The lack of Prox1 activity results in the complete absence of lymphatic endothelial cells (LECs). Here, Srinivasan et al. identified Vegfr3, the cognate receptor of the lymphangiogenic growth factor Vegfc, as a dosage-dependent, direct in vivo target of Prox1. Vegfr3 regulates Prox1 by establishing a feedback loop necessary to maintain the identity of LEC progenitors, and Vegfc-mediated activation of Vegfr3 signaling is necessary to maintain Prox1 expression in LEC progenitors.
Keywords: Prox1, Vegfr3, lymphatics, endothelial cell, progenitor, mouse
Abstract
The mammalian lymphatic vasculature is important for returning fluids from the extracellular tissue milieu back to the blood circulation. We showed previously that Prox1 dosage is important for the development of the mammalian lymphatic vasculature. The lack of Prox1 activity results in the complete absence of lymphatic endothelial cells (LECs). In Prox1 heterozygous embryos, the number of LECs is reduced because of a decrease in the progenitor pool in the cardinal vein. This reduction is caused by some progenitor cells being unable to maintain Prox1 expression. In this study, we identified Vegfr3, the cognate receptor of the lymphangiogenic growth factor Vegfc, as a dosage-dependent, direct in vivo target of Prox1. Using various mouse models, we also determined that Vegfr3 regulates Prox1 by establishing a feedback loop necessary to maintain the identity of LEC progenitors and that Vegfc-mediated activation of Vegfr3 signaling is necessary to maintain Prox1 expression in LEC progenitors. We propose that this feedback loop is the main sensing mechanism controlling the number of LEC progenitors and, as a consequence, the number of budding LECs that will form the embryonic lymphatic vasculature.
In the mammalian embryo, lymphatic endothelial cells (LECs) originate from the embryonic veins during a brief period (mouse embryonic days 9.75–13.5 [E9.75–E13.5]) (Srinivasan et al. 2007). The nuclear hormone receptor Coup-TFII and the Sry-related homeobox transcription factor Sox18 are both required to activate the expression of the prospero-related homeobox transcription factor Prox1 in a subpopulation of venous ECs. This step leads to the formation of the initial Prox1-expressing LEC progenitors in the cardinal vein (CV) (Francois et al. 2008; Srinivasan et al. 2010). Subsequently, most of these progenitors bud from the CV and express additional genes such as podoplanin (Yang et al. 2012; Hagerling et al. 2013). The small fraction of LEC progenitors that remain inside the CV help form the lymphovenous valve, which is responsible for returning lymph fluid to the blood circulation (Srinivasan and Oliver 2011).
In vivo and in vitro data have highlighted the key roles of Prox1 during different stages of LEC differentiation and lymphatic vasculature formation. In Prox1-null embryos, LECs are absent (Wigle and Oliver 1999), and conditional deletion of Prox1 at any developmental or postnatal time point results in the loss of LEC identity and the reversal of these cells’ identity to a blood EC (BEC)-like fate (Johnson et al. 2008). Ectopic expression of Prox1 in BECs maintained in culture activates several LEC-specific genes, including podoplanin and Vegfr3 (Hong et al. 2002; Petrova et al. 2002). At the molecular level, the analysis of Prox1-null embryos in which the ORF of Prox1 was replaced with either LacZ or GFP reporter gene constructs revealed that Prox1 maintains its own expression in venous LEC progenitors (Wigle et al. 2002; Srinivasan et al. 2010). The expression of these reporter genes is normally activated in Prox1-null embryos but cannot be maintained (Wigle et al. 2002; Srinivasan et al. 2010).
Prox1+/− (Prox1+/LacZ and Prox1+/GFPCre) embryos display edema and occasionally develop blood-filled lymphatics (Harvey et al. 2005; Johnson et al. 2008; Srinivasan and Oliver 2011). Most of these animals die at birth due to chylothorax and chylous ascites (Harvey et al. 2005; Johnson et al. 2008; Srinivasan and Oliver 2011), and those that survive become obese with age (Harvey et al. 2005). We recently showed that Prox1+/− mice have a reduced number of LEC progenitors and LECs and that the lymphovenous valves are absent (Srinivasan and Oliver 2011). Furthermore, we determined that the autoregulation of Prox1 expression is dose-dependent, and the number of Prox1-expressing LEC progenitors in the CV of Prox1+/− mice is reduced due to the decreased amount of available Coup-TFII/Prox1 protein complex during the early stages of Prox1 regulation in these cells (Srinivasan and Oliver 2011). This protein complex is known to regulate several LEC-specific genes, including the lymphangiogenic receptor tyrosine kinase Vegfr3 (Lee et al. 2009; Yamazaki et al. 2009; Aranguren et al. 2013).
Despite these facts, how Prox1 regulates itself or how Prox1 expression is regulated in LECs remains unknown. This knowledge is important, as it might provide us with clues about how the embryo controls the number of specified LEC progenitors in the CV and their subsequent budding. Our previous work failed to identify direct binding of Prox1 to the Coup-TFII-binding site in the Prox1 regulatory element, excluding the possibility that the Coup-TFII/Prox1 protein complex directly regulates Prox1 expression. Alternatively, downstream targets of Prox1 might regulate Prox1 expression in a feedback manner; however, in vivo, Prox1 targets in LECs have not yet been identified. Earlier in vitro work suggested that Vegfr3 is a Prox1 target (Petrova et al. 2002). Vegfr3 is expressed in all BECs until around E10.5, and, in its absence, mouse embryos die at around E10.0 with severe cardiovascular defects (Dumont et al. 1998). Later, its expression becomes restricted to LECs and the tips of growing blood vessels (Kaipainen et al. 1995; Dumont et al. 1998; Tammela et al. 2008; Nakayama et al. 2013). Vegfc is the best-characterized Vegfr3 ligand, and, in its absence, Prox1+ LEC progenitors fail to bud from the CV (Karkkainen et al. 2004). Similar to Prox1+/− embryos, the number of LEC progenitors and LECs is reduced in both Vegfr3+/− and Vegfc+/− embryos (Hagerling et al. 2013), suggesting a functional relationship between Prox1 and Vegfr3.
In this study, we provide conclusive in vivo evidence to demonstrate that Vegfr3 is a dosage-dependent, direct Prox1 target gene. We also report for the first time that Vegfr3 regulates Prox1 expression and establish the existence of a positive feedback loop between Prox1 and Vegfr3 in LECs in vivo and in vitro. Finally, we show that Vegfc signaling helps to maintain LEC progenitor identity and that, in Vegfc−/− embryos, Prox1 expression fails to be maintained, resulting in the reduction in the number of Prox1+ LEC progenitors and hence the number of LECs. We conclude that a defective Prox1–Vegfr3 regulatory loop is responsible for the reduction in the number of LEC progenitors in Prox1+/−, Vegfr3+/−, and Vegfc+/− embryos. We also show that this reduction is even more severe in Prox1+/−;Vegfr3+/− and Prox1+/;Vegfc+/− embryos. We propose that in the mammalian embryo, this feedback loop is the main regulator of the number of LEC progenitors produced in the CV and ultimately the number of budding LECs that will participate in the formation of the mammalian lymphatic vasculature.
Results
Vegfr3 is a dosage-dependent target of Prox1
Because the number of LEC progenitors on the CV and the number of LECs outside the vein are reduced in Prox1+/− embryos (Srinivasan and Oliver 2011), we hypothesized that, in LEC progenitors, Prox1 regulates at least some of its target genes in a dosage-sensitive manner and that the reduced levels of expression of these targets results in fewer LECs.
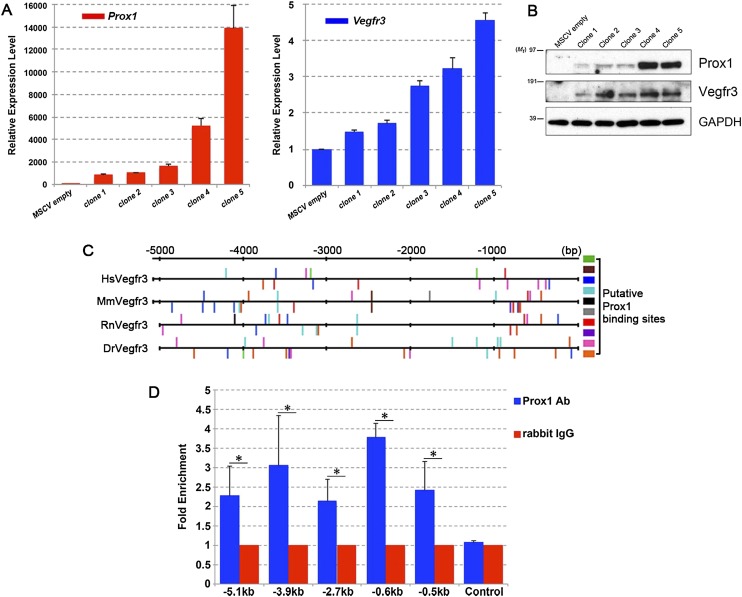
To identify some of those target genes, we first generated retroviral particles expressing Prox1, as previously reported (Srinivasan et al. 2010). We infected the mouse EC line H5V (Garlanda et al. 1994) with the particles and then selected from the stably integrated viral genome individual clones expressing Prox1. We reasoned that the clones would have variable numbers of retroviral integration events, resulting in a variable amount of Prox1 expression. Following RNA isolation from these clones, we quantified the amount of Prox1 expression by using real-time PCR. As expected, the clones had a wide range of Prox1 expression levels (Fig. 1A). Similar variability in expression levels was also observed at the protein level using Western blot analysis (Fig. 1B).
Figure 1.
Vegfr3 is a dosage-dependent target of Prox1. (A) H5V cells were infected with Prox1-expressing retroviruses. Individual clones were picked following positive selection with antibiotics. RNA extracted from the clones was analyzed by real-time PCR for the expression of Prox1 (left graph) and Vegfr3 (right graph). (B) Protein levels of Vegfr3 appear to correlate directly with those of Prox1. (C) DNA regions located 5 kb upstream of exon 1 of human (Hs), mouse (Mm), rat (Rn), and zebrafish (Dr) Vegfr3 were analyzed for putative Prox1-binding sites by TRANSFAC bioinformatics software. Upward bars indicate the sites in the sense orientation, and downward bars indicate those in the antisense orientation. Colored boxes identify the different Prox1 recognition sites (see Supplemental Table 1). (D) ChIP was carried out on mouse LECs collected by flow cytometry. A rabbit polyclonal antibody against Prox1 or control IgG was used to pull down the DNA fragments. Real-time PCR was carried out using primers specific for the various regions along the 5-kb DNA element of mouse Vegfr3. Substantial Prox1 binding was observed at the indicated sites. These data are from three independent experiments. (*) P < 0.05.
Podoplanin is not expressed in venous Prox1+ LEC progenitors; instead, its expression is turned on once the cells bud from the vein as differentiating LECs at around E11.5 (Yang et al. 2012). Thus, we decided to identify the genes whose expression is regulated by Prox1 in a dosage-dependent manner prior to the activation of podoplanin expression. As expected and consistent with previous reports that used a similar strategy (Hong et al. 2002; Srinivasan et al. 2010), the infected H5V cells expressed podoplanin but only in clones expressing the highest amounts of Prox1 (Supplemental Fig. 1). Next, we used a targeted approach and quantified the expression levels of various BEC- and LEC-specific genes (Foxc2, Integrin α9, Coup-TFII, Reelin, Tie2, Nrp1, Nrp2, PECAM1, VE-Cadherin, Lyve1, and Tie2) (Supplemental Fig. 1) in the different clones. Among those, the only gene whose expression level showed a tight correlation with that of Prox1 was Vegfr3 (Fig. 1A). This correlation with Vegfr3 is also seen at the protein level (Fig. 1B).
Because of the identified role of Vegfr3/Vegfc in early lymphangiogenesis and the compelling in vitro data suggesting that Vegfr3 is regulated by Prox1, we decided to validate this regulation further. To determine whether, in this context, Prox1 is a direct regulator of Vegfr3, we performed a computational analysis to identify putative Prox1-binding sites. It was previously reported that a 3.6-kb DNA fragment located upstream of the mouse Vegfr3 translational initiation site is sufficient to recapitulate its endogenous expression pattern (Iljin et al. 2001), and Prox1 was also shown to activate luciferase reporters containing fragments of this regulatory element (Flister et al. 2010). Accordingly, we used the TRANSFAC bioinformatic software (Matys et al. 2006) to compare a 5-kb genomic region encompassing that DNA fragment across humans, mice, rats, and zebrafish. This software considers 12 different recognition sequences as putative Prox1 DNA-binding sites (Supplemental Table 1). Ten of the sites were identified within the different Vegfr3 regulatory regions of different species (Fig. 1C). To validate this initial analysis, we next sorted LECs from E14.5 mouse embryos and performed chromatin immunoprecipitation (ChIP) using an antibody specific for Prox1 followed by real-time PCR to identify various fragments within the region. Five of the seven binding sites (located 5.1 kb, 3.9 kb, 2.7 kb, 0.6 kb, and 0.5 kb upstream of the ORF of mouse Vegfr3) that we tested showed substantial enrichment of Prox1 binding when compared with their IgG control and an independent region devoid of putative Prox1-binding sites (Fig. 1D). In conclusion, from this initial analysis, we identified Vegfr3 as a gene that is directly regulated by Prox1 in a dosage-sensitive manner.
Lymphatic vasculature is defective in Prox1+/GFPCre;Vegfr3+/LacZ embryos
Prox1+/− mice develop edema embryonically, and nearly 70% die soon after birth (Srinivasan et al. 2010). If Vegfr3 is a dosage-dependent target of Prox1, then further reducing Vegfr3 levels would most likely aggravate the phenotype of Prox1+/GFPCre (Prox1 heterozygous) embryos. To better understand the functional significance of this regulation, we crossed Vegfr3+/LacZ mice (Dumont et al. 1998) with Prox1+/GFPCre mice (Srinivasan et al. 2010) to generate Prox1+/GFPCre;Vegfr3+/LacZ double-heterozygous embryos. Genotyping of nearly 300 live pups at the time of weaning identified no double-heterozygous animals, indicating that those animals died in utero or shortly after birth. To determine their cause of death, we collected E13.5 and E15.5 embryos. As described previously, Vegfr3+/LacZ embryos are normal (Fig. 2A), and, at those stages, most of the Prox1+/GFPCre embryos exhibit edema (Fig. 2B), and some display blood-filled lymphatics (data not shown). In the case of Prox1+/GFPCre;Vegfr3+/LacZ embryos, the severity of the phenotype was variable: Most of them exhibited severe edema and an accumulation of blood in different body regions (Fig. 2C), others showed a phenotype that is characteristic of blood-filled dermal lymphatics (Supplemental Fig. 2C), and some were almost devoid of lymphatic vessels in the skin (Supplemental Fig. 2D)
Figure 2.
LEC progenitors and LECs are reduced in Prox1+/GFPCre;Vegfr3+/LacZ embryos. Vegfr3+/LacZ mice were bred with Prox1+/GFPCre to generate Prox1+/GFPCre;Vegfr3+/LacZ double-heterozygous embryos. (A–C) At E13.5 Vegfr3+/LacZ embryos were phenotypically normal (A), Prox1+/GFPCre embryos had mild edema (B), and severe edema and regions of hemorrhagic accumulation were observed in most Prox1+/GFPCre;Vegfr3+/LacZ embryos (C). Prox1+/GFPCre and Prox1+/GFPCre;Vegfr3+/LacZ embryos were collected at E11.5 (D–F), E13.5 (G,H), or E15.5 (I,J) and analyzed by immunohistochemistry for the LEC markers Prox1 and podoplanin together with the pan-EC marker PECAM1. (D,E,G,H) Transverse sections, with the neural tube and heart at the right and left, respectively, of the figures. (I,J) Whole mounts on the peripheral skin. (D,E) Compared with Prox1+/GFPCre embryos, the expression of Prox1 and podoplanin is reduced in Prox1+/GFPCre;Vegfr3+/LacZ littermates at E11.5. (F) Furthermore, the number of Prox1+ LECs is reduced in Prox1+/GFPCre;Vegfr3+/LacZ embryos at E11.5 (n = 3 for each genotype). At E13.5, while a clear lymph sac (LS) was seen in Prox1+/GFPCre embryos (G), scattered and mispatterned blood-filled structures were seen in Prox1+/GFPCre;Vegfr3+/LacZ embryos (H; arrows). At E15.5, lymphatic vessels are seen in Prox1+/GFPCre embryonic skin (I); however, in Prox1+/GFPCre;Vegfr3+/LacZ embryos, the few Prox1+ structures (dashed lines) failed to express podoplanin (J). P-values are as follows: (**) P = 0.0068; (*) P = 0.0128. Bars, 100 μm.
The number of LEC progenitors and differentiating LECs is severely reduced in Prox1+/GFPCre;Vegfr3+/LacZ embryos
To further evaluate the lymphatic phenotype of Prox1+/GFPCre;Vegfr3+/LacZ embryos and determine whether the cause of their severe phenotype is a reduction in the number of LEC progenitors even greater than that seen in Prox1 heterozygous embryos (Srinivasan and Oliver 2011), we performed a detailed analysis at three key developmental time points. E11.5 and E13.5 Prox1+/GFPCre;Vegfr3+/LacZ embryos were sectioned and immunostained for Prox1, podoplanin, and PECAM1. The same antibodies were used to immunostain the skin of E15.5 embryos. In E11.5 Prox1+/GFPCre;Vegfr3+/LacZ embryos, the number of LEC progenitors in the CV and differentiating LECs outside the vein was reduced even more drastically than in Prox1+/GFPCre embryos (Fig. 2D–F). In addition, the level of podoplanin expression was down-regulated in the few scattered Prox1+ ECs seen outside of the CV (Fig. 2D,E). At E13.5, relatively normal-looking lymph sacs were seen in Prox1+/GFPCre embryos (Fig. 2G); in contrast, several scattered blood-containing enlarged structures were seen in Prox1+/GFPCre;Vegfr3+/LacZ embryos at this stage (Fig. 2H, arrows). The levels of Prox1 and podoplanin expression were also drastically reduced at E13.5 in these double-heterozygous embryos (Fig. 2H). At E15.5, mostly normal-looking lymphatic vessels were seen in the skin of Prox1 heterozygous embryos (Fig. 2I; Supplemental Fig. 2B,F). Instead, as mentioned above, many Prox1+/GFPCre;Vegfr3+/LacZ embryos exhibited blood-filled dermal lymphatics (Supplemental Fig. 2C), and, consistent with the X-gal staining data (Supplemental Fig. 2D), some Prox1+/GFPCre;Vegfr3+/LacZ embryos were almost devoid of lymphatic vessels in the skin, as shown by the lack of Prox1 and Vegfr3 staining (Supplemental Fig. 2H). The few Prox1+ vessels detected in those embryos were devoid of podoplanin expression (Fig. 2J, dashed lines).
LEC progenitor identity is lost in Prox1+/GFPCre;Vegfr3+/LacZ embryos
The reduced number of LECs in Prox1+/GFPCre;Vegfr3+/LacZ embryos could be caused by a reduction in cell proliferation, an increase in apoptosis, or the loss of LEC identity. Prox1 heterozygous embryos that have fewer LECs do not exhibit altered cell proliferation or apoptosis (Srinivasan and Oliver 2011). Instead, they fail to maintain LEC fate. This led us to believe that something similar could happen in Prox1+/GFPCre;Vegfr3+/LacZ embryos; however, proliferation and apoptosis assays did not reveal obvious alterations in these embryos at E11.5 (data not shown).
In the case of Prox1 heterozygous embryos, we previously used a lineage-tracing approach to follow the fate of LEC progenitors. To that end, we crossed the Prox1+/GFPCre mice with R26+/YFP mice in which R26 was ubiquitously expressed, and an YFP reporter cassette was targeted into the locus downstream from a transcription stop cassette that was flanked by LoxP sites (Soriano 1999; Jeong et al. 2004). In Prox1+/GFPCre animals, Cre recombinase is expressed where Prox1 is expressed (Srinivasan et al. 2010). Cre-mediated deletion of the stop cassette in R26+/YFP mice activates the expression of YFP in all Prox1-expressing cells and their descendants irrespective of the subsequent presence or absence of the expression of Prox1 or Cre. Using this approach, we previously identified numerous Prox1−;YFP+ ECs on the CV of E13.5 Prox1 heterozygous embryos (Srinivasan and Oliver 2011). This finding conclusively argued that the Prox1+ LEC progenitors had reverted to their original venous EC fate by losing Prox1 expression.
To determine whether Vegfr3 is also involved in maintaining LEC fate, we performed a similar lineage-tracing experiment in the Vegfr3+/LacZ background. We used the R26mT/mG mice (Muzumdar et al. 2007) that express a fluorescent membrane-tagged GFP that fluoresces stronger than the YFP of R26+/YFP mice. Numerous GFP+;Prox1− cells were detected in the CV of E10.5 Prox1+/GFPCre;R26mT/mG embryos (Fig. 3A–C, white dotted line). However, the number of GFP+;Prox1− ECs detected was greater (nonsignificant but with a trend P-value = 0.065) in Prox1+/GFPCre;Vegfr3+/LacZ;R26mT/mG littermates (Fig. 3D–F [white dotted line], G). Thus, Vegfr3 is likely involved in maintaining LEC progenitor fate.
Figure 3.
Prox1+/GFPCre;Vegfr3+/LacZ embryos fail to maintain Prox1 expression in LEC progenitors. Prox1+/GFPCre mice were bred with Vegfr3+/LacZ;R26mT/mG mice, and the resulting E10.5 Prox1+/GFPCre;R26mT/mG and Prox1+/GFPCre; Vegfr3+/LacZ;R26mT/mG embryos were analyzed by immunohistochemistry for Prox1 and GFP. Upon Cre-mediated activation, the R26mT/mG reporter expresses a membrane-tagged GFP that allows the visualization of the cell surface. (A–C) In Prox1+/GFPCre;R26mT/mG embryos, numerous Prox1+ cells were seen on the CV. All of these cells and a few Prox1− cells on the CV were GFP+ (white dotted line). (D–F) In contrast, in Prox1+/GFPCre; Vegfr3+/LacZ;R26mT/mG embryos, fewer Prox1+ cells were seen on the CV, although most of these cells were GFP+ (white dotted line). Bars, 100 μm. (G) For cell counting, the number of GFP+ Prox1−DAPI+ or GFP+ Prox1+DAPI+ cells were quantitated in stained sections (n = 3 embryos for each genotype). Cell counting showed that the number of Prox1− GFP+ LEC progenitors (P-value = 0.065) was higher in double heterozygous, indicating that the Vegfr3–Prox1 interaction is required to maintain LEC fate.
Vegfr3 regulates Prox1 expression in LEC progenitors and LECs
One possible mechanism by which Vegfr3 maintains LEC fate could be by regulating Prox1, a known regulator of LEC progenitor identity. To evaluate this possibility, we isolated E11.5 LEC progenitors (inside the CV) and differentiating LECs (outside the CV) using a laser capture microdissection approach from wild-type, Prox1 heterozygous, and Vegfr3 heterozygous embryos. Prox1 and Vegfr3 mRNA levels were compared in these different cell populations by quantitative PCR (qPCR). In wild-type embryos, Prox1 and Vegfr3 levels were higher in differentiating LECs than in progenitors (Fig. 4). As expected, Vegfr3 expression in LEC progenitors was reduced in Prox1 heterozygous embryos (Fig. 4B). In addition, we found that Prox1 expression was also reduced in Vegfr3 heterozygotes (Fig. 4A). Interestingly, the reduction in the expression of these two genes was in not only LEC progenitors but also differentiating LECs outside of the CV (Fig. 4).
Figure 4.
Vegfr3 regulates Prox1 expression in LEC progenitors and differentiating LECs. Wild-type (WT) [Tg(Prox1-tdTomato)], Prox1+/−[Tg(Prox1-tdTomato);Prox1+/LacZ], and Vegfr3+/− [Tg(Prox1-tdTomato); Vegfr3+/LacZ] embryos were collected at E11.5 (one embryo per genotype). Using laser capture microdissection, LEC progenitors (inside the CV) and differentiating LECs (outside the CV) were collected separately, and the levels of Prox1 (A) and Vegfr3 (B) were analyzed by quantitative real-time PCR. mRNA levels are shown as fold of increase and were normalized to PECAM1 levels. These qPCR results were generated from a single laser capture experiment using pooled mRNA extracted from cells captured from 10-μm sections obtained from the entire embryo for each genotype.
Due to the severely reduced numbers of LECs, we were unable to perform a similar laser capture and qPCR analysis in Prox1+/GFPCre;Vegfr3+/LacZ embryos. However, as reported above, we used image analysis software to quantify the fluorescent intensity of Vegfr3 and Prox1 as a measure of their expression levels in semiquantitative immunohistochemical analysis. As before, we grouped the cells as progenitors (on the vein) or differentiating LECs (outside). Consistent with the qPCR data, Vegfr3 expression was reduced in LEC progenitors and differentiating LECs in E11.5 Prox1+/GFPCre embryos (Fig. 5I,J). Prox1 expression was also reduced in both populations in Vegfr3+/LacZ embryos (Fig. 5I,J). Moreover, Vegfr3 and Prox1 levels were even more reduced in Prox1+/GFPCre;Vegfr3+/LacZ (double-heterozygous) embryos than in their Prox1+/GFPCre or Vegfr3+/LacZ littermates (Fig. 5I,J). Expression of the pan-endothelial marker PECAM1 was not significantly different between the samples, although, as expected, its expression was higher in progenitors relative to differentiated LECs (Fig. 5I,J). These in vivo results strongly support the presence of a positive feedback loop between Prox1 and Vegfr3 in LECs.
Figure 5.
Prox1 and Vegfr3 levels are reduced in Prox1+/GFPCre;Vegfr3+/LacZ embryos. (A–H′) Immunohistochemistry for Prox1, Vegfr3, and PECAM1 was carried out in E11.5 wild-type (WT) (A,A′,E,E′), Vegfr3+/LacZ (B,B′,F,F′), Prox1+/GFPCre (C,C′,G,G′), and Prox1+/GFPCre;Vegfr3+/LacZ (D,D′,H,H′) embryos. The expression levels of Prox1, Vegfr3, and PECAM1 in venous LEC progenitors (A–D′) and differentiating LECs outside of the CV (E–H′) were quantified using the Slidebook image analysis software (I,J). Prox1 and Vegfr3 expression levels were significantly reduced in Prox1+/GFPCre;Vegfr3+/LacZ embryos compared with their single heterozygous littermates. At least three embryos for each genotype were used for quantification. Bars: A–H, 100 μm.
Blood flow was shown to down-regulate the expression of LEC-specific genes, particularly that of Prox1 (Chen et al. 2012). To evaluate whether venous blood flow could be responsible for the observed down-regulation of Prox1 levels in Vegfr3 heterozygous embryos, we used an in vitro approach in which Prox1 or Vegfr3 activity was knocked down using specific siRNAs in hLECs. By using Alexa-Fluor-564-conjugated siRNA and performing flow cytometry, we determined that the siRNA transfection efficiency was ∼85% (data not shown). Quantitative real-time PCR analysis demonstrated that, as expected and in agreement with previous results (Mishima et al. 2007; Lee et al. 2009; Pan et al. 2009), Vegfr3 levels were down-regulated when Prox1 is knocked down (Fig. 6A). Likewise, Prox1 mRNA levels decreased ∼40% after siVegfr3 transfection in hLECs (Fig. 6A). At the protein level, endogenous Prox1 protein was detected in the nuclei of hLECs transfected with control siRNA, whereas Prox1 protein was decreased or not detected in LECs transfected with siProx1 or siVegfr3 (Fig. 6B, arrowheads) These results argue that the feedback loop regulation between Prox1 and Vegfr3 is at the RNA and protein levels, that it is most likely independent of blood flow, and that it takes place in LEC progenitors and differentiating LECs.
Figure 6.
Feedback loop regulation between Prox1 and Vegfr3 is at the RNA and protein levels. (A) hLECs were transfected with siRNAs and cultured in conditioned medium. After 48 hm mRNA was extracted, and qPCR was carried out to quantify Prox1 and Vegfr3 expression levels. siProx1 down-regulates both Prox1 and Vegfr3 levels (P < 0.0001 and P = 0.0108, respectively). Likewise, siVegfr3 down-regulates both Vegfr3 and Prox1 levels (P = 0.0003 and P = 0.0051, respectively). These data are the result of three independent experiments. (B) hLECs transfected with siRNA were cultured and immunostained for Prox1. Prox1 expression was down-regulated in those cells that were successfully transfected with fluorescently labeled siRNAs directed against either Prox1 or Vegfr3 (arrowheads).
Vegfc signaling helps maintain LEC progenitor identity
In Vegfc−/− embryos, LEC progenitors remain inside the CV; thus, Vegfc activity is required for these cells to bud from the vein (Karkkainen et al. 2004). The data presented above showed that a Prox1–Vegfr3 feedback loop in LEC progenitors helps maintain Prox1 expression. Accordingly, as Vegfc activates Vegfr3 signaling, the number of venous LEC progenitors in Vegfc−/− embryos should be lower than in their control littermates. To test this possibility, we immunostained E10.5 Vegfc+/− and Vegfc−/− embryos (Karkkainen et al. 2004) against the LEC markers Prox1 and Lyve1. At this stage, in Vegfc heterozygous embryos, Prox1+Lyve1+ LEC progenitors lined the anterior CV, and Prox1+ LECs cells were budding from the vein (Supplemental Fig. 3A). However, fewer Prox1+ LEC progenitors remained inside the CV of Vegfc−/− embryos (Supplemental Fig. 3B,C). Furthermore, compared with wild-type littermates, the number of LEC progenitors and LECs was reduced in E11.5 Prox1+/GFPCre and Vegfc+/− embryos, as previously described (Fig. 7A–C; Srinivasan and Oliver 2011; Hagerling et al. 2013). These numbers were more dramatically reduced at E11.5 in Prox1+/GFPCre;Vegfc+/− embryos when compared with Prox1+/GFPCre or Vegfc+/− littermates, a result providing further support to the proposed regulatory interaction between Prox1 and Vegfc/Vegfr3 signaling (Fig. 7D,E). No surviving Prox1+/GFPCre;Vegfc+/− pups were found from these crosses. Finally, to determine whether the reduction in LEC numbers seen in Prox1,Vegfc double-heterozygous embryos is due to the reversal of LEC progenitor fate into venous ECs, we generated Prox1+/GFPCre;Vegfc+/−;R26mT/mG embryos as described previously. As expected, several GFP+Prox1− DAPI+ cells were seen on the veins of Prox1+/GFPCre;R26mT/mG embryos (Supplemental Fig. 4A, white dotted line); however, these numbers were higher in Prox1+/GFPCre;Vegfc+/−;R26mT/mG embryos (Supplemental Fig. 4D, white dotted line), indicating that Vegfc also cooperates with Prox1 in the maintenance of LECs progenitors.
Figure 7.
Prox1 and Vegfc genetically interact with each other to regulate the number of LEC progenitors and LECs. (A–D) Prox1+/GFPCre and Vegfc+/− mice were bred with each other, and embryos were collected at E11.5 and analyzed by immunohistochemistry for the LEC markers Prox1 and podoplanin together with the pan-EC marker PECAM1. Compared with wild-type (WT) embryos (A), Vegfc+/− (B) and Prox1+/GFPCre (C) embryos had fewer Prox1+ LEC progenitors and LECs. (D) Prox1+/GFPCre;Vegfc+/− embryos had even fewer LECs. (E) Quantification of the cell numbers revealed that the differences in differentiating LEC numbers are statistically significant ([***] P ≤ 0.001; n = 3 embryos for each genotype). Regarding LEC progenitors, there is no significant difference; however, there is a trend that shows that the number of LECs decreases in the double-heterozygous embryos (P = 0.0925). Bar, 100 μm.
Although these results provide further support for a Prox1–Vegfr3 feedback loop in LECs, it could still be argued that the observed results are the consequence of the loss of LEC fate and therefore of the expression of LEC-specific genes upon Prox1 or Vegfr3 inactivation. To address this possibility, we cultured hLECs in the presence of Vegfc and found that Vegfr3 and Prox1 expression was up-regulated 3.0-fold and 2.8-fold above baseline, respectively (Supplemental Fig. 5). These quantifications were done with internal controls to normalize for cell numbers (data not shown).
Together, these results argue that Prox1+ LEC progenitors require Vegfr3 to maintain their identity and bud from the CV and that this regulation is mediated through the activation of Vegfr3 by Vegfc. Alterations in Vegfc–Vegfr3 signaling leads to the loss of Prox1 expression in LEC progenitors and their reversal to venous EC fate.
Discussion
We showed previously that Prox1 heterozygous embryos exhibit a reduction in the number of LEC progenitors and, consequently, LECs because of a defect in maintaining Prox1 expression (Srinivasan and Oliver 2011). To better characterize the molecular steps leading to the formation and maintenance of LEC progenitors, we identified direct in vivo downstream targets of Prox1 that are regulated in a dosage-dependent manner. Using a combination of in vitro and in vivo approaches, we identified Vegfr3 as a dosage-dependent target of Prox1 and demonstrated that, in turn, Vegfc–Vegfr3 signaling is necessary to maintain Prox1 in LECs. Consistent with these data, Vegfr3+/− and Vegfc+/− embryos have reduced numbers of LEC progenitors and LECs (Hagerling et al. 2013). Our lineage-tracing experiments further revealed that this reduction is due to the reversal of LEC progenitor fate into venous ECs. We conclude that a feedback loop between Prox1 and Vegfr3 is important for maintaining LEC fate and therefore for regulating the number of LEC progenitors being specified in the CV and their subsequent budding to form the complete lymphatic vasculature.
Feedback loops that operate between transcription factors and signaling molecules are known to regulate the normal development of many tissues. Such feedback loops most likely play an important role in rapidly amplifying the molecular programs that control lineage differentiation. Based on the generated data, we propose a simple model to explain the functional significance of the newly identified Prox1–Vegfr3 feedback loop in lymphatic vascular development (Fig. 8). According to this model, Coup-TFII and Sox18 provide the initial “spark” for LEC specification by initiating the expression of Prox1 in the CV at around E9.75. In turn, this expression of Prox1 will be necessary to maintain Vegfr3 expression in venous LEC progenitors. Vegfc, whose expression is stronger in the mesenchyme on the dorso–lateral side of the anterior CVs where LECs bud directly (Karkkainen et al. 2004), will trigger the Prox1–Vegfr3 feedback loop by stabilizing Prox1 and therefore Vegfr3 expression on that side of the vein and will be responsible for the budding of those progenitors. The lack of Vegfr3 expression in ECs of the lymphovenous valves (Srinivasan and Oliver 2011) may explain why those cells remain inside the vein.
Figure 8.
Model of the feedback loop regulating the specification of Prox1-expressing LEC progenitors in the embryonic veins. Between E9.75 and E13.5, in some venous EC cells with low or no Notch signaling, CoupTFII is up-regulated; then, in combination with Sox18, it induces Prox1 expression such that LEC progenitors start to be specified. Notch signaling is repressed in the specified LEC progenitors by Coup-TFII. Prox1 in turn activates the expression of Vegfr3 in a dosage-dependent manner. Activation of Vegfr3 signaling by Vegfc will also maintain Prox1 expression in LEC progenitors and differentiating LECs. Coup-TFII also interacts with Prox1 to maintain Prox1 expression. Coup-TFII and Prox1 also likely maintain Notch signaling at low levels in LEC progenitors at this stage. Prox1+ LEC progenitors will subsequently bud off from the CV and intersomitic vessels (ISV) and start to express differentiation markers such as podoplanin. After E13.5, Notch signaling levels are increased in venous ECs such that Notch will suppress Coup-TFII to prevent further specification of Prox1+ LEC progenitors. At the same time, Notch signaling also likely inhibits Vegfr3 expression via Hey1/2, thereby short-circuiting the Prox1–Vegfr3 feedback loop and stopping the generation of LEC progenitors in the veins. It could be speculated that most likely the Prox1–Vegfr3 feedback loop does not operate in differentiated LECs.
Reduction in Vegfr3-mediated signaling in Prox1+/−, Prox1−/−, Vegfr3+/−, Vegfc+/−, Vegfc−/− Prox1+/−;Vegfc+/−, and Prox1+/−;Vegfr3+/− embryos results in the silencing of Prox1 expression in some or all LEC progenitors and therefore results in the reduction in the number of LECs. It is worth pointing out that Vegfc-mediated signals could be modulated by multiple mechanisms. Mice that carry a deletion in the Vegfc-binding domain of Vegfr3 (Vegfr3LBD/LBD) still retain their ability to form lymph sacs (Zhang et al. 2010). In this context, Neuropilin-2 (Nrp-2) could bind Vegfc and function as a coreceptor for Vegfr3 (Zhang et al. 2010). Vegfr3LBD/LBD embryos are nevertheless severely edematous due to reasons that remain unknown (Zhang et al. 2010). Based on our data, we predict that it is a defect in the Prox1–Vegfr3 feedback loop that leads to the reduction in the number of LEC progenitors and LECs in these embryos. The Prox1–Vegfr3 feedback loop could also be modulated by CCBE1, a protein that proteolytically processes Vegfc (Jeltsch et al. 2014). Any of the aforementioned proteins could also affect the timing and number of specified LEC progenitors, and it could be possible that Prox1 might also control the expression of some of them. In fact, our transfection assay showed that Prox1 up-regulates Nrp-2 expression in H5V cells (Supplemental Fig. 1).
It has been proposed that Jagged1/Notch1 signaling is responsible for the down-regulation of Coup-TFII expression in a subset of venous ECs in the early CV such that LEC progenitors are not specified in that subpopulation (Murtomaki et al. 2013). At the same time, in neighboring venous EC cells with low or no Notch signaling, Coup-TFII is up-regulated (Murtomaki et al. 2013). In these cells, Coup-TFII in turn inhibits Notch signaling but activates Prox1 (You et al. 2005; Srinivasan et al. 2010). This argument is in agreement with results from Murtomaki et al. (2013) showing that Notch1 is capable of suppressing Prox1 expression (likely via repression of Coup-TFII) in early embryonic LECs and that deletion of one allele of Notch1 rescues Prox1 haploinsufficiency. In the context of our findings (see Fig. 8), it could be speculated that early on in the CV, some venous ECs are devoid of Notch signaling, or its threshold is below the one necessary to repress Coup-TFII. Therefore, in those venous ECs, Coup-TFII together with Sox18 induce Prox1 expression such that LEC specification takes place, and the Prox1/Vegfr3 feedback loop is initiated and maintained in LEC progenitors. Interestingly, we found that Notch1 levels were increased in LEC progenitors of Prox1 heterozygous embryos as well as in hLECs transfected with Prox1 siRNA (data not shown), a result suggesting that there could also be cross-regulation between Notch signaling and Prox1 in LECs such that, at those early stages, Prox1 expression in LEC progenitors maintains Notch expression at low levels. Later on (after approximately E13.5) (see Fig. 8), for reasons that we still do not understand, the threshold of Notch signaling in venous ECs increases to a level sufficient to directly repress Coup-TFII and, as a consequence, shut off Prox1 expression. At the same time, this increase in Notch signaling levels is probably also sufficient to inhibit Vegfr3 activity via its effectors, Hey1 and Hey2 (Murtomaki et al. 2013). As a consequence (see Fig. 8), it is likely that the Prox1–Vegfr3 feedback loop will be disrupted and so will the generation of LEC progenitors in the CV at those later stages.
Another level of complexity is added by the fact that Coup-TFII and Prox1 could interact to form a protein complex (Lee et al. 2009; Yamazaki et al. 2009; Srinivasan et al. 2010). We previously determined that the reduction in the number of Prox1-expressing LEC progenitors in Prox1+/− embryos was the consequence of a decrease in the amount of the available Coup-TFII/Prox1 protein complex (Srinivasan and Oliver 2011). This complex is known to activate Vegfr3 in LECs (Lee et al. 2009; Yamazaki et al. 2009). Coup-TFII also activates Nrp-2, although it is currently unknown whether this is via the Coup-TFII/Prox1 complex (Lin et al. 2010). Alternatively, it could then be argued that the Coup-TFII/Prox1 protein complex might regulate the expression of additional LEC-specific genes such as Hey1/2, as recently reported (Aranguren et al. 2013), and these genes in turn inhibit Vegfr3 expression.
We found that the Prox1–Vegfr3 feedback loop is operational in not only LEC progenitors but also differentiating LECs at least until E13.5. How is this loop operating in functional lymphatics and in what context? Are there other players involved? It was previously shown that the expression of Prox1 and Vegfr3 is down-regulated in collecting lymphatic vessels during maturation (Norrmen et al. 2009). While the functional relevance of this down-regulation is not yet clear, this down-regulation appears not to take place in Foxc2−/− embryos that fail to undergo proper collecting vessel maturation (Norrmen et al. 2009). It is possible that a subtle interference with the Prox1–Vegfr3 feedback loop by Foxc2 results in a sudden and dramatic reduction in their expression levels and the subsequent maturation of the lymphatic vessel. A genetic interaction between Foxc2 and Vegfr3 has been reported (Petrova et al. 2004).
In summary, we propose that the Prox1–Vegfr3 feedback loop is a simple but necessary sensing mechanism required to maintain LEC fate and regulate the number of specified LEC progenitors and, subsequently, the number of budding LECs. Clearly, we only solved a small portion of a jigsaw puzzle, and future research with the advancement of appropriate technologies will help us put together the other pieces.
Materials and methods
Mice
R26+/YFP, R26mT/mG, and Tg(Prox1-tdTomato) mice were obtained from the Jackson Laboratory (Jeong et al. 2004; Muzumdar et al. 2007; Truman et al. 2012). Vegfc+/−, Prox1+/LacZ, Prox1+/GFPCre, and Vegfr3+/LacZ mice have been reported previously (Dumont et al. 1998; Wigle et al. 1999; Karkkainen et al. 2004; Srinivasan et al. 2010). All mouse experiments were approved by the International Animal Care and Use Committee (IACUC) at St. Jude Children’s Research Hospital.
Viral constructs and generation of viral particles
Avitag-Prox1 was cloned into a two-promoter MSCV-fl-sv-Puro retroviral vector containing the LTR promoter and an SV40-PAC selection cassette. Viral particles pseudotyped with VSV-G and PEQ-PAM were generated by transient transfection in 293T cells. Briefly, 293T cells were plated at 2 × 106 cells per 100-mm-diameter tissue culture dish and transfected with the retroviral vector (empty or containing avitag-Prox1) by using FuGENE 6 (Roche Applied Science). At 12 h post-transfection, the medium was replaced with fresh DMEM containing 10% FBS, and cells were grown for an additional 24 h. The conditioned medium containing recombinant retroviruses was collected and filtered through polysulfonic filters (pore size, 0.45 µm; Corning).
Viral transduction and selection of H5V clones expressing Prox1
For retroviral infections, H5V cells were transduced with viral particles for 24 h. Samples of retrovirus supernatants were applied to H5V cells, which had been plated 18 h prior to infection at a density of 106 cells per tissue culture dish (100-mm diameter). Polybrene (Sigma) was added to a final concentration of 8 mg/mL, and the supernatants were incubated with the cells for 12 h. After infection, cells were placed in fresh growth medium and maintained in culture as usual. Selection with 1.5 mg of puromycin per milliliter was initiated 12 h after infection. About 7 d after selection, 1 × 104 cells were seeded into each tissue culture dish (100-mm diameter) to form individual clones. The individual clones picked from microplates of the retrovirus-infected cells were transferred to 24 microtiter wells and expanded to generate cell clones stably expressing different levels of avitag-Prox1.
siRNA analysis
Human dermal LECs (HDLECs) were purchased from PromoCell and cultured in endothelial basal medium (EBM) complemented with supplement mix (C-39225, PromoCell) according to the supplier’s instructions. These cells were used between passages 2 and 6. HDLECs were transfected with ON-TARGETplus SMARTpool human for PROX1 (J-016913-08-0005), VEGFR3 (L-003138-00-0005), or a control siRNA (D-001810-01-05) purchased from Dharmacon (Thermo Scientific). siRNA transfections (50 nM final concentration) were performed using Lipofectamine 2000 according to the manufacturer’s recommendations. After 5 h of incubation, cells were rinsed twice with EBM and then incubated in complete EBM for an additional 24 and 48 h in the presence of 100 ng/mL recombinant Vegfc (R&D Systems). The knockdown of endogenous Prox1 and Vegfr3 was examined by Western blot analysis (data not shown), immunofluorescence, and qPCR.
RNA isolation and quantification of gene expression
Total RNA was extracted with the RNeasy minikit (Qiagen) according to the manufacturer’s instructions. To quantify gene expression, 1 µg of total RNA was used for cDNA synthesis (Clontech), and 1 μL of cDNA was used for real-time PCR using LEC- and BEC-specific primers. Primer sequences are available in Supplemental Table 2. PCR (SYBR Green, ABI) analysis was performed on an Applied Biosystems 7500 real-time PCR system. Ct values for each gene were normalized to the endogenous control gene GAPDH using the ΔΔCt method. All experiments were performed in triplicate.
Immunohistochemistry
For immunohistochemical analysis, whole embryos were fixed in 4% PFA for 1 h at room temperature, embedded in 7% low-melting agarose, sectioned on a vibratome (100 µm), and immunostained. For immunohistochemical analysis of cryosections, embryos were fixed in 4% PFA overnight at 4°C, embedded in OCT, sectioned in a cryostat (10 µm), and immunostained. The sections were mounted using Prolong Gold mounting medium containing DAPI (Life Technologies), and confocal microscopy was performed.
Antibodies
The following primary antibodies were used: rabbit anti-β-gal (MP Biomedicals), rabbit anti-Prox1 (AngioBio and ProteinTech Group), goat anti-Prox1 (R&D Systems), hamster anti-podoplanin (Hybridoma Bank, Developmental Studies, University of Iowa), rat anti-PECAM1 (BD Pharmingen), goat anti-Vegfr3 (R&D Systems), rabbit anti-GFP (Molecular Probes), and chicken anti-GFP (Abcam). The following secondary antibodies were used: Alexa 488-conjugated donkey anti-rabbit (Molecular Probes), Alexa 488-conjugated donkey anti-guinea pig (Molecular Probes), Alexa 488-conjugated goat anti-hamster (Molecular Probes), Alexa 488-conjugated donkey anti-goat (Molecular Probes), Cy3-conjugated donkey anti-rabbit (Jackson ImmunoResearch Laboratories), Cy3-conjugated donkey anti-goat (Jackson ImmunoResearch Laboratories), and Cy5-conjugated donkey anti-rat (Jackson ImmunoResearch Laboratories).
Image analysis
Tissue sections prepared on a vibratome were immunostained with antibodies and then visualized with a confocal laser-scanning microscope (Zeiss LSM510). For images to be compared quantitatively, the same laser intensity settings were used, and images were acquired on the same day. Quantification of the fluorescence intensity was performed using Slidebook image analysis software.
Statistical analysis
Data analyses and statistical analyses were performed using Prism 5 software (Graph Pad Software, Inc.). Values are presented as group mean ± SEM. For statistical analyses in siRNA experiments, we used one-way analysis of variance (ANOVA) followed by ANOVA test, followed by Bonferroni’s multiple comparison test. P-values <0.05 were considered statistically significant (P ≤ 0.05 [*], P ≤ 0.01 [**],and P ≤ 0.001 [***]). For experiments where intensity of fluorescence was measured, Microsoft Excel was used to evaluate the statistical significance by unpaired Student’s t-test.
ChIP
Mouse primary LECs were isolated from E14.5 embryos by flow cytometry. Lyve-1+, CD31+, and CD45− populations were used in the ChIP assay. ChIP was carried out with 105 cells by using the LowCell ChIP kit (Diagenode). Rabbit anti-Prox1 antibody (ProteinTech Group) and rabbit IgG were used in the ChIP. Following the pull-down and isolation of the chromatin fragments, real-time PCR was performed using primers that were specific for the multiple Prox1-binding sites and the nonspecific control site (data not shown).
Whole-mount immunohistochemistry
Whole-mount immunohistochemistry was performed as previously reported (James et al. 2013)
Laser capture microdissection
E11.5 Tg(Prox1-tdTomato) (wild type), Tg(Prox1-tdTomato); Prox1+/LacZ (Prox1+/−), and Tg(Prox1-tdTomato); Vegfr3+/LacZ (Vegfr3+/−) embryos were generated and rapidly dissected at 4°C under RNase-free conditions. Embryos were fixed in 2% PFA for 15 min at 4°C, washed twice with PBS, and frozen in OCT on dry ice immediately. The blocks were stored at −80°C until sectioning in the cryostat. Sections were subsequently fixed for 30 sec by immersion in cold methanol, dehydrated in graded ethanol solutions, and cleared in xylene. After air-drying, laser capture was performed under direct fluorescence microscopic visualization. Tomato+ cells on the CV and Tomato+ cells outside the CV were microdissected from the same frozen sections and kept separately. Cells were captured using ArcturusXT LCM instrument (Applied Biosystems). RNA was isolated using the RNeasy FFPE kit (Qiagen) followed by cDNA synthesis and amplification using Ovation Pico WTA System V2 (NuGEN) according to the manufacturer’s instructions. RNA quality was assessed using a Bioanalyzer picochip (Agilent Technologies).
Acknowledgments
We thank Dr. Elizabetta Dejana for the H5V cells, the members of our laboratories for their helpful suggestions and discussions, Dr. Angela McArthur for scientific editing of this manuscript, and Julie Gholson Groff for the generation of Figure 8. This work was supported by National Institutes of Health grant R01-HL073402, the Leducq Foundation, and the American Lebanese Syrian Associated Charities (ALSAC) to G.O.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.216226.113.
References
- Aranguren XL, Beerens M, Coppiello G, Wiese C, Vandersmissen I, Nigro AL, Verfaillie CM, Gessler M, Luttun A. 2013. COUP-TFII orchestrates venous and lymphatic endothelial identity by homo- or heterodimerisation with PROX1. J Cell Sci 126: 1164–1175 [DOI] [PubMed] [Google Scholar]
- Chen CY, Bertozzi C, Zou Z, Yuan L, Lee JS, Lu M, Stachelek SJ, Srinivasan S, Guo L, Vicente A, et al. 2012. Blood flow reprograms lymphatic vessels to blood vessels. J Clin Invest 122: 2006–2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont DJ, Jussila L, Taipale J, Lymboussaki A, Mustonen T, Pajusola K, Breitman M, Alitalo K. 1998. Cardiovascular failure in mouse embryos deficient in VEGF receptor-3. Science 282: 946–949 [DOI] [PubMed] [Google Scholar]
- Flister MJ, Wilber A, Hall KL, Iwata C, Miyazono K, Nisato RE, Pepper MS, Zawieja DC, Ran S. 2010. Inflammation induces lymphangiogenesis through up-regulation of VEGFR-3 mediated by NF-κB and Prox1. Blood 115: 418–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Caprini A, Hosking B, Orsenigo F, Wilhelm D, Browne C, Paavonen K, Karnezis T, Shayan R, Downes M, et al. 2008. Sox18 induces development of the lymphatic vasculature in mice. Nature 456: 643–647 [DOI] [PubMed] [Google Scholar]
- Garlanda C, Parravicini C, Sironi M, De Rossi M, Wainstok de Calmanovici R, Carozzi F, Bussolino F, Colotta F, Mantovani A, Vecchi A. 1994. Progressive growth in immunodeficient mice and host cell recruitment by mouse endothelial cells transformed by polyoma middle-sized T antigen: implications for the pathogenesis of opportunistic vascular tumors. Proc Natl Acad Sci 91: 7291–7295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F. 2013. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J 32: 629–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey NL, Srinivasan RS, Dillard ME, Johnson NC, Witte MH, Boyd K, Sleeman MW, Oliver G. 2005. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat Genet 37: 1072–1081 [DOI] [PubMed] [Google Scholar]
- Hong YK, Harvey N, Noh YH, Schacht V, Hirakawa S, Detmar M, Oliver G. 2002. Prox1 is a master control gene in the program specifying lymphatic endothelial cell fate. Dev Dyn 225: 351-357 [DOI] [PubMed] [Google Scholar]
- Iljin K, Karkkainen MJ, Lawrence EC, Kimak MA, Uutela M, Taipale J, Pajusola K, Alhonen L, Halmekyto M, Finegold DNet al. 2001. VEGFR3 gene structure, regulatory region, and sequence polymorphisms. FASEB J 15: 1028-1036 [DOI] [PubMed] [Google Scholar]
- James JM, Nalbandian A, Mukouyama YS. 2013. TGFβ signaling is required for sprouting lymphangiogenesis during lymphatic network development in the skin. Development 140: 3903–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeltsch M, Jha SK, Tvorogov D, Anisimov A, Leppanen VM, Holopainen T, Kivela R, Ortega S, Karpanen T, Alitalo K. 2014. CCBE1 enhances lymphangiogenesis via a disintegrin and metalloprotease with thrombospondin motifs-3-mediated vascular endothelial growth factor-C activation. Circulation 129: 1962–1971 [DOI] [PubMed] [Google Scholar]
- Jeong J, Mao J, Tenzen T, Kottmann AH, McMahon AP. 2004. Hedgehog signaling in the neural crest cells regulates the patterning and growth of facial primordia. Genes Dev 18: 937–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. 2008. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev 22: 3282–3291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. 1995. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci 92: 3566–3570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, et al. 2004. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol 5: 74–80 [DOI] [PubMed] [Google Scholar]
- Lee S, Kang J, Yoo J, Ganesan SK, Cook SC, Aguilar B, Ramu S, Lee J, Hong YK. 2009. Prox1 physically and functionally interacts with COUP-TFII to specify lymphatic endothelial cell fate. Blood 113: 1856–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FJ, Chen X, Qin J, Hong YK, Tsai MJ, Tsai SY. 2010. Direct transcriptional regulation of neuropilin-2 by COUP-TFII modulates multiple steps in murine lymphatic vessel development. J Clin Invest 120: 1694–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matys V, Kel-Margoulis OV, Fricke E, Liebich I, Land S, Barre-Dirrie A, Reuter I, Chekmenev D, Krull M, Hornischer K, et al. 2006. TRANSFAC and its module TRANSCompel: transcriptional gene regulation in eukaryotes. Nucleic Acids Res 34: D108–D110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishima K, Watabe T, Saito A, Yoshimatsu Y, Imaizumi N, Masui S, Hirashima M, Morisada T, Oike Y, Araie M, et al. 2007. Prox1 induces lymphatic endothelial differentiation via integrin α9 and other signaling cascades. Mol Biol Cell 18: 1421–1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtomaki A, Uh MK, Choi YK, Kitajewski C, Borisenko V, Kitajewski J, Shawber CJ. 2013. Notch1 functions as a negative regulator of lymphatic endothelial cell differentiation in the venous endothelium. Development 140: 2365–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. 2007. A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605 [DOI] [PubMed] [Google Scholar]
- Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, Itoh N, Hirose T, Breier G, Vestweber D, et al. 2013. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol 15: 249–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrmen C, Ivanov KI, Cheng J, Zangger N, Delorenzi M, Jaquet M, Miura N, Puolakkainen P, Horsley V, Hu J, et al. 2009. FOXC2 controls formation and maturation of lymphatic collecting vessels through cooperation with NFATc1. J Cell Biol 185: 439–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan MR, Chang TM, Chang HC, Su JL, Wang HW, Hung WC. 2009. Sumoylation of Prox1 controls its ability to induce VEGFR3 expression and lymphatic phenotypes in endothelial cells. J Cell Sci 122: 3358–3364 [DOI] [PubMed] [Google Scholar]
- Petrova TV, Makinen T, Makela TP, Saarela J, Virtanen I, Ferrell RE, Finegold DN, Kerjaschki D, Yla-Herttuala S, Alitalo K. 2002. Lymphatic endothelial reprogramming of vascular endothelial cells by the Prox-1 homeobox transcription factor. EMBO J 21: 4593–4599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, et al. 2004. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med 10: 974–981 [DOI] [PubMed] [Google Scholar]
- Soriano P 1999. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet 21: 70–71 [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Oliver G. 2011. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev 25: 2187–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. 2007. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev 21: 2422–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Geng X, Yang Y, Wang Y, Mukatira S, Studer M, Porto MP, Lagutin O, Oliver G. 2010. The nuclear hormone receptor Coup-TFII is required for the initiation and early maintenance of Prox1 expression in lymphatic endothelial cells. Genes Dev 24: 696–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, et al. 2008. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature 454: 656–660 [DOI] [PubMed] [Google Scholar]
- Truman LA, Bentley KL, Smith EC, Massaro SA, Gonzalez DG, Haberman AM, Hill M, Jones D, Min W, Krause DS, et al. 2012. ProxTom lymphatic vessel reporter mice reveal Prox1 expression in the adrenal medulla, megakaryocytes, and platelets. Am J Pathol 180: 1715–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. 1999. Prox1 function is required for the development of the murine lymphatic system. Cell 98: 769–778 [DOI] [PubMed] [Google Scholar]
- Wigle JT, Chowdhury K, Gruss P, Oliver G. 1999. Prox1 function is crucial for mouse lens-fibre elongation. Nat Genet 21: 318–322 [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. 2002. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J 21: 1505–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Yoshimatsu Y, Morishita Y, Miyazono K, Watabe T. 2009. COUP-TFII regulates the functions of Prox1 in lymphatic endothelial cells through direct interaction. Genes Cells 14: 425–434 [DOI] [PubMed] [Google Scholar]
- Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. 2012. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood 120: 2340–2348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. 2005. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature 435: 98–104 [DOI] [PubMed] [Google Scholar]
- Zhang L, Zhou F, Han W, Shen B, Luo J, Shibuya M, He Y. 2010. VEGFR-3 ligand-binding and kinase activity are required for lymphangiogenesis but not for angiogenesis. Cell Res 20: 1319–1331 [DOI] [PubMed] [Google Scholar]