Abstract
Amblyomma americanum is the most commonly-encountered tick species in southeastern Virginia, representing approximately 95% of the human-biting tick population in this area. Here we investigated the prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in questing Amblyomma americanum and Dermacentor variabilis ticks collected from multiple sites in southeastern Virginia from 2010–2011. Although both Ehrlichia species were detected in Amblyomma americanum, no evidence of either pathogen was found in Dermacentor variabilis. Prevalence of E. chaffeensis varied by location, ranging from 0 – 5.08% among Amblyomma americanum populations. Ehrlichia ewingii prevalence was slightly higher, ranging from 0 – 8.20% among A. americanum populations. We conclude that both pathogens are established in southeastern Virginia A. americanum populations, and that although there are no apparent temporal trends in Ehrlichia prevalence, there is variation among locations, suggesting the potential for disease hotspots.
Keywords: Amblyomma americanum, Dermacentor variabilis, Ehrlichia chaffeensis, Ehrlichia ewingii
Introduction
The lone star tick, Amblyomma americanum (L.) (Acari: Ixodidae), is found throughout the southeastern United States with populations extending west to central Texas and north to Iowa (Childs and Paddock, 2003). The eastern range of A. americanum extends through the mid-Atlantic region, with populations intermittently reported in New England states including Maine (Kierans and Lacombe, 1998), Connecticut and Rhode Island (Ijdo et al., 2000). Amblyomma americanum is the most commonly reported tick species collected from humans in the southeastern and mid-Atlantic U.S., representing over 60% of ticks collected from humans from New Jersey, Maryland, Virginia, Kentucky and South Carolina from 2004–2010 (Stromdahl and Hickling, 2012). In southeastern Virginia, A. americanum is the most commonly encountered human-biting tick, constituting over 95% of questing ticks collected from 2010–2012 (Nadolny et al., 2014). Because of the abundance of this tick in the southeastern U.S. and its propensity to feed on humans, pathogens transmitted by A. americanum pose an important threat to human health.
Ehrlichia chaffeensis and Ehrlichia ewingii are the causative agents of human ehrlichiosis and are transmitted to humans and animals by infected A. americanum (Anziania et al., 1990 and Ewing et al., 1995). These Ehrlichia spp. have also been found in the American dog tick, Dermacentor variabilis (Say) (Murphy et al., 1998 and Steiert and Gilfoy, 2002), although it is unclear whether D. variabilis is capable of transmitting these pathogens. Here we describe the prevalence of Ehrlichia chaffeensis and Ehrlichia ewingii in ticks collected from southeastern Virginia.
Materials and Methods
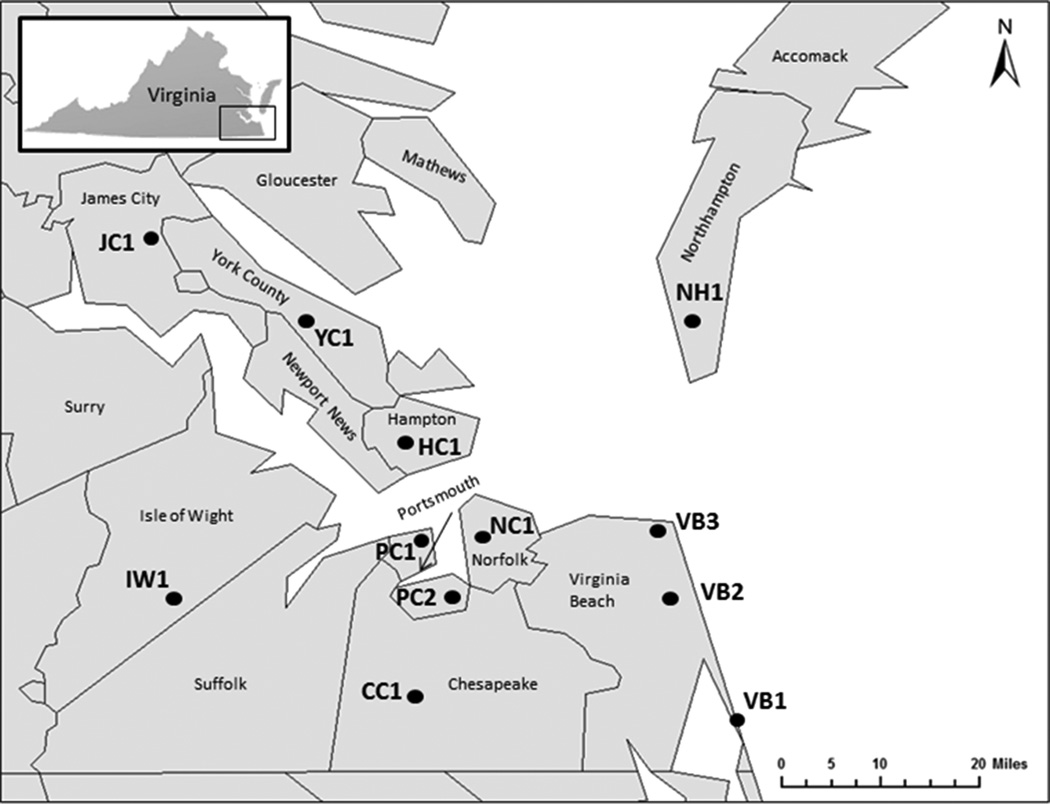
Questing adult and nymphal A. americanum and adult D. variabilis were collected on flags from April through September of 2010 and 2011 from multiple locations representing 11 independent cities and counties in southeastern Virginia (Fig. 1). Nine sites were sampled on a weekly basis in 2010 and 12 sites were sampled on a weekly basis in 2011 (Nadolny et al., 2014). Within each site, the area of each transect was recorded so that density of host-seeking ticks encountered during each sampling event could be determined. Ticks were identified to species morphologically (Keirans and Litwak, 1989; Keirans and Durden, 1998) and individuals were pooled prior to DNA extraction. Adult A. americanum and D. variabilis collected at the same location in the same week were morphologically identified and then pooled into groups of up to 10. Amblyomma americanum nymphs were pooled into groups of up to 25. Prior to extraction all adult ticks were cut in half, one half was used for DNA extraction and the other stored at −80°C for future use. All ticks were homogenized by bead-beating with 1 mm glass beads. DNA was extracted using the DNeasy Blood and Tissue Kit (Qiagen, Inc. Valencia, CA) following the manufacturer’s protocol and stored at −20°C.
Figure 1.
Map of southeastern Virginia showing the location of the sites where ticks were collected in 2010 and 2011.
Samples were tested separately for E. chaffeensis and E. ewingii DNA using real-time quantitative PCR (qPCR) assays specific to each species. Both E. chaffeensis and E. ewingii were tested for using TaqMan qPCR assays targeting the 16S rRNA gene (Loftis et al., 2003 and Killmaster et al., 2014). A subset of qPCR-positive samples were confirmed by sequencing either the groEL gene of E. chaffeensis (Tabara et al., 2007) or the p28 gene of E. ewingii (Gusa et al., 2001) using a nested PCR assay. A total of 38 E. chaffeensis positive samples and 6 E. ewingii positive samples were sequence-confirmed. Sequences were analyzed by performing a BLAST search on GenBank.
Because tick samples were pooled prior to extraction, a maximum likelihood estimation (MLE) was used to approximate the true prevalence of E. chaffeensis and E. ewingii in the tick population. The software used to perform MLE (Biggerstaff, 2008) was acquired from the Centers for Disease Control and Prevention website (CDC).
Results
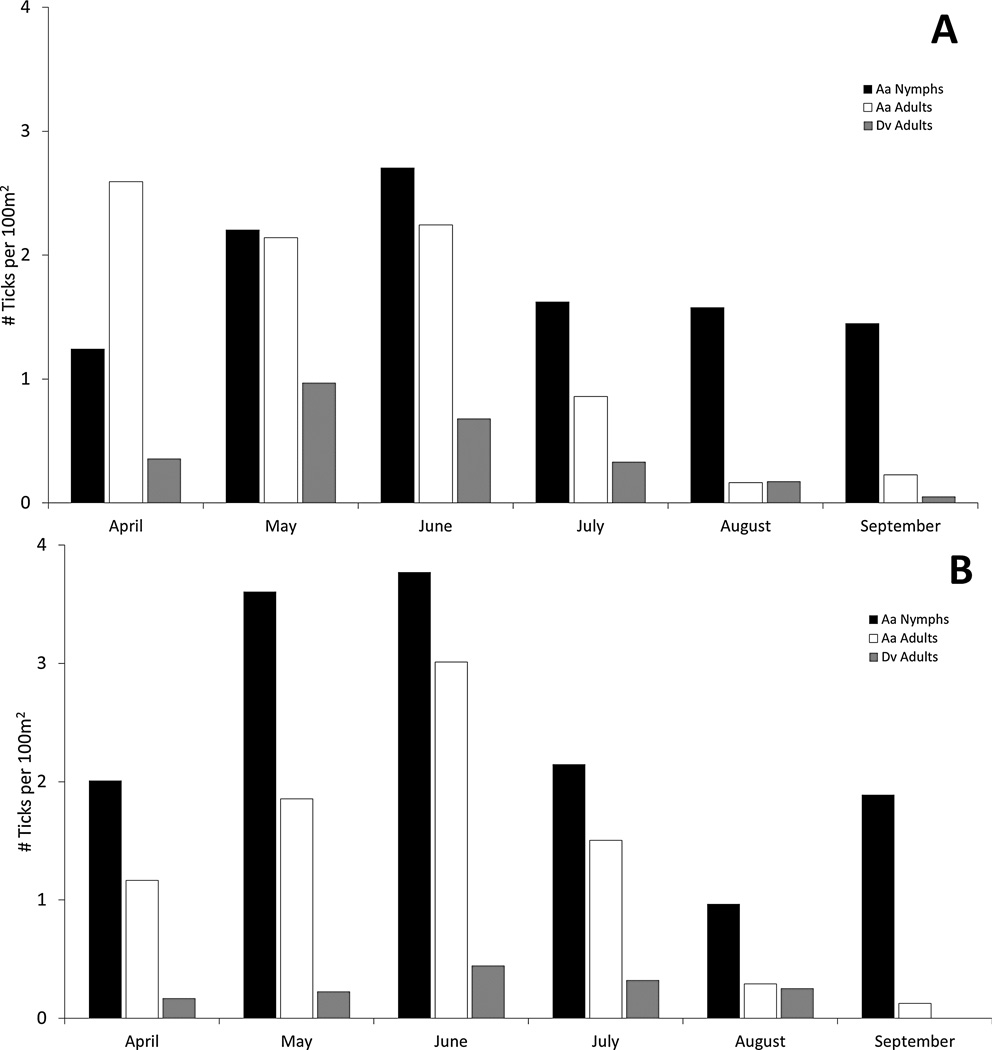
A total of 605 D. variabilis adults and 8700 A. americanum adults and nymphs were collected during 2010 and 2011. The highest numbers of both species were collected in May and June in both years (Fig. 2). Although both E. chaffeensis and E. ewingii were detected in A. americanum, no evidence of either pathogen was found in the D. variabilis tested. Sequence confirmation of 44 positive samples showed either ≥99% match to E. chaffeensis or 100% match to E. ewingii in a BLAST search. A total of 967 and 981 A. americanum pools were tested for E. chaffeensis and E. ewingii, respectively. Because testing for each pathogen was performed at different times, not every sample was available for testing in both assays. Overall prevalence of E. chaffeensis in A. americanum adults and nymphs was 0.9% in 2010 and 0.6% in 2011; E. ewingii prevalence was 1.5% and 1.3% in 2010 and 2011, respectively (Table 1). A higher prevalence of both Ehrlichia spp. was found in adults than in nymphs, with adults having an approximate ten-fold greater prevalence of both pathogens (Table 1). In adults, prevalence of E. chaffeensis varied by location (Table 2), ranging from 0 – 4.3% (mean = 1.6 ± 1.4) in 2010 and 0 – 5.1% (mean = 1.1 ± 1.6) in 2011. Prevalence of E. ewingii in adults also varied by location, ranging from 0 – 8.2% (mean = 3.1 ± 2.6) in 2010 and 0 – 7.7% (mean = 2.8 ± 2.8) in 2011. The higher prevalence of E. ewingii relative to that of E. chaffeensis in adult A. americanum was mainly driven by one site in Virginia Beach, which had the highest prevalence of all sites (8.2% in 2010 and 7.7% in 2011). Although greater numbers of both A. americanum and D. variabilis were collected during May and June, there were no apparent spatial or temporal trends in prevalence of either Ehrlichia spp. (Table 2).
Figure 2.
Phenology of A. americanum nymphs and adults and D. variabilis adults collected during 2010 (A) and 2011 (B) from southeastern Virginia.
Table 1.
Pooled and maximum likelihood estimated (MLE) prevalence of Ehrlichia spp. in questing adult and nymphal A. americanum and adult D. variabilis collected on flags from multiple sites within southeastern Virginia. To assess true pathogen prevalence from pooled DNA samples, a MLE calculation was used (Biggerstaff, 2008).
| Tick | Year | Organism | Life Stage |
Number Pools Positive |
Number Pools Tested |
# of Ticks |
Pools Positive (%) |
MLE (%) |
|---|---|---|---|---|---|---|---|---|
| A. americanum | 2010 | E. chaffeensis | All | 28 | 417 | 3134 | 6.7 | 0.9 |
| Adult | 25 | 221 | 1363 | 11.3 | 2.0 | |||
| Nymph | 3 | 196 | 1771 | 1.5 | 0.2 | |||
| E. ewingii | All | 45 | 426 | 3095 | 10.5 | 1.5 | ||
| Adult | 42 | 231 | 1343 | 18.2 | 3.4 | |||
| Nymph | 3 | 195 | 1752 | 1.5 | 0.2 | |||
| 2011 | E. chaffeensis | All | 26 | 550 | 4814 | 4.7 | 0.6 | |
| Adult | 20 | 254 | 1483 | 7.8 | 1.4 | |||
| Nymph | 6 | 296 | 3331 | 2.1 | 0.2 | |||
| E. ewingii | All | 59 | 555 | 4813 | 10.6 | 1.3 | ||
| Adult | 48 | 262 | 1532 | 18.3 | 3.5 | |||
| Nymph | 11 | 293 | 3281 | 3.2 | 0.3 | |||
| D. variabilis | 2010 | E. chaffeensis | Adult | 0 | 69 | 259 | 0 | - |
| E. ewingii | Adult | 0 | 69 | 259 | 0 | - | ||
| 2011 | E. chaffeensis | Adult | 0 | 76 | 228 | 0 | - | |
| E. ewingii | Adult | 0 | 76 | 228 | 0 | - |
Table 2.
Maximum likelihood estimated (MLE) prevalence of Ehrlichia chaffeensis (left) and Ehrlichia ewingii (right) in adult and nymphal A. americanum collected from various locations (Fig. 1) within southeastern Virginia in 2010 and 2011. The total number of individuals represented within pooled DNA samples is indicated.
| Year | Site | Ehrlichia chaffeensis | Ehrlichia ewingii | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MLE Prevalence (%) | # Ticks Represented | MLE Prevalence (%) | # Ticks Represented | ||||||
| Adults | Nymphs | Adults | Nymphs | Adults | Nymphs | Adults | Nymphs | ||
| 2010 | All | 1.95 | 0.17 | 1363 | 1771 | 3.44 | 0.17 | 1343 | 1752 |
| CC1 | 4.33 | 0.85 | 50 | 121 | 3.50 | 0 | 59 | 99 | |
| IW1 | 2.43 | 0 | 40 | 52 | 2.40 | 0 | 41 | 53 | |
| JC1 | 1.09 | 0 | 92 | 155 | 5.80 | 0 | 82 | 153 | |
| NH1 | 0 | 0 | 15 | 3 | 0 | 0 | 15 | 3 | |
| PC1 | 2.23 | 0.27 | 630 | 746 | 2.10 | 0.13 | 624 | 733 | |
| PC2 | 0 | 0 | 21 | 0 | 0 | 0 | 21 | 0 | |
| VB1 | 1.04 | 0 | 93 | 183 | 3.50 | 0 | 91 | 185 | |
| VB2 | 1.30 | 0 | 239 | 302 | 8.2 | 0.65 | 233 | 317 | |
| YC1 | 2.30 | 0 | 183 | 208 | 2.40 | 0 | 177 | 208 | |
| 2011 | All | 1.41 | 0.18 | 1483 | 3331 | 3.47 | 0.34 | 1532 | 3281 |
| CC1 | 0 | 0 | 203 | 495 | 2.09 | 0.41 | 203 | 495 | |
| HC1 | 0 | 0 | 15 | 33 | 2.22 | 0 | 95 | 41 | |
| IW1 | 5.08 | 0.65 | 41 | 152 | 5.58 | 0 | 38 | 48 | |
| JC1 | 0 | 0 | 22 | 31 | 0 | 0 | 22 | 31 | |
| NC1 | 0 | 0 | 4 | 2 | 0 | 0 | 4 | 2 | |
| NH1 | 2.59 | 0 | 209 | 119 | 4.40 | 0 | 209 | 119 | |
| PC1 | 1.31 | 0.06 | 393 | 1760 | 1.13 | 0.18 | 366 | 1707 | |
| PC2 | 0 | 0 | 4 | 1 | 0 | 0 | 4 | 0 | |
| VB1 | 2.05 | 0 | 101 | 83 | 7.06 | 1.31 | 97 | 77 | |
| VB2 | 1.94 | 0.95 | 216 | 451 | 7.72 | 0.91 | 217 | 451 | |
| VB3 | 0 | 0 | 1 | 3 | 0 | 0 | 1 | 3 | |
| YC1 | 0.74 | 0 | 274 | 201 | 3.18 | 0 | 276 | 202 | |
To validate the accuracy of the maximum likelihood estimation, leftover individual adult A. americanum halves from pools which tested positive for E. chaffeensis were extracted and tested by qPCR for E. chaffeensis. A MLE was then performed to determine E. chaffeensis prevalence within these individually extracted samples. Pooled samples had an overall E. chaffeensis prevalence of 1.95% in 2010, whereas when MLE analysis of individually extracted samples indicated a prevalence of 2.01%. Since these prevalence values are similar, this experiment validates the accuracy of the MLE calculation, which has been used extensively to estimate the prevalence of vector-borne disease agents in studies with pooled samples.
Discussion
We describe the collection of both A. americanum and D. variabilis in southeastern Virginia, as well as the presence of both E. chaffeensis and E. ewingii in questing A. americanum nymphs and adults. Although D. variabilis has occasionally been shown to harbor these pathogens, we found no evidence of either pathogen in any D. variabilis collected in this study. The prevalence of E. chaffeensis (1.4 – 2.0%) and E. ewingii (3.4 – 3.5%) in adult A. americanum is comparable to the prevalence of E. chaffeensis (2.2%) and E. ewingii (2.2%) determined in another study assessing the rate of Ehrlichia spp. infection in A. americanum adults collected in 2012 (Gaines et al., 2014). The study, which assessed the prevalence of Ehrlichia spp. in A. americanum adults collected throughout the state of Virginia, found that E. chaffeensis prevalence ranged from 0 – 24.5% and E. ewingii prevalence ranged from 0 – 14.3% (Gaines et al., 2014). The lower infection prevalence in A. americanum nymphs is consistent with other studies assessing the prevalence of Ehrlichia spp. in questing ticks. Amblyomma americanum nymphs collected in Maryland were determined to have an E. chaffeensis minimum infection rate (MIR) of just 0.8%, while adults showed a MIR prevalence of 3.5% (Stromdahl et al., 2000). Given that the white-tailed deer (Odocoileus virginianus) is a known reservoir of Ehrlichia spp. (Ewing et al., 1995, Lockhart et al., 1997) it is not surprising that questing A. americanum adults, which have taken two bloodmeals in their lifetime, would have a greater prevalence than questing nymphs, which would have taken just one bloodmeal.
Other studies have noted the great abundance of A. americanum present in the southeastern and south-central United States in relation to other sympatric tick species (Stromdahl and Hickling, 2012 and Nadolny et al., 2014). All three A. americanum life stages (larva, nymph and adult) are known to aggressively parasitize humans and multiple concurrent tick bites of this species are often reported. Stromdahl and Hickling (2012) observed that approximately 15% of persons submitting A. americanum to the DOD for testing submitted multiple specimens. Because of the high proportion of A. americanum in this area and the propensity of this species to seek out human hosts in both the nymphal and adult stages, pathogens present even in low numbers within these populations warrant attention as concerns to public health. Furthermore, this study found no uniformity in geographic distribution of either Ehrlichia species in A. americanum, indicating a potential for disease “hotspots” in areas where these pathogens are more abundant.
Acknowledgements
We would like to thank all of the field assistants for their efforts in collecting ticks.
The project described was supported by Grant Number K25AI067791 (H. Gaff, PI) from the National Institute of Allergy and Infectious Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Allergy and Infectious Diseases or the National Institutes of Health.
We would also like to acknowledge the Nature Conservancy, the Back Bay Wildlife Refuge and Elizabeth River Project for permission to use their land.
References Cited
- 1.Anziania OS, Ewing SA, Barker RW. Experimental transmission of a granulocytic form of the tribe Ehrlichieae by Dermacentor variabilis and Amblyomma americanum to dogs. Am J Vet Res. 1990;51:929–931. [PubMed] [Google Scholar]
- 2.Biggerstaff BJ. Confidence intervals for the difference of two proportions estimated from pooled samples. J Agric Biol Envir S. 2008;13(4):478–496. [Google Scholar]
- 3.CDC. Mosquito Surveillance Software. [accessed 13.06.13];2013 < http://www.cdc.gov/westnile/resourcepages/mosqSurvSoft.html>.
- 4.Childs JE, Paddock CD. The Ascendancy of Amblyomma americanum as a Vector of Pathogens Affecting Humans in The United States. Annu Rev Entomol. 2003;48:307–337. doi: 10.1146/annurev.ento.48.091801.112728. [DOI] [PubMed] [Google Scholar]
- 5.Ewing SA, Dawson JE, Kocan AA, Barker RW, Warner CK, Panciera RJ, Fox JC, Kocan KM, Blouin EF. Experimental transmission of Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) among white-tailed deer by Amblyomma americanum (Acari: Ixodidae) J Med Entomol. 1995;32:368–374. doi: 10.1093/jmedent/32.3.368. [DOI] [PubMed] [Google Scholar]
- 6.Gaines DN, Operario DJ, Stroup S, Stromdahl E, Wright C, Gaff H, et al. Ehrlichia and Spotted Fever Group Rickettsiae Surveillance in Amblyomma americanum in Virginia Through Use of a Novel Six-Plex Real-Time PCR Assay. Vector-Borne Zoonot. 2014;14(5):307–316. doi: 10.1089/vbz.2013.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gusa AA, Buller RS, Storch GA, Huycke MM, Machado LJ, Slater LN, Stockham SL, Massung RF. Identification of a p28 gene in Ehrlichia ewingii: evaluation of gene for use as a target for a species-specific PCR diagnostic assay. J Clin Microbiol. 2001;39:3871–3876. doi: 10.1128/JCM.39.11.3871-3876.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ijdo JW, Wu C, Magnarelli LA, Stafford KC, Anderson JF, Fikrig E. Detection of Ehrlichia chaffeensis DNA in Amblyomma americanum Ticks in Connecticut and Rhode Island. J Clin Microbiol. 2000;38:4655–4656. doi: 10.1128/jcm.38.12.4655-4656.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keirans JE, Lacombe EH. First records of Amblyomma americanum, Ixodes (Ixodes) dentatus, and Ixodes (Ceratixodes) uriae (Acari: Ixodidae) from Maine. J Parasitol. 1998;84:629–631. [PubMed] [Google Scholar]
- 10.Keirans JE, Litwak TR. Pictorial key to the adults of hard ticks, family Ixodidae (Ixodida: Ixodoidea), east of the Mississippi River. J Med Entomol. 1989;26(5):435–448. doi: 10.1093/jmedent/26.5.435. [DOI] [PubMed] [Google Scholar]
- 11.Keirans JE, Durden LA. Illustrated key to nymphs of the tick genus Amblyomma (Acari: Ixodidae) found in the United States. J Med Entomol. 1998;35(4):489–495. doi: 10.1093/jmedent/35.4.489. [DOI] [PubMed] [Google Scholar]
- 12.Killmaster LF, Loftis AD, Zemtsova GE, Levin ML. Detection of Bacterial Agents in Amblyomma americanum (Acari: Ixodidae) from Georgia, USA, and the use of a Multiplex Assay to Differentiate Ehrlichia chaffeensis and Ehrlichia ewingii. J Med Entomol. 2014;51(4):868–872. doi: 10.1603/me13225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lockhart JM, Davidson WR, Stallknecht DE, Dawson JE, Howerth EW. Isolation of Ehrlichia chaffeensis from wild white-tailed deer (Odocoileus virginianus) confirms their role as natural reservoir hosts. J Clin Microbiol. 1997;35(7):1681–1686. doi: 10.1128/jcm.35.7.1681-1686.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftis AD, Massung RF, Levin ML. Quantitative real-time PCR assay for detection of Ehrlichia chaffeensis. J Clin Microbiol. 2003;41:3870–3872. doi: 10.1128/JCM.41.8.3870-3872.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy GL, Ewing SA, Whitworth LC, Fox JC, Kocan AA. A molecular and serologic survey of Ehrlichia canis, E. chaffeensis, and E. ewingii in dogs and ticks from Oklahoma. Vet Parasitol. 1998;79:325–339. doi: 10.1016/s0304-4017(98)00179-4. [DOI] [PubMed] [Google Scholar]
- 16.Nadolny RM, Wright CL, Sonenshine DE, Hynes WL, Gaff HD. Ticks and Spotted Fever Group Rickettsiae of Southeastern Virginia. Ticks Tick Borne Dis. 2014;5:53–57. doi: 10.1016/j.ttbdis.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Steiert JG, Gilfoy F. Infection rates of Amblyomma americanum and Dermacentor variabilis by Ehrlichia chaffeensis and Ehrlichia ewingii in southwest Missouri. Vector-Borne Zoonot. 2002;2:53–60. doi: 10.1089/153036602321131841. [DOI] [PubMed] [Google Scholar]
- 18.Stromdahl EY, Hickling GJ. Beyond Lyme: Aetiology of Tick-borne Human Diseases with Emphasis on the South-Eastern United States. Zoonoses Public Hlth. 2012;59:48–64. doi: 10.1111/j.1863-2378.2012.01475.x. [DOI] [PubMed] [Google Scholar]
- 19.Stromdahl EY, Randolph MP, O'Brien JJ, Gutierrez AG. Ehrlichia chaffeensis (Rickettsiales: Ehrlichieae) infection in Amblyomma americanum (Acari: Ixodidae) at Aberdeen Proving Ground, Maryland. J Med Entomol. 2000;37(3):349–356. doi: 10.1093/jmedent/37.3.349. [DOI] [PubMed] [Google Scholar]
- 20.Tabara K, Arai S, Kawabuchi T, Itagaki A, Ishihara C, Satoh H, Okabe N, Tsuji M. Molecular survey of Babesia microti, Ehrlichia species and Candidatus Neoehrlichia mikurensis in wild rodents from Shimane Prefecture, Japan. Microbiol Immunol. 2007;51:359–367. doi: 10.1111/j.1348-0421.2007.tb03923.x. [DOI] [PubMed] [Google Scholar]