Abstract
Introduction: California has experienced its worst outbreak of pertussis in 50 y. In preparing for such outbreaks of pertussis, vaccine providers in the state play a key role in educating patients about the public health implications of vaccination, explaining the benefits to immunization, and facilitating patients' receipt of recommended immunizations.
Methods: We conducted a survey of 800 California vaccine providers to investigate provider level response to recent pertussis outbreaks and regulation by provider type and geography.
Results: Sixty-nine percent (533/777) of vaccine providers within the state of California responded to the survey. Fifty-three percent (278/527) of vaccine providers indicated that it was part of standard care at their practice or pharmacy location to ask adult patients about pertussis vaccine (Table 1) and this varied across practice types (P < 0.0001). Fifty-seven percent of providers (270/476) indicated that the information they received from the state about pertussis during the 2010 California pertussis outbreak was very useful or useful, while 52% of providers indicated this information was neutral, not useful, not at all useful. Vaccine administration, patient groups seen, and challenges varied by provider type however meaningful differences among subpopulations to which the vaccine was administered were found between provider types (P < 0.001, Table 2).
Conclusion: The 2010 pertussis outbreak in California challenged vaccine providers in a way that changed the preparation, promotion, and planning for future outbreaks and emergency situations. Adaptability to the new state law and increased awareness of pertussis in the physician community were important in the number of patients receiving the vaccine. Also, forming partnerships with schools and health agencies were important in facilitating and promoting wide spread vaccination.
Keywords: pertussis, vaccine providers, preparedness, California
Introduction
In 2010, the state of California experienced its worst outbreak of pertussis in 50 y.1 From January through June of 2010, 1337 cases were reported, a 4-fold increase from the previous year.2 That year 10 infants died and 9000 Californians were diagnosed with pertussis. The Centers for Disease Control and Prevention (CDC) reported that elementary, middle, and high school students were at greater risk of an outbreak due to possible waning effectiveness of the childhood vaccine.3 In preparing for such outbreaks of pertussis, vaccine providers in the state play a key role in educating patients about the public health implications of vaccination, explaining the benefits to immunization, and facilitating patients’ receipt of recommended immunizations.
Due to the resurgence of pertussis in the state, the California Assembly Bill 354 was passed in September of 2010 requiring all incoming seventh through twelfth grade students to be vaccinated with the tetanus, diphtheria, and acellular pertussis (Tdap) booster, already an ACIP (Advisory Committee on Immunization Practices) recommendation4 for the 2011–12 academic year.5 Accordingly, as a result of the passage of Assembly Bill 354, the number of adolescents in need of getting a pertussis vaccine booster by July 1, 2011 was over one million.6
The pertussis epidemic in California and previous vaccine shortages have created a situation of high stress for some vaccine providers due to increased demand and problems with vaccine supply and distribution. Vaccine providers are encouraged to follow the CDC recommendation for ages of vaccination, times to receive boosters, and populations most at risk,7 but challenges occur when the demand for vaccines surpasses the supply due to distribution issues, lack of vaccine availability, or because vaccines were not ordered early enough.8
Currently, there are few published studies that explain how vaccine providers are impacted when there is a sudden increase in demand of vaccines, such as during times of an outbreak or an epidemic, and none specifically relate to pertussis. We administered a survey in California among vaccine providers aimed at understanding practice level responses and challenges to ongoing outbreaks of pertussis and legislative action. This survey examined potential themes in how providers respond to vaccine-related public health emergencies. In addition, we examined differences in response between vaccine provider types, especially perceived barriers to administering Tdap, and which providers were most impacted by the law.
Methods
Sample
We drew a representative, random sample of 800 vaccine providers from 9071 eligible practices who ordered H1N1 vaccine from the California Department of Public Health (CDPH) during the 2009–2010 H1N1 influenza pandemic; methods are previously described.9 The sample size of 800 was based upon a minimum expected response rate of 50% and to obtain survey estimates accurate within ± 5% for all measures.10,11 A meta-analysis of 178 articles published in 1991 indicated the response rates of mailed physician surveys ranged from 20% to 90%; in this analysis the mean response rate was 54% thus our target response rate was 50%.10 Women’s health providers and pharmacies were oversampled for subsequent pooled analysis with surveys in other states. Group categories were determined by the investigators for the remaining 6 categories of provider types. The latter 6 categories were proportionally represented in the sample, and included non-traditional vaccinators (e.g., alternative medicine, rehabilitation, occupational health, specialists), under-25-y-old priority group practices (e.g., pediatrics, college health services), pharmacies, government providers (e.g., Indian Health Service, local health jurisdictions, Veterans Affairs), hospitals and acute care, and traditional family practices. After eliminating 23 out-of-date, duplicate, or incorrect addresses, 777 surveys were delivered by FedEx to the person identified by the CDPH as the primary contact for ordering H1N1.
Materials
Printed and online survey instruments with identical questions were used to collect data. The survey instrument consisted of 40 questions in 7 sections. One of the 7 sections included questions pertaining to pertussis vaccine administration. Questions were asked in a variety of ways including yes/no, multiple choice, Likert-like scales, and open-ended responses.
Non-respondents received multiple reminders via phone and fax. Each provider also received a $25 gift card to thank them for their time. Missing, incomplete, or outdated information was updated during telephone follow-up with the vaccine provider.
The online survey tool was administered using Feedback Server version 2008.1.
Measures and analysis
We characterized the provider level response to recent pertussis outbreaks and regulation by provider type and geography.
Demographic and practice level data about providers was obtained from the CDPH, including physical address, participation in Vaccines for Children (VFC) and number of H1N1 doses ordered through the state. We categorized geographic regions in California as defined by the regional Immunization Information Systems (IIS) practice reports in an effort to evaluate variations in outbreak response among the regions.11 Survey respondents self-reported type of practice, size of practice, and all information about their response to pertussis outbreaks. Degree of urbanicity was determined using Rural-Urban Commuting Area (RUCA) codes obtained by ZIP code approximation through the Rural Health and Research Center (RHRC).12
The survey gathered perceptions of the usefulness of pertussis information from state and local health departments. The survey used a 5-point scale with options including “very useful,” “useful,” “neutral,” “not useful,” “not at all useful,” with a supplementary option “I cannot recall.” In the analysis, the responses of usefulness of information questions were dichotomized with responses “very useful” and “useful” being one group while the other category included “neutral,” “not useful,” and “not at all useful.” Categorical comparisons were performed using a Rao-Scott Chi Square test performed in SAS version 9.3 and SUDAAN version x32. Bivariate analyses were stratified by provider type as well as by IIS region. Statistical significance was determined with an α level of 0.05. SUDAAN was used for exact tests where expected cell counts were small (<5). To determine perceived level of difficulty in adapting to new laws and guidelines using the aforementioned dichotomization for ability to adapt, number of patients seen was categorized into groups of increases in number of patients seen within a given practice. Using logistic regression, we assessed the odds of increased difficulty for adapting to new laws and guidelines after controlling for provider type and number of providers within the practice.
Qualitative analysis
Open-ended questions were analyzed through thematic analysis using Microsoft Excel 2011 version 14.1.0. Qualitative coding was dependent upon emerging ideas and themes reported by the providers. Prior to coding the data, the codebooks for each question were developed and revised by the research team. All researchers agreed on each of the coding themes prior to coding. The primary investigator coded 100% of the data and a second investigator coded 20% of the data. Based on the qualitative codes, we analyzed the proportion of providers who indicated the given theme in their response. Bar charts were developed as a visual tool to demonstrate the range of responses and respective proportion for each code. The Cohen Kappa Index of Inter-rater reliability was 78% and the coder agreement was 87% or greater for all questions.
Means and proportions
Proportions were evaluated using survey procedures in SAS and weighted due to the different selection probabilities among provider type. Frequencies were generated using SURVEY procedures in SAS. Comparisons made between groups of variables were performed using Rao-Scott Chi-Square analyses. All data management and statistical analyses were performed primarily using SAS v. 9.3 however we used SUDAAN to generate exact tests for stratified tables that had zero cell counts in at least one cell. Results are considered statistically significant at an α level of 0.05 for all tests.
Ethics
The Emory University Institutional Review Board deemed the study as exempt (#00044917). The California Department of Public Health determined that the project is not research but program evaluation. Informed consent was obtained via courier delivery of a Frequently Asked Questions document included with the survey which addressed the purpose, risks and benefits, confidentiality, incentives, and voluntary nature of the survey.
Results
Vaccine administration practices
Sixty-nine percent (533/777) of vaccine providers within the state of California responded to the survey. Our survey garnered a higher response rate of 69% in comparison to average from the meta-analysis. Fifty-three percent (278/527) of vaccine providers indicated that it was part of standard care at their practice or pharmacy location to ask adult patients about pertussis vaccine (Table 1) and this varied across practice types (P < 0.0001). Fifty-seven percent of providers (270/476) indicated that the information they received from the state about pertussis during the 2010 California pertussis outbreak was very useful or useful, while 52% of providers indicated this information was neutral, not useful, not at all useful. Vaccine administration, patient groups seen, and challenges varied by provider type however meaningful differences among subpopulations to which the vaccine was administered were found between provider types (P < 0.001, Table 2).
Table 1. Provider differences.
| Pertussis vaccine is part of standard of care§§ | Usefulness of pertussis information targeted toward providers from state health department§§ | Usefulness of pertussis information targeted toward providers from local health department §§ | Perceived ability to adapt to new law and guidelines regarding pertussis vaccine administration | |||||
|---|---|---|---|---|---|---|---|---|
| Yes (n = 531) (weighted %) |
P | Very useful/ useful (crude %) | P | Very useful/ useful (crude %) | P | Not Easy/ Difficult (crude %) | P | |
| Provider type | <0.001 | 0.222 | 0.070 | 0.034 | ||||
| Non-traditional | 39.36 | 74.37 | 77.39 | 34.00 | ||||
| Under 25 | 36.78 | 85.37 | 83.79 | 18.52 | ||||
| Pharmacy | 52.59 | 72.80 | 60.90 | 30.41 | ||||
| Government | 72.34 | 88.89 | 71.43 | 51.85 | ||||
| Hospital | 74.21 | 100.00 | 69.32 | 60.74 | ||||
| Family practice | 71.68 | 82.66 | 82.77 | 24.30 | ||||
| Women's health | 42.17 | 86.81 | 87.71 | 39.78 | ||||
| Correctional facilities *§§ | 0.00 | 100.0 | 100.0 | 0.00 | ||||
| Weighted total | 54.26% | 82.34% | 80.74% | |||||
Provider types with less than 10 providers were not included to preserve anonymity. P-values ≤ 0.05 statistically significant are bolded. §§Removed Corrections and Hospitals from the analysis to run the Chi-square statistic except with perceived adaptability.
Table 2. Reported pertussis vaccine administration to subpopulations and perceived challenges in pertussis vaccine administration to subpopulations among provider types.
| Provider Type | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-traditional (%) | Under 25 (%) | Pharmacy (%) | Government (%) | Hospital (%) | Family practice (%) | Women's health (%) | Weighted total |
P | |
| To whom did your practice or pharmacy location administer pertussis vaccine (e.g., DTaP, Tdap) in 2010? | |||||||||
| Adults with close contact with infants | 50.73 | 48.15 | 82.59 | 92.57 | 59.18 | 79.81 | 87.43 | 55.60% | <0.001 |
| Healthcare workers | 58.26 | 60.49 | 73.46 | 100.00 | 91.84 | 65.42 | 60.30 | 53.05% | <0.001 |
| Middle School and High School aged adolescents | 13.91 | 92.59 | 68.44 | 62.86 | 47.62 | 78.44 | 12.27 | 52.21% | <0.001 |
| Older adults aged 65 and above | 53.51 | 12.35 | 55.46 | 85.14 | 34.69 | 60.99 | 15.07 | 39.05% | <0.001 |
| Children Under age 6 | 1.39 | 83.95 | 15.64 | 70.28 | 23.13 | 48.66 | 6.54 | 34.15% | <0.001 |
| Infants | 0.64 | 80.25 | 14.17 | 70.28 | 19.73 | 42.49 | 0.00 | 30.81% | <0.001 |
| What new challenges regarding pertussis vaccination have your practice or pharmacy location faced as a result of the law requiring Tdap boosters?* | |||||||||
| Scheduling | 8.06 | 38.16 | 22.53 | 37.76 | 9.76 | 26.59 | 1.78 | 19.05% | <0.001 |
| Reimbursement | 18.42 | 5.26 | 41.75 | 6.92 | 9.76 | 26.59 | 17.80 | 17.60% | <0.001 |
| Documentation for schools | 5.76 | 44.74 | 23.08 | 34.58 | 19.51 | 19.21 | 7.42 | 17.89% | <0.001 |
| Ability to recall or remind patients | 8.06 | 19.74 | 17.58 | 34.58 | 9.76 | 18.89 | 0.00 | 13.16% | 0.019 |
| Patient education | 14.96 | 23.68 | 23.08 | 20.75 | 37.40 | 23.79 | 19.58 | 18.99% | 0.298 |
| Increased vaccine exemptions | 3.45 | 6.58 | 4.12 | 10.10 | 29.27 | 11.19 | 0.00 | 6.72% | 0.086 |
The new law in California for 2011–2012 academic school year requires 7th–12th graders to receive a Tdap booster prior to beginning school (California General Assembly AB 354, 2010). ∧Rao Chi Square calculated using SUDAAN for each of the provider types.
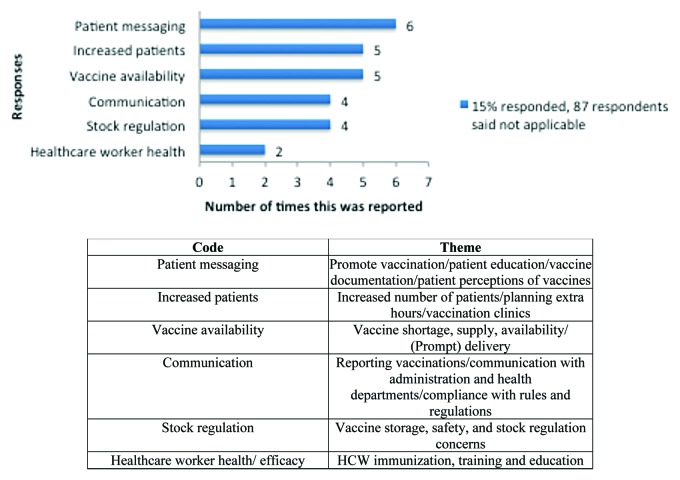
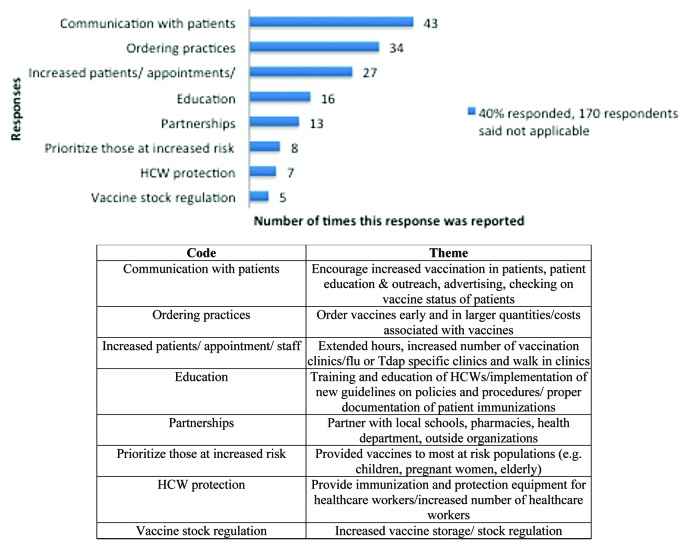
When asked about greatest concerns among members of the practice regarding vaccine administration for pertussis vaccination, providers indicated patient messaging, increased patient load, and vaccine availability as top concerns; communication and the health and efficiency of healthcare workers were additional concerns (Fig. 1). As a result of responding to recent pertussis outbreaks, providers indicated the main changes that have been made to their practice or pharmacy location included improved communication with patients, ordering practices, and increased patients, appointments, and staff; other changes were healthcare worker protection and vaccine stock management (Fig. 2).
Figure 1. Changes in practices and pharmacies as a result of a new law requiring Tdap booster shots.
Figure 2. Plans among members of practices or pharmacies regarding future vaccine shortages or emergencies.
Impact during outbreaks or epidemics
Vaccine providers were asked whether or not they had seen an increase in demand for the pertussis vaccine within the year of 2010. Approximately 70% of providers (358/508) overall indicated an increase in demand for the vaccine during 2010. Seventy percent of providers among those located in a metropolitan area (326/464) and approximately 73% (32/44) of providers outside of metropolitan areas indicated an increase in demand for the vaccine. There does not appear to be an association between whether the vaccine provider experienced an increase in demand for the pertussis vaccine in 2010 and the urbanicity of the area in which they are located (e.g., metropolitan, micropolitan, small town, or rural) (P = 0.728) nor was there an association between increased demand among providers and IIS region (P = 0.800).
Between January 1 and December 31, 2010, 38% of providers (187/498) said that they saw patients with pertussis. Thirty eight percent (172/455) of providers located in metropolitan areas and 35% (15/43) of providers outside of the metropolitan area indicated that they had seen pertussis patients. There were no meaningful associations observed between seeing pertussis patients in 2010 and metropolitan vs. non-metropolitan practices (P = 0.579) nor were there differences among IIS regions (P = 0.257). Among providers reporting seeing pertussis patients, 70% of providers surveyed indicated experiencing increased demand for pertussis vaccines. There was no significant difference in pertussis vaccine demand among regions (P = 0.661).
Adaptations and challenges due to legislation
Providers indicated that the top challenges regarding the new law concerning adolescent vaccination were those related to scheduling appointments (22%), vaccine reimbursement (20%), proper documentation for school records (21%), ability to remind patients about appointments (15%), educating patients (22%), and an increase in vaccine exemptions (8%) (Table 2). Meaningful differences among providers’ challenges faced were found between provider types (P < 0.001, Table 2). Among IIS regions, meaningful differences among challenges including scheduling (P = 0.022), reimbursement (0.009), and patient education and increased vaccine exemptions (<0.001) were found between IIS regions. Overall, reported challenges also varied among IIS regions (Table 3).
Table 3. Reported pertussis vaccine administration to subpopulations and perceived challenges in pertussis vaccine administration to subpopulations among IIS regions.
| IIS Regions (n = 421) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Northern CA (n = 15) (%) | 2 Greater Sacramento (n = 19) (%) |
3 Bay Area (n = 65) (%) |
4 San Joaquin (n = 13) (%) |
5 Central Valley (n = 44) (%) |
6 Central Coast (n = 14) (%) |
7 Los Angeles (n = 195) (%) |
8 Inland Empire (n = 31) (%) |
9 San Diego (n = 24) (%) |
Weighted Total |
P | |
| To whom did your practice or pharmacy location administer pertussis vaccine (e.g., DTaP, Tdap) in 2010? | |||||||||||
| Adults with close contact with infants | 81.56 | 67.45 | 74.36 | 77.73 | 65.25 | 65.70 | 64.70 | 68.41 | 61.39 | 55.60% | 0.585 |
| Healthcare workers | 84.71 | 67.60 | 67.21 | 76.63 | 55.23 | 81.72 | 60.74 | 59.05 | 80.05 | 53.05% | 0.287 |
| Middle School and High School aged adolescents | 58.65 | 49.61 | 50.50 | 75.81 | 71.05 | 64.58 | 65.32 | 76.69 | 59.37 | 52.21% | 0.049 |
| Older adults aged 65 and above | 71.96 | 57.50 | 50.93 | 65.08 | 43.62 | 41.90 | 43.85 | 48.56 | 40.24 | 39.05% | 0.498 |
| Children Under age 6 | 47.09 | 30.67 | 33.26 | 67.07 | 51.33 | 47.43 | 37.44 | 47.29 | 55.25 | 34.15% | 0.211 |
| Infants | 47.09 | 28.13 | 29.56 | 58.33 | 44.75 | 39.88 | 33.92 | 36.07 | 61.49 | 30.81% | 0.228 |
| 1 (n = 16) (%) | 2 (n = 19) (%) | 3 (n = 69) (%) | 4 (n = 15) (%) | 5 (n = 46) (%) | 6 (n = 17) (%) | 7 (n = 216) (%) | 8 (n = 31) (%) | 9 (n = 25) (%) | |||
| What new challenges regarding pertussis vaccination have your practice or pharmacy location faced as a result of the above law? | |||||||||||
| Scheduling | 16.59 | 24.22 | 8.55 | 43.20 | 27.36 | 12.45 | 23.06 | 33.25 | 24.08 | 19.05% | 0.022 |
| Reimbursement | 12.15 | 14.52 | 22.86 | 22.96 | 13.53 | 9.61 | 25.22 | 17.89 | 4.73 | 17.60% | 0.009 |
| Documentation for schools | 13.16 | 12.91 | 12.27 | 34.93 | 21.69 | 28.46 | 21.04 | 29.86 | 25.86 | 17.89% | 0.118 |
| Ability to recall or remind patients | 25.31 | 26.51 | 16.50 | 23.94 | 12.82 | 6.22 | 12.16 | 19.21 | 22.97 | 13.16% | 0.664 |
| Patient education | 27.33 | 27.84 | 21.84 | 23.94 | 21.36 | 0.00 | 22.28 | 26.50 | 21.66 | 18.89% | <0.001 |
| Increased vaccine exemptions | 6.96 | 0.00 | 6.55 | 23.94 | 8.76 | 0.00 | 7.54 | 10.41 | 9.40 | 6.72% | <0.001 |
The new law in California for 2011–2012 academic school year requires 7th–12th graders to receive a Tdap booster prior to beginning school.
Certain practice types had more difficulty than other practice types in adapting to laws and guidelines related to administering the pertussis vaccine. Fifty-two percent of government and 61% of hospital providers indicated it was not easy to adapt to new laws and guidelines regarding pertussis vaccine administration while approximately 34% of non-traditional practices and 30% of pharmacy providers indicated the same (Table 1).
Providers qualitatively reported changes occurring as a result of a new law. Namely, themes of preparation and plans for future emergencies and shortages, concerns regarding vaccine shortages, and changes that occurred within the practice due to emergencies and vaccine shortages were reported.
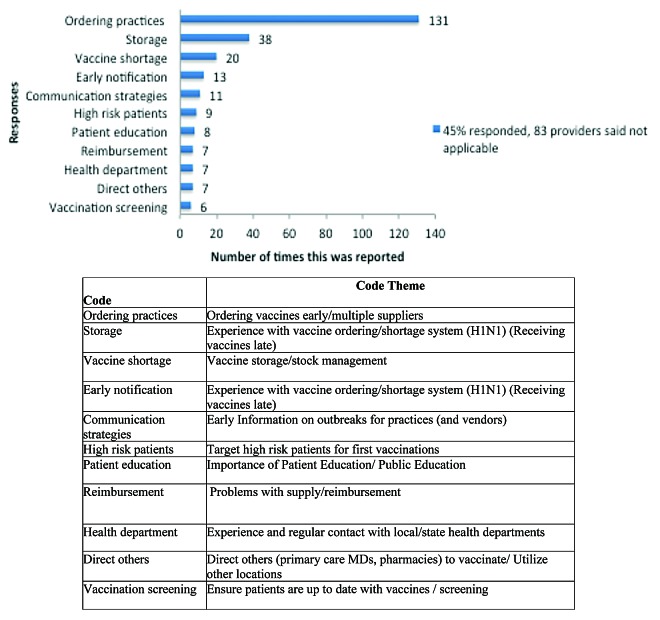
The top three adaptations related to this law cited by providers included ordering practices, patient messaging, and the demand for more appointments. The three least cited changes included partnerships (such as with schools or local organizations), an extended age range for required vaccination (ranging from seventh through twelfth as opposed to no booster requirement prior to beginning seventh grade as previously done), and immunization of healthcare workers (Fig. 3). Providers indicated the top 3 lessons learned from previous vaccine shortages were related to better ordering practices, storage, and vaccine shortages (such as problems with supply, availability/prompt delivery) (Fig. 4). Other challenges included documentation (e.g., immunization records or additional paperwork as proof of being vaccinated to provide to schools), increased appointments, and immunizing employees.
Figure 3. Concerns among members of practices and pharmacies regarding vaccine administration for pertussis outbreak.
Figure 4. Changes to practices and pharmacies regarding vaccine administration as a result of experiences responding to pertussis outbreaks.
Discussion
Nationwide, the incidence of reported pertussis has been on the rise since the 1970s, and the 2010 pertussis outbreak was the worst outbreak of pertussis in California in 50 y.13 Vaccine providers had to be strategic in decision-making regarding the subpopulations to which the vaccine would be administered, managing a steady supply to keep up with demand, and strengthening healthcare worker capacity. Prevention and control efforts are focused on vulnerable populations at greatest risk including infants, pregnant women, and those at greatest risk for severe disease. In this survey, provider types focused on different patient populations. It is important to target the correct provider type for each at-risk patient group.
Approximately half of all providers surveyed reported it being part of their standard care practices to ask adult patients about the pertussis vaccine which may suggest room for improvement in pertussis awareness in the physician community. Pertussis is a re-emergent public health issue that is preventable with the appropriate vaccines. Increased awareness and patient education are important factors in reducing outbreaks of pertussis.
Providers experienced challenges regarding an increased demand for the Tdap booster among middle and high school aged adolescents not only due to the pertussis outbreak, but also due to the implementation of the middle school entry law. A variety of adaptations were reported in practices and pharmacies as a result of this increased demand for pertussis vaccine, primarily including ordering vaccines early and ordering more supplies. Providers also reported the importance of patient messaging, monitoring vaccination status, promoting vaccination at any time, and encouraging patients to get vaccinated. Health agencies should be aware of the differences in the ability to adapt to the new law and increased demand for the vaccine, especially larger practices. Currently 42 states require Tdap vaccination for all students entering the seventh grade and as more states adopt such laws there will be increased demand for Tdap/DTaP vaccines and potentially increased strain on practices.
The high response rate for this survey of California vaccine providers may reduce the potential for non-response bias. When categorizing providers based on provider type and IIS Regions we found vaccine administration to certain subpopulations was significant for most providers and challenges regarding vaccines were almost all significant. Should researchers consider the indicated challenges encountered when administering Tdap/DTap vaccines to specific subpopulations, the method by which providers are categorized (provider type vs. providers within IIS region vs. providers divided by the Northern and Southern Region of California) should also be considered given the varied results for vaccine administration per provider type and IIS region.
In a Massachusetts Chapter of the American Academy of Pediatrics (MCAAP) Immunization Initiative Survey on vaccine reimbursement issues and barriers to the provision of immunizations, members indicated experiencing the following barriers to providing immunizations to their patients: patient concern regarding vaccine safety, cost of obtaining/purchasing vaccines, low payment for administration of vaccines, challenges associated with vaccine storage, and lack of clarity regarding the recommended immunization schedule and indications. Providers also indicated that barriers included supply shortages, manufacturer supply issues (e.g., calling patients back who were unable to get the scheduled vaccine because of a supply shortage), vaccine supply variation across the country, and state covered vs. purchased vaccines.14
Our survey results may help providers and health agencies be more prepared for future outbreaks. They might adopt successful adaptations such as vaccination at regular visits, promotion of vaccination within their practices, partnering with outside organizations such as schools or clinics, or implementing a referral system if the given practice has depleted their supply of vaccine. The referral system would still give patients an opportunity to receive the vaccine. Implementing these changes before another outbreak or shortage could help future preparedness and response efforts.
Conclusion
The 2010 pertussis outbreak in California challenged vaccine providers in a way that changed the preparation, promotion, and planning for future outbreaks and emergency situations. Adaptability to the new state law and increased awareness of pertussis in the physician community were important in the number of patients receiving the vaccine. Also, forming partnerships with schools and health agencies were important in facilitating and promoting widespread vaccination. As providers continue to learn from such challenges and implementing changes such as those indicated in this survey, providers will be more equipped and prepared to response to future vaccine-related emergencies.
Disclosure of Potential Conflicts of Interest
Source of support: This study was supported by a grant from the Centers for Disease Control and Prevention (CDC), 5P01TP000300, to the Emory Preparedness and Emergency Response Research Center, Emory University (Atlanta, GA). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the CDC.
Acknowledgments
We thank the California vaccine providers for participating and responding to the survey as well as graduate research assistants Krista Valenzuela, Jennifer Richards, Meghan Griffin, and Katharina van Santen for their assistance with data collection and cleaning related to the survey. We would also like to thank Abdiasiis Omar of Liverpool School of Tropical Medicine and Jennifer Chang of Emory University for their assistance in editorial critiques of early drafts of the manuscript.
References
- 1.Rohani P, Drake JM. The decline and resurgence of pertussis in the US. Epidemics. 2011;3:183–8. doi: 10.1016/j.epidem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Notes from the Field: Pertussis—California, January–June 2010. Morbidity and Mortality Weekly Report. 2010;59:817. [Google Scholar]
- 3.Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, et al. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices. Healthcare Infection Control Practices Advisory Committee Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep. 2006;55(RR-17):1–37. [PubMed] [Google Scholar]
- 4.Kretsinger K, Broder KR, Cortese MM, Joyce MP, Ortega-Sanchez I, Lee GM, Tiwari T, Cohn AC, Slade BA, Iskander JK, et al. Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices. Healthcare Infection Control Practices Advisory Committee Preventing tetanus, diphtheria, and pertussis among adults: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine recommendations of the Advisory Committee on Immunization Practices (ACIP) and recommendation of ACIP, supported by the Healthcare Infection Control Practices Advisory Committee (HICPAC), for use of Tdap among health-care personnel. MMWR Recomm Rep. 2006;55(RR-17):1–37. [PubMed] [Google Scholar]
- 5.California General Assembly. AB 354. In: State of California, ed2010. [Google Scholar]
- 6.California Immunization Coalition. Director's Update. 2012; http://www.immunizeca.org/about/directors-update Accessed December 5, 2012.
- 7.Centers for Disease Control and Prevention (CDC) Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis (Tdap) vaccine from the Advisory Committee on Immunization Practices, 2010. MMWR Morb Mortal Wkly Rep. 2011;60:13–5. [PubMed] [Google Scholar]
- 8.Chamberlain AT, Wells K, Seib K, Kudis A, Hannan C, Orenstein WA, Whitney EA, Hinman AR, Buehler JW, Omer SB, et al. Lessons learned from the 2007 to 2009 Haemophilus influenzae type B vaccine shortage: implications for future vaccine shortages and public health preparedness. J Public Health Manag Pract. 2012;18:E9–16. doi: 10.1097/PHH.0b013e31821dce27. [DOI] [PubMed] [Google Scholar]
- 9.Van Otterloo JSK, Omer SB. Study protocol for surveying vaccine providers– achieving a high response rate using gift card incentives. Emory University Preparedness and Emergency Response Research Center, Atlanta, Georgia, USA; 2012. [Google Scholar]
- 10.Van Otterloo J, Richards JL, Seib K, Weiss P, Omer SB. Gift card incentives and non-response bias in a survey of vaccine providers: the role of geographic and demographic factors. PLoS One. 2011;6:e28108. doi: 10.1371/journal.pone.0028108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.California Immunization Registry. CAIR Regions. 2012; http://cairweb.org/cair-regions/, 2012.
- 12.Rural Health Research Center. RUCA Data Zip Code RUCA Approximation. 2012; http://depts.washington.edu/uwruca/ruca-approx.php Accessed August 15, 2012.
- 13.Centers for Disease Control and Prevention. Grantee immunization websites. 2012; http://www.cdc.gov/vaccines/spec-grps/prog-mgrs/grantee-imz-websites.htm
- 14.MCAAP. MCAAP Immunization Survey on Vaccine Reimbursement Issues and Barriers to the Provision of Immunization 2012; http://www.mcaap.org/MCAAP%20II%20Survey%20Results%20-%20Vaccine%20Reimbursement%20and%20Barriers%20to%20Immunization%20-%20June%202012(1).pdf, 2013.