Abstract
We previous reported that miR-27a regulates lipid metabolism and cell proliferation during hepatic stellate cells (HSCs) activation. To further explore the biological function and underlying mechanisms of miR-27a in HSCs, global protein expression affected by overexpression of miR-27a in HSCs was analyzed by a cleavable isotope-coded affinity tags (cICAT) based comparative proteomic approach. In the present study, 1267 non-redundant proteins were identified with unique accession numbers (score ≥1.3, i.e. confidence ≥95%), among which 1171 were quantified and 149 proteins (12.72%) were differentially expressed with a differential expression ratio of 1.5. We found that up-regulated proteins by miR-27a mainly participate in cell proliferation and myogenesis, while down-regulated proteins were the key enzymes involved in de novo lipid synthesis. The expression of a group of six miR-27a regulated proteins was validated and the function of one miR-27a regulated protein was further validated. The results not only delineated the underlying mechanism of miR-27a in modulating fat metabolism and cell proliferation, but also revealed a novel role of miR-27a in promoting myogenic tans-differentiation during HSCs activation. This study also exemplified proteomics strategy as a powerful tool for the functional study of miRNA.
Introduction
microRNAs (miRNAs) regulate gene expression post-transcriptionally by binding primarily to the 3′untranslated region (3′UTR) of their target mRNAs, resulting in mRNA destabilization or translational repression[1]. Genes encoding 2042 mature human miRNAs have so far been identified (miRBase v.19) [2] and miRNAs are predicted to regulate the expression of up to 60% of human protein-encoding genes [3]. The best way to understand the biological function of a miRNA is to identify the genes that it regulates. Several bioinformatics methods have been developed for miRNA target prediction, including TargetScan (www.targetscan.org), miRanda (www.microrna.org), TarBase (diana.cslab.ece.ntua.gr), PicTar (pictar.mdcberlin. de) et al. However since the mechanism of miRNA target recognition is still not fully understood, target gene prediction is not accurate and sometimes over predict [4]. In addition, a single miRNA can target hundreds of proteins and a single protein can be influenced by multiple miRNAs [5]. Thus comprehensive understanding of the phenotypic effects of miRNAs at the cellular level is currently difficult.
The use of quantitative proteomic strategies to characterize targets of miRNAs has opened new avenues to miRNA biology study [6]. The method of cleavable isotope-coded affinity tags (cICAT) coupling with nano LC-MS/MS is a quantitative proteomic approach that enables rapid, comprehensive and reliable analysis of the proteomes of two comparable samples [7]. More importantly, compared with other quantitative proteomic strategies, cICAT based approach could greatly reduce the sample complexity, therefore those low abundance proteins could be readily identified.
We have previously reported that miR-27a,b suppresses fat accumulation and promotes cell proliferation during hepatic stellate cells (HSCs) activation [8]. Thereafter, miR-27 has been evidenced to act as negative regulator of adipocyte differentiation [9] or lipid metabolism [10], and positive regulator of cell proliferation [11] by several groups. It has also been regarded as an oncogene in some malignant tumor [12], [13]. To further explore the possible functions and underlying mechanism of miR-27a during HSCs activation, human stellate cell line LX2/miR-27a stable transfectants was established and validated. Global protein expression profiles were compared between LX2/miR-27a and LX2/miR-neg control by cICAT-based proteomic approach. We found that out of 1267 identified proteins, 149 proteins were differentially expressed, and 75 were repressed by miR-27a overexpression among which, 15 proteins were predicted miR-27a targets. The bio-significance of miR-27a was analyzed based on the functional annotation of miR-27a regulated proteins. Individual siRNA mediated knock-down of one miR-27a regulated protein was performed to demonstrate the phenotypic effects.
Materials and Methods
1. Plasmid constructions
To construct miRNA expression plasmid, miR-27a expression fragments designed according to manufactures’ instructions, miR-27a, sense 5′-TGCTGTTCACAGTGGCTAAGTTCCGCGTTTTGGCCACTGACTGACGCGGAACTGCCACTGTGAA-3′, anti-sense 5′-CCTGTTCACAGTGGCAGTTCCGCGTCAGTCAGTGGCCAAAACGCGGAACTTAGCCACTGTGAAC-3′; were cloned into pcDNA6.2-GW/EmGFP-mir vector (Invitrogen, Carlsbad, CA) after annealing the oligonucleotides, termed as pcDNA6.2-GW/EmGFP-mir-27a. The pcDNA6.2-GW/EmGFP-mir-neg vector was provided by Invitrogen. DNA sequencing analyses confirmed the nucleotide sequences of the constructed plasmids.
2. Establishment of stable transfectants
Human hepatic stellate cell line LX2 cells [14] were maintained in DMEM (Invitrogen), supplemented with 10% FBS (Invitrogen), and were incubated in a humidified atmosphere of 5% CO2 and 95% air at 37°C. The medium was changed every 48 hours. Stable transfectants were constructed using LX2 cells that had been plated at approximately 1×105 per 60-mm diameter culture dish and cultured overnight. The cells were transfected with 5 µg pcDNA6.2-GW/EmGFP-mir-27a or mir-neg control plasmids by Lipofectamine 2000 (Invitrogen). Transfection efficiencies were checked by EmGFP expression under fluorescent microscope. Clones were selected and maintained in DMEM supplemented with 10 µg/ml Blasticidin (Invitrogen). Two stably transfected cell lines, LX2/miR-27a and LX2/miR- neg were isolated after 28 days’ selection.
3. Real-time reverse transcription PCR (RT-PCR)
Total RNA from LX2 cells was extracted using Trizol reagent (Invitrogen). cDNAs and the first-strand cDNAs of miRNA were produced according to the manufacturer’s instructions for Thermoscript RT-PCR system (Invitrogen) or NCode miRNA First-Strand cDNA Synthesis kits (Invitrogen). For the quantitative detection of miR-27a and mRNAs of interested genes, the templates and primer sets (Table S1) were mixed with SYBR qPCR master mix (TaKaRa, Dalian, China), and real-time PCR was performed using Rotor-Gene 3000 (Corbett Research, Sydney). The cycling parameters were: initial denaturing at 94°C for 15 sec, followed by 40 cycles of 94°C denaturing for 10 sec, primer annealing and extension at 60°C for 40 sec. To ensure the specificity of the amplification reaction, melting curve analysis was performed. The expression of miR-27a was normalized to U6snRNA, and mRNAs were normalized to GAPDH. Relative gene expression was presented by comparative CT method.
4. Quantitative proteomic analysis
Global protein expression profile changes of LX2/miR-27a transfectants were analyzed by a cleavable isotope-coded affinity tags (cICAT) labeling coupled with online 2D nanoLC-MS/MS based quantitative proteomic approach. cICAT reagents were from Applied Biosystems (Foster City, CA).
(A) cICAT labeling
Proteins from LX2/miR-27a and LX2/miR-neg control were labeled with isotopically heavy (H) and light (L) cICAT reagents respectively following the manufacture’s protocol. Briefly 100 µg total protein collected from LX2/miR-27a and negative control LX2/miR-neg were labeled, respectively, with isotopically light (12C for LX2/miR-neg) and heavy (13C for LX2/miR-27a ) cICAT reagents at 37°C for 2 hours. The labeled preparations were combined and digested with trypsin (Promega, madison, WI) overnight at 37°C using an enzyme-to-protein ratio of 1∶50 w/w. The resulting peptides were subsequently purified by cation exchange chromatography and avidin affinity chromatography (Applied Biosystems). The biotin group on the tag was removed by acid cleavage and the peptides were dried by vacuum-evaporation using a Speedvac™ system (Thermo Scientific).
(B) 2D nanoLC-MS/MS analysis
The dried peptides were resuspended in 80 ul of an aqueous solution containing 0.1% FA and 5% acetonitrile, the resulting solution was loaded onto a 30*0.5 mm strong cation exchange column (Agilent Technologies) and separated into 17 fractions with a step gradient of 0 mM, 10 mM, 20 mM, 30 mM, 40 mM, 50 mM, 60 mM, 70 mM, 80 mM, 90 mM, 100 mM, 125 mM 150 mM, 200 mM, 300 mM, 400 mM, 500 mM and 900 mM, 0.1% FA, 5% acetonitrile. The elutions from SCX column were further separated on a 150*0.075 mm Vydac C18 reverse phase column (Grace, inc) in line after a nanotrap column (Agilent Technologies) using a nanoHPLC 1100 system (Agilent Technologies). Separation of the peptides was performed at 400 nl/min and was coupled to online analysis by tandem mass spectrometry using a QstarXL MS/MS system (Applied Biosystems) equipped with a nanospray ion source (Applied Biosystems). Elution of the peptides into the mass spectrometer was performed with a linear gradient from 95% mobile phase A (0.1% FA, 99.9% water) to 35% mobile phase B (0.1% FA, 99.9% acetonitrile) over 120 minutes followed by 80% mobile phase B for 10 min. The peptides were detected in positive ion mode using an IDA (information dependent acquisition) method in which three most abundant ions detected in a MS scan were selected for MS/MS analysis. Two independent analyses were performed.
(C) Data Analysis
For protein identification and quantification, all MS/MS spectra were searched against the IPI human protein database (V3.83) using ProteinpilotTM 3.0.1 (Applied Biosystem). The software compares relative intensity of proteins present in samples based on the intensity of reporter ions released from each labeled peptide and automatically calculates protein ratios and p-values for each protein. For protein identification, 95% confidence was used and the corresponding FDR <1%.
5. Bio-functional analysis of differentially expressed proteins
GOfact (http://61.50.138.118/gofact/cgi/gofact2009.cgi) strategy [15], [16] which based on the structured and controlled vocabularies - Gene Ontology (GO), and the GO annotation from related databases was used to identify the functional distribution and the enriched functional categories of miR-27a regulated proteins in LX2 cells. The subcellular locations and bio-functions of proteins were also annotated by Protein Knowledgebase (UniprotKB) (http://www.uniprot.org/).
6. Transfection of siRNA
Transfection of siRNA was performed according to the manufacturer’s protocol (Sigma, Saint Louis, MO). LX2 and LX2/miR-27a transfectants cultured in 24-well plates or 6-cm dishes were transfected at 50–70% confluence with siRNA targeting human four and a half LIM domains 1 (FHL1) by means of the siRNA transfection reagent RNAiMAX (Invitrogen). NTC (Non-targeting control) siRNA was transfected simultaneously as negative control. After 48 h transfection, the efficiency of siRNA-mediated mRNA degradation was assessed by real-time RT–PCR.
7. Proliferation and migration assays
The effects of siRNA transfection on LX2/miR-27a transfectants migration were measured by using a modified Boyden chamber assay. Two days after transfection, 2×104 cells in serum-free DMEM were plated on the upper chamber of each Transwell (Costar, Corning Inc., NY) with 8 µm pores, while the lower chamber contained 800 µl completed medium. Transfected cells were incubated for 16 h at 37°C in 5% CO2. Non-migrating cells were carefully removed from the upper surface of the membrane with cotton swabs. Membranes were stained with crystal violet and mounted onto glass slides. Migration was quantified by counting cells in eight 200x microscopic fields.
Forty-eight hours after siRNAs transfection, the cell proliferation of LX2 cells was detected by incorporation of 5-ethynyl-2′-deoxyuridine (EdU) with the Cell-Light EdU Apollo 567 Cell Proliferation Kit (Ruibo Biotech, Guangzhou, China). According to the kit’s protocol, cells were incubated with 10 µM EdU for 16 h before fixation, permeabilization, and EdU staining. EdU was detected by Apollo fluorescent dye at 567 nm wave length and nuclei were counterstained with 5 µg/ml Hoechst 33342. For each well, eight fields were counted at a 200x magnification. The results were expressed as the labeling index according to the following formula: number of EdU-positive nuclei×100/number of total nuclei.
8. Statistics assay
Data are expressed as the mean ± SD. Comparison between groups were made by Student’s t test (two tailed) or one-way ANOVA followed by Tukey's multiple comparison test. The relationship between two data sets was analyzed by linear regression. Differences were considered significant if P<0.05. Unless otherwise specified, all assays were performed in triplicate.
Results and Discussion
1. Biological characterization of LX2/miR-27a stable transfectants
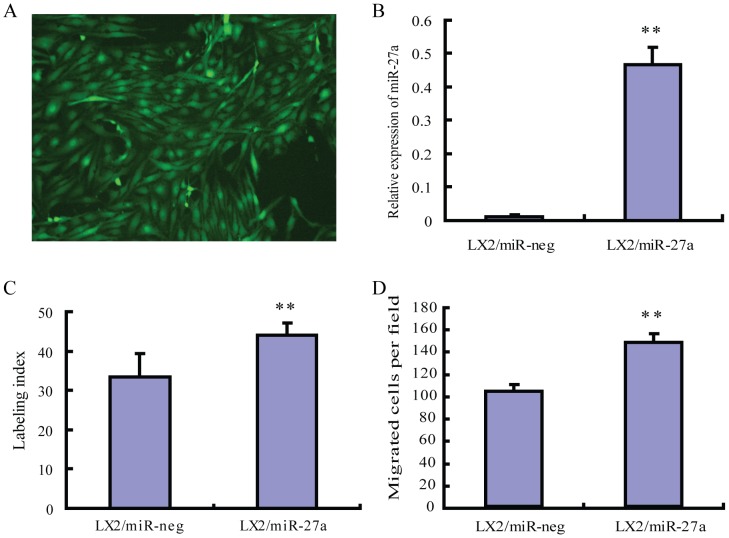
To explore the biological effects of miR-27a overexpression on HSCs, we established a LX2/miR-27a stable transfectants (Figure 1A). The expression of mature miR-27a increased significantly in LX2/miR-27a stable transfectants (Figure 1B). As it was expected, LX2/miR-27a stable transfectants showed increased cell proliferation and migration compared to LX2/miR- neg stable transfectants (Figure 1C and D). The influence of miR-27a over expression on lipid metabolism was not measurable due to the already activated HSC phenotype of LX2 cell line.
Figure 1. Establishment and biological characters of LX2/miR-27a, LX2/miR-neg stable transfectants.
(A) Almost all cells in the positive clone expressed EmGFP (green), original magnification ×200. (B) The expression of miR-27a in LX2/miR-27a, LX2/miR-neg stable transfectants. (C) Over-expression of miR-27a promoted LX2 cell proliferation. (D) miR-27a over-expression facilitated LX2 migration. **P<0.01 compared with LX2/miR-neg.
2. Identification of miR-27a regulated proteins by cICAT-based proteomic analyses
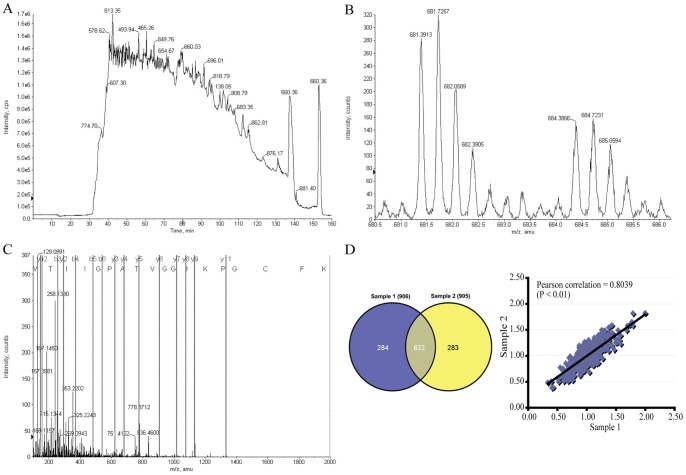
Global protein expression profiles were compared between LX2/miR-27a and LX2/miR-neg stable transfectants by a cICAT-based quantitative proteomic approach (Figure 2A–C). Two biological replications were analyzed (Table S2). To estimate the analytical reproducibility of our proteomics study, linear regression analyses were performed on H/L ratios of duplicate analyses of samples 1 and 2 (Figure 2D). Pearson correlation coefficient for sample 1 and 2 was 0.8039 (P<0.01). Thus, the ratios of the two duplicate analyses were significantly positively correlated, indicating the good analytical reproducibility of the on-line 2D LC/MS/MS system. Thereby, spectral data from two duplicate analyses were merged and searched again to enhance the coverage of protein identification and to “average” the expression ratios of proteins identified in samples 1 and 2 (Table S3).
Figure 2. Protein samples from LX2/miR-27a and LX2/miR-neg were compared by cleavable isotope-coded affinity tag (cICAT)-based quantitative proteomic analysis - identification and quantitation of ATP-citrate synthase.
(A) Total ion chromatogram (TIC) indicating cICAT-labeled peptides eluting from a reverse phase column. (B) Expanded MS spectrum view of a pair of peaks showing the differential expression between peptides labeled with the isotopically light and heavy cICAT reagent. (C) MS/MS spectrum analysis of the light-cICAT labeled triply charged peptide (681.4 m/z) showed in (B) led to identification of a peptide with sequence GVTIIGPATVGGIKPGCFK (ICAT-C(C)@17), unique to the ATP-citrate synthase (ACLY), a predicted target of miR-27a. The labels b and y designated the N- and C- terminal fragments, respectively, of the peptide produced by breakage at the peptide bond in the mass spectrometer. The number represents the number of N- or C- terminal residues present in the peptide fragment. (D) Venn diagram depicting the overlap of proteins identified in two independent cICAT experiments. Numbers in parentheses indicate the number of identified proteins for each sample. To examine the biological reproducibility, linear regression analyses were performed on H/L ratios (LX2/miR-27a/LX2/miR-neg) of two independent analyses. Pearson correlation coefficient between samples 1 and 2 was 0.8039, P<0.01.
In the present study, 1267 non-redundant proteins were identified with unique accession numbers (score ≥1.3, i.e. confidence ≥95%), among which 1171 were quantified (Table S3). In the present study, based on the expression ratio of housekeeping proteins such as β-actin (ACTB, H/L = 1.0637) and tubulin β chain (TUBB, H/L = 1.0274), a differential protein expression ratio of 1.5 was selected as significant threshold [17], thus 149 (12.72%) proteins were differentially expressed. Of these 149 proteins, 74 were up-regulated (i.e. H/L ≥1.5) and 75 were down-regulated (i.e. H/L ≤0.6667), the number of up-regulated proteins was almost equal to that of down-regulated (Table S4). Compared with our previous study on HSCs activation [18], the extent of protein expression changes is relatively small in miR-27a overexpressed LX2, only 6 proteins increase up to 3-fold (i.e. H/L ≥3.0) and 2 proteins reduced below 3-fold (i.e. H/L ≤0.3333). The results also corroborated a recent finding that a single miRNA could regulate the production of hundreds of proteins, but the regulation was typically relatively mild [5].
3. Correlation between miR-27a target prediction and down-regulated proteins in LX2/miR-27a identified by cICAT
Next, we tried to figure out how miR-27a target prediction correlated with miR-27a down-regulated proteins in HSCs identified by cICAT-based proteomics analyses. TargetScan is one of the widely recognized databases for biological targets prediction of miRNAs [19]. By searching TargetScan Human Release 6.2 (http://www.targetscan.org/vert_61/), we found that only 2 out of the 75 down-regulated proteins were predicted targets of miR-27a, namely SMAD5 (mothers against decapentaplegic homolog 5) and ACLY (ATP-citrate synthase). SMAD5, a key component of TGF-beta signaling pathway, is an experimentally confirmed target of miR-27 [20]. ACLY is the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA in many tissues and has a central role in de novo lipid synthesis. We further searched the predicted consequential pairing of miR-27a target region in the 3′ UTR of the remaining 73 down-regulated proteins in TargetScan Human Release 6.2. As shown in Table 1, 15 (20%) out of 75 down-regulated proteins could be potential targets of miR-27a, while the other 60 (80%) down-regulated proteins did not have consequential pairing of miR-27a target region in the 3′ UTR. Moreover, 74 proteins were even up-regulated in LX2/miR-27a stable transfectants. These findings suggested that the miRNA responsive proteins were not necessarily the predicted endogenous targets, they also reflected indirect effects. The underlying mechanisms deserve further investigation, as it has also been reported that miRNAs can even stimulate gene expression post transcriptionally by direct and indirect mechanisms [21].
Table 1. Predicted miR-27a Targets among Down-regulated Proteins in LX2/miR-27a Identified by cICAT.
| Gene symbol | Accession | Predicted consequentialpairing of target region(top) and miRNA (bottom) | Seed match | Context score | Context scorepercentile | PCT * | H/L | |
| ACLY | NM_001096 | Position 697–703 of ACLY 3' UTR | 5′ …UGGAAAUGCAGAAAGCUGUGAAA… | 7mer-1A | −0.13 | 73 | 0.67 | 0.6597 |
| |||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| AP3D1 | NM_001077523 | Position 187–193 of AP3D1 3′ UTR | 5′ …UGACCAUCCUUUUUUACUGUGAC… | 7mer-m8 | −0.20 | 87 | <0.1 | 0.5462 |
| ||| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| ATP2A2 | NM_170665 | Position 2249–2256 of ATP2A2 3′ UTR | 5′ …AAAAAAAUCAGCCUUACUGUGAA… | 8mer | >−0.03 | 2 | <0.1 | 0.6095 |
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| COPA | NM_001098398 | Position 1233–1239 of COPA 3′ UTR | 5′ …UGAGGACCUAAACUGCUGUGAAA… | 7mer-1A | −0.11 | 63 | <0.1 | 0.6641 |
| |||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| DYNLL2 | NM_080677 | Position 535–541 of DYNLL2 3′ UTR | 5′ …AGAAUAUUCCACUGAACUGUGAU… | 7mer-m8 | −0.12 | 71 | 0.34 | 0.4487 |
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| FN1 | NM_002026 | Position 431–437 of FN1 3′ UTR | 5′ …AAGCAUGAUCUUGUU-ACUGUGAU… | 7mer-m8 | −0.22 | 89 | <0.1 | 0.5669 |
| ||| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| Position 742–748 of FN1 3′ UTR | 5′ …CGGGGGAAAUAAUUCCUGUGAAU… | 7mer-1A | −0.13 | 71 | <0.1 | |||
| |||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| GNPNAT1 | NM_198066 | Position 175–181 of GNPNAT1 3′ UTR | 5′ …GGCUGGUGGGACAUGCUGUGAAU… | 7mer-1A | −0.12 | 68 | <0.1 | 0.5175 |
| |||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| Position 668–674 of GNPNAT1 3′ UTR | 5′ …UACCACUUGUCUUUUCUGUGAAU… | 7mer-1A | −0.10 | 60 | <0.1 | |||
| |||| |||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGU–GACACUU | |||||||
| H6PD | NM_004285 | Position 1513–1519 of H6PD 3′ UTR | 5′ …GAGCAUAGGUUGGGGACUGUGAU… | 7mer-m8 | > −0.02 | 0 | <0.1 | 0.5198 |
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| Position 5755–5761 of H6PD 3′ UTR | 5′ …UGUGCCGGAGUGGGAACUGUGAU… | 7mer-m8 | −0.02 | 27 | <0.1 | |||
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| HSD17B12 | NM_016142 | Position 1071–1078 of HSD17B12 3′ UTR | 5′ …AAGAAAGAAUUCAAUACUGUGAA… | 8mer | −0.33 | 97 | <0.1 | 0.3966 |
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| PAK2 | NM_002577 | Position 2076–2082 of PAK2 3′ UTR | 5′ …CAACGAGAUGAGAAGACUGUGAU… | 7mer-m8 | > −0.02 | 2 | <0.1 | 0.5688 |
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| PPM1B | NM_001033557 | Position 177–184 of PPM1B 3′ UTR | 5′ …AUUAAACUUUAAAUGACUGUGAA… | 8mer | −0.40 | 99 | <0.1 | 0.4537 |
| ||||| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGG–UGACACUU | |||||||
| RAB23 | NM_016277 | Position 982–988 of RAB23 3′ UTR | 5′ …GUCAUUCAGGAGGUCCUGUGAAG… | 7mer-1A | −0.01 | 23 | <0.1 | 0.6407 |
| |||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| SEC61A1 | NM_013336 | Position 197–204 of SEC61A1 3′ UTR | 5′ …GCACUGGCAAAAAGAACUGUGAA… | 8mer | −0.30 | 95 | <0.1 | 0.5849 |
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| SMAD5 | NM_001001419 | Position 72–78 of SMAD5 3′ UTR | 5′ …ACUUUGAGUACAGAUACUGUGAG… | 7mer-m8 | −0.20 | 87 | 0.75 | 0.6113 |
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| Position 2427–2433 of SMAD5 3′ UTR | 5′ …UUAUUGGUGUUUUCUACUGUGAG… | 7mer-m8 | −0.03 | 31 | <0.1 | |||
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
| SPTBN1 | NM_178313 | Position 2130–2136 of SPTBN1 3′ UTR | 5′ …UCAUUUGAUCAUAGCACUGUGAU… | 7mer-m8 | −0.16 | 81 | <0.1 | 0.6351 |
| ||||||| | ||||||||
| hsa-miR-27a | 3′ CGCCUUGAAUCGGUGACACUU | |||||||
* PCT, the probability of conserved targeting.
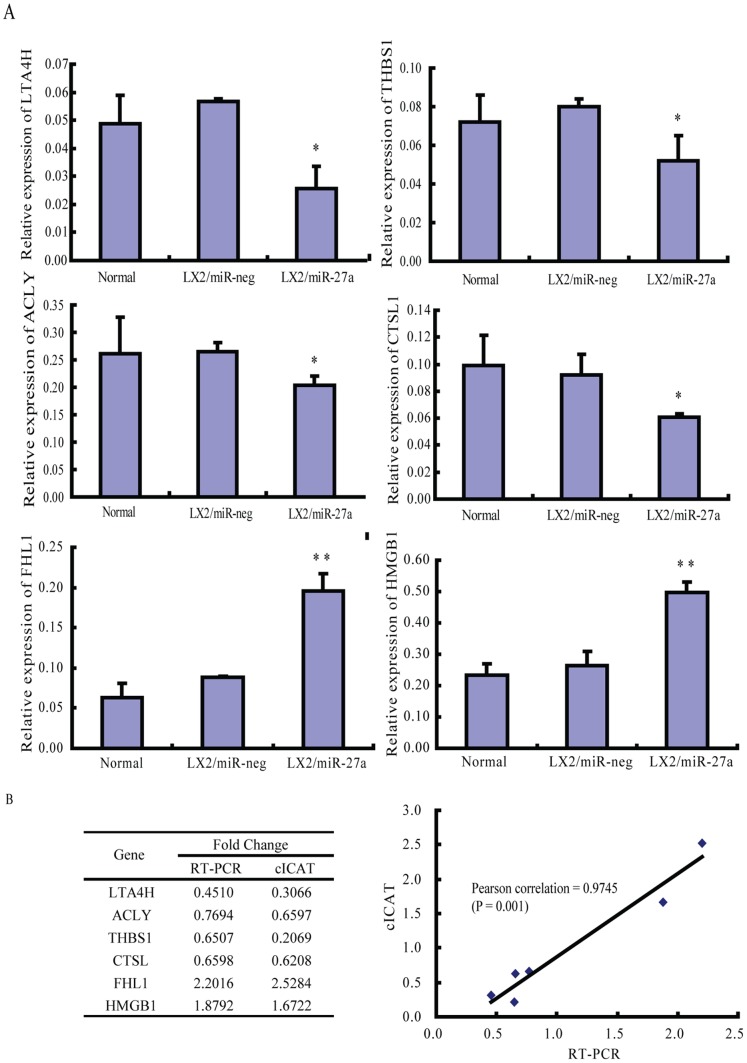
4. Validation of proteomic findings by real-time RT-PCR
Six of the differentially expressed proteins identified in two replicate cICAT assays, ATP-citrate synthase (ACLY), leukotriene A4 hydrolase (LTA4H), cathepsin L1 (CTSL1), thrombospondin-1 precursor (THBS1), four and a half LIM domains 1 (FHL1) and high-mobility group box 1(HMGB1), were validated by real-time RT-PCR. The relationship between fold changes of protein detected by cICAT and fold changes of protein encoding gene detected by PCR was assessed by linear regression analysis. Pearson correlation coefficient for cICAT and real-time RT-PCR expression data was 0.9745 (P = 0.001). The PCR results confirmed the expression pattern observed in cICAT quantitative proteomics analysis (Figure 3).
Figure 3. Validation of cICAT proteomic findings by real-time RT-PCR.
(A) The expression of 6 genes encoding selected proteins in LX2/miR-27a stable transfectants. (B) Linear regression analysis of the fold change of protein and encoding gene in LX2/miR-27a detected by cICAT and RT-PCR respectively. ACLY, ATP-citrate synthase; LTA4H, leukotriene A4 hydrolase; CTSL1, cathepsin L1; THBS1, thrombospondin-1 precursor; FHL1, four and a half LIM domains 1; HMGB1, high-mobility group box 1. *P<0.05, **P<0.01 compared with LX2/miR-neg.
5. Overall distribution of miR-27a regulated proteins in LX2 cells
The subcellular location and bio-function of miR-27a regulated proteins in LX2 cells were categorized by using Protein Knowledgebase (UniprotKB) (Table S4). The subcellular localization of miR-27a regulated proteins is wide, including cytoplasm, nucleus, plasma membrane and extracellular space (Figure 4A). Enzymes, kinase, peptidase and phosphatase constituted the largest part of miR-27a regulated proteins in LX2 cells (49 out of 134 annotated differentially expressed proteins, 37%), followed by transcription regulator (11 out of 134, 8%). Therefore, by preferentially influencing the expression of enzymes and transcription regulators, miR-27a could perform its bio-function with high efficiency (Figure 4B).
Figure 4. Overall distribution of miR-27a regulated proteins in LX2 cells.
(A) Cell location and (B) Functional distribution of all the 134 differentially expressed proteins.
6. Bio-functional analysis of differentially expressed proteins in LX2/miR-27a stable transfectants
GOfact was used to identify the enriched functional categories. The data of functional categorizing was inspiring, according to their molecular functions, most of the altered proteins could be well assigned into the categories involved in de novo lipid synthesis, cell proliferation, apoptosis, cell adhesion and migration, which were closely associated with the mechanisms participating in HSCs activation (Table 2, 3).
Table 2. Functional Categories of Down-regulated Proteins in LX2/miR-27a Compared with LX2/miR-neg (H/L ≤0.6667).
| Functional Categories | Accession | Gene Symbol | Name | H/L | FunctionalCategories | Accession | Gene Symbol | Name | H/L |
| Lipid metabolism | Cell adhesionand mobility | ||||||||
| IPI00021290.5 | ACLY | ATP-citrate synthase | 0.6597 | IPI00394837.2 | RAC1 | ras-related C3 botulinum toxin substrate 1 isoform Rac1c | 0.6298 | ||
| IPI00219077.4 | LTA4H | Isoform 1 of Leukotriene A-4 hydrolase | 0.3066 | IPI00031008.1 | TNC | Isoform 1 of Tenascin precursor | 0.6217 | ||
| IPI00007676.3 | HSD17B12 | Estradiol 17-beta-dehydrogenase 12 | 0.3966 | IPI00845263.1 | FN1 | fibronectin 1 isoform 2 preproprotein | 0.5669 | ||
| IPI00022793.5 | HADHB | Trifunctional enzyme subunit beta, mitochondrial precursor | 0.4545 | IPI00218803.2 | FBLN1 | Isoform B of Fibulin-1 precursor | 0.4012 | ||
| IPI00169285.5 | P76 | Putative phospholipase B-like 2 precursor | 0.6120 | IPI00296099.6 | THBS1 | Thrombospondin-1 precursor | 0.2069 | ||
| Glycolysis and TCA | IPI00011285.1 | CAPN1 | Calpain-1 catalytic subunit | 0.5367 | |||||
| IPI00217143.3 | SDHA | 57 kDa protein | 0.6594 | IPI00844394.1 | CYR61 | 42 kDa protein | 0.5468 | ||
| IPI00790739.1 | ACO2 | Aconitase 2, mitochondrial | 0.4723 | IPI00872386.1 | BCAR1 | Breast cancer anti-estrogen resistance protein 1 | 0.5436 | ||
| IPI00291006.1 | MDH2 | Malate dehydrogenase, mitochondrial precursor | 0.5272 | IPI00009198.3 | TFPI2 | Tissue factor pathway inhibitor 2 precursor | 0.4616 | ||
| IPI00607861.2 | H6PD | GDH/6PGL endoplasmic bifunctional protein precursor | 0.5198 | IPI00007117.1 | SERPINB2 | Plasminogen activator inhibitor 2 precursor | 0.5357 | ||
| IPI00643196.1 | PFKP | Phosphofructokinase, platelet | 0.5484 | Cytoskeleton | |||||
| IPI00418262.4 | ALDOC | Fructose-bisphosphate aldolase C | 0.5835 | IPI00871932.1 | SPTBN1 | 276 kDa protein | 0.6351 | ||
| Cell growth related | IPI00456969.1 | DYNC1H1 | Cytoplasmic dynein 1 heavy chain 1 | 0.6607 | |||||
| IPI00869040.1 | NUBP1 | Isoform 2 of Nucleotide-binding protein 1 | 0.6392 | IPI00062037.1 | DYNLL2 | Dynein light chain 2, cytoplasmic | 0.4487 | ||
| IPI00419273.5 | CUL4A | Isoform 1 of Cullin-4A | 0.5050 | IPI00146935.4 | DNM1L | Isoform 1 of Dynamin-1-like protein | 0.4586 | ||
| IPI00788802.1 | TKT | Transketolase variant (Fragment) | 0.6588 | Ubl conjugation pathway | |||||
| Transcription/translation regulator | IPI00871372.1 | HECTD1 | HECT domain containing 1 | 0.3967 | |||||
| IPI00025091.3 | RPS11 | 40S ribosomal protein S11 | 0.6222 | IPI00645078.1 | UBA1 | Ubiquitin-like modifier-activating enzyme 1 | 0.5802 | ||
| IPI00219156.7 | RPL30 | 60S ribosomal protein L30 | 0.6370 | Miscellaneous | |||||
| IPI00738381.2 | EEF1G | Elongation factor 1-gamma | 0.6504 | IPI00384428.3 | BPHL | Isoform 1 of Valacyclovir hydrolase precursor | 0.4093 | ||
| IPI00017730.1 | SMAD5 | Mothers against decapentaplegic homolog 5 | 0.6113 | IPI00746782.1 | MPST | 3-mercaptopyruvate sulfurtransferase variant (Fragment) | 0.4171 | ||
| IPI00215888.4 | SRP72 | Signal recognition particle 72 kDa protein | 0.6129 | IPI00026612.1 | PPM1B | Isoform Beta-1 of Protein phosphatase 1B | 0.4537 | ||
| IPI00376317.4 | EDC4 | Isoform 1 of Enhancer of mRNA-decapping protein 4 | 0.5609 | IPI00019568.1 | F2 | Prothrombin precursor (Fragment) | 0.5520 | ||
| Transport | IPI00019903.1 | CCDC44 | Coiled-coil domain-containing protein 44 | 0.5392 | |||||
| IPI00008034.1 | RAB23 | Ras-related protein Rab-23 | 0.6407 | IPI00554521.2 | FTH1 | Ferritin heavy chain | 0.6172 | ||
| IPI00791106.2 | SCAMP4 | Isoform 3 of Secretory carrier-associated membrane protein 4 | 0.6565 | IPI00291136.4 | COL6A1 | Collagen alpha-1(VI) chain precursor | 0.5397 | ||
| IPI00060287.3 | C3orf31 | MMP37-like protein, mitochondrial precursor | 0.6380 | IPI00872430.1 | RPS8 | 25 kDa protein | 0.5161 | ||
| IPI00029557.3 | GRPEL1 | GrpE protein homolog 1, mitochondrial precursor | 0.6625 | IPI00827508.2 | RPL10A | 25 kDa protein | 0.5912 | ||
| IPI00646493.1 | COPA | coatomer protein complex, subunit alpha isoform 1 | 0.6641 | IPI00061525.3 | GNPNAT1 | Glucosamine 6-phosphate N-acetyltransferase | 0.5175 | ||
| IPI00219078.5 | ATP2A2 | Isoform SERCA2B of Sarcoplasmic/endoplasmic reticulum calcium ATPase 2 | 0.6095 | IPI00873294.1 | BLMH | 61 kDa protein | 0.6072 | ||
| IPI00026530.4 | LMAN1 | Protein ERGIC-53 precursor | 0.4662 | IPI00289159.3 | GLS | Isoform KGA of Glutaminase kidney isoform, mitochondrial precursor | 0.6137 | ||
| IPI00178314.1 | STXBP6 | Isoform 1 of Syntaxin-binding protein 6 | 0.5278 | IPI00219029.3 | GOT1 | Aspartate aminotransferase, cytoplasmic | 0.6165 | ||
| IPI00411453.3 | AP3D1 | Isoform 1 of AP-3 complex subunit delta-1 | 0.5462 | IPI00012887.1 | CTSL1 | Cathepsin L1 precursor | 0.6208 | ||
| IPI00218466.6 | SEC61A1 | Isoform 1 of Protein transport protein Sec61 subunit alpha isoform 1 | 0.5849 | IPI00022334.1 | OAT | Ornithine aminotransferase, mitochondrial precursor | 0.6457 | ||
| IPI00022881.1 | CLTCL1 | Isoform 1 of Clathrin heavy chain 2 | 0.5929 | IPI00295386.7 | CBR1 | Carbonyl reductase [NADPH] 1 | 0.6148 | ||
| IPI00550382.2 | SLC29A1 | Equilibrative nucleoside transporter 1 | 0.5941 | IPI00413986.2 | Ribosomal protein L1 | 0.5311 | |||
| IPI00328181.1 | TCIRG1 | T-cell, immune regulator 1 isoform a | 0.5663 | Hypothetical proteins | |||||
| Apoptosis | IPI00738655.2 | LOC653781 | similar to protein expressed in prostate, ovary, testis, and placenta 2 | 0.6075 | |||||
| IPI00010277.1 | TNFRSF12A | Isoform 1 of Tumor necrosis factor receptor superfamily member 12A precursor | 0.6016 | IPI00788011.2 | LOC728622 | similar to S-phase kinase-associated protein 1A | 0.5591 | ||
| IPI00419979.3 | PAK2 | Serine/threonine-protein kinase PAK 2 | 0.5688 | IPI00888100.1 | LOC390956 | similar to peptidylprolyl isomerase A-like | 0.5376 | ||
| IPI00847689.1 | HTATIP2 | HIV-1 Tat interactive protein 2, 30kDa isoform a | 0.6114 | IPI00847300.1 | Similar to Voltage-dependent anion-selective channel protein 1 | 0.5335 | |||
| IPI00888597.1 | LOC100129762 | similar to KIAA0367 | 0.5103 | ||||||
| IPI00737530.1 | LOC653888 | similar to p41-Arc | 0.4929 | ||||||
Proteins from LX2/miR-27a were labeled with heavy isotope (H) tagging and those from LX2/miR-neg were labeled with light isotope (L) tagging. Data were from two independent cICAT-based quantitative analyses.
Table 3. Functional Categories of Up-regulated Proteins in LX2/miR-27a Compared with LX2/miR-neg (H/L ≥1.5).
| Functional Categories | Accession | Gene Symbol | Name | H/L | Functional Categories | Accession | Gene Symbol | Name | H/L |
| Lipid metabolism | Apoptosis | ||||||||
| IPI00872459.2 | PRKAA1 | Uncharacterized protein PRKAA1 | 1.9474 | IPI00893062.1 | XRCC6 | X-ray repair complementing defective repair in Chinese hamster cells 6 | 1.5110 | ||
| DNA replication and cell growth | IPI00010882.3 | DFFA | Isoform DFF45 of DNA fragmentation factor subunit alpha (Fragment) | 2.0058 | |||||
| IPI00163608.1 | PARD3 | Isoform 5 of Partitioning-defective 3 homolog | 1.5964 | IPI00006904.1 | AVEN | Cell death regulator Aven | 1.5283 | ||
| IPI00219420.3 | SMC3 | Structural maintenance of chromosomes protein 3 | 1.5081 | Cell adhesion and mobility | |||||
| IPI00791117.1 | TK1 | 29 kDa protein | 1.7692 | IPI00010676.1 | PLAUR | Isoform 1 of Urokinase plasminogen activator surface receptor precursor | 1.5458 | ||
| IPI00465044.2 | RCC2 | Protein RCC2 | 1.7793 | Cytoskeleton | |||||
| IPI00419258.4 | HMGB1 | High mobility group protein B1 | 1.6722 | IPI00220278.5 | MYL9 | Myosin regulatory light chain 2, smooth muscle isoform | 1.5910 | ||
| IPI00031517.1 | MCM6 | DNA replication licensing factor MCM6 | 1.6907 | IPI00328113.2 | FBN1 | Fibrillin-1 precursor | 1.5611 | ||
| IPI00013679.1 | DUT | Isoform DUT-M of Deoxyuridine 5′-triphosphate nucleotidohydrolase, mitochondrial precursor | 1.6977 | IPI00013991.1 | TPM2 | Isoform 1 of Tropomyosin beta chain | 1.6519 | ||
| IPI00384967.3 | ALDH1A3 | Putative uncharacterized protein DKFZp686G1675 (Fragment) | 1.8431 | IPI00442894.3 | TPM1 | Tropomyosin alpha-1 chain | 1.8151 | ||
| IPI00002135.1 | TACC3 | Transforming acidic coiled-coil-containing protein 3 | 1.6166 | IPI00336047.5 | MYO9B | Isoform Long of Myosin-IXb | 2.3887 | ||
| IPI00014572.1 | SPARC | SPARC precursor | 1.7071 | IPI00398735.3 | CNN2 | calponin 2 isoform b | 1.6890 | ||
| IPI00034181.1 | RBBP9 | Isoform 1 of Retinoblastoma-binding protein 9 | 1.7084 | IPI00844425.1 | C3orf10 | Isoform 2 of Probable protein BRICK1 | 2.0215 | ||
| IPI00014398.2 | FHL1 | Four and a half LIM domains 1 variant | 2.5284 | IPI00183002.6 | PPP1R12A | Isoform 1 of Protein phosphatase 1 regulatory subunit 12A | 1.9959 | ||
| Transcription/translation regulator | IPI00478231.2 | RHOA | Transforming protein RhoA precursor | 1.5511 | |||||
| IPI00011675.1 | SP100 | Isoform Sp100-HMG of Nuclear autoantigen Sp-100 | 1.5817 | Ubl conjugation pathway | |||||
| IPI00604620.3 | NCL | NCL Isoform 1 of Nucleolin | 1.6097 | IPI00874175.1 | UBE2G2 | Ubiquitin carrier protein (Fragment) | 1.8507 | ||
| IPI00647163.1 | TCEAL4 | Isoform 2 of Transcription elongation factor A protein-like 4 | 1.5207 | Miscellaneous | |||||
| IPI00219097.4 | HMGB2 | High mobility group protein B2 | 1.7124 | IPI00163230.5 | COPS6 | COP9 signalosome complex subunit 6 | 6.9577 | ||
| IPI00853059.2 | FUBP1 | Isoform 2 of Far upstream element-binding protein 1 | 1.7293 | IPI00477962.3 | UAP1L1 | Isoform 1 of UDP-N-acetylhexosamine pyrophosphorylase-like protein 1 | 2.0940 | ||
| IPI00167985.5 | ZNF579 | Zinc finger protein 579 | 1.8441 | IPI00296141.3 | DPP7 | Dipeptidyl-peptidase 2 precursor | 1.8415 | ||
| IPI00007941.4 | HEXIM1 | Protein HEXIM1 | 1.8459 | IPI00026087.1 | BANF1 | Barrier-to-autointegration factor | 1.6141 | ||
| IPI00028122.1 | PSIP1 | Isoform 1 of PC4 and SFRS1-interacting protein | 1.9394 | IPI00807702.1 | TNIP1 | NEF-associated factor 1 | 1.5713 | ||
| IPI00855957.2 | KHSRP | Isoform 2 of Far upstream element-binding protein 2 | 2.0065 | IPI00101968.3 | DBNL | Isoform 3 of Drebrin-like protein | 1.6175 | ||
| IPI00215801.1 | RBM39 | Isoform 2 of RNA-binding protein 39 | 2.0987 | IPI00093057.6 | CPOX | Coproporphyrinogen III oxidase, mitochondrial precursor | 1.5958 | ||
| IPI00871695.1 | DEK | 48 kDa protein | 4.8877 | IPI00103925.2 | IRGQ | Immunity-related GTPase family Q protein | 1.5803 | ||
| IPI00024662.1 | CBX5 | Chromobox protein homolog 5 | 1.8359 | IPI00894202.1 | C2orf30 | chromosome 2 open reading frame 30 isoform 2 | 1.5903 | ||
| IPI00297579.4 | CBX3 | Chromobox protein homolog 3 | 1.7487 | IPI00550308.1 | RBM12 | RNA-binding protein 12 | 1.5255 | ||
| IPI00021417.3 | SART1 | U4/U6.U5 tri-snRNP-associated protein 1 | 1.5333 | IPI00031622.3 | CHCHD6 | Coiled-coil-helix-coiled-coil-helix domain-containing protein 6 | 3.5705 | ||
| IPI00555857.1 | SFRS5 | CS0DF038YO05 variant (Fragment) | 1.7597 | IPI00178750.3 | NIP30 | NEFA-interacting nuclear protein NIP30 | 2.2462 | ||
| IPI00026957.1 | WBP4 | WW domain-binding protein 4 | 1.7331 | IPI00304922.1 | LSMD1 | Isoform 1 of LSM domain-containing protein 1 | 12.1912 | ||
| IPI00215884.4 | SFRS1 | Isoform ASF-1 of Splicing factor, arginine/serine-rich 1 | 1.5994 | IPI00396321.1 | LRRC59 | Leucine-rich repeat-containing protein 59 | 1.7094 | ||
| IPI00290461.3 | EIF3J | Eukaryotic translation initiation factor 3 subunit J | 1.5853 | IPI00297263.6 | HEG1 | Isoform 1 of Protein HEG homolog 1 precursor | 1.9231 | ||
| IPI00552639.2 | EIF4G1 | Isoform 1 of Eukaryotic translation initiation factor 4 gamma 1 | 1.6356 | IPI00419836.1 | DCBLD2 | Isoform 1 of Discoidin, CUB and LCCL domain-containing protein 2 precursor | 1.8740 | ||
| Transport | Hypothetical proteins | ||||||||
| IPI00848342.1 | LTF | Lactotransferrin precursor | 1.6590 | IPI00006932.3 | LUC7L2 | Isoform 1 of Putative RNA-binding protein Luc7-like 2 | 1.5778 | ||
| IPI00303402.7 | RNUXA | RNA U small nuclear RNA export adapter protein | 1.5796 | IPI00333014.3 | C13orf3 | Isoform 1 of Uncharacterized protein C13orf3 | 1.6993 | ||
| IPI00449201.2 | ATG3 | Isoform 2 of Autophagy-related protein 3 | 1.5491 | IPI00013832.3 | GATC | GatC-like protein | 1.5144 | ||
| IPI00871988.1 | SFXN3 | Uncharacterized protein SFXN3 | 1.6101 | IPI00795769.1 | 52 kDa protein | 2.0541 | |||
| IPI00641384.2 | SEC16A | SEC16 homolog A | 3.0693 | IPI00472879.3 | Novel protein similar to Pre-B cell enhancing factor | 1.5245 | |||
| IPI00872163.1 | ATP2A1 | Similar to ATPase, Ca++ transporting, cardiac muscle, fast twitch 1 | 3.2500 | ||||||
Proteins from LX2/miR-27a were labeled with heavy isotope (H) tagging and those from LX2/miR-neg were labeled with light isotope (L) tagging. Data were from two independent cICAT-based quantitative analyses.
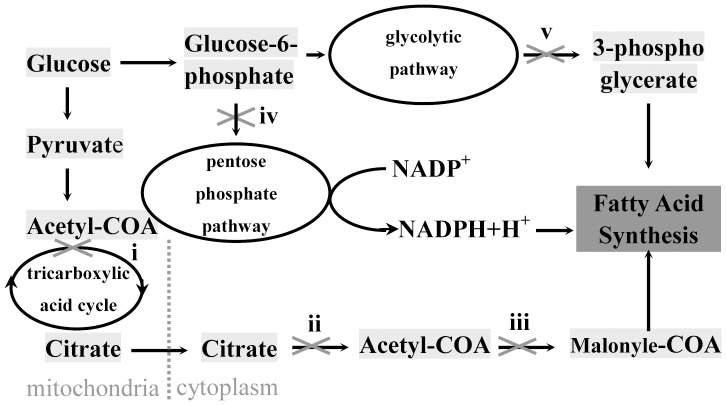
A large number of the down-regulated proteins were involved in de novo lipid synthesis (Figure 5), among which three groups were most concerned: (1) aconitase (ACO2), malate dehydrogenase (MDH2), and ATP-citrate synthase (ACLY), which are important enzymes participating in tricarboxylic acid cycle and favor the production of acetyl-CoA; (2) glucose 1-dehydrogenase/6-phosphogluconolactonase (H6PD), the rate-limiting enzyme for pentose phosphate pathway that supplies NADPH; (3) 6-phosphofructokinase type C (PFKP) and fructose-bisphosphate aldolase C (ALDOC), are involved in glycolytic pathway that provides glycerol-3-phosphate, and the former is a rate-limiting enzyme (Table 2). Acetyl-CoA, NADPH and glycerol-3-phosphate are all required in de novo lipid synthesis. On the other hand, one negative regulator of lipid synthesis called 5′-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1) was significantly up-regulated(Table 3). By phosphorylation, PRKAA1 can inactivate acetyl-CoA carboxylase that catalyzes the rate-limiting reaction in the biosynthesis of long-chain fatty acids [22], [23]. So miR-27a may affect HSCs fat accumulation by directly regulating a group of genes that are involved in the biosynthesis of triglyceride.
Figure 5.
Altered proteins that are involved in metabolism processes related to de novo lipid synthesis: aconitase 2 (ACO2) and malate dehydrogenase (MDH2), which participate in tricarboxylic acid cycle (TAC) (i) decreased; ATP-citrate synthase (ACLY), the primary enzyme responsible for the synthesis of cytosolic acetyl-CoA (ii) decreased; 5′-AMP-activated protein kinase catalytic subunit alpha-1 (PRKAA1) that repress the synthesis of malonyl-CoA (iii) by phosphorylation of acetyl-CoA carboxylase increased; glucose 1-dehydrogenase/6-phosphogluconolactonase (H6PD), the rate-limiting enzyme in pentose phosphate pathway (PPP) (iv) decreased; 6-phosphofructokinase type C (PFKP) that acts as the rate-limiting enzyme, fructose-bisphosphate aldolase C (ALDOC), which are involved in glycolytic pathway(v) decreased.
Proteins involved in cell adhesion and mobility constituted another major group of down-regulated proteins (10 out 75), including Tenascin (TNC) [24], fibronectin 1 (FN1) [25] and Fibulin-1 (FBLN1) [26], which correlated with reduced adhesion and increased migration of miR-27a stable transfectants (Figure 1D).
Over expression of miR-27a also up-regulated a group of factors that favorite proliferation of HSCs. Twelve out of 74 up-regulated proteins were DNA replication and growth-related, and 19 proteins were important transcription/translation regulators, e.g. DNA replication licensing factor MCM6 (MCM6), transcription elongation factor A protein-like 4 (TCEAL4), eukaryotic translation initiation factor 3 subunit J (EIF3J), eukaryotic translation initiation factor 4 gamma 1 (EIF4G1), retinoblastoma-binding protein 9 (RBBP9) [27] and FHL1 [28].
The present proteomic study not only provided the possible mechanism underlying the previously reported miR-27 function in HSCs, but also casted new light on a novel role of miR-27a in myogenesis, which was consistent with the myofibroblast trans-differentiation during HSCs activation. In 9 up-regulated cytoskeleton related proteins, 4 are structural constituents of muscle, including tropomyosin alpha-1 chain (TPM1), tropomyosin beta chain (TPM2), myosin-IXb (MYO9B) and myosin regulatory light chain 2 (MYL9); 4 are in regulation of actomyosin structure and function, including protein phosphatase 1 regulatory subunit 12A (PPP1R12A) [29]; calponin 2 (CNN2) [30]; transforming protein RhoA (RHOA) [31] and FHL1 [32]. The up-regulation of TPM1, MYO9B and MYL9 by miR-27a in LX2 cells was further validated by RT-PCR (Figure S1). In a previous study, it has also been evidenced that miR-27a can up-regulate cardiac myosin heavy chain (MHC) gene (β-MHC) expression via thyroid hormone signaling [33]. And miR-27a has also been reported to be able to influence muscle stem cell behavior [34]. It is the first time for us to recognize a novel role of miR-27a in promoting myogenic tans-differentiation in HSCs. The finding also suggested similar bio-functions of the same miRNA in different types of tissues or cells. However, further effort is needed to determine the role of miR-27a in myogenic trans-differentiation of activated HSCs.
7. The biological significance of miR-27a regulated protein in HSCs
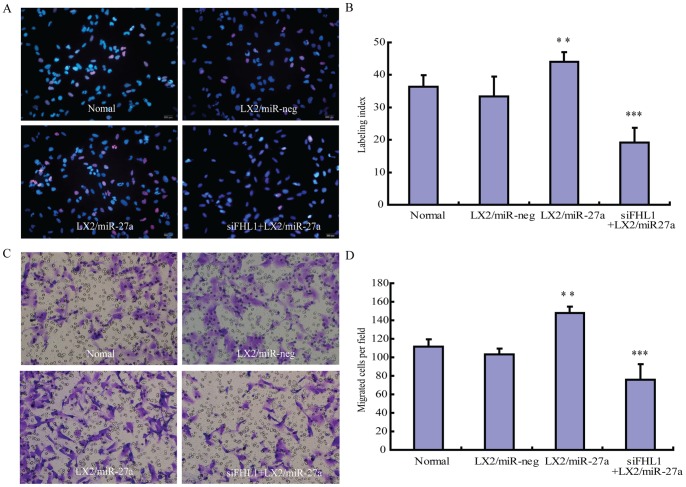
In order to validate the biological significance of miR-27a regulated proteins identified by cICAT proteomic strategy, the function of FHL1, one of the highest increased proteins which not only related to cell growth [28] but also played a crucial role in embryonic skeletal muscle myogenesis [32], was evaluated in miR-27a transfectants. Three different siRNA targeting FHL1 were compared. The one possessed the highest knockdown efficiency (Figure S2) was used in the following experiment. Our data showed that FHL1 involved in miR-27a related HSCs proliferation and migration, knockdown of FHL1 significantly inhibited the proliferation and migration of LX2/miR-27a transfectants (Figure 6). Interestingly, in a recent study based on 2-dimensional polyacrylamide gel electrophoresis (2D-PAGE) proteomic approach, FHL-1 was identified as one of the most prominently up-regulated proteins in pulmonary hypertension mouse model, and a similar effects of FHL-1 on promoting pulmonary arterial smooth muscle cell migration and proliferation has also been evidenced [35].
Figure 6. Involvement of FLH1 in miR-27a related HSCs proliferation and migration.
Knockdown of FLH1 suppressed cell proliferation in LX2/miR-27a transfectants. (A) EdU cell proliferation assay. EdU was detected by Apollo 567 fluorescent dye (red) and nuclei were counterstained with Hoechst 33342 (blue) (original magnification ×200). (B) Statistical results of three independent experiments. The results are expressed as the labeling index according to the following formula: number of EdU-positive nuclei x 100/number of total nuclei. FHL1 was required for increased migration in LX2/miR-27a transfectants. (C) Migration assays. LX2/miR-27a transfectants were plated on 8-lm pore size Transwell inserts for 16 hours. The number of migrated cells was counted manually (original magnification ×200). (D) The statistical results of three independent experiments. Each image is a representative of three independent experiments. ***P<0.001, **P<0.01 compared with LX2/miR-neg.
Conclusions
The data of present study indicated that miR-27a influenced the activation of HSCs by affecting several groups of proteins. These results not only explained our previous finding that over-expression of miR-27a promoted HSC activation with reduced cytoplasmic lipid drops and increased cell proliferation [8], but also revealed a novel role of miR-27a in promoting the myogenic trans-differentiation of activated HSC into myofibroblast. The pattern of miR-27a regulation on protein expression might well reflect the emerging picture of miRNA regulation in animals is far richer and more complex than the crisp linear pathways [1]. Our study also validated proteomic strategy as a promising tool for functional study of miRNA. In the future, it will be interesting to uncover the mechanisms underlying the regulation of miR-27a on these functionally related genes.
Supporting Information
Validation of myogenesis related genes found by cICAT proteomic analyses. The expression of TPM1, MYO9B and MYL9 encoding mRNA was evaluated by RT-PCR in LX2/miR-27a stable transfectants. *P<0.05, compared with LX2/miR-neg.
(TIF)
Knockdown efficiency of FHL1 siRNA, LX2 cells were transfected with FHL1 specific siRNA or with NTC siRNA, after 48 hours, their mRNA levels were determined by quantitative polymerase chain reaction. GAPDH was used as housekeeping gene. NTC, non-targeting control siRNA transfected cells. **P<0.01 compared with NTC.
(TIF)
Primer Sets for Real-time PCR. *Sense primers for mature miR-27a were provided here, anti-sense primer was provided by Invitrogen as Universal q-PCR Primer.
(DOC)
Protein List of 2 Independent 2D nano-LC-MS/MS Analysis of LX2/miR-27a and LX2/miR-neg.
(XLS)
List of Proteins Identified and Quantified in LX2/miR-27a and LX2/miR-neg.
(XLS)
List of Proteins Up-or Down-regulated in LX2/miR-27a Compared with LX2/miR-neg.
(XLS)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its supporting information files.
Funding Statement
This research is supported by grants from the Natural Science Foundation of China (NSFC, http://www.nsfc.gov.cn/publish/portal1/), No. 81141048 and 30900563 to JJL, No. 81272027 to JYH, Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teachers and Presidents from Jiangsu Provincial Department of Education (http://english.jsjyt.gov.cn/) to JJL, a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions and Natural Science Foundation of the Higher Education Institutions of Jiangsu Province No. 13KJA180005 to JYH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ (2008) miRBase: tools for microRNA genomics. Nucleic Acids Res 36: D154–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thomas M, Lieberman J, Lal A (2010) Desperately seeking microRNA targets. Nat Struct Mol Biol 17: 1169–74. [DOI] [PubMed] [Google Scholar]
- 5. Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, et al. (2008) Widespread changes in protein synthesis induced by microRNAs. Nature 455: 58–63. [DOI] [PubMed] [Google Scholar]
- 6. Huang TC, Pinto SM, Pandey A (2013) Proteomics for understanding miRNA biology. Proteomics 13: 558–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shiio Y, Aebersold R (2006) Quantitative proteome analysis using isotope-coded affinity tags and mass spectrometry. Nat Protoc 1: 139–45. [DOI] [PubMed] [Google Scholar]
- 8. Ji J, Zhang J, Huang G, Qian J, Wang X, et al. (2009) Over-expressed microRNA-27a and 27b influence fat accumulation and cell proliferation during rat hepatic stellate cell activation. FEBS Lett 583: 759–66. [DOI] [PubMed] [Google Scholar]
- 9. Wang T, Li M, Guan J, Li P, Wang H, et al. (2011) MicroRNAs miR-27a and miR-143 Regulate Porcine Adipocyte Lipid Metabolism. Int J Mol Sci 12: 7950–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vickers KC, Shoucri BM, Levin MG, Wu H, Pearson DS, et al. (2013) MicroRNA-27b is a regulatory hub in lipid metabolism and is altered in dyslipidemia. Hepatology 57: 533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xu W, Liu M, Peng X, Zhou P, Zhou J, et al. (2013) miR-24–3p and miR-27a-3p promote cell proliferation in glioma cells via cooperative regulation of MXI1. Int J Oncol 42: 757–66. [DOI] [PubMed] [Google Scholar]
- 12. Guttilla IK, White BA (2009) Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J Biol Chem 284: 23204–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Acunzo M, Romano G, Palmieri D, Lagana A, Garofalo M, et al. (2013) Cross-talk between MET and EGFR in non-small cell lung cancer involves miR-27a and Sprouty2. Proc Natl Acad Sci U S A 110: 8573–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xu L, Hui AY, Albanis E, Arthur MJ, O’Byrne SM, et al. (2005) Human hepatic stellate cell lines, LX-1 and LX-2: new tools for analysis of hepatic fibrosis. Gut 54: 142–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dong L, Jianqi L, Shuguang O, Jian W, Xiaojie X, et al. (2005) An Integrated Strategy for Functional Analysis in Large-scale Proteomic Research by Gene Ontology. Progress in Biochemistry and Biophysics 32: 1026–1029. [Google Scholar]
- 16. Dong L, Jianqi L, Shuguang O, Songfeng W, Jian W, et al. (2005) An integrated strategy for functional analysis in large scale proteomic research by gene ontology. Molecular & Cellular Proteomics. 4: S34–S34. [Google Scholar]
- 17. Baek D, Villen J, Shin C, Camargo FD, Gygi SP, et al. (2008) The impact of microRNAs on protein output. Nature 455: 64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ji J, Yu F, Ji Q, Li Z, Wang K, et al. (2012) Comparative proteomic analysis of rat hepatic stellate cell activation: a comprehensive view and suppressed immune response. Hepatology 56: 332–49. [DOI] [PubMed] [Google Scholar]
- 19. Lewis BP, Burge CB, Bartel DP (2005) Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell 120: 15–20. [DOI] [PubMed] [Google Scholar]
- 20. Rogler CE, Levoci L, Ader T, Massimi A, Tchaikovskaya T, et al. (2009) MicroRNA-23b cluster microRNAs regulate transforming growth factor-beta/bone morphogenetic protein signaling and liver stem cell differentiation by targeting Smads. Hepatology 50: 575–84. [DOI] [PubMed] [Google Scholar]
- 21. Ma F, Liu X, Li D, Wang P, Li N, et al. (2010) MicroRNA-466l upregulates IL-10 expression in TLR-triggered macrophages by antagonizing RNA-binding protein tristetraprolin-mediated IL-10 mRNA degradation. J Immunol 184: 6053–9. [DOI] [PubMed] [Google Scholar]
- 22. Carlson CA, Kim KH (1973) Regulation of hepatic acetyl coenzyme A carboxylase by phosphorylation and dephosphorylation. J Biol Chem 248: 378–80. [PubMed] [Google Scholar]
- 23. Towler MC, Hardie DG (2007) AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res 100: 328–41. [DOI] [PubMed] [Google Scholar]
- 24. Mackie EJ, Tucker RP, Halfter W, Chiquet-Ehrismann R, Epperlein HH (1988) The distribution of tenascin coincides with pathways of neural crest cell migration. Development 102: 237–50. [DOI] [PubMed] [Google Scholar]
- 25. Akiyama SK, Yamada SS, Chen WT, Yamada KM (1989) Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol 109: 863–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Timpl R, Sasaki T, Kostka G, Chu ML (2003) Fibulins: a versatile family of extracellular matrix proteins. Nat Rev Mol Cell Biol 4: 479–89. [DOI] [PubMed] [Google Scholar]
- 27. Shields DJ, Niessen S, Murphy EA, Mielgo A, Desgrosellier JS, et al. (2010) RBBP9: a tumor-associated serine hydrolase activity required for pancreatic neoplasia. Proc Natl Acad Sci U S A 107: 2189–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schawalder SB, Kabani M, Howald I, Choudhury U, Werner M, et al. (2004) Growth-regulated recruitment of the essential yeast ribosomal protein gene activator Ifh1. Nature 432: 1058–61. [DOI] [PubMed] [Google Scholar]
- 29. Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR (2009) Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Winder SJ, Allen BG, Clement-Chomienne O, Walsh MP (1998) Regulation of smooth muscle actin-myosin interaction and force by calponin. Acta Physiol Scand 164: 415–26. [DOI] [PubMed] [Google Scholar]
- 31. Wei L, Zhou W, Croissant JD, Johansen FE, Prywes R, et al. (1998) RhoA signaling via serum response factor plays an obligatory role in myogenic differentiation. J Biol Chem 273: 30287–94. [DOI] [PubMed] [Google Scholar]
- 32. Arber S, Halder G, Caroni P (1994) Muscle LIM protein, a novel essential regulator of myogenesis, promotes myogenic differentiation. Cell 79: 221–31. [DOI] [PubMed] [Google Scholar]
- 33. Nishi H, Ono K, Horie T, Nagao K, Kinoshita M, et al. (2011) MicroRNA-27a regulates beta cardiac myosin heavy chain gene expression by targeting thyroid hormone receptor beta1 in neonatal rat ventricular myocytes. Mol Cell Biol 31: 744–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Crist CG, Montarras D, Pallafacchina G, Rocancourt D, Cumano A, et al. (2009) Muscle stem cell behavior is modified by microRNA-27 regulation of Pax3 expression. Proc Natl Acad Sci U S A 106: 13383–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kwapiszewska G, Wygrecka M, Marsh LM, Schmitt S, Trosser R, et al. (2008) Fhl-1, a new key protein in pulmonary hypertension. Circulation 118: 1183–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Validation of myogenesis related genes found by cICAT proteomic analyses. The expression of TPM1, MYO9B and MYL9 encoding mRNA was evaluated by RT-PCR in LX2/miR-27a stable transfectants. *P<0.05, compared with LX2/miR-neg.
(TIF)
Knockdown efficiency of FHL1 siRNA, LX2 cells were transfected with FHL1 specific siRNA or with NTC siRNA, after 48 hours, their mRNA levels were determined by quantitative polymerase chain reaction. GAPDH was used as housekeeping gene. NTC, non-targeting control siRNA transfected cells. **P<0.01 compared with NTC.
(TIF)
Primer Sets for Real-time PCR. *Sense primers for mature miR-27a were provided here, anti-sense primer was provided by Invitrogen as Universal q-PCR Primer.
(DOC)
Protein List of 2 Independent 2D nano-LC-MS/MS Analysis of LX2/miR-27a and LX2/miR-neg.
(XLS)
List of Proteins Identified and Quantified in LX2/miR-27a and LX2/miR-neg.
(XLS)
List of Proteins Up-or Down-regulated in LX2/miR-27a Compared with LX2/miR-neg.
(XLS)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its supporting information files.