Abstract
Background & objectives:
National Anti-retroviral treatment (ART) programme in India was launched in 2004. Since then, there has been no published country representative estimate of suboptimal adherence among people living with HIV (PLHIV) on first line ART in public settings. Hence a multicentric study was undertaken in 15 States of India to assess the level of suboptimal adherence and its determinants among PLHIV.
Methods:
Using a prospective observational study design, 3285 PLHIV were enrolled and followed up to six months across 30 ART centres in India. Adherence was assessed using pill count and self-reported recall method and determinants of suboptimal adherence were explored based on the responses to various issues as perceived by them.
Results:
Suboptimal adherence was found in 24.5 per cent PLHIV. Determinants of suboptimal adherence were illiteracy (OR-1.341, CI-1.080-1.665), on ART for less than 6 months (OR-1.540, CI- 1.280-1.853), male gender (OR for females -0.807, CI- 0.662-0.982), tribals (OR-2.246, CI-1.134-4.447), on efavirenz (EFA) regimen (OR- 1.479, CI - 1.190 - 1.837), presence of anxiety (OR- 1.375, CI - 1.117 - 1.692), non-disclosure of HIV status to family (OR- 1.549, CI - 1.176 - 2.039), not motivated for treatment (OR- 1.389, CI - 1.093 - 1.756), neglect from friends (OR-1.368, CI-1.069-1.751), frequent change of residence (OR- 3.373, CI - 2.659 - 4.278), travel expenses (OR- 1.364, CI - 1.138-1.649), not meeting the PLHIV volunteer/community care coordinator at the ART center (OR-1.639, CI-1.330-2.019).
Interpretation & conclusions:
To enhance identification of PLHIV vulnerable to suboptimal adherence, the existing checklist to identify the barriers to adherence in the National ART Guidelines needs to be updated based on the study findings. Quality of comprehensive adherence support services needs to be improved coupled with vigilant monitoring of adherence measurement.
Keywords: Antiretroviral therapy (ART), epidemiologic determinants, HIV, India, medication adherence, PLHIV
India is a home to 2.1 million PLHIV1 of whom 0.5 million were receiving free antiretroviral therapy (ART) until January 20122. Initiated in 2004, free ART is available through 355 fully functional ART centres and 725 Link ART Centres (LAC) in medical colleges, district hospitals and non-profit charitable institutions providing counselling, care, support and treatment services to PLHIV2. The PLHIV volunteer/community care coordinator at the ART centre along with community care centers /NGOs provides adherence support programmes like forming self-support groups or adherence clubs, providing home visits, undertaking therapeutic education programmes for drug adherence, nutrition and facilitating access to treatment and social support services. The National AIDS Control Organisation (NACO) guidelines (2007) recommended first-line ART regimen consisting of two nucleoside reverse transcriptase inhibitors (NRTIs) and one non-nucleoside reverse transcriptase inhibitor (NNRTIs) for patients with disease stage one and two, having CD4 counts less than 200 cells/μl, for stage 3 if CD4 counts less than 350 and for stage 4 irrespective of CD4 status3.
Optimal adherence is fundamental to reducing the likelihood of the emergence and spread of drug-resistant pathogens4. There is a need to optimize ART, especially when HIV/AIDS has transitioned into a chronically manageable disease. Optimization of adherence to ART is a shared responsibility of the programme, patient, health care providers, family and community5. The current National ART programme in India considers an optimum adherence level of ≥ 95 per cent3.
After introduction of the National ART programme in India, there have been no published country representative data in public settings that have holistically assessed prevalence of suboptimal adherence and its determinants. Hence a multicentric study was undertaken to bridge this knowledge gap that could help in improving the ART programme. Determining the factors associated with the suboptimal adherence at various levels could help the programme in identifying vulnerability, generating support for PLHIV on medication, improving the counselling component and quality of care at the centres.
The measurement of adherence to treatment has been a major challenge because of the subjective and private nature of pill taking behaviour of PLHIV4. Although there is no gold standard method for measuring ART adherence, numerous methodologies such as electronic devices i.e. Medical Event Monitoring System (MEMS), monitoring CD4/CD8 counts or viral loads and assessment of plasma concentrations of antiretroviral drugs have been used in research settings. However, these are not operationally feasible methods to assess adherence in resource poor settings and many times not accurate. Self-reported recall has been used in resource limited settings because it is feasible to use it in routine clinical practice. The pill count method might not be the best standard for assessment of adherence as it does not match with self-reporting by patients6. Hence, use of more than one ART adherence measures to capture more accurate information has been recommended7.
Material & Methods
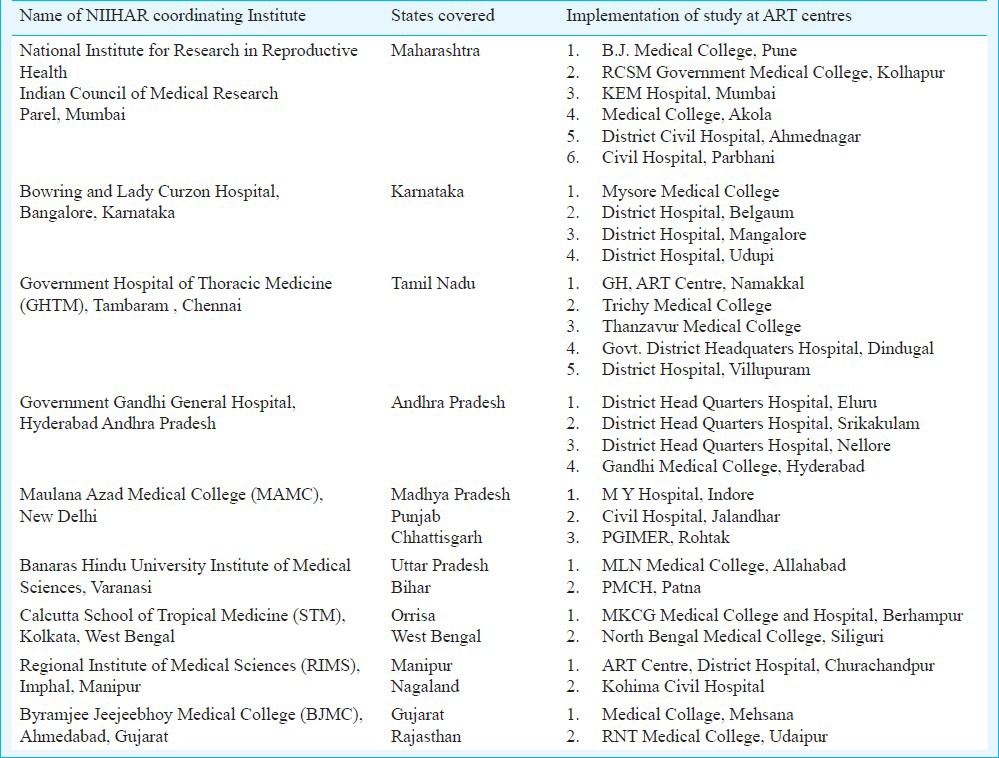
Study design: A six month prospective observational study of PLHIV on first line ART was undertaken between July 2009 and August 2010. The ART centres that were functional for at least one year with PLHIV load of 500 or more patients were considered in the sampling frame. As on September 2008, 179 ART centres were functioning, of which 114 fulfilled the above two criteria. Considering the geographical distribution and proportionate to number of ART centres in a State, 30 ART centres (13 from south, 4 from north, 7 from west, 2 from northeast, 2 from central part and 2 from east zone) representing 15 States of India were selected (Table I).
Table I.
Study sites in different States in India
Sample size: Sample size was calculated conservatively based on the average number of clients visiting the ART centre per month and the operational feasibility to have adequate numbers enrolled in one month. Thus, a total sample of 3300 was calculated assuming a 10 per cent dropout. Using a simple random sampling technique, 110 eligible PLHIV were enrolled at each centre consecutively.
Inclusion criteria: Both males and females above 18 yr, new (started ART for first time) and old (>6 months on ART) cases on first line ART and willing to participate in the study were included. All cases were enrolled within one month and followed up every month for six months when they came to the centre to collect their monthly drug stock. Each ART centre was designated to Network of Indian Institutes for HIV/AIDS Research (NIIHAR) in India (Table I). National Institute for Research in Reproductive Health (NIRRH), Mumbai, Maharashtra, India, was the nodal agency for the study.
The study protocol was approved by the Technical Review Group and Ethics Committee of NACO and NIRRH and discussed with the collaborating centres of excellence. All the study participants provided written informed consent. All data were treated confidential. Anonymous data were pooled and analysed centrally by the nodal agency.
Data collection and measurement
Factors explored: Various factors explored were broadly categorized as new or old case, personal, financial, socio-cultural, medical and psychological conditions, drug related factors (dosage, side effects, pill burden) and institutional factors which were categorised as (i) infrastructure, (ii) waiting time (iii) interaction with various service providers, (iv) quality of counselling, and (v) cordial environment at the centre. Questions included under infrastructure were related to the availability of drugs, communication aids, separate room for counselling and overcrowded OPDs. Waiting time was assessed at each level such as for registration, to consult the doctor, counsellor and overall time spent at ART. Interaction with doctor/ counsellor was a composite indicator derived by assessment of the comfort level felt by participants to discuss matters related to illness, perception that they listened to their problems and were available when they needed them and doctor had physically examined them. Meeting the PLHIV volunteer and nurse were single questions with Yes / No response. Quality of counselling was a composite indicator assessed on the type of counselling and adequacy of information provided. Issues were regarding one to one counselling, family counselled, pros and cons of taking treatment regularly, informing about PLHIV network and other referral services. Cordial environment at the centre included their experiences on maintenance of confidentiality, experience of stigma both from providers and other patients and guidance to services. Self-reported responses of the patients to all questions associated with adherence, as perceived by them, were recorded every month.
Tools: Eligible participants were interviewed using a semi-structured questionnaire (separate at enrolment and during every follow up) that was adapted from Adult AIDS Clinical Trials Group (AACTG)8. Psychological status for the past one month was assessed every month using questions adapted from Hospital Anxiety and Depression Scale9. To overcome recall, and social desirability bias10, a research team that was not a part of the ART service delivery was recruited and trained. Qualitative data were collected by conducting Focus Group Discussions (FGD) and Key Informant interviews to understand the system level barriers in greater details but this information was not included in the analysis for the present study.
Adherence measurement: Considering the feasibility, adherence measurement was done using a combination of methods, i.e. self-reported recall including time of pill consumption and pill count. The number of unused pills in the bottle was noted for each participant. If the patient came late or did not consume pills within the scheduled time (+2 h), then those numbers of pills were added to the number of remaining pills and a final number of missing pills was calculated. The missing pills were also cross checked with the self-reported recall. Every month, the triangulated value of drug intake was used to calculate percentage of drug adherence by dividing the total number of pills taken on time for the month, by total number of pills expected to be taken by the patient, multiplied by 100. An average estimate of adherence was obtained for the six months duration. Adherence was classified as optimal (≥95%) and suboptimal (<95%) as per the national ART guidelines3.
Statistical analysis: Reponses were elicited for each factor every month using a couple of questions that were dichotomous in nature (Yes and No). These responses were then given a weighted score (1 for unfavourable response and 0 for a favourable response) and an average score for six months was obtained by totalling the responses of each visit and dividing by number of visits (Minimum score - 0, Maximum score - 1). For example, if the patient felt confidentiality was not maintained that would be scored as 1 or if ART drugs were available that would be scored as zero. Cumulative values of time spent at each level were divided by the number of visits and then converted into quartiles. Value less than 25th percentile was scored as low (score 0) and above 75th percentile as high (score 3). The middle two quartiles were considered as intermediate (score 2). These scores for each category were then totalled and dichotomised into “satisfied” or “unsatisfied” considering the median value. Symptoms of anxiety and depression were assessed by asking a list of five questions in each category. Each question had grades to elicit severity of the problem (normal as 1, sometimes 2 and always 3). Cumulative scores were derived from the responses of each visit. An average score for six months was obtained by totalling the responses of each visit and dividing by number of visits (Min score - 5, Max score - 15). These were then dichotomised based on the median values.
Data were entered and analysed in SPSS version 16 (IBM, India). Frequencies and percentages were used for categorical data. Median and inter quartile range (IQR) were calculated for continuous variables. The magnitude of the association between ART adherence and selected independent variables was measured by odds ratio (OR) and their 95% confidence intervals (CI) obtained with simple logistic regression models. All variables that were significant at a P value of <0.05 were entered in univariate analysis. The step-up model was used for multivariate analysis.
Results
Overall, 3285 cases were enrolled in the study, of whom 2924 cases were considered for analysis. The remaining 361 participants who did not complete three follow up visits (derived by calculating median number of visits completed by the study population) were excluded from the study due to reasons such as: not willing to continue in the study (n=28), deaths (n=80), transferred to link ART centres (n=43), stopped treatment (n=11) and lost to follow up (n=199). The lost to follow up cases were categorized by the ART centres after following their routine tracing procedures. Table II shows the characteristics of HIV positive participants during enrolment. The median age of the participants was 36 yr (IQR 31-41 yr). About 20 per cent of the study population were illiterate, 64. 2 per cent belonged to rural areas. The median per capita income was 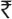 1000/ month (IQR-
1000/ month (IQR- 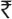 571-1600). During enrolment, 82 per cent of the participants were on nevirapine based regimen and 18 per cent on efavirenz (EFV) regimen. Among those on EFV regimen, 98 per cent were on treatment for tuberculosis (TB). Nearly half of the participants reported moderate to severe forms of psychological disturbance (both anxiety and depression). Disclosure of HIV status to family members was 90 per cent with spouse being reported as the main caretaker (52.7%).
571-1600). During enrolment, 82 per cent of the participants were on nevirapine based regimen and 18 per cent on efavirenz (EFV) regimen. Among those on EFV regimen, 98 per cent were on treatment for tuberculosis (TB). Nearly half of the participants reported moderate to severe forms of psychological disturbance (both anxiety and depression). Disclosure of HIV status to family members was 90 per cent with spouse being reported as the main caretaker (52.7%).
Table II.
Baseline characteristics of enrolled PLHIV (N=2924)
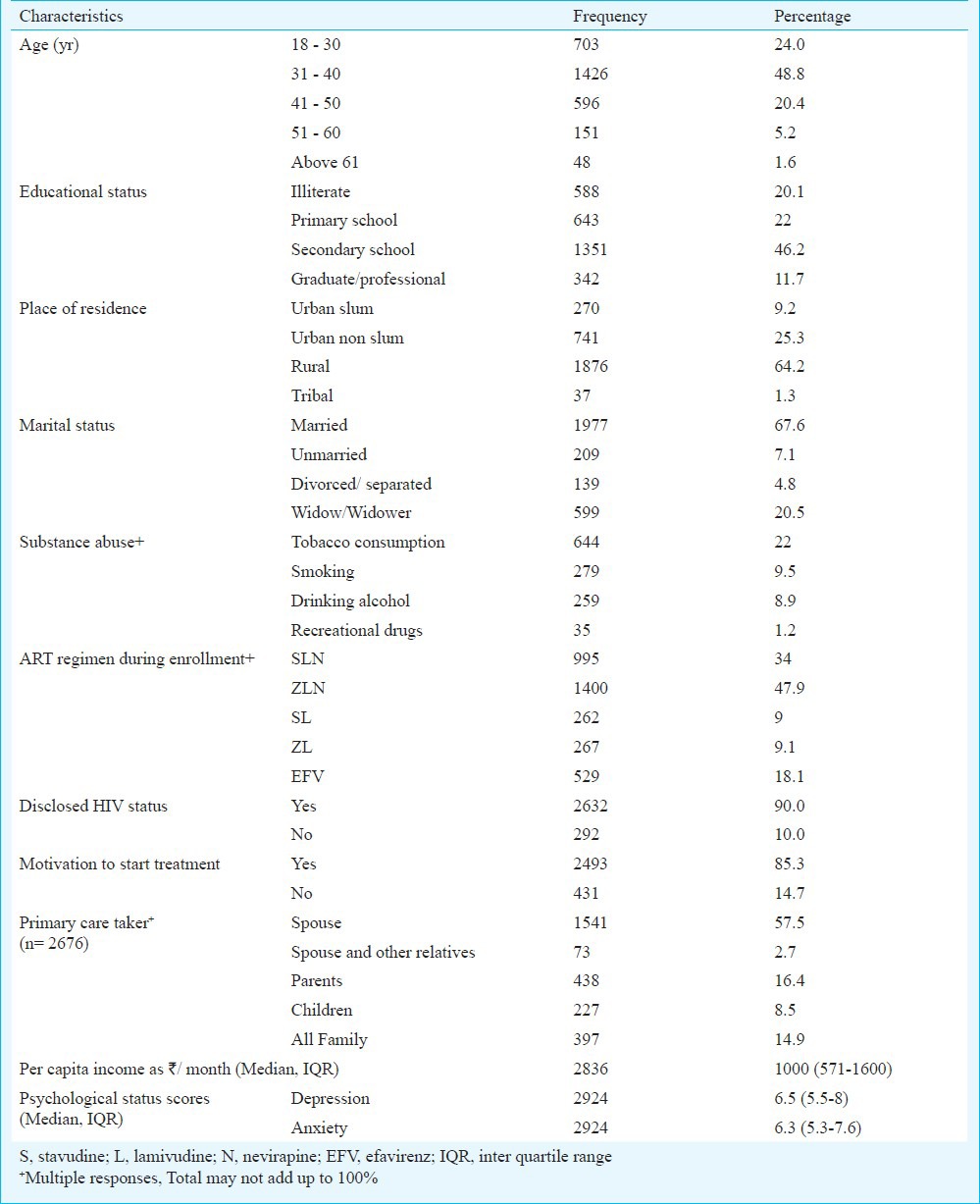
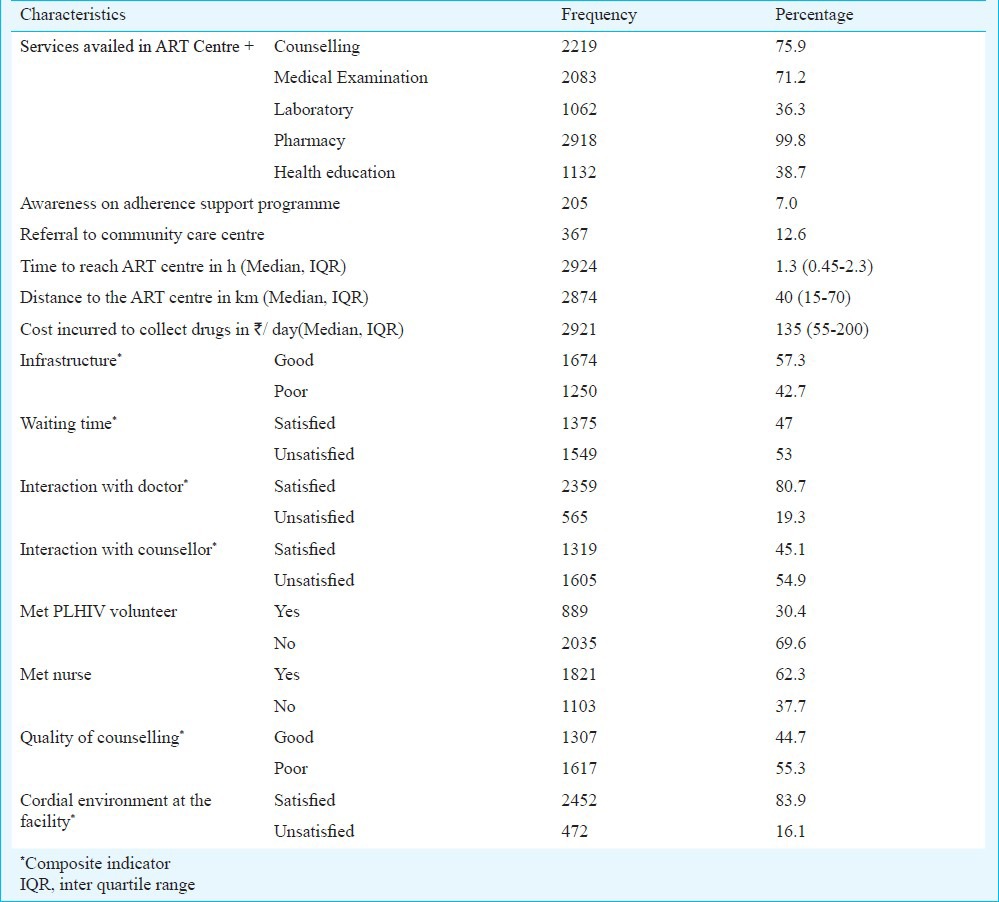
Perceptions and experiences of participants attending ART centres are presented in Table III. Median distance travelled to reach ART centre was 40 km (IQR 15-70) and median time taken was 1 h 30 min (IQR 0.45-2.3). The median cost incurred for a single visit to ART center was  135/day (IQR 55-200). The mean total time spent at the ART center was about 1 h 10 min. The mean waiting time to consult the doctor and counsellor was 27 and 25 min, respectively. However, the mean time spent with doctor and counsellor each was about 5 min. Only 7 per cent of the enrolled PLHIV were aware of adherence support programmes being held at the ART center and less than a quarter among them participated in these programmes. Satisfactory interaction with counsellor was resorted only by half of the participants. One quarter of the patients never met the counsellor and only collected drugs from pharmacist. Majority (84%) felt that the environment at the facility was comfortable. Overall only 4.1 per cent reported stigma and discrimination of which 1.6 per cent reported it to be from other patients in the hospital and 2 per cent from service providers of other referral departments, and 0.4 per cent from both groups. Availability of ART and drugs for opportunistic infections (OI) was 100 and 68 per cent, respectively.
135/day (IQR 55-200). The mean total time spent at the ART center was about 1 h 10 min. The mean waiting time to consult the doctor and counsellor was 27 and 25 min, respectively. However, the mean time spent with doctor and counsellor each was about 5 min. Only 7 per cent of the enrolled PLHIV were aware of adherence support programmes being held at the ART center and less than a quarter among them participated in these programmes. Satisfactory interaction with counsellor was resorted only by half of the participants. One quarter of the patients never met the counsellor and only collected drugs from pharmacist. Majority (84%) felt that the environment at the facility was comfortable. Overall only 4.1 per cent reported stigma and discrimination of which 1.6 per cent reported it to be from other patients in the hospital and 2 per cent from service providers of other referral departments, and 0.4 per cent from both groups. Availability of ART and drugs for opportunistic infections (OI) was 100 and 68 per cent, respectively.
Table III.
Perceptions and experiences of enrolled PLHIV at ART centres (N=2924)
The study data revealed an overall optimal adherence (>95%) of 75.5 per cent among PLHIV on first line ART. If the lost to follow up (LFU) cases are also considered, this estimate would further decline. However, CMIS reports submitted by most of these ART centres during the study period revealed an average adherence level of above 90 per cent. More number of old cases (81.1%) and female (78.7%) study participants reported optimal adherence compared to new cases and males. About 15.4 per cent of the participants did not take ART on scheduled time. Fifty seven per cent of the participants came late to collect drugs at least once during the study period with mean number of days missed being 2.3 (SD – 4.1). The major reasons for missing pills, as perceived by the participants were due to personal reasons, forgetting to take pills, irregularity in collecting drugs due to financial problems and difficulty to travel due to illness, inconvenience to carry medicines to work place and drug related factors (pill burden, size of tablets).
Univariate analysis (Table IV) revealed significant association (P<0.05) of suboptimal adherence with new cases, male PLHIV, illiterates, participants residing in tribal regions, those addicted to smoking, on EFV based regimen, those complaining of pill burden and symptoms due to drug toxicity. Psychological ill health, difficulty to visit ART centre to collect drugs due to OIs were health related factors significantly associated with suboptimal adherence. Non-disclosure of HIV status, less motivation for treatment, neglect from family, lack of support from friends, frequent change of residence due to work/personal reasons were some of the personal factors that were significantly associated with suboptimal adherence. Financial factors associated with suboptimal adherence were mainly related to travel expenses and cost of medication other than ART. Death in the family or any natural calamity, fasting and religious beliefs did not have any association with suboptimal adherence. Of the various institutional level factors, not meeting the PLHIV volunteer and absence of cordial environment at the ART centre were significantly (P<0.05) associated with suboptimal adherence.
Table IV.
Factors associated with suboptimal ART adherence among enrolled PLHIV - Univariate analysis
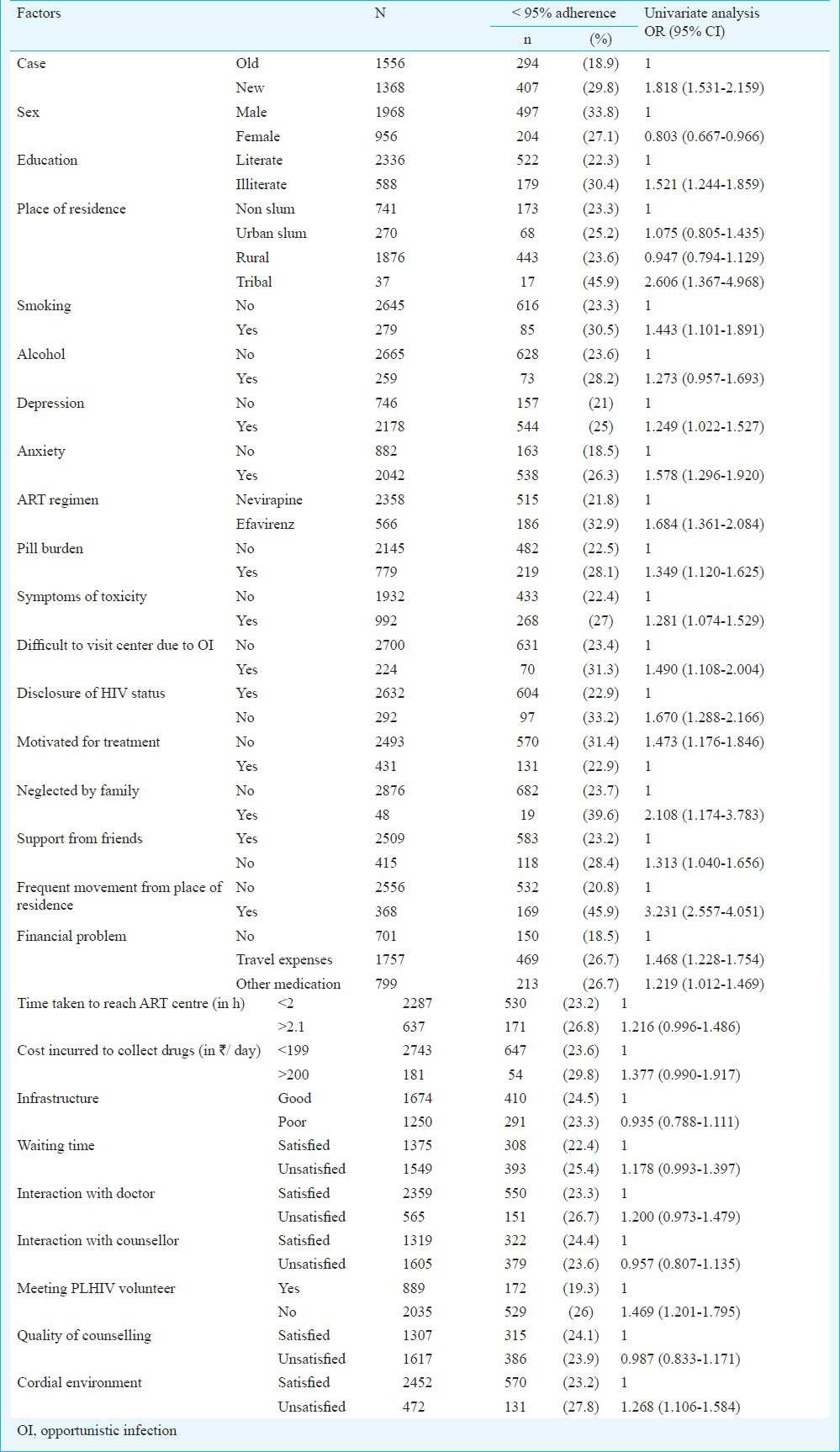
Table V.
Factors associated with suboptimal ART adherence among enrolled PLHIV - Multivariate analysis
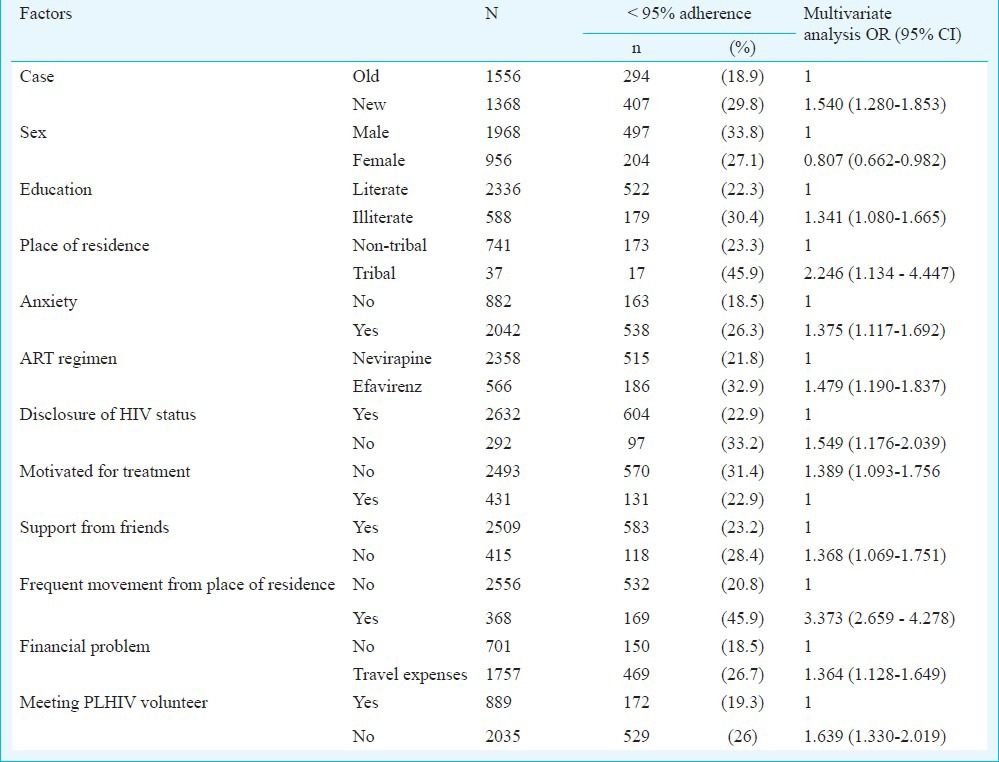
On multivariate analysis (Table IV), illiteracy (OR- 1.341, CI – 1.080 - 1.665), living in tribal region (OR-2.246, CI – 1.134-4.447) and male gender (OR for female gender -0.807, CI- 0.662-0.982) emerged as background predictors of suboptimal adherence. Treatment and health related predictors were, if one was on ART for less than 6 months i.e. new case (OR- 1.540, CI- 1.280 -1.853), on efavirenz regimen (OR- 1.479, CI - 1.190 - 1.837) and presence of anxiety (OR- 1.375, CI - 1.117 - 1.692). Social and behaviour related factors were non-disclosure of HIV status (OR- 1.549, CI - 1.176 - 2.039), not motivated for treatment (OR- 1.389, CI - 1.093 - 1.756) and poor support from friends (OR-1.368, CI -1.069-1.751). Access to treatment related factors were frequent change of residence (OR- 3.373, CI – 2.659-4.278) and travel expenses incurred to reach ART centres (OR- 1.364, CI - 1.128-1.649). Not meeting the PLHIV volunteer (OR-1.639, CI -1.330-2.019) was the sole institution related factor. Post hoc assessment of model fit (Hosmer and Lemeshow test, P=0.146) showed good fit of the mathematical model.
Discussion
This study generated an overall estimate of 76 per cent optimal adherence among PLHIV in India who were on free first line ART. This is quite encouraging given the large geographical coverage and diverse population of India catered by the ART programme. In comparison to international studies, the levels reported in the current study were superior to the pooled estimate of 55 per cent adherence from the North American studies, but similar to the 77 per cent pooled estimated adherence data in studies from Africa6. ART adherence estimate in this study was more reliable compared to the one time adherence levels quoted by various studies in India, either through interviews or through existing records11,12,13,14,15,16,17. In the present study, the estimate was a calculated average over six months, the information was elicited by an independent research team using assessment techniques which included both self-report for meaningful medication taking pattern10 coupled with pill count. Several regional studies in limited settings in India have estimated adherence rates ranging from 41 to 97.3 per cent using different assessment methods11,12,13,17,18,19.
Programme evaluation studies in countries including India have revealed missing the scheduled monthly appointments as an important barrier for optimal adherence5,7,18,20,21, and in our study 57 per cent came late to collect drugs at least once in six months and 15 per cent did not consume drugs on scheduled time. Various studies have explored barriers to optimal adherence such as social, economic, lifestyle factors20,22,23 knowledge related to general health, HIV24,25 and opportunistic infections (OIs), awareness regarding importance of ART adherence26, patient provider relationship27, high pill burden and inability to integrate the treatment regimen into patient's daily routine21.This study has further added on to the existing knowledge by identifying more intricate barriers in Indian settings at various levels.
Males and those residing in tribal areas had high vulnerability for suboptimal adherence in multi-variate analysis. Although male sex emerged as a significant determinant, it is important to recognize that gender and household dynamics prevent some women from successfully adhering to ART. As suggested by a few researchers, new ways need to be explored to identify couple-based strategies to increase adherence to ART28,29. Tribals, probably due to higher levels of illiteracy, coupled with frequent change of residence and long commuting distance to reach ART centres have been found to be more vulnerable to suboptimal adherence. Strategies to improve skills of health care providers at ART centres to effectively communicate to these groups and facilitate easy access to care needs further strengthening.
As reported in a number of studies12,17,19, drug toxicity faced by patients in the initial days of treatment contributes to suboptimal adherence among new cases more so if they were on EFA treatment. PLHIV on EFA regimen complained of increased pill burden as 98 per cent were on TB treatment resulting in sub-optimal adherence in the current study. Anxiety and lack of self-motivation to treatment contributing to suboptimal adherence justifies the importance of social support that PLHIV on treatment need. This support is possible only when one discloses the positive status. It was noted that people who disclosed their status had better adherence. Support from friends played a crucial role in improving adherence, re-emphasising the role of positive social environment beyond families. These findings were consistent with studies reported in a systematic review on factors affecting ART adherence in Asian countries21. The role of support programmes is very crucial to encourage disclosure and seek support from family and friends. Hence all PLHIV must be referred to community care centres and linked with PLHIV networks.
Apart from socio-economic and drug related factors, this study also identified key institutional factors affecting ART adherence. Data revealed that, about a quarter of patients visited the centre and did not meet the counsellors but just collected the drugs from pharmacy and left. This calls for urgent attention. Meeting the PLHIV at the centre emerged as important factor that improved adherence among patients. PLHIV volunteers/community care coordinators are facilitators who interact with PLHIV during the group counselling sessions and also coordinate their linkages with community care centres and guidance to other services. Hence, there is a need to acknowledge the supportive role played by these volunteers at the centre to improve adherence. Nurse although a part of the ART centre staff has only a supportive role in managing the centre and is not trained in adherence counselling. Her skills could be used to counsel on adherence when managing patients at the ART centre or dispensing drugs in absence of pharmacist by imparting training on adherence counselling. Non-cordial environment at the centre appeared as an important variable associated with suboptimal adherence in univariate analysis. However, since only a fraction of PLHIV reported stigma and discrimination mainly from staff at referral departments, it probably did not stand out in the multivariate analysis. It is important to note that continued sensitization of all service providers must be an ongoing process to deliver quality services that include reduced waiting time, efficient patient provider interaction, maintaining confidentiality and absence of stigma discrimination of any kind.
Frequent change of residence and cost of travelling to the ART centre was a major barrier for optimal adherence. All the selected ART centers were located in urban tertiary care hospitals, and most patients were from the rural areas. Hence, to ensure long term treatment goals and good compliance, ART should be delivered close to where people reside. ART and drugs for OI are expected to be available free of cost for the PLHIV at all the centres. Although availability of drugs was not projected as the determinant of suboptimal adherence, it was important to note that drugs for OI were unavailable in one third of the facilities leading to the out of pocket expenses. Inspite of a dramatic decrease in the cost of HIV treatment through the scale-up of government programmes in India, other HIV associated treatment and care costs, coupled with low income and productivity can drain the already limited financial resources among low-income Indian households30.
The study had several limitations. Although external investigators were employed to administer the questionnaire, the study was conducted within the premises of the ART centres which might have influenced their responses to certain questions like provider interactions and waiting time which were related to institutional level issues. Plasma viral load or ART drug level measurements to correlate with the adherence data or test drug resistance were not done because these tests were not standard of care in the current settings or were either unreliable or operationally not feasible. Although the study participants included a diverse population of HIV positive Indians from urban and rural locations, from varying socio-economic status, caution is warranted when extrapolating results to different sub population like intra venous drug users, commercial sex workers. Data from cases that were lost to follow-up could not be collected as there was no provision for research team to follow them up at their place of residence. Some LACs were made functional during the study period, leading to transfer of the patients from the study centre. With the limited time frame of the study, the impact of LACs to address the problem of long distance and associated expenses among PLHIV could not be studied. The standard of care at the ART centres which were assumed to be homogeneous was different at some places. The reasons for discrepancy in the adherence figures noted in the study and the CMIS of the respective centres were not explored.
In view of these findings, National ART programme needs to identify determinants of suboptimal adherence that are amicable for programme improvement. Based on the study findings, the existing checklist to identify barriers to adherence in the national ART guidelines3 needs to be updated, to include new cases, on EFV regimen, frequent change of residence, institutional barriers such as poor interaction with community care coordinator/PLHIV volunteer. Vulnerable subjects who are more prone to have suboptimal adherence as listed in the study must be identified through a mandatory checklist depicted in the Patient Monitoring Card and extra effort must go into counselling them to enhance their adherence to treatment. Strategies are needed to improve quality of comprehensive adherence support services coupled with vigilant monitoring of adherence measurement to achieve optimal adherence to ART treatment.
Acknowledgment
Authors acknowledge the support extended by NACO, ICMR (NIRRH/M/24/12), the Centres of Excellence (NIIHAR), authorities and staff of ART centres where the study was conducted. Authors thank Shrimati Renuka Panchal and Shri Narsihma Rao, who assisted in data management and the entire research team involved in data collection and Dr Shahina Begum for expert suggestions in the statistical aspects of the study. The cooperation extended by PLHIV enrolled in the study, for sharing information and sparing time during participation in the study, is duly acknowledged.
References
- 1.Pandey A, Sahu D, Bakkali T, Reddy D, Venkatesh S, Kant S, et al. Estimate of HIV prevalence and number of people living with HIV in India 2008-2009. BMJ Open. 2012;2:1–16. doi: 10.1136/bmjopen-2012-000926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National AIDS Control Organization (NACO) New Delhi: NACO; 2012. National AIDS Control Programme, Phase III. State fact sheets. Available from: www.naco.gov.in>publications-factsheet . [Google Scholar]
- 3.National AIDS Control Organisation. Ministry of Health and Family Welfare, Government of India, Antiretroviral therapy guidelines for HIV-infected adults and adolescents including post-exposure prophylaxis. 2007. [accessed on April 30, 2013]. Available from: http://www.nacoonline.org .
- 4.Steel G, Nwokike J, Joshi M. Submitted to the U.S. Agency for International Development by the Rational Pharmaceutical Management Plus Program. Arlington, VA: Management Sciences for Health; 2007. Development of a multi-method tool to measure ART adherence in resource-constrained settings: the South Africa experience. Available from: www.pdf.usaidgov/pdf_docs/Pnadk153.pdf . [Google Scholar]
- 5.Bachani D, Garg R, Rewari BB, Hegg L, Rajasekaran S, Deshpande A, et al. Two-year treatment outcomes of patients enrolled in India's national first-line antiretroviral therapy programme. Natl Med J India. 2010;23:7–12. [PubMed] [Google Scholar]
- 6.Mills EJ, Nachega JB, Buchan I, Orbinski J, Attaran A, Singh S, et al. Adherence to antiretroviral therapy in sub-Saharan Africa and North America: a meta-analysis. JAMA. 2006;296:679–90. doi: 10.1001/jama.296.6.679. [DOI] [PubMed] [Google Scholar]
- 7.Vitolins MZ, Rand CS, Rapp SR, Ribisl PM, Sevick MA. Measuring adherence to behavioral and medical interventions. Control Clin Trials. 2000;21(Suppl 5):188S–94S. doi: 10.1016/s0197-2456(00)00077-5. [DOI] [PubMed] [Google Scholar]
- 8.Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–66. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 9.American Psychiatric Association. text revision. 4th ed. Washington DC: American Psychiatric Association; 2000. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- 10.Nieuwkerk PT, Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: a meta-analysis. J Acquir Immun Defic Syndr. 2005;38:445–8. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- 11.Shet A, DeCosta A, Heylen E, Shastri S, Chandy S, Ekstrand M. High rates of adherence and treatment success in a public and public-private HIV clinic in India: potential benefits of standardized national care delivery systems. BMC Health Ser Res. 2011;11:277. doi: 10.1186/1472-6963-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatesh KK, Srikrishnan AK, Mayer KH, Kumarasamy N, Raminani S, Thamburaj E, et al. Predictors of non adherence to highly active antiretroviral therapy among HIV-infected South Indians in clinical care: Implications for developing adherence interventions in resource-limited settings. AIDS Patient Care STDs. 2010;24:795–803. doi: 10.1089/apc.2010.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cauldbeck MB, O’ Connor C, O’Connor MB, Saunders JA, Rao B, Mallesh VG, et al. Adherence to anti-retroviral therapy among HIV patients in Bangalore, India. AIDS Res Ther. 2009;6:7. doi: 10.1186/1742-6405-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murri R, Ammassari A, De Luca A, Cingolani A, Marconi P, Wu AW, et al. Self-reported non adherence with antiretroviral drugs predicts persistent condition. HIV Clin Trials. 2001;2:323–9. doi: 10.1310/KDM0-RU5W-NVTW-N9MC. [DOI] [PubMed] [Google Scholar]
- 15.Rajasekaran S, Jeyaseelan L, Vijila S, Gomathi C, Raja K. Predictors of failure of first-line antiretroviral therapy in HIV-infected adults: Indian experience. AIDS. 2007;21(Suppl 4):S47–S53. doi: 10.1097/01.aids.0000279706.24428.78. [DOI] [PubMed] [Google Scholar]
- 16.Sogarwal R, Bachani D. Assessment of ART centres in India: client perspectives. J Indian Med Assoc. 2009;107:276–80. [PubMed] [Google Scholar]
- 17.Shah B, Walshe L, Saple DG, Mehta SH, Ramnani JP, Kharkar RD, et al. Adherence to antiretroviral therapy and virologic suppression among HIV-infected persons receiving care in private clinics in Mumbai, India. Clin Infect Dis. 2007;44:1235–44. doi: 10.1086/513429. [DOI] [PubMed] [Google Scholar]
- 18.Sharma M, Singh RR, Laishram P, Kumar B, Nanao H, Sharma C, et al. Access, adherence, quality and impact of ARV provision to current and ex-injecting drug users in Manipur (India): an initial assessment. Int J Drug Policy. 2007;18:319–25. doi: 10.1016/j.drugpo.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Safren S, Kumarasamy N, James R, Raminani S, Solomon S, Mayer KH. ART adherence, demographic variables and CD4 outcome among HIV-positive patients on antiretroviral therapy in Chennai, India. AIDS Care. 2005;17:853–62. doi: 10.1080/09540120500038439. [DOI] [PubMed] [Google Scholar]
- 20.Sarna A, Pujari S, Sengar AK, Garg R, Gupta I, Dam J. Adherence to antiretroviral therapy & its determinants amongst HIV patients in India. Indian J Med Res. 2008;127:28–36. [PubMed] [Google Scholar]
- 21.Wasti SP, van Teijlingen E, Simkhada P, Randall J, Baxter S, Kirkpatrick P, et al. Factors influencing adherence to antiretroviral treatment in Asian developing countries: a systematic review. Trop Med Int Health. 2012;17:71–81. doi: 10.1111/j.1365-3156.2011.02888.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumarasamy N, Safren SA, Raminani SR, Pickard R, James R, Krishnan AK, et al. Barriers and facilitators to antiretroviral medication adherence among patients with HIV in Chennai, India: a qualitative study. AIDS Patient Care STDs. 2005;19:526–37. doi: 10.1089/apc.2005.19.526. [DOI] [PubMed] [Google Scholar]
- 23.Pujari SN, Patel AK, Naik E, Patel KK, Dravid A, Patel JK, et al. Effectiveness of generic fixed-dose combinations of highly active antiretroviral therapy for treatment of HIV infection in India. J Acquir Immune Defic Syndr. 2004;37:1566–9. doi: 10.1097/00126334-200412150-00005. [DOI] [PubMed] [Google Scholar]
- 24.Weiss L, French T, Finkelstein R, Waters M, Mukherjee R, Agins B. HIV-related knowledge and adherence to HAART. AIDS Care STDs. 2003;15:673–9. doi: 10.1080/09540120310001595159. [DOI] [PubMed] [Google Scholar]
- 25.van Servellen G, Nyamathi A, Carpio F, Pearce D, Garcia-Teague L, Herrera G, et al. Effects of a treatment adherence enhancement program on health literacy, patient-provider relationships and adherence to HAART among low-income HIV positive Spanish speaking Latinos. AIDS Patient Care STDs. 2005;19:745–59. doi: 10.1089/apc.2005.19.745. [DOI] [PubMed] [Google Scholar]
- 26.Mannheimer S, Friedland G, Matts J, Child C, Chesney M. The consistency of adherence to antiretroviral therapy predicts biologic outcomes for human immunodeficiency virus-infected persons in clinical trials. Clin Infect Dis. 2002;34:1115–21. doi: 10.1086/339074. [DOI] [PubMed] [Google Scholar]
- 27.Ickovics JR, Meade CS. Adherence to antiretroviral therapy among patients with HIV: A critical link between behavioral and biomedical sciences. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):98–102. doi: 10.1097/00126334-200212153-00002. [DOI] [PubMed] [Google Scholar]
- 28.Skovdal M, Campbell C, Nyamukapa C, Gregson S. When masculinity interferes with women's treatment of HIV infection: a qualitative study about adherence to antiretroviral therapy in Zimbabwe. J Int AIDS Soc. 2011;9:14–29. doi: 10.1186/1758-2652-14-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remien RH, Stirratt MJ, Dolezal C, Dognin JS, Wagner GJ, Carballo-Dieguez A, et al. Couple-focused support to improve HIV medication adherence: a randomized controlled trial. AIDS. 2005;19:807–14. doi: 10.1097/01.aids.0000168975.44219.45. [DOI] [PubMed] [Google Scholar]
- 30.Kumarasamy N, Venkatesh KK, Mayer KH, Freedberg K. Financial burden of health services for people with HIV/AIDS in India. Indian J Med Res. 2007;126:509–17. [PMC free article] [PubMed] [Google Scholar]


