Abstract
Background & objectives:
Diabetes is a metabolic pro-inflammatory disorder characterized by chronic hyperglycaemia and increased levels of circulating cytokines suggesting a causal role for inflammation in its aetiology. In order to decipher the role of interleukin-6 (IL-6) in type 2 diabetes mellitus (T2DM) we analyzed two promoter polymorphisms -597 A/G (rs1800797) and -174 G/C (rs1800795) in T2DM cases from north India, and in healthy controls.
Methods:
DNA was isolated from venous blood samples of T2DM patients (n=213) and normal healthy controls (n=145). Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) was performed after biochemical analysis. The genotypic and allelic frequency distributions were analyzed.
Results:
The clinical/biochemical parameters of T2DM cases when compared to controls showed a significant difference. No significant association was observed with -597A/G polymorphism while, -174 G/C showed a highly significant association (P<0.001). In haplotypic analysis, combination of -597G*/-174C* showed significant association (P=0.010).
Interpretation & conclusions:
Our data suggest that IL-6 gene polymorphisms play a prominent role in T2DM disease susceptibility in population from north India.
Keywords: Gene polymorphism, interleukin-6, PCR-RFLP, SNP, T2DM
Type 2 diabetes mellitus (T2DM) accounts for 90 per cent of diabetes cases worldwide. Over the past 30 years, the status of diabetes has changed from being considered as a mild disorder of old age to one of the major causes of morbidity and mortality affecting the young and middle-aged people. It has been estimated that in India 101.3 million people would be suffering from diabetes by 20301.
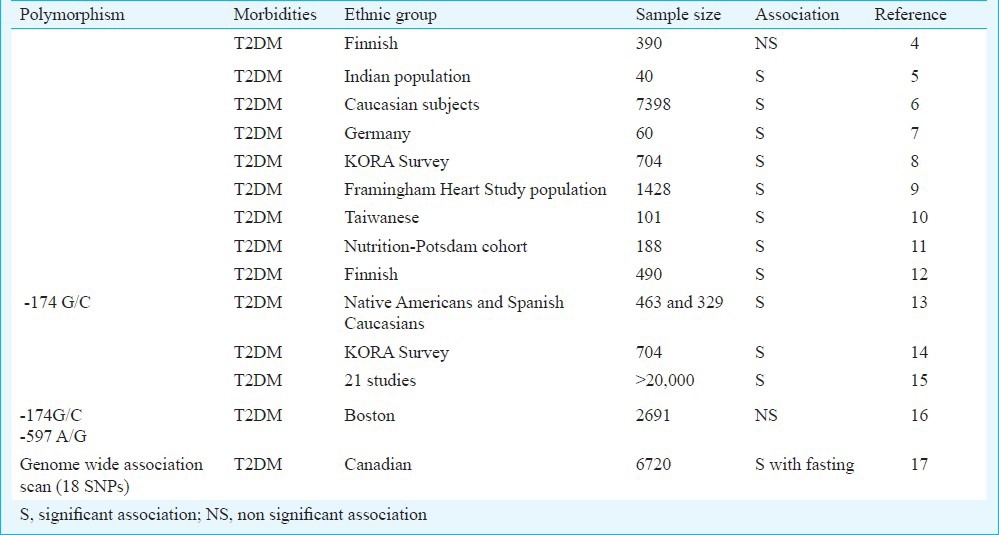
In recent years, evidences have shown that T2DM is associated with a subclinical systemic inflammation attributable to the dysregulation of innate immune system2. This immune response is characterized by elevated blood levels of certain acute phase markers, the principal mediator being interleukin-6(IL-6)2. Augmented levels of IL-6 are associated not only with T2DM but also with impaired glucose tolerance (IGT), indicating a potential role of this cytokine in its aetiology3. A high rate of plasma clearance of IL-6 suggested that concentration is regulated at the levels of transcription and translation4. Studies on single nucleotide polymorphisms (SNPs) in the promoter region of IL-6 gene in different populations worldwide suggested its possible role in T2DM susceptibility (Table I). Therefore, we attempted to analyze the association of two IL-6 promoter polymorphisms viz. -597 A/G (rs1800797) and -174 G/C (rs1800795) with T2DM patients from north India.
Table I.
Variants of IL-6 gene and their association with T2DM in different populations
Material & Methods
Patient selection and clinical evaluation: T2DM cases (n=213; 128 males; 85 females) with an age ranging from 22-76 yr were randomly enrolled from the outpatient Diabetes Clinic of King George's Medical University (KGMU), Lucknow, India, according to the National Diabetes Data Group (NDDG) and World Health Organization (WHO) inclusion-exclusion criteria18. Age/sex-matched normal controls (n=145; 96 males; 49 females) were screened from healthy staff members of both KGMU and University of Lucknow during January 2011 to January 2012. Sample size was calculated using QUANTO software, USA using 2 tailed analysis (Power: 90; Disease prevalence: 3%; error: 5%). The study was approved by the Institutional Ethical Committee of KGMU and a written informed consent was taken from all subjects enrolled in the study. Plasma glucose (Fasting blood sugar; FBS and Post-prandial; PPBS) (mg/dl) and lipid profile viz. total cholesterol (TC), triglycerides (TGL), high density lipoproteins (HDL) and serum creatinine (SCRT) were estimated using commercially available Ecoline kits (Merck, Germany) by UV-Vis spectrophotometer followed by low density lipoproteins (LDL) and very low density lipoproteins (VLDL) LDL and VLDL were calculated by known formulae. Height, weight and waist circumference were measured to calculate body mass index (BMI) and waist hip ratio (WHR).
DNA extraction and genotyping by PCR-RFLP: Blood samples (5 ml) were collected in 0.5M ethylene diamine tetra acetic acid (EDTA) and genomic DNA was extracted using the salting out method with slight modifications19,20. DNA was amplified with primers specific for -597 A/G (rs1800797) and -174 G/C (rs1800795) (Fig. 1) in a 15μl reaction mixture containing 100 ng of template DNA, buffer (100 mM Tris, pH 9.0; 500 mM KCl; 15 mM MgCl2; 0.1% gelatin), 200μM deoxynucleotide triphosphate (dNTP), 10pmol of each primer (Integrated DNA Technologies, USA) and 1.5 units Taq DNA polymerase (Banglore Genei, India). PCR for -597 A/G (rs1800797) was performed in 35 cycles (95°C for 30 sec; 61.5°C for 40 sec and 72°C for 50 sec) and -174 G/C (rs1800795) in 44 cycles (95°C for 30 sec; 57°C for 30 sec and 72°C for 60 sec) with an initial denaturation at 95°C for 5 min and a final extension at 72°C for 10 min. The PCR products were digested with respective restriction enzymes (FokI and NlaIII, Fermentas, USA) and electrophoresed on 12 per cent polyacrylamide gel. To ensure the quality of genotyping, random duplicates were performed in 20 per cent of the samples and 98 per cent concordance was found.
Fig. 1.
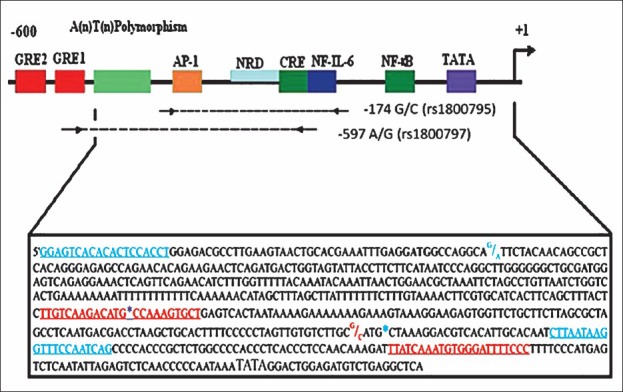
Schematic representation of promoter region of IL-6 gene from -600 to +1. Nucleotide sequence shown in text box, the 5’ and 3’ primers for -597A/G SNP are shown in blue while those for -174G/C are shown in red. GATG, FokI recognition site; *, Nla III restriction site; AP-1, activator protein-1; NRD, negative regulatory domain; CRE, cAMP responsive element; GRE, glucocorticoid responsive element; TATA, TATA box; NF-kB, nuclear factor - kB.
Statistical analysis: Allele frequencies and carriage rates were compared in controls and T2DM patients using 2×2 contingency table. Genotype frequencies were compared using 2×3 contingency table by Fisher's exact test. Carriage reates were calculated to know the inheritance frequency of individual alleles involved in disease manifestation. The Hardy-Weinberg equilibrium at individual locus was assessed by chi-square (χ2) statistics using SPSS (v15.0) Inc., USA. Student t-test and multivariate logistic regression analysis were performed for anthropometric and biochemical parameters of each genotype. Bonferroni correction was carried out for multiple testing camparisons. Haplotypes were analyzed by SHEsis software21.
Results
The association between clinical/biochemical parameters of controls and T2DM patients showed a significant difference in FBS, PPBS, TC, TGL, HDL, LDL and VLDL (P<0.001) (Table II). The IL-6 -597 A/G (rs1800797) and -174 G/C (rs1800795) polymorphisms were successfully genotyped in 213 T2DM cases and 145 healthy controls. Results of genotypic patterns by PCR-RFLP are shown in Fig. 2. The allele and genotype frequency distributions and carriage rates of both polymorphisms are shown in Table III. All frequencies were found to be in Hardy-Weinberg equilibrium (HWE).
Table II.
Clinical characteristics of controls and T2DM cases
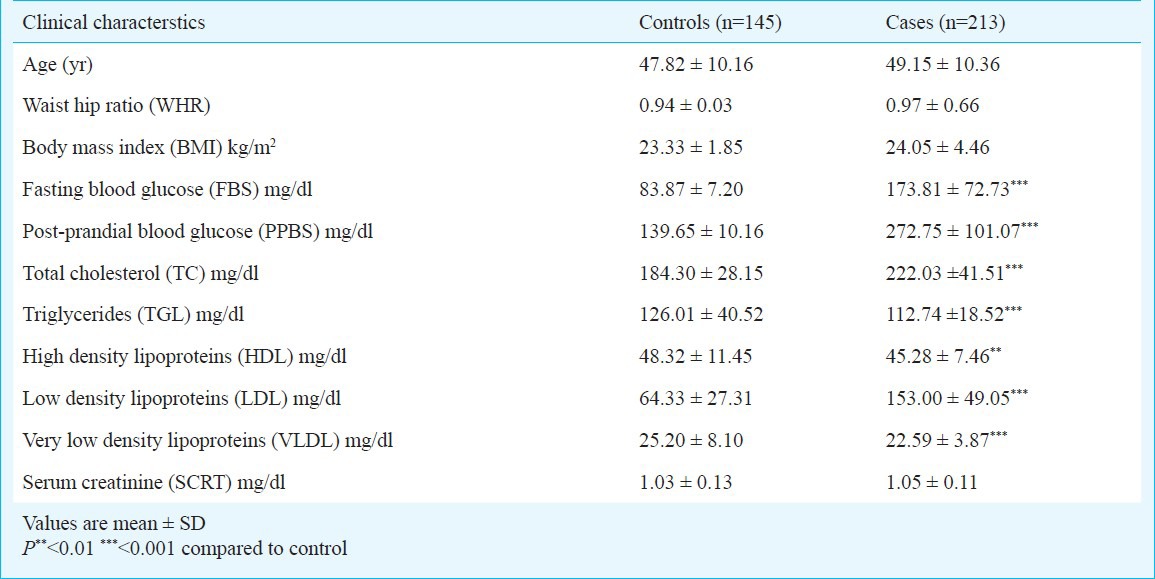
Fig. 2.
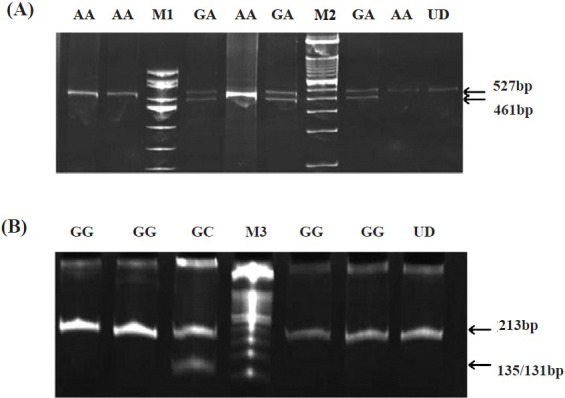
Polyacrylamide gel (12%) photographs of PCR-RFLP products (amplicons digested with restriction enzymes) depicting the genotypes of IL-6 polymorphisms. A. PCR products with IL-6 -597 A/G SNP digested with FokI showing the genotypes AA (527 bp) and GA (527 and 461 bps). B PCR products with IL-6-174 G/C SNP digested with NlaIII showing the genotypes GG (213 bp) and GC (213 and 135/131 bps). M1, ΦX174/HinfI digest; M2, 100bp Ladder; M3, 50bp Ladder; UD, Undigested.
Table III.
Distribution of genotype, allele frequencies and carriage rates of IL-6 -597 A/G and -174 G/C polymorphisms among controls and T2DM cases
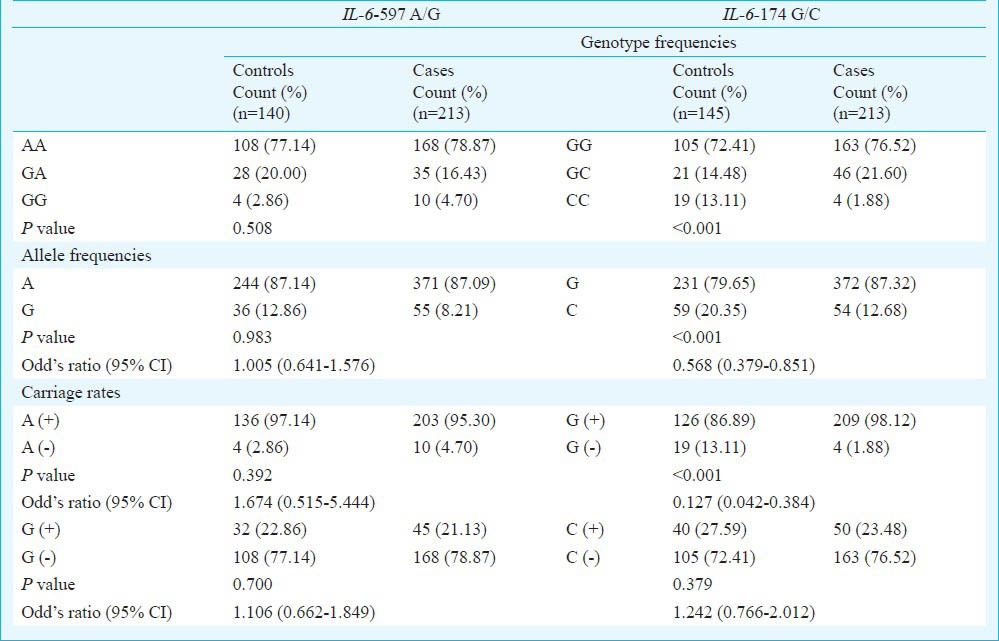
In case of IL-6 -597 A/G (rs1800797), no genotypic and allelic association was observed in the present study. Genotype GG of -597 A/G was rare in both controls and T2DM cases (2.86 and 4.70 %, respectively) while, GA accounted for 20.00 and 16.43 per cent in controls and T2DM cases, respectively. Individuals with AA genotype were major in our population. No association was observed in carriage rates of ‘A’ and ‘G’ alleles in controls and T2DM cases (Table III).
IL-6 -174 G/C (rs1800795) polymorphism showed significant genotypic and allelic associations (P<0.001; 0.006) while, CC genotype was rare and GG was most prevalent in our population. GC genotype was present in 14.48 per cent controls and 21.60 per cent T2DM cases (Table III). The carriage rate of ‘G’ showed significant association (P<0.001; OR 0.127; CI 0.042-0.384) with T2DM when compared to controls but ‘C’ allele showed no association (Table III).
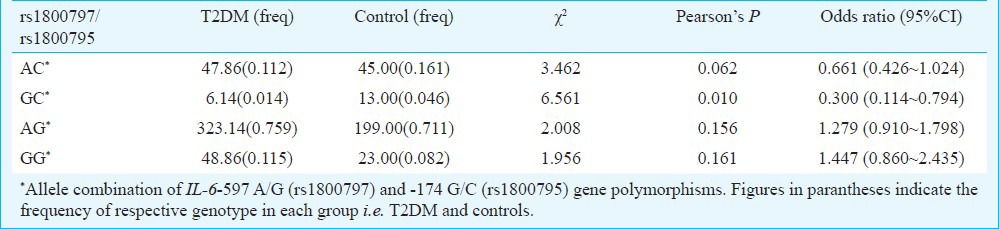
Analysis of genotypic associations with biochemical parameters using multivariate logistic regression showed that subjects with GG genotype had significant association with TC, TGL, LDL and VLDL (P=0.005, 0.021, <0.001 and 0.021 respectively). In case of ‘GA’ of -597 A/G polymorphism, significant association was observed with age, BMI, FBS, PPBS, TC, TGL, LDL and VLDL while AA showed significant association with all clinical parameters except age, BMI, WHR and SCRT (Table IV). None of the genotypes showed association with WHR and SCRT. GG genotype of IL-6-174 G/C polymorphism showed significant association with FBS, PPBS, TC, TGL, HDL, LDL and VLDL. While GC showed significant association with all biochemical parameters except total cholesterol and CC showed significant association with FBS, PPBS and LDL (Table IV). In haplotypic analysis, -597G* and -174C* allele combination showed significant association (P=0.010). The ‘D’ and r2 values were calculated in haplotypic analysis and found to be 0.070 and 0.004, respectively. Another important observation was that the combination of -597G* and -174G* alleles also showed upto 1.5 times increased risk of developing T2DM (Table V).
Table IV.
Association of anthropometric/biochemical parameters with IL-6 -597 A/G and -174 G/C genotypes
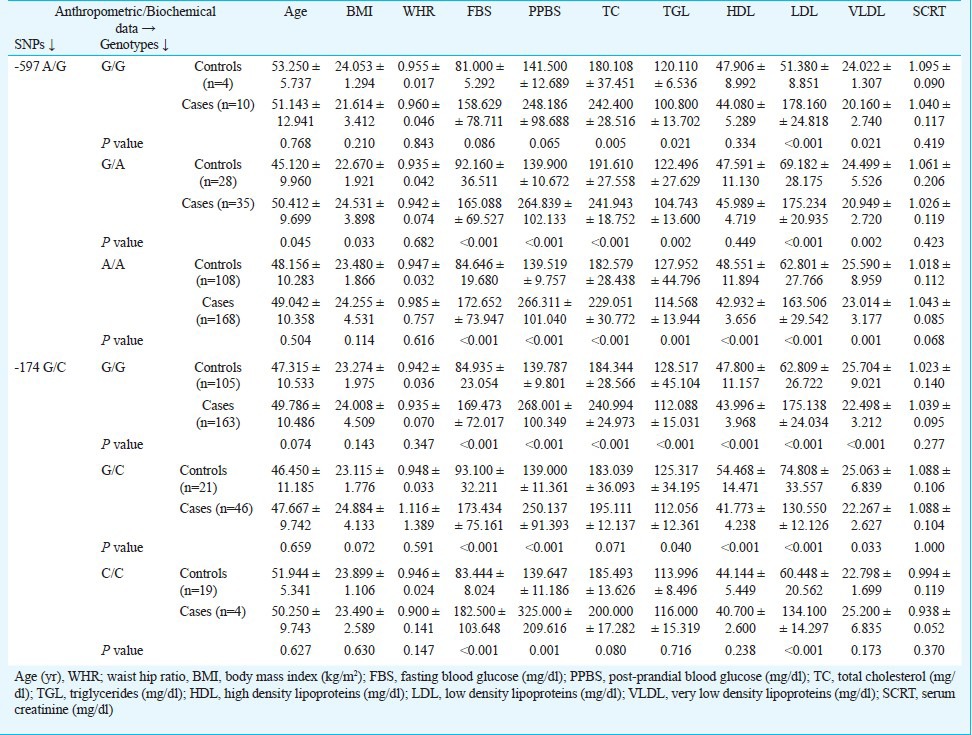
Table V.
Distribution of haplotypes of IL-6 genotypes in controls and T2DM cases
Discussion
Earlier studies on the association of -597 A/G (rs1800797) and -174 G/C (rs1800795) SNPs with T2DM and insulin resistance (IR) have demonstrated varied results among different populations. Analyses of small cohorts of native Americans and Spanish Caucasians showed the ‘G’ allele of -174 G/C SNP to be associated with higher risk of T2DM22, but this SNP was not linked with diabetes in the Finnish Diabetes Prevention Study (DPS)23. In another study, non diabetic subjects showed an association of IL-6-174 C/C genotype with higher insulin sensitivity14,24. However, neither this nor -597 A/G polymorphism was investigated in detail with respect to diabetes status, parameters of metabolic syndrome and subclinical systemic inflammation in comprehensive, population-based studies.
A Korean population was screened for IL-6 gene promoter polymorphisms viz. -174G/C, -572C/G, -597G/A and -1363G/T and correlated with serum concentrations of IL-6 and high-sensitivity C-reactive protein (hs-CRP)25. The study suggested that the IL-6 -572G/G genotype was associated with higher serum IL-6 and hs-CRP concentrations and increased risk for T2DM. A significant interaction has been identified between body mass index (BMI) and rs1800795 polymorphism of the IL-6 gene that influences both insulin resistance and onset of T2DM. The obese individuals homozygous for the ‘C’ allele demonstrated the highest level of IR and greatest risk of T2DM3. Moreover, FBS and PPBS showed significant association with all genotypes except GG of -597 A/G (rs1800797). However, genotypes of -597 A/G (rs1800797) showed significant associations with TC and only GG genotype of -174 G/C (rs1800795) showed significant association with TC. In case of HDL only AA of -597 A/G (rs1800797) and GG/GC showed significant association. GA genotype of -597 A/G (rs1800797) showed significant associations with age and BMI and no other genotypes of -174 G/C (rs1800795) and -597 A/G (rs1800797) showed any significant association with age & BMI. The similar negative association of WHR and SCRT was observed with genotypes of -597 A/G (rs1800797) and -174 G/C (rs1800795). The prominent finding in respect to biochemical parameters was that LDL showed significant association with all genotypes of both -597 A/G (rs1800797) and -174 G/C (rs1800795) gene polymorphisms while TGL and VLDL showed association with all genotypes except CC genotype of -174 G/C (rs1800795).
Genotyping in the present study showed that unlike -597 A/G, the -174 G/C SNP was significantly associated with T2DM in north Indian population. Our -174 G/C (rs1800795) results were in support of the metagenomic study in an Indian population26 which showed the prevalence of GG genotype. ‘C’ allele and CC genotype at -174 G/C SNP were found to be protective for diabetes as frequency of this genotype was more in control. Haplotype analysis showed that in combination, the -174C* with -597G* allele increased the risk and susceptibility of developing T2DM in the north Indian population. The results showed that the allele ‘G’ was involved in disease prevalence and manifestation. No significant association of genotypes, allele and carriage rates was observed in -597 A/G (rs1800797) polymorphism. However, in case of -174 G/C (rs1800795) genotypic and allelic frequencies showed significant association. The carriage rate of ‘A’ allele of -174 G/C (rs1800795) also showed significant association. However, it is important that the study groups are matched with respect to age, degree of obesity, glucose tolerance and sex distribution5,27.
The power for analyzing binary traits of IL-6 -597 A/G (rs1800797) polymorphism associated with T2DM in this study was the major limitation of our study. IL-6 -174 G/C (rs1800795) polymorphism has been studied in T2DM and other diseases as well as with pre and post disease complications in almost all ethnic groups and has shown controversial results. Moreover, till now only a few studies on -597 A/G (rs1800797) gene polymorphism have been reported and showed negative correlation with T2DM28.
Our data suggest that IL-6 gene polymorphisms play a prominent role in determining susceptibility to T2DM. The present study provides a lead to the contribution of cytokine gene heterogeneity to the susceptibility and development of T2DM but it is essential to find out the degree of association in an individual population.
Acknowledgment
The work was supported by research grants from the Indian Council of Medical Research and the Department of Science and Technology, New Delhi, India. The first author (MS) thanks Indian Council of Medical Research for Senior Research fellowship.
References
- 1.Anjana RM, Pradeepa R, Deepa M, Dutta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: Phase I results of the Indian Council of Medical Research–INdia DIABetes (ICMR–INDIAB) study. Diabetologia. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 2.Joshi SV, Tambwekar SR, Khadalia K, Dhar HL. Role of inflammatory marker Interleukin 6 (IL-6) and insulin in diabetes and diabetic neuropathy. Bombay Hosp J. 2008;50:466–71. [Google Scholar]
- 3.Underwood PC, Chamarthi B, Williams JS, Sun B, Vaidya A, Raby BA, et al. Replication and meta-analysis of the gene-environment interaction between body mass index and the interleukin-6 promoter polymorphism with higher insulin resistance. Metabolism. 2012;61:667–71. doi: 10.1016/j.metabol.2011.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mendoza-Carrera F, Ramirez-Lopez G, Ayala-Martinez NA, Garcia-Zapien AG, Flores-Martinez SE, Sanchez-Corona J, et al. Influence of CRP, IL-6, and TNF-α gene polymorphisms on circulating levels of C-reactive protein in Mexican adolescents. Arch Med Res. 2010;41:472–7. doi: 10.1016/j.arcmed.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 5.Popko K, Gorska E, Demkow U. Influence of interleukin-6 and G174C polymorphism in IL-6 gene on obesity and energy balance. Eur J Med Res. 2010;15(Suppl 2):123–7. doi: 10.1186/2047-783X-15-S2-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kilpeläinen TO, Laaksonen DE, Lakka TA, Herder C, Koenig W, Lindstrom J, et al. Finnish Diabetes Prevention Study. The rs1800629 polymorphism in the TNF gene interacts with physical activity on the changes in C-reactive protein levels in the Finnish Diabetes Prevention Study. Exp Clin Endocrinol Diabetes. 2010;118:757–9. doi: 10.1055/s-0030-1249686. [DOI] [PubMed] [Google Scholar]
- 7.Bouhaha R, Baroudi T, Ennafaa H, Vaillant E, Abid H, Sassi R, et al. Study of TNFalpha -308G/A and IL6 -174G/C polymorphisms in type 2 diabetes and obesity risk in the Tunisian population. Clin Biochem. 2010;43:549–52. doi: 10.1016/j.clinbiochem.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Rudofsky G, Jr, Schlotterer A, Reismann P, Engel J, Grafe IA, Tafel J, et al. The -174G>C IL-6 gene promoter polymorphism and diabetic microvascular complications. Horm Metab Res. 2009;41:308–13. doi: 10.1055/s-0028-1119373. [DOI] [PubMed] [Google Scholar]
- 9.Testa R, Olivieri F, Bonfigli AR, Sirolla C, Boemi M, Marchegiani F, et al. Interleukin-6-174 G > C polymorphism affects the association between IL-6 plasma levels and insulin resistance in type 2 diabetic patients. Diabetes Res Clin Pract. 2006;71:299–305. doi: 10.1016/j.diabres.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Herbert A, Liu C, Karamohamed S, Schiller J, Liu J, Yang Q, et al. The -174 IL-6 GG genotype is associated with a reduced risk of type 2 diabetes mellitus in a family sample from the National Heart, Lung and Blood Institute's Framingham Heart Study. Diabetologia. 2005;48:1492–5. doi: 10.1007/s00125-005-1830-3. [DOI] [PubMed] [Google Scholar]
- 11.Mostafazadeh A, Herder C, Haastert B, Hanifi-Moghaddam P, Schloot N, Koenig W, et al. KORA Group. Association of humoral immunity to human Hsp60 with the IL-6 gene polymorphism C-174G in patients with type 2 diabetes and controls. Horm Metab Res. 2005;37:257–63. doi: 10.1055/s-2005-861209. [DOI] [PubMed] [Google Scholar]
- 12.Chang YH, Huang CN, Shiau MY. The C-174G promoter polymorphism of the interleukin-6 (IL-6) gene that affects insulin sensitivity in Caucasians is not involved in the pathogenesis of Taiwanese type 2 diabetes mellitus. Eur Cytokine Netw. 2004;15:117–9. [PubMed] [Google Scholar]
- 13.Möhlig M, Boeing H, Spranger J, Osterhoff M, Kroke A, Fisher E, et al. Body mass index and C-174G interleukin-6 promoter polymorphism interact in predicting type 2 diabetes. J Clin Endocrinol Metab. 2004;89:1885–90. doi: 10.1210/jc.2003-031101. [DOI] [PubMed] [Google Scholar]
- 14.Fernández-Real JM, Broch M, Vendrell J, Gutierrez C, Casamitjana R, Pugeat M, et al. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49:517–20. doi: 10.2337/diabetes.49.3.517. [DOI] [PubMed] [Google Scholar]
- 15.Xiao LM, Yan YX, Xie CJ, Fan WH, Xuan DY, Wang CX, et al. Association among interleukin-6 gene polymorphism, diabetes and periodontitis in a Chinese population. Oral Dis. 2009;15:547–53. doi: 10.1111/j.1601-0825.2009.01584.x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Ma L, Peng F, Wu Y, Chen Y, Yu L, et al. The endothelial dysfunction in patients with type 2 diabetes mellitus is associated with IL-6 gene promoter polymorphism in Chinese population. Endocrine. 2011;40:124–9. doi: 10.1007/s12020-011-9442-9. [DOI] [PubMed] [Google Scholar]
- 17.Huth C, Heid IM, Vollmert C, Gieger C, Grallert H, Wolford JK, et al. IL6 gene promoter polymorphisms and type 2 diabetes: joint analysis of individual participants’ data from 21 studies. Diabetes. 2006;55:2915–21. doi: 10.2337/db06-0600. [DOI] [PubMed] [Google Scholar]
- 18.Modan M, Harris MI, Halkin H. Evaluation of WHO and NDDG criteria for impaired glucose tolerance: results from two national samples. Diabetes. 1989;38:1630–5. doi: 10.2337/diab.38.12.1630. [DOI] [PubMed] [Google Scholar]
- 19.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautam S, Agrawal CG, Bid HK, Banerjee M. Preliminary studies on CD36 gene in type 2 diabetic patients from north India. Indian J Med Res. 2011;134:107–12. [PMC free article] [PubMed] [Google Scholar]
- 21.Shi YY, He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res. 2005;15:97–8. doi: 10.1038/sj.cr.7290272. [DOI] [PubMed] [Google Scholar]
- 22.Vozarova B, Fernandez-Real JM, Knowler WC, Gallart L, Hanson RL, Gruber JD, et al. The interleukin-6 (-174) G/C promoter polymorphism is associated with type-2 diabetes mellitus in native Americans and Caucasians. Hum Genet. 2003;112:409–13. doi: 10.1007/s00439-003-0912-x. [DOI] [PubMed] [Google Scholar]
- 23.Kubaszek A, Pihlajamaki J, Komarovski V, Lindi V, Lindstrom J, Eriksson J, et al. Finnish Diabetes Prevention Study. Promoter polymorphisms of the TNF-α (G-308A) and IL-6 (C-174G) genes predict the conversion from impaired glucose tolerance to type 2 diabetes : the Finnish Diabetes Prevention Study. Diabetes. 2003;52:1872–6. doi: 10.2337/diabetes.52.7.1872. [DOI] [PubMed] [Google Scholar]
- 24.Kubaszek A, Pihlajamaki J, Punnonen K, Karhapaa P, Vauhkonen I, Laakso M. The C-174G promoter polymorphism of the IL-6 gene affects energy expenditure and insulin sensitivity. Diabetes. 2003;52:558–61. doi: 10.2337/diabetes.52.2.558. [DOI] [PubMed] [Google Scholar]
- 25.Koh SJ, Jang Y, Hyun YJ, Park JY, Song YD, Shin KK, et al. Interleukin-6 (IL-6) -572C/G promoter polymorphism is associated with type 2 diabetes risk in Koreans. Clin Endocrinol (Oxf) 2008;70:238–44. doi: 10.1111/j.1365-2265.2008.03315.x. [DOI] [PubMed] [Google Scholar]
- 26.Mukhopadhyaya PN, Acharya A, Chavan Y, Purohit SS, Mutha A. Metagenomic study of single-nucleotide polymorphism within candidate genes associated with type 2 diabetes in an Indian population. Genet Mol Res. 2010;9:2060–8. doi: 10.4238/vol9-4gmr883. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira AP, Ferreira CB, Souza VC, Furioso AC, Toledo JO, Moraes CF, Cordova C, et al. Risk of glycemic disorder in elderly women adjusted by anthropometric parameters and cytokine genotypes. Rev Assoc Med Bras. 2011;57:565–9. doi: 10.1590/s0104-42302011000500016. [DOI] [PubMed] [Google Scholar]
- 28.Qi L, van Dam RM, Meigs JB, Manson JE, Hunter D, Hu FB. Genetic variation in IL6 gene and type 2 diabetes: tagging-SNP haplotype analysis in large-scale case-control study and meta-analysis. Hum Mol Genet. 2006;15:1914–20. doi: 10.1093/hmg/ddl113. [DOI] [PubMed] [Google Scholar]


