Abstract
Background & objectives:
The emergence of drug resistance tuberculosis (TB) is a significant challenge for TB control and prevention programmes, and the major problem is multidrug resistant tuberculosis (MDR-TB). The present study was carried out to determine the frequency of drug resistant Mycobacterium tuberculosis isolates among newly and retreated TB lymphadenitis patients and risk factors for acquiring this infection.
Methods:
Two hundred twenty five M. tuberculosis isolates from TB lymphadenitis patients who were diagnosed as new and retreated tuberculosis cases between April 2012 and May 2012 were included in this study. Isolates were tested for susceptibility to isoniazed (INH), rifampicin (RMP), streptomycin (SM), ethambutol (EMB) and pyrazinamide (PZA) using the BacT/AlerT 3D system protocol.
Results:
Among 225 isolates, 15 (6.7%) were resistant to at least one first line anti-TB drug. Three (1.3%) were MDR-TB. Resistance to INH, RMP, SM, and EMB was found in 8 (3.6%), 4 (1.8%), 10 (4.4%), and 4 (1.8%) isolates, respectively. Of the 212 new TB lymphadenitis cases three (1.4%) were MDR-TB. A rifampicin resistant M. tuberculosis isolate was diagnosed from smear and culture negative newly treated cases. All isolates were susceptible to PZA. Matted cervical lymph nodes were the prominent sites involved. Newly treated TB lymphadenitis patients had a greater risk for presenting resistance to anti-TB drugs (P=0.046).
Interpretation & conclusions:
Our study showed that TB lymphadenitis patients harboured drug resistant TB and MDR-TB, although at a low rate. Resistance was not associated with age, sex, patients’ education and contact history. Further research is required to determine transmission dynamics of drug resistant strains.
Keywords: Anti-tuberculosis drug resistance, Ethiopia, Mycobacterium tuberculosis, TB lymphadenitis
Globally around 8.8 million people develop tuberculosis (TB) and 1.45 million die every year as a consequence of TB, of whom 0.35 million deaths are associated with HIV-TB co-infection1. Ethiopia is badly harmed by the tuberculosis pandemic and is ranked second after Nigeria and seventh among the 22 high TB burden countries worldwide2.
At present, the country is involved in early case detection, provision of enough treatment and prevention of transmission of TB in the community. In spite of these efforts, the development of drug resistance in the bacterium has remained a continuous challenge for TB control and prevention programme in Ethiopia3. Drug resistance is primary when it occurs in an individual who has not taken anti-TB drugs treatment in the past, acquired when it develops in patients previously treated with inadequate or partial chemotherapy4.
Chemotherapy regimens that are efficient in pulmonary tuberculosis (PTB) are also efficient in TB lymphadenitis5. According to the Ministry of Health (MOH) of Ethiopia5, the treatment regimens for the new TB cases consist of isonized (INH) - rifampicin (RMP) - pyrazinamide (PZA) - ethambutol (EMB) for the first two months followed by INH-RMP for four months whereas for previously treated TB cases an eight month regimen containing streptomycin (SM)-RMP-INH-PZA and EMB for two months followed by INH-RMP-PZA-EMB for one month during the intensive phase, followed by five months INH-RMP-EMB, is recommended. The standard treatment regimen for MDR-TB consists of EMB-PZA kanamycin (KM) or amikacin (AMK)-levofloxacin (LVX)-ethionamide (ETH)-cycloserine (CS) for six months followed by EMB-PZA-LVX-ETH-CS for 12 months. Single drug resistance to INH, RMP, SM and EMB has been reported to be 13.8, 5.8, 10.0 and 7.3 per cent respectively in Ethiopia6. According to WHO Global TB Report 2011, Ethiopia has been ranked 15th among 27 high TB burden MDR-TB countries1. In Ethiopia, among newly and retreated cases there were an estimated 1600 and 580 MDR-TB cases in Ethiopia, respectively5.
In Ethiopia, drug susceptibility tests (DST) for Mycobacterium species are not available as routine tests, not even for patients with suspected infection by drug resistant strains. There is a lack of data on the frequency of drug resistant Mycobacterium tuberculosis isolates from TB lymphadenitis patients in Ethiopia. This study was, therefore, aimed to determine the prevalence of anti-TB drug resistance and risk factors for acquiring infection to drug resistant M. tuberculosis among newly and retreated TB lymphadenitis patients in Ethiopia.
Material & Methods
Study design and subjects: This cross-sectional prospective study was conducted at four main hospitals (Felege Hiwot, Gamby, Gondar and Dessie) and at Bikat Diagnostic Clinic located in northern Ethiopia between April and May 2012. Consecutive patients with enlarged lymph nodes who were not responding to a two-week course of broad spectrum antibiotics and who were clinically and cytologically diagnosed as TB lymphadenitis were included in this study. Pyogenic abscesses were excluded based on the clinical and cytomorphological features.
A structured and pretested questionnaire was administered to collect socio-demographic characteristics and data on treatment history for TB, and the patients were classified as new cases and retreated TB cases. The study was conducted with the written consent of the patients after obtaining an institutional ethical clearance from research and publication committee ethical review board of the Bahir Dar University, Bahir Dar, Ethiopia.
Specimen collection, storage and transport: Fine needle aspirates (FNAs) were collected from lymph nodes of all patients. FNAs were divided into two halves for cytology and culture. The cytological criteria for diagnosis of TB lymphadenitis have been made based on the presence of epitheloid cell granuloma with or without multinucleated giant cells and with or without caseous necrosis or degenerate caseous necrosis and liquefied necrotic material with marked degenerating and viable inflammatory cell infiltration without epitheloid granuloma7. The culture FNAs was placed in to 1ml of phosphate buffer saline (pH=6.8) and stored at -80°C.
Culture and identification of mycobacteria: Four hundred thirty seven FNAs were processed for culture according to the German Institute for Standardization [Deutsches Institute fur Normung (DIN)] recommendations for the detection of Mycobacteria by culture methods8. For species identification and differentiation of M. tuberculosis complex strains from cultures, the GeneType MTBC assay was done according to the instructions of the manufacturer (Hain Life Science GmbH, Nehren, Germany).
Anti-TB drug resistant assay: As previously described9, DST against INH, RMP, SM, EMB and PZA was conducted on culture positive 225 M. tuberculosis isolates using BacT/AerT 3D protocol (BioMerieux, S.A, France). The final test concentration for INH, RMP and SM were 1, 2 μg/ml for EMB and pyrazinamide had test concentration 200 μg/ml, adjusted to (pH-6) by the addition of 1 ml of 0.75 m monopotassium phosphate. DST inoculum (0.5 ml) was inoculated into drug containing bottles and subsequently bottles were incubated at 37°C. Two controls, 1 per cent control (0.5 ml of the 1:100 diluted test organisms suspension) and original control without drug were prepared for interpretation of the test results. M. tuberculosis isolate was determined to be resistant to an antibiotic when the drug containing bottle had a time to detection (TTD) that was less than or equal to the TTD of the 1 per cent control. If a valid result was not obtained for an isolates, the DST was repeated. Samples that were culture negative were examined by GeneXpert MTB/RIF assay (Cepheid, Sunnyvale, CA) for susceptibility to RMP.
According to the recommendations by the Ministry of Health of Ethiopia5, the following definitions were used for resistant cases:
Resistance in new cases: the presence of resistant strain of M. tuberculosis with a patient who had no or less than one month of anti-TB treatment.
Resistance in previously treated cases: the presence of resistant strain of M. tuberculosis with a patient who had been treated for one month or more for TB only with first line anti-TB dugs.
Mono resistance: only resistance to first line anti-TB drugs.
MDR-TB: M. tuberculosis isolates resistant to at least INH and RMP, while resistance to more than one drug except INH and RMP was named as poly drug resistance TB.
Statistical analysis: All data were entered, cleared, and analyzed using the SPSS statistical software package, Version 16 (SPSS Inc., Chicago, IL, USA). Descriptive data analysis was done to visualize differences within data. Logistic regression model was applied to assess factors associated with drug resistance TB in terms of the odds ratio and 95% confidence interval (CI).
Results
The study included 225 M. tuberculosis isolates from TB lymphadenitis patients for DST. Patient ages ranged from 1 to 70 yr, with a mean age (± SD) of 28 (± 12.8) years. Of the 225 study subjects, 130 (57.8 %) were females, 25 (11.1%) were paediatric age group (≤14 yr old), 13 (5.8%) were previously treated cases, 159 (70.7%) were rural residents, 109 (48.4%) had contact history to TB patients and 119 (52.9%) were illiterate (Figure). Fifteen isolates showed resistance to any one of the five anti-TB drugs was and three isolates III (1.3 %) were MDR. Eight isolates (3.6%) were resistant to INH, 10 (4.4%) to Sn and four each (1.8 %) to RMP and EMB, respectively.
Fig.
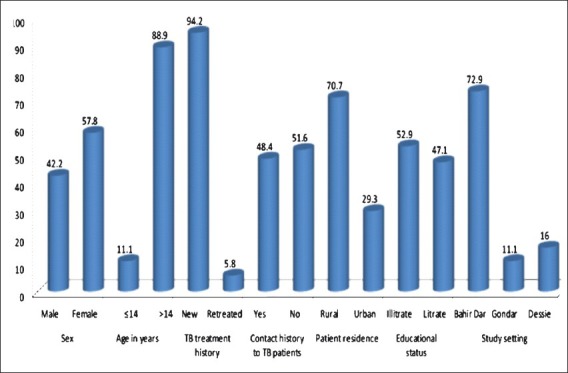
Socio-demographic characteristics of study subjects (n= 225).
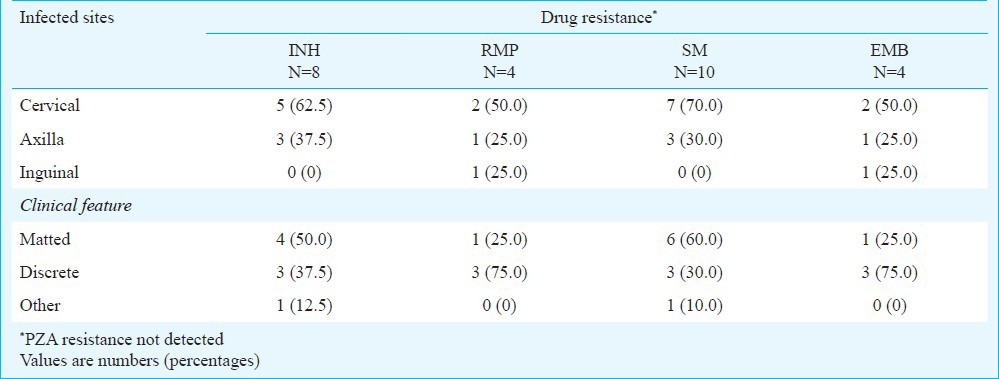
Of the 212 new cases, resistant isolates were found in 12 (5.7%) patients and three (1.4%) of these were MDR isolates. Mono resistance to RMP, SM and EMB were diagnosed in two, three and one patient, respectively. Of the 13 previously treated patients, resistant isolates were detected in three (23.1%) cases. Among these, one isolate was resistant to INH and two were resistant to both INH and SM. MDR-TB was not found in retreated patients in this study (Table I). Of the three MDR isolates resistant to RMP, one was detected from smear and culture negatives. In this study, resistance to PZA was not observed. Five of eight INH resistant isolates, seven of 10 SM resistant isolates, and two each of the four RMP and EMB resistant isolates were diagnosed from the cervical lymph node region and most of these were from TB lymphadenitis cases with matted clinical feature (Table II).
Table I.
Prevalence of drug resistance among 225 clinical M.tuberculosis isolates*
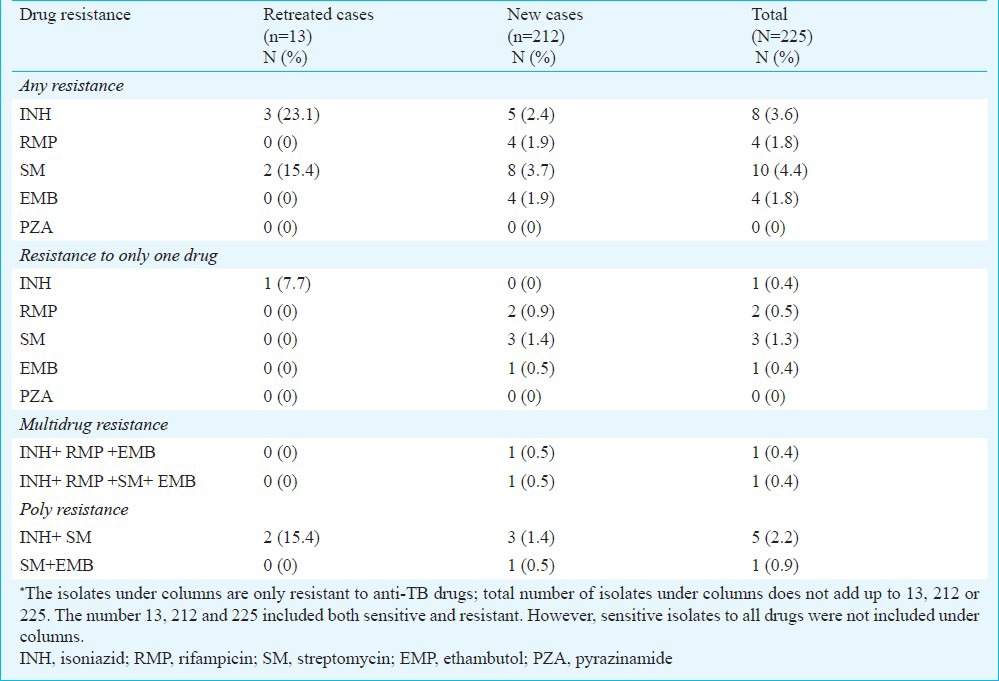
Table II.
Distribution of drug resistance by lymph node region and clinical feature
Among the three study settings 6 (40%) of the resistant isolates were from BahirDar, one (7.1%) from Gondar and eight (57.2%) from Dessie. Of the three total MDR isolates, two were from Dessie and one was from Bahir Dar.
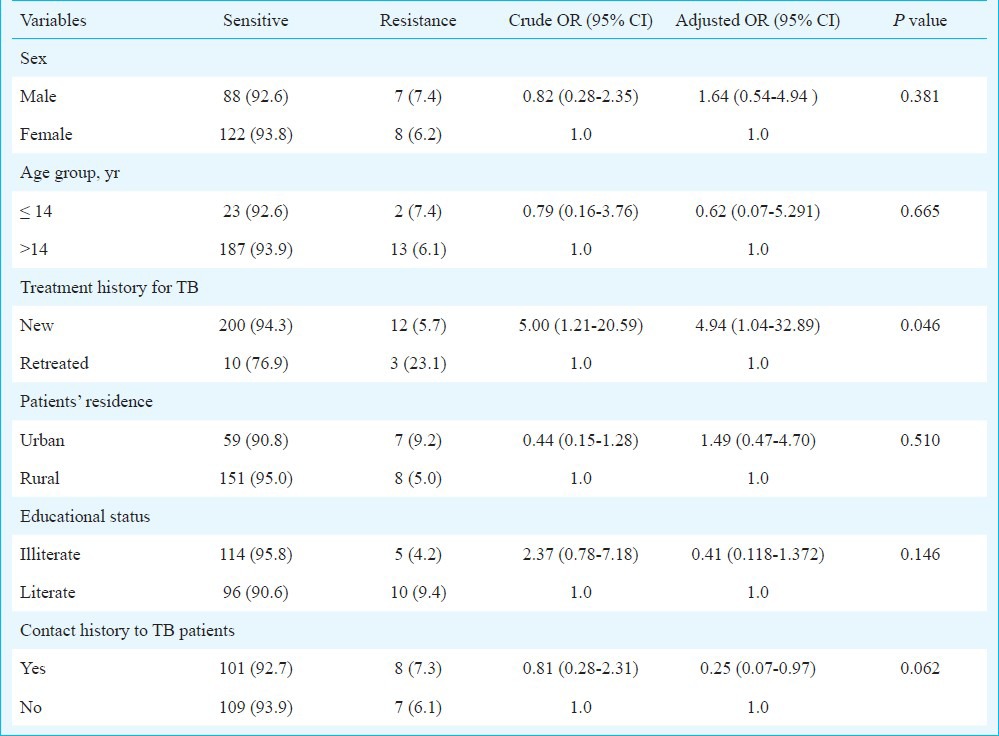
The history of newly treated cases for TB was identified as a significant risk factor for drug resistance (P=0.046). PZA was not found to be a significant risk factor for drug resistance in this study. Resistance was not significantly associated with age, sex, patients’ residence, education, and contact history to TB patients (Table III).
Table III.
Association of different characteristics with the occurrence of drug resistance tuberculosis
Discussion
In the present study, 93.3 per cent of the M.tuberculosis isolates were sensitive to all of the first line anti-TB drugs tested. The magnitude of first line anti-TB drug resistance and MDR-TB was 6.7 and 1.3 per cent, respectively. The overall level of resistance to first line anti-TB drugs among retreated TB lymphadenitis cases (23.1 %) was lower to the rates reported in Ethiopia (39.1%6, 48.7%5, and 85.7%10). Resistance to first line anti-TB drugs among retreated cases has been reported to be 56 per cent in Nigeria11 and 58.2 per cent in cameron12.
The resistance to any one of the first line anti-TB drugs among new cases was lower in the present study compared with reports in Ethiopia6,10,13,14 and in the central African republic15. However, a comparable prevalence of drug resistance among newly treated cases was reported from India (5.2%)16.
In the present study, the resistance level observed for INH among retreated cases was 7.7 per cent, which agreed well with the findings reported from PTB patients (6.5%) in Ethiopia6. In a study conducted in north India mono-resistance to INH among retreated cases was reported to be 29.7 per cent17. Increased resistance to INH may affect the success of TB control programme since the drug is used both in intensive and continuous phase of anti-TB treatment14. The resistance to SM in this study (14%) was lower compared with previous reports in Ethiopia1,14,18,19 and in other African countries20,21. Ethambutol is a drug that enhances the effect of many other drugs including beta-lactam drugs on mycobacterial species22. In this study, the resistance observed for EMB among new cases was in agreement with earlier observations reported in Ethiopia1,6,19.
In the present study, MDR-TB was seen in three new TB lymphadenitis patients only. A recent study conducted in northwest Ethiopia showed 3.7 and 10.9 per cent MDR-TB in new and previously treated PTB patients6. Yimer et al14 reported 1 per cent MDR-TB in the same study area. A report from Addis Ababa indicated 2.3 per cent MDR-TB among new and 71.4 per cent among previously treated cases10. In Ethiopia, among new cases lower levels of MDR-TB were reported by Asmamaw et al (0.6%)23 and 0 per cent by Gebeyehu et al24. Studies among new cases in Africa indicated 1.3 per cent MDR-TB in Tanzania25, 1.6 per cent in Benin26, 2.6 per cent in South Africa27 and 3.4 per cent in Mozambique20.
In the patients described here, matted cervical lymph node regions were the prominent sites involved, indicating the late arrival of patients in seeking medical care28. The health care seeking behaviour could be low for TB lymphadenitis compared with that of pulmonary TB.
New cases of TB lymphadenitis currently on anti-TB drugs at the time of diagnosis showed a high risk for presenting resistance to one or more anti-TB drugs in the present study. This finding was different from that reported in different studies6,10,29. The high level of drug resistance among new cases in this study might be due to the exposure of patients to anti-TB drugs before they visited health institutions or exposure to drug resistant M. tuberculosis strain in the community.
In conclusion, the present findings indicated that the TB lymphadenitis patients harboured drug resistant TB and MDR-TB, although at a low prevalence. Identifying undiagnosed rifampicin resistance TB lymphadenitis cases remains a challenge to prevent the spread of MDR-TB in TB endemic setting. Further research is required to determine transmission dynamics of drug resistant strains.
Acknowledgment
This study was carried out with financial support from the German Federal Ministry of Education and Research (BMBF, PtJ-Bio, 0315883), the Institute of Medical Microbiology and Epidemiology of Infectious Diseases, University Hospital Leipzig, Germany, Translational Centre for Regenerative Medicine (TRM), University Hospital Leipzig, Germany, the German Academic Exchange Service (DAAD) and University of Bahir Dar, Ethiopia. Authors acknowledge Dr Jörg Beer and Elisabeta Krawczyk for their kindly assistance during drug susceptibility testing, and thank all pathologists and data collectors.
References
- 1.World Health Organization. WHO HTM/TB/2011.16. Geneva, Switzerland: WHO; 2011. Global tuberculosis control. WHO report 2011. Available from: http://whqlibdoc.who.int/publications/2011/9789241564380_eng.pdf . [Google Scholar]
- 2.World Health Organization. WHO/HTM/TB/2010. Geneva, Switzerland: WHO; 2010. WHO Report 2011. Global Tuberculosis Control: Surveillance, planning, Financing. [Google Scholar]
- 3.1st ed. Addis Ababa, Ethiopia: Federal Ministry of Ethiopia; 2009. Ministry of Health of Ethiopia. Guideline for program and clinical management of drug resistant tuberculosis. [Google Scholar]
- 4.Zhang Y, Yew WW. Mechanisms of drug resistance in M.tuberculosis. Int J Tuberc Lung Dis. 2009;13:1320–30. [PubMed] [Google Scholar]
- 5.Addis Ababa: MOH; 2012. Ministry of Health of Ethiopia. Tuberculosis, leprosy and TB/HIV prevention and control programme manual. [Google Scholar]
- 6.Tessema B, Beer J, Emmrich F, Sack U, Rodloff AC. First and second line anti-tuberculosis drug resistance in Northwest Ethiopia. Int J Tuberc Lung Dis. 2012;16:805–11. doi: 10.5588/ijtld.11.0522. [DOI] [PubMed] [Google Scholar]
- 7.Sulaiman A, Afshan S, Tazeen M, Talat M, Akbar A, Rafiq k0. A comparison of fine needle aspiration cytology with Ziehl Neelsen staining in diagnosis of tuberculosis lymphadenitis. comprehensive cytol Pathol. 2010;16:707–9. [Google Scholar]
- 8.German Institute for Standardization (Deutsces Institute für Normung) DIN58943-3. Beuth Verlag, Berlin, Germany: DIN; 1986. Medical microbiology-diagnosis of tuberculosis. Part 3: detection of mycobacteria by culture methods. [Google Scholar]
- 9.Beer J, KÜchler R, Rodloff AC. Investigations about the possibility for testing the susceptibility of mycobacteria with MB/BacT culture system. J lab Med. 1997;21:390–8. [Google Scholar]
- 10.Agonafir M, Lemma E, Wolde-Meskel D, Goshu S, Santhanam A, Girmachew F, et al. Phenotypic and genotypic analysis of MDR-TB in Ethiopia. Int J Tuberc Lung Dis. 2010;14:1259–65. [PubMed] [Google Scholar]
- 11.Idigbe EO, Duque JP, John EK, Annam O. Resistance to anti-tuberculosis drugs in treated patients in Lagos, Nigeria. J Trop Med Hyg. 1992;95:186–91. [PubMed] [Google Scholar]
- 12.Kuaban C, Bercion R, Jifon G, Cunin P, Blackett KN. Acquired anti-tuberculosis drug resistance in Yaounde, Cameroon. Int J Tuberc Lung Dis. 2000;4:427–32. [PubMed] [Google Scholar]
- 13.Demise M, Gebeyehu M, Berhane Y. Primary resistance to anti-TB drugs in Addis Ababa, Ethiopia. Int J Tuberc Lung Dis. 1997;1:64–7. [PubMed] [Google Scholar]
- 14.Yimer SA, Agonafir M, Derese Y, Sani Y, Bjune GA, Holm-Hansen C. Primary drug resistance to anti-TB drugs in major towns of Amhara region, Ethiopia. APMIS. 2011;120:503–9. doi: 10.1111/j.1600-0463.2011.02861.x. [DOI] [PubMed] [Google Scholar]
- 15.Garin B, Di Costanzo B, Kassa Kelembho E, Legot B, Guerin D, Ouapou-Lena F, et al. Drug resistance M.tuberculosis strains in TB patients in Bangui, Central African Republic. AIDS. 1995;9:213–4. [PubMed] [Google Scholar]
- 16.Dhingra VK, Rajpal S, Bhalla P, Yadav A, Jain SK, Hanif M. Prevalence of initial drug resistance in new sputum positive revised national TB control programme(RNTCP) patients. J Commun Dis. 2003;35:82–9. [PubMed] [Google Scholar]
- 17.Gupta A, Mathuria JP, Singh SK, Gulati AK, Anupurba S. Anti tubercular drug resistance in four health care facilities in North India. J Health Popul Nutr. 2011;29:583–92. [PMC free article] [PubMed] [Google Scholar]
- 18.Abate D, Taye B, Abisino M, Bidglign S. Epidemiology of anti-tuberculosis drug resistance patterns and trends in tuberculosis referral hospital in Addis Ababa, Ethiopia. BMC Res notes. 2012;5:462. doi: 10.1186/1756-0500-5-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Desta K, Asrat D, Lemma E, Gebeyehu M, Feleke B. Drug susceptibility of M.tuberculosis isolates from smear negative pulmonary tuberculosis patients, Addis Ababa, Ethiopia. Ethiop J Health Dev. 2008;2:212–5. [Google Scholar]
- 20.Mac-Arthur A, Gloyd S, Perdigão P, Noya A, Sacarlal J, Kreiss J. Characteristics of drug resistance and HIV among tuberculosis in Mozambique. Int J Tuberc Lung Dis. 2001;5:894–902. [PubMed] [Google Scholar]
- 21.Kuaban C, Bercion R, Noeske J, Cunin P, Nkamsse P, Ngo Niobe S. Anti-tuberculosis drug resistance in the West Province of Cameroon. Int J Tuberc Lung Dis. 2000;4:356–60. [PubMed] [Google Scholar]
- 22.Abate G, Miorner H. Susceptibility of MDR strains of M.tuberculosis to amoxicillin in combination with clavulanic acid and ethambutol. J Antimicob Chemother. 1998;42:735–40. doi: 10.1093/jac/42.6.735. [DOI] [PubMed] [Google Scholar]
- 23.Asmamaw D, Seyoum B, Makonnen E, Atsebeha H, Woldemeskel D, Yamuah L, et al. Primary drug resistance in newly diagnosed smear positive tuberculosis patients in Addis Ababa, Ethiopia. Ethiop Med J. 2008;46:367–74. [PubMed] [Google Scholar]
- 24.Gebeyehu M, Lemma E, Eyob G. Prevalence of drug resistance tuberculosis in Arsi Zone, Ethiopia. Ethiop J Health Dev. 2001;15:11–6. [Google Scholar]
- 25.Chonde TM, Basra D, Mfinanga SG, Range N, Lwilla F, Shirima RP, et al. National anti-tuberculosis drug resistance study in Tanzania. Int J Tuberc Lung Dis. 2010;14:967–72. [PubMed] [Google Scholar]
- 26.Affolabi D, Adjagba OA, Tanimomo-Kledjo B, Gninafon M, Anagonou SY, Portaels F. Anti-TB drug resistance among new and previously treated pulmonary tuberculosis patients in Cotonou, Bennin. Int J Tuberc Lung Dis. 2007;11:1221–4. [PubMed] [Google Scholar]
- 27.Green E, Obi CL, Nchabeleng M, de Villiers BE, Sein PP, Letsoalo T, et al. Drug susceptibility patterns of M.tuberculosis in Mpumalanga Province, South Africa: Regimen. J Health Popul Nutr. 2010;28:7–13. doi: 10.3329/jhpn.v28i1.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bezabih M, Mariam DW, Solomon GS. Fine needle aspiration of suspected tuberculous lymphadnitis. Cytopathology. 2002;13:284–90. doi: 10.1046/j.1365-2303.2002.00418.x. [DOI] [PubMed] [Google Scholar]
- 29.Faustini A, Hall AJ, Perucci CA. Risk factors for MDR-TB in Europe: A systematic review. Thorax. 2006;61:158–63. doi: 10.1136/thx.2005.045963. [DOI] [PMC free article] [PubMed] [Google Scholar]


