMed] [Google Scholar]
- 7.Schlegel A, et al. Anionic polymers for decreased toxicity and enhanced in vivo delivery of siRNA complexed with cationic liposomes. J. Control. Release. 2011;152:393–401. doi: 10.1016/j.jconrel.2011.03.031. [DOI] [PubMed] [Google Scholar]
- 8.Liu DX, Gao X, Kim KS. Nonviral gene delivery: What we know and what is next. Am. Assoc. Pharm. Sci. J. 2007;9:E92–E104. doi: 10.1208/aapsj0901009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 10.Gao X, et al. The association of autophagy with polyethylenimine-induced cytotoxity in nephritic and hepatic cell lines. Biomaterials. 2011;32:8613–8625. doi: 10.1016/j.biomaterials.2011.07.047. [DOI] [PubMed] [Google Scholar]
- 11.Felgner JH, et al. Enhanced gene delivery and mechanism studies with a novel series of cationic lipid formulations. J. Biol. Chem. 1994;269:2550–2561. [PubMed] [Google Scholar]
- 12.Kafil V, Omidi Y. Cytotoxic impacts of linear and branched polyethylenimine nanostructures in A431 cells. BioImpacts. 2011;1:23–30. doi: 10.5681/bi.2011.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv H, Zhang S, Wang B, Cui S, Yan J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release. 2006;114:100–109. doi: 10.1016/j.jconrel.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Jiang Z, Zhou J, Zhang SM, Saltzman WM. Enzyme-synthesized poly(amine-co-esters) as nonviral vectors for gene delivery. J. Biomed. Mater. Res. A. 2011;96A:456–465. doi: 10.1002/jbm.a.32994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang Z. Lipase-catalyzed synthesis of poly(amine-co-esters) via copolymerization of diester with amino-substituted diol. Biomacromolecules. 2010;11:1089–1093. doi: 10.1021/bm1000586. [DOI] [PubMed] [Google Scholar]
- 16.Wang Y, Wang LS, Goh SH, Yang YY. Synthesis and characterization of cationic micelles self-assembled from a biodegradable copolymer for gene delivery. Biomacromolecules. 2007;8:1028–1037. doi: 10.1021/bm061051c. [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, et al. The self-assembly of biodegradable cationic polymer micelles as vectors for gene transfection. Biomaterials. 2007;28:5358–5368. doi: 10.1016/j.biomaterials.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Kiang T, Wen J, Lim HW, Leong KW. The effect of the degree of chitosan deacetylation on the efficiency of gene transfection. Biomaterials. 2004;25:5293–5301. doi: 10.1016/j.biomaterials.2003.12.036. [DOI] [PubMed] [Google Scholar]
- 19.DeRouchey J, Netz RR, Radler JO. Structural investigations of DNA-polycation complexes. Eur. Phys. J. E. 2005;16:17–28. doi: 10.1140/epje/e2005-00003-4. [DOI] [PubMed] [Google Scholar]
- 20.Piest M, Engbersen JF. Effects of charge density and hydrophobicity of poly(amido amine)s for non-viral gene delivery. J. Control. Release. 2010;148:83–90. doi: 10.1016/j.jconrel.2010.07.109. [DOI] [PubMed] [Google Scholar]
- 21.Liu Z, Zhang Z, Zhou C, Jiao Y. Hydrophobic modifications of cationic polymers for gene delivery. Prog. Polym. Sci. 2010;35:1144–1162. [Google Scholar]
- 22.Kuhn PS, Levin Y, Barbosa MC. Charge inversion in DNA-amphiphile complexes: Possible application to gene therapy. Physica A. 1999;274:8–18. [Google Scholar]
- 23.Alvarez-Lorenzo C, et al. Biophysical characterization of complexation of DNA with block copolymers of poly(2-dimethylaminoethyl) methacrylate, poly(ethylene oxide), and poly(propylene oxide) Langmuir. 2005;21:5142–5148. doi: 10.1021/la050170v. [DOI] [PubMed] [Google Scholar]
- 24.Takigawa DY, Tirrell DA. Interactions of synthetic-polymers with cell-membranes and model membrane systems .6. Disruption of phospholipid packing by branched poly(ethylenimine) derivatives. Macromolecules. 1985;18:338–342. [Google Scholar]
- 25.Thomas M, Klibanov AM. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc. Natl Acad. Sci. USA. 2002;99:14640–14645. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bajaj A, Kondaiah P, Bhattacharya S. Synthesis and gene transfection efficacies of PEI-cholesterol-based lipopolymers. Bioconjug. Chem. 2008;19:1640–1651. doi: 10.1021/bc700381v. [DOI] [PubMed] [Google Scholar]
- 27.Eliyahu H, et al. Novel dextran-spermine conjugates as transfecting agents: Comparing water-soluble and micellar polymers. Gene Ther. 2005;12:494–503. doi: 10.1038/sj.gt.3302395. [DOI] [PubMed] [Google Scholar]
- 28.Gabrielson NP, Pack DW. Acetylation of polyethylenimine enhances gene delivery via weakened polymer/DNA interactions. Biomacromolecules. 2006;7:2427–2435. doi: 10.1021/bm060300u. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Z. Lipase-catalyzed synthesis of aliphatic polyesters via copolymerization of lactone, dialkyl diester, and diol. Biomacromolecules. 2008;9:3246–3251. doi: 10.1021/bm800814m. [DOI] [PubMed] [Google Scholar]
- 30.Mazzocchetti L, Scandola M, Jiang ZZ. Enzymatic synthesis and structural and thermal properties of poly(omega-pentadecalactone-co-butylene-co-succinate) Macromolecules. 2009;42:7811–7819. [Google Scholar]
- 31.Stridsberg KM, Ryner M, Albertsson AC. Controlled ring-opening polymerization: Polymers with designed macromolecular architecture. Degrad. Aliphatic Polyest. 2002;157:41–65. [Google Scholar]
- 32.Nomura R, Ueno A, Endo T. Anionic ring-opening polymerization of macrocyclic esters. Macromolecules. 1994;27:620–621. [Google Scholar]
- 33.Hefetz A, Fales HM, Batra SWT. Natural polyesters—dufours gland macrocyclic lactones form brood cell laminesters in colletes bees. Science. 1979;204:415–417. doi: 10.1126/science.204.4391.415. [DOI] [PubMed] [Google Scholar]
- 34.Hefetz A, Bergstrom G, Tengo J. Species, individual and Kin specific blends in dufours gland secretions of halictine bees—chemical evidence. J. Chem. Ecol. 1986;12:197–208. doi: 10.1007/BF01045603. [DOI] [PubMed] [Google Scholar]
- 35.Duffield RM, Laberge WE, Cane JH, Wheeler JW. Exocrine secretions of bees. 4. Macrocyclic lactones and isopentenyl esters in dufours gland secretions of nomia bees (hymenoptera, halictidae) J. Chem. Ecol. 1982;8:535–543. doi: 10.1007/BF00987801. [DOI] [PubMed] [Google Scholar]
- 36.Sugahara KN, et al. Tissue-penetrating delivery of compounds and nanoparticles into tumors. Cancer Cell. 2009;16:510–520. doi: 10.1016/j.ccr.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Teesalu T, Sugahara KN, Kotamraju VR, Ruoslahti E. C-end rule peptides mediate neuropilin-1-dependent cell, vascular, and tissue penetration. Proc. Natl Acad. Sci. USA. 2009;106:16157–16162. doi: 10.1073/pnas.0908201106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Green JJ, et al. Electrostatic ligand coatings of nanoparticles enable ligand-specific gene delivery to human primary cells. Nano Lett. 2007;7:874–879. doi: 10.1021/nl062395b. [DOI] [PubMed] [Google Scholar]
- 39.Harris TJ, et al. Tissue-specific gene delivery via nanoparticle coating. Biomaterials. 2010;31:998–1006. doi: 10.1016/j.biomaterials.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kagawa S, et al. Antitumor activity and bystander effects of the tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene. Cancer Res. 2001;61:3330–3338. [PubMed] [Google Scholar]
- 41.Matsubara H, et al. Gene therapy with TRAIL against renal cell carcinoma. Mol. Cancer Ther. 2006;5:2165–2171. doi: 10.1158/1535-7163.MCT-05-0522. [DOI] [PubMed] [Google Scholar]
- 42.Cantarella G, et al. TRAIL inhibits angiogenesis stimulated by VEGF expression in human glioblastoma cells. Br. J. Cancer. 2006;94:1428–1435. doi: 10.1038/sj.bjc.6603092. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.