Abstract
Background & objectives:
Multidrug-resistance of methicillin-resistant Staphylococcus aureus (MRSA) is a serious therapeutical problem. Chalcones belong to a group of naturally occurring flavonoids, usually found in various plant species, and have potent antibacterial, antiviral and antifungal activities. The goal of this study was to evaluate the antibacterial effect of three newly-synthesized chalcones against clinical isolates of MRSA, and their synergism with β-lactam and non- β-lactam antibiotics.
Methods:
Antimicrobial activity of the three newly-synthesized chalcones was tested against 19 clinical isolates of MRSA and a laboratory control strain of MRSA (ATCC 43300). The synergism with β-lactams: cefotaxime (CFX), ceftriaxone (CTX), and non-β-lactam antibiotics: ciprofloxacin (CIP), gentamicin (GEN) and trimethoprim/sulphamethoxazole (TMP-SMX) was investigated by checkerboard method.
Results:
All evaluated compounds showed significant anti-MRSA activity with MIC values from 25-200 μg/ml. Observed synergism with antibiotics demonstrated that chalcones significantly enhanced the efficacy of CIP, GEN and TMP-SMX.
Interpretation & conclusions:
Our study demonstrated that three newly-synthesized chalcones exhibited significant anti-MRSA effect and synergism with non-β-lactam antibiotics. The most effective compound was 1,3-Bis-(2-hydroxy-phenyl)-propenone. Our results provide useful information for future research of possible application of chalcones in combination with conventional anti-MRSA therapy as promising new antimicrobial agents.
Keywords: Chalcones, checkerboard method, MRSA, multidrug-resistance, synergy
Staphylococcus aureus is a potentially pathogenic bacterium that causes a broad spectrum of diseases, ranging from minor infections of the skin and soft tissue to severe nosocomial infections like endocarditis, bacteraemia and sepsis. Therapeutic usage of penicillin in early 1940s decreased the mortality rate due to infections caused by susceptible strains. The first penicillin-resistant β-lactamase-producing strains of staphylococci were reported several years after1. Decades after, continuous increase in the number of multidrug-resistant strains of staphylococci, particularly resistant to methicillin and vancomycin, again resulted in high mortality rate due to staphylococcal infections. Therapeutic effect of antibiotics commonly used in treatment of these infections often fails due to problems with pharmacokinetics of the drug, or adaptation of the bacteria through the mechanisms of inducible resistance to antibiotics and mutations of regulatory staphylococcal genes2,3,4. Administration of high doses of antibiotics, or combination of antibiotics is useful therapeutic approach often limited by high toxicity of the drugs.
Besides conventional antimicrobial agents, numerous studies reported antibacterial activity of various plant extracts and their synthesized counterparts. Antimicrobial activity of plants is mainly caused by small molecules like terpenoids, flavonoids and polyphenols5. Chalcones belong to a group of naturally occurring flavonoids with chemical structure made of two aryl rings linked by a α,β-unsaturated ketone. Although these compounds are usually isolated from natural sources like diverse plant species, fruits and vegetables, many chalcones and their analogues can be obtained by the methods of classical and combinatorial synthesis. Both naturally and synthetic chalcones exhibit a broad spectrum of biological activities: antibacterial, antiviral, antifungal, antiangiogenic, anticancer, antiproliferative and anti-inflammatory6,7,8,9,10.
We undertook this study to investigate the antimicrobial activity and synergism with antibiotics of three newly-synthesized chalcones against 19 clinical isolates and one laboratory control strain of MRSA (ATCC 43300).
Material & Methods
The study was conducted in the department of Microbiology and Immunology, University of Belgrade, Belgrade, Serbia in 2012.
Bacterial strains and culture media: Antibacterial activity of chalcones was tested against 19 clinical isolates of MRSA and one laboratory control strain of methicillin-resistant S. aureus ATCC 43300 (KWIK-STIK™, Microbiologics, USA) as positive control.
The clinical isolated were obtained from blood (3), wound (6), sputum (3), endotracheal tube (2), abdominal drain (1), nose (1), skin (1), urine (1), and external auditory canal (1). Identification of the isolates and methicillin resistance were determined by VITEK 2 test cards GP and AST-P580 (bioMérieux, France) and confirmed by PCR for nuc11 and mecA12 genes. SCCmec typing was performed according to Kondo et al13. The clinical isolates of MRSA were stored at -70°C in brain heart infusion broth (BHI; Lab M Limited, UK) with the addition of 10 per cent sterile glycerol. Prior to experiment, bacteria were defrosted, inoculated on tryptic soy agar (TSA; Lab M Limited) and cultivated in aerobic conditions for 18-24 h at 35°C.
Chalcones: The newly synthesized chalcones 1,3- Bis-(2-hydroxy-phenyl)-propenone (further referred as O-OH), 3-(3-hydroxy-phenyl)-1-(2-hydroxy-phenyl)-propenone (further referred as M-OH) and 3-(4-hydroxy-phenyl)-1-(2-hydroxy-phenyl)-propenone (further referred as P-OH) were obtained from the Department of Pharmaceutical Chemistry, University of Belgrade-Faculty of Pharmacy, Belgrade, Serbia. Chalcones were prepared through base catalyzed Claisen-Schmidt condensation of ortho, metha or para hydroxy substituted benzaldehydes with 2-hydroxy acetophenones. Synthesized compounds were characterized by infra red (IR), nuclear magnetic resonance (NMR) and mass spectrometry. The purity of the compounds was checked using high performance liquid chromatography (HPLC) and thin layer chromatography (TLC) methods.
Prior to experiments, chalcones were dissolved in sterile dimethyl sulphoxide (DMSO; Sigma-Aldrich Chemical Company Inc, USA) to a stock solution of 1000 μg/ml and subsequently diluted to the desired concentrations with medium.
Susceptibility testing: Antibiotic resistance profile of MRSA isolates was determined by VITEK 2 test card AST-P580 and further supplemented with disc diffusion test according to CLSI (Clinical Laboratory Standards Institute) guidelines14. Disk diffusion test was performed on Mueller-Hinton agar (Oxoid Limited, Basingstoke, Hampshire, UK) with the following antibiotic discs: amikacin (30 μg), gentamicin (10 μg), kanamycin (30 μg), tobramycin (10 μg), netilmicin (30 μg), streptomycin (10 μg), lincomycin (15 μg), clindamycin (2 μg), erythromycin (15 μg), clarithromycin (15 μg), azithromycin (15 μg), spiramycin (100 μg), pristinamycin (15 μg), tetracycline (30 μg), doxycycline (30 μg), minocycline (30 μg) and chloramphenicol (30 μg) (BioRad, Hercules, California, USA).
Antimicrobial activity of chalcones was determined by broth microdilution test according to CLSI guidelines14. In brief, one colony of the overnight cultures of bacterial isolates was diluted in saline to adjust the turbidity of the bacterial suspension to 0.5 McFarland standard (approximately 108 cfu/ml). Chalcones were prepared in fresh Mueller-Hinton broth in concentrations ranging from 3.12-500 μg/ml with addition of 0.05 per cent triphenyl tetrazolium chloride (Sigma-Aldrich). Triphenyl tetrazolium chloride is a growth indicator that is enzymatically reduced by metabolically active cells into red colour indicating for positive growth of bacteria. Each dilution of chalcones was poured in triplicates into 96-well microtiter plate and inoculated with 5×105 cfu/ml of previously prepared bacterial suspension. Positive growth controls of each isolate (bacteria in medium without presence of chalcones) were incubated under the same conditions. Negative control for each plate was medium only. After incubation for 24 h at 35°C in aerobic conditions minimum inhibitory concentration (MIC) was identified as the lowest concentration of the chalcones showing no visible growth of tested microorganisms (yellow coloured medium). To determine minimum bactericidal concentration (MBCs), each well showing no visible growth of bacteria was inoculated onto Mueller-Hinton agar and incubated for additional 24 h at 35 °C in aerobic conditions. MBC was identified as the lowest concentration of the chalcones that performed bactericidal effect. Each test was repeated three times.
Synergy testing: The synergism between chalcones and commercial antibacterial drugs was investigated in 96-well microtiter plates by checkerboard method15. Five antibiotics that represent different groups of antimicrobial agents were used: β-lactams cefotaxime (CFX) and ceftriaxone (CTX), and non β-lactam antibiotics ciprofloxacin (CIP), gentamicin (GEN) and trimethoprim/sulphamethoxazole (TMP-SMX) (Sigma-Aldrich). Synergistic effect of the combination was investigated in one concentration above and several concentrations below the MIC of each compound (both antibiotics and tested chalcones). The interaction between the two antimicrobial agents was estimated by calculating the fractional inhibitory concentration (FIC) indices (FICI). The FIC of each compound was calculated by dividing the concentration of the compound in effective MIC of the combination, with the MIC of the drug alone (e.g. FICchalcone = MICchalcone-antibiotic combination /MICchalcone). FICI values were calculated as the sum of the FICchalcone and FICantibiotic and interpreted as follows: FICI ≤ 0.5 synergy; 0.5 < FICI ≤ 1 additivity; 1 < FICI ≤ 2 indifference (no effect) and FICI ≥ 2 antagonism16,17. Each test was repeated three times.
Statistical analysis: The data obtained were analyzed in SPSS statistical program (PASW statistics 18.0 version) (SPSS Inc., Chicago, USA) using methods of descriptive statistics and Mann-Whitney U test.
Results
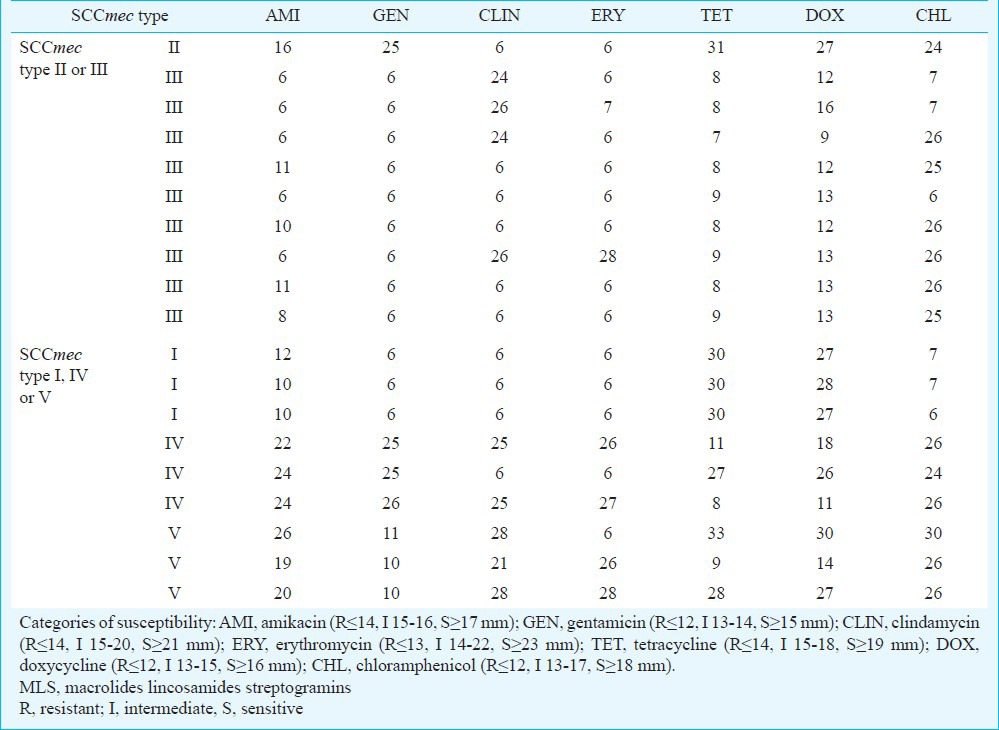
Clinical isolates of MRSA expressed SCCmec type as follows: SCCmec I (3), SCCmec II (1), SCCmec III (9), SCCmec IV (3) and SCCmec V (3). Susceptibility testing revealed that all MRSA isolates had multidrug-resistant phenotype (resistance to more than one different class of antibiotics beside members of β-lactams). All tested isolates (including ATCC43300) were 100 per cent sensitive to the following antibiotics: vancomycin, teicoplanin, trimethoprim/sulphamethoxazole, pristinamycin, linezolid, mupirocin, nitrofurantoin, and tigecycline. The next two were netilmicin (97% S, 3% I) and fosfomycin (90% S). Most of the isolates (95-100%) were resistant to benzylpenicillin, ampicillin and combinations with beta-lactamase inhibitors, second and third-generation cephalosporins, oxacillin and imipenem. Sixty four per cent isolates were resistant to members of aminoglycoside class of antibiotics (of which 41.2% SCCmec type II and III). Among members of aminoglycosides, highest frequency of resistance occurred against kanamycin (14.9%), tobramycin (14.0%) and gentamicin (13.2%). Most of the isolates (54.9%) were resistant to MLS (macrolides, lincosamides and streptogramins) group of antibiotics (of which 33.8% SCCmec type II and III), with highest frequency of resistance against erythromycin (10.5%), clarithromycin (10.5%) and azithromycin (10.5%). Resistance to tetracycline class of antibiotics occurred in 33.3 per cent of the isolates (of which 26.3% SCCmec type II and III), mostly against tetracycline (21.1%). Results of the zones of inhibition (mm) for the antibiotics representing different classes of antibiotics obtained by the agar diffusion method are presented in Table I.
Table I.
Disc diffusion method-zones of inhibition (mm) of the antibiotics representing different classes of antibiotics (aminoglycosides, MLS, tetracyclines) and chloramphenicol
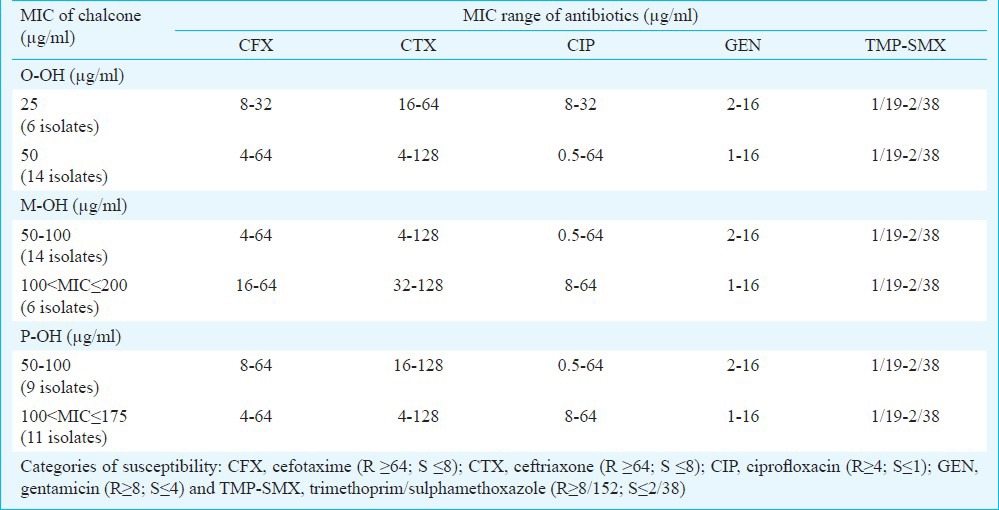
Preliminary investigation of antimicrobial activity of chalcones was performed with 13 newly-synthesized chalcones with diverse chemical structure, against seven laboratory control strains of Gram-positive and Gram-negative bacteria and two laboratory control strains of yeasts (data not shown). Three of the tested compounds (1,3- Bis-(2-hydroxy-phenyl)-propenone, 3-(3-hydroxy-phenyl)-1-(2-hydroxy-phenyl)-propenone and 3-(4-hydroxy-phenyl)-1-(2-hydroxy-phenyl)-propenone) exerted most prominent antistaphylococcal activity and were chosen for further investigation of antimicrobial activity against clinical isolates of MRSA. Anti-MRSA activities of the three tested chalcones and five antibiotics alone against 19 clinical isolates and one laboratory control strain of MRSA is shown in Table II. Chalcone with highest anti-MRSA activity was O-OH with MIC values ranging from 25-50 μg/ml and MBC values from 50-100 μg/ml. The order of potency of chalcones (average MIC±SD) was: O-OH (MIC=42.5±11.8 μg/ml)> M-OH (MIC=98.7±43.3 μg/ml) > P-OH (MIC=108.7±29.6 μg/ml.
Table II.
Minimal inhibitory concentrations (MIC) of chalcones and antibiotics
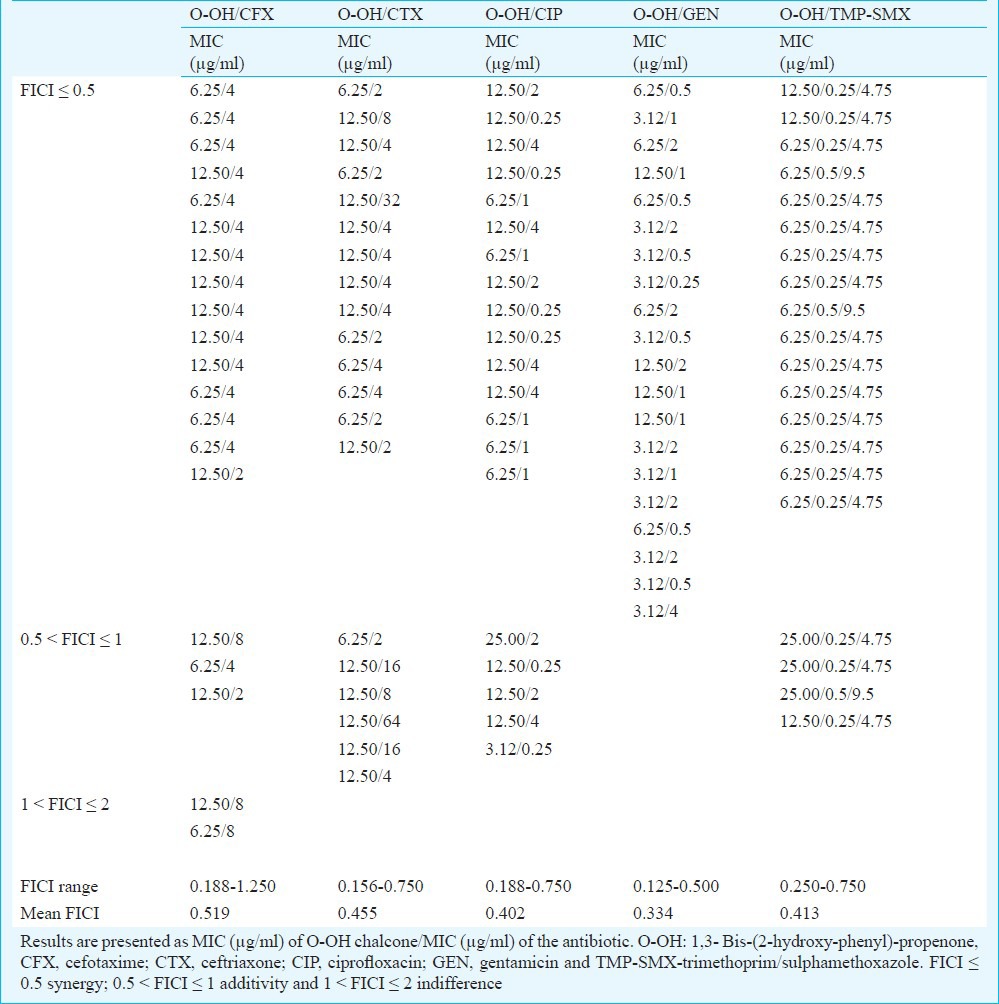
The checkerboard method was performed with five antibiotics that represent different groups of antimicrobial agents: β-lactam antibiotics cephalosporins (CFX, CTX), fluoroquinolone (CIP), aminoglycoside (GEN) and inhibitor of folate synthesis (TMP-SMX). MIC values of antibiotics were in the range of 4-64 μg/ml (CFX), 4-128 μg/ml (CTX), 0.5-64 μg/ml (CIP), 1-16 μg/ml (GEN) and 1/19-2/38 μg/ml (TMP-SMX). Overall effect of antibiotic-chalcone combination varied from synergistic (FICI ≤ 0.5) to indifferent (1 < FICI ≤ 2). The most significant synergistic effect was observed in combination of O-OH chalcone and GEN (FICI=0.125-0.500), CIP (FICI=0.188-0.750) and TMP-SMX (FICI=0.250-0.750) against all 20 tested MRSA isolates, respectively. The effects were exhibited in O-OH/GEN combinations at concentration of 1/16-1/8 MIC (3.12-12.50 μg/ml of O-OH) and 1/16-1/4 MIC (0.5-4 μg/ml of GEN), in O-OH/CIP combinations at concentration of 1/16-1/2 MIC (3.12-25.00 μg/ml of O-OH) and 1/256-1/2 MIC (0.25-4 μg/ml of CIP) and in O-OH/TMP-SMX combinations at concentration of 1/8-1/2 MIC (6.25-25.00 μg/ml of O-OH) and 1/8-1/4 MIC (0.25/4.75-0.5/9.5 μg/ml of TMP-SMX), respectively (Table III). The order of synergy potency (mean % of MIC reduction, mean FICI) was GEN (80.3%, 0.334) > CIP (87.6%, 0.402) > TMP-SMX (83.1%, 0.413) > CTX (80.2%, 0.455) > CFX (71.9%, 0.519). All O-OH/GEN combinations were synergistic; 80 per cent of O-OH/TMP-SMX combinations showed synergistic and 20 per cent additive effect; 75 per cent of O-OH/CIP combinations were synergistic and 25 per cent additive and combinations of O-OH with cephalosporins were mostly synergistic (70-75%) (Table III).
Table III.
Minimum inhibitory concentrations (MICs) and fractional inhibitory concentration indices (FICIs) of O-OH chalcone in combination with antibiotics
The effect of M-OH chalcone resembled to the one of O-OH, with order of synergy potency (mean % of MIC reduction, mean FICI): CIP (96.0%, 0.229) > GEN (78.4%, 0.349) > TMP-SMX (83.1%, 0.421) > CTX (79.7%, 0.449) > CFX (70.9%, 0.503). The weakest synergistic effect was observed with P-OH combinations with cephalosporins (average FICI CFX=0.700, CTX=0.527) and GEN (average FICI =0.576) with mostly additive and a few indifferent effects, while combinations with CIP (average FICI=0.305) and TMP-SMX (average FICI=0.489) were still in the range of synergy (90% of P-OH/CIP and 65% of P-OH/TMP-SMX combinations).
Discussion
Resistance to methicillin is determined by the function of penicillin-binding protein 2’ (PBP2’, or PBP2a) that binds to β-lactam antibiotics with much lower affinity than the intrinsic set of PBPs of S. aureus. PBP2’ is encoded by the methicillin resistance gene mecA located in the chromosome of MRSA on mobile genetic element designated staphylococcal cassette chromosome mec (SCCmec)18. Besides mecA gene, SCCmec element often contains genes responsible for resistance to antibiotics other than β-lactams. Currently, eleven SCCmec types have been described by the “International Working Group on the Classification of Staphylococcal Cassette Chromosome (SCC) Elements (IWG-SCC)19. SCCmec types I, IV and V encode exclusively for resistance to β-lactam antibiotics, while SCCmec types II and III determine multidrug-resistance owing to the presence of additional drug resistance genes on integrated plasmids pUB110 (resistance to kanamycin, tobramycin and bleomycin) and pT181 (resistance to tetracyclin) or transposon Tn554 (inducible MLS resistance)18,20,21. Resistance to antibiotics other than β-lactams in strains SCCmec type I, IV and V can be assigned to resistance genes inserted at other sites of the chromosome and on plasmids, besides the resistance genes situated on SCCmec18.
Antimicrobial activity of different classes of flavonoids is well known and has been extensively reviewed6,7,22. Chalcones with highest anti-MRSA activity are open chain flavonoids whose basic structure includes two aromatic rings bound by an α,β-unsaturated carbonyl group. Antibacterial effect of these compounds is often resulted by the presence of -OH groups in various positions of B ring23. Our results revealed that chalcone with free hydroxyl group in 2’ position of ring B (O-OH) exerted the most significant anti-MRSA effect, as reported by other investigators23,24. Other feature necessary for antistaphylococcal activity of chalcones is lipophilicity of the A ring7. Dihydroxy-25 and trihydroxychalcones26, also possess inhibitory activity against MRSA strains with MIC values in the range of 15-45 and 25-50 μg/ml, respectively.
There has been a growing evidence of synergistic effect between flavonoids/chalcones and antibiotics commonly used in the treatment of staphylococcal infections25,27,28,29. This approach allows the reduction of MICs, of both chalcones and antibiotics, making them more suitable for therapeutic usage. Heterocyclic chalcone analogues possess moderate antistaphylococcal activity alone (MIC=32-512 μg/ml), however, in combination with antibiotics, MIC values of chalcones reduce 2-4-fold and MIC values of antibiotics up to 16-fold29.
Structure-activity relationship for synergism of chalcones and antibiotics has not been elucidated yet. Several studies reported the synergistic and antibiotic resistance-modulating activity of flavonoids, suggesting that modulation of β-lactam resistance mainly originates from alterations of PBP2’30,31. However, synergism of chalcones and non- β-lactam antibiotics cannot be easily explained. Other mechanisms could be inhibition of β-lactamase, inactivation of efflux pumps, destabilization of cytoplasmic membrane, disruption of PBP2’ synthesis and inhibition of topoisomerase22.
In conclusion, results obtained in this study suggest that tested compounds possess strong anti-MRSA activity, with chalcone bearing hydroxyl group at 2’ position of B ring as the most effective one. Observed synergism with antibiotics demonstrated that O-OH and M-OH chalcone significantly enhanced the efficacy of CIP, GEN and TMP-SMX, and P-OH chalcone of CIP and TMP-SMX. Further research needs to be done to find the possible application of chalcones in combination with conventional anti-MRSA therapy as promising new antimicrobial agents.
Acknowledgment
The work of the first and the last authors (DDB, IC) was supported by the Ministry of Science, Republic of Serbia (project grant no. 175039).
References
- 1.Kirby WM. Extraction of a highly potent penicillin inactivator from penicillin resistant staphylococci. Science. 1944;99:452–3. doi: 10.1126/science.99.2579.452. [DOI] [PubMed] [Google Scholar]
- 2.Fowler VG, Jr, Sakoulas G, McIntyre LM, Meka VG, Arbeit RD, Cabell CH, et al. Persistent bacteremia due to methicillin-resistant Staphylococcus aureus infection is associated with agr dysfunction and low-level in vitro resistance to thrombin-induced platelet microbicidal protein. J Infect Dis. 2004;190:1140–9. doi: 10.1086/423145. [DOI] [PubMed] [Google Scholar]
- 3.Lewis JS, 2nd, Jorgensen JH. Inducible clindamycin resistance in staphylococci: should clinicians and microbiologists be concerned? Clin Infect Dis. 2005;40:280–5. doi: 10.1086/426894. [DOI] [PubMed] [Google Scholar]
- 4.Garau J, Bouza E, Chastre J, Gudiol F, Harbarth S. Management of methicillin-resistant Staphylococcus aureus infections. Clin Microbiol Infect. 2009;15:125–36. doi: 10.1111/j.1469-0691.2009.02701.x. [DOI] [PubMed] [Google Scholar]
- 5.Mahady GB. Medicinal plants for the prevention and treatment of bacterial infections. Curr Pharm Des. 2005;11:2405–27. doi: 10.2174/1381612054367481. [DOI] [PubMed] [Google Scholar]
- 6.Batovska D I, Todorova IT. Trends in utilization of the pharmacological potential of chalcones. Curr Clin Pharmacol. 2010;5:1–29. doi: 10.2174/157488410790410579. [DOI] [PubMed] [Google Scholar]
- 7.Nowakowska Z. A review of anti-infective and anti-inflammatory chalcones. Eur J Med Chem. 2007;42:125–37. doi: 10.1016/j.ejmech.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Sharma V, Singh G, Kaur H, Saxena AK, Ishar MP. Synthesis of β-ionone derived chalcones as potent antimicrobial agents. Bioorg Med Chem Lett. 2012;22:6343–6. doi: 10.1016/j.bmcl.2012.08.084. [DOI] [PubMed] [Google Scholar]
- 9.Mojzis J, Varinska L, Mojzisova G, Kostova I, Mirossay L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol Res. 2008;57:259–65. doi: 10.1016/j.phrs.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 10.Kontogiorgis C, Mantzanidou M, Hadjipavlou-Litina D. Chalcones and their potential role in inflammation. Mini Rev Med Chem. 2008;8:1224–42. doi: 10.2174/138955708786141034. [DOI] [PubMed] [Google Scholar]
- 11.Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol. 1992;30:1654–60. doi: 10.1128/jcm.30.7.1654-1660.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bignardi GE, Woodford N, Chapman A, Johnson AP, Speller DC. Detection of the mec-A gene and phenotypic detection of resistance in Staphylococcus aureus isolates with borderline or low-level methicillin resistance. J Antimicrob Chemother. 1996;37:53–63. doi: 10.1093/jac/37.1.53. [DOI] [PubMed] [Google Scholar]
- 13.Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, Etienne J, et al. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr and major differences in junkyard regions. Antimicrob Agents Chemother. 2007;51:264–74. doi: 10.1128/AAC.00165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clinical and Laboratory Standards Institute (CLSI) Wayne, PA, USA: CLSI; 2007. Performance standards for antimicrobial susceptibility testing; Seventeenth Informational Supplement: CLSI document M100-S17. [Google Scholar]
- 15.White RL, Burgess DS, Manduru M, Bosso JA. Comparison of three different in vitro methods of detecting synergy: time-kill, checkerboard, and E test. Antimicrob Agents Chemother. 1996;40:1914–8. doi: 10.1128/aac.40.8.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu ZQ, Zhao WH, Asano N, Yoda Y, Hara Y, Shimamura T. Epigallocatechin gallate synergistically enhances the activity of carbapenems against methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 2002;46:558–60. doi: 10.1128/AAC.46.2.558-560.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Orhan G, Bayram A, Zer Y, Balci I. Synergy tests by E test and checkerboard methods of antimicrobial combinations against Brucella melitensis. J Clin Microbiol. 2005;43:140–3. doi: 10.1128/JCM.43.1.140-143.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deurenberg RH, Vink C, Kalenic S, Friedrich AW, Bruggeman CA, Stobberingh EE. The molecular evolution of methicillin-resistant Staphylococcus aureus. Clin Microbiol Infect. 2007;13:222–35. doi: 10.1111/j.1469-0691.2006.01573.x. [DOI] [PubMed] [Google Scholar]
- 19.International Working Group on the Staphylococcal Cassette Chromosome elements (IWG-SCC) Currently identified SCCmec types in S.aureus strains. [accessed on February 15, 2013]. Available from: http://www.sccmec.org/Pages/SCC_TypesEN.html .
- 20.Ito T, Okuma K, Ma XX, Yuzawa H, Hiramatsu K. Insights on antibiotic resistance of Staphylococcus aureus from its whole genome: genomic island SCC. Drug Resist Updat. 2003;6:41–52. doi: 10.1016/s1368-7646(03)00003-7. [DOI] [PubMed] [Google Scholar]
- 21.Leclercq R. Mechanisms of resistance to macrolides and lincosamides: nature of the resistance elements and their clinical implications. Clin Infect Dis. 2002;34:482–92. doi: 10.1086/324626. [DOI] [PubMed] [Google Scholar]
- 22.Cushine TP, Lamb AJ. Recent advances in understanding the antibacterial properties of flavonoids. Int J Antimicrob Agents. 2011;38:99–107. doi: 10.1016/j.ijantimicag.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 23.Alcaraz LE, Blanco SE, Puig ON, Tomas F, Ferretti FH. Antibacterial activity of flavonoids against methicillin-resistant Staphylococcus aureus strains. J Theor Biol. 2000;205:231–40. doi: 10.1006/jtbi.2000.2062. [DOI] [PubMed] [Google Scholar]
- 24.Kromann H, Larsen M, Boesen T, Schønning K, Nielsen SF. Synthesis of prenylated benzaldehydes and their use in the synthesis of analogues of licochalcone A. Eur J Med Chem. 2004;39:993–1000. doi: 10.1016/j.ejmech.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Talia JM, Debattista NB, Pappano NB. New antimicrobial combinations: substituted chalcones-oxacillin against methicillin resistant Staphylococcus aureus. Braz J Microbiol. 2011;42:470–5. doi: 10.1590/S1517-838220110002000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sato M, Tsuchiya H, Miyazaki T, Fujiwara S, Yamaguchi R, Kureshiro H, et al. Antibacterial activity of hydroxychalcone against methicillin-resistant Staphylococcus aureus. Int J Antimicrob Agents. 1996;6:227–31. doi: 10.1016/0924-8579(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 27.Williamson EM. Synergy and other interactions in phytomedicines. Phytomedicine. 2001;8:401–9. doi: 10.1078/0944-7113-00060. [DOI] [PubMed] [Google Scholar]
- 28.Hemaiswarya S, Kruthiventi AK, Doble M. Synergism between natural products and antibiotics against infectious diseases. Phytomedicine. 2008;15:639–52. doi: 10.1016/j.phymed.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 29.Tran TD, Nguyen TT, Do TH, Huynh TN, Tran CD, Thai KM. Synthesis and antibacterial activity of some heterocyclic chalcone analogues alone and in combination with antibiotics. Molecules. 2012;17:6684–96. doi: 10.3390/molecules17066684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bernal P, Zloh M, Taylor PW. Disruption of D-alanyl esterification of Staphylococcus aureus cell wall teichoic acid by the β-lactam resistance modifier (-)-epicatechin gallate. J Antimicrob Chemother. 2009;63:1156–62. doi: 10.1093/jac/dkp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernal P, Lamaire S, Pinho MG, Mobashery S, Hinds J, Taylor PW. Insertion of epicatechin gallate into the cytoplasmic membrane of methicillin-resistant Staphylococcus aureus disrupts penicillin-binding protein (PBP) 2a-mediated β-lactam resistance by delocalizing PBP2. J Biol Chem. 2010;285:24055–65. doi: 10.1074/jbc.M110.114793. [DOI] [PMC free article] [PubMed] [Google Scholar]


