Abstract
Aim:
Neurocognitive tests can provide reliable endophenotypes for bipolar disorder (BD). The aim of this study was to compare the neurocognitive functioning of unaffected siblings of patients of bipolar affective disorder (BPAD) with that of patients with BD and a group of healthy controls.
Materials and Methods:
A total of 20 unaffected siblings of patients with BD-I, 20 patients of BD-I who were currently in remission and a group of 20 healthy control subjects were assessed for neurocognitive functions using Wisconsin Card Sorting Test, Brief Visuospatial Memory Test-Revised, Hopkins Verbal Learning Test-Revised and Wechsler Adult Intelligence Scale Digit Symbol Test.
Results:
Compared to healthy controls, unaffected siblings of patients with BD performed poorly on tests of verbal learning, but no significant differences were seen between the two groups for executive functions, visual learning and psychomotor speed, concentration and graphomotor abilities. Compared to unaffected siblings, patients with BD performed poorly on the tests of executive functions, visual memory, verbal memory, psychomotor speed, concentration, and graphomotor abilities.
Conclusion:
Verbal memory can serve as an endophenotype of bipolar disorder.
Keywords: Bipolar disorder, cognitive functions, endophenotype, unaffected siblings
INTRODUCTION
Endophenotypic markers are understood as those vulnerability factors, which are present as a trait in the asymptomatic phase of the illness in those with an illness, are heritable and are also present in the unaffected relatives.[1] Among the various endophenotypes, neurocognitive dysfunctions have received considerable attention as potential endophenotypic markers for bipolar disorder (BD) as these are seen in patients (during the euthymic phase) and in the first degree relatives. The various neurocognitive deficits in patients with BD include deficits in attention, processing speed, set shifting, verbal learning, and memory.[2,3,4] Data arising out of various studies and meta-analysis have also shown that unaffected siblings of patients with BD also have neurocognitive deficits.[5,6,7,8,9,10,11] Unaffected siblings of BD patients have been shown to have deficits in set shifting, attention, processing speed, and memory functions, a profile similar to that of patients with BDs.[8,9,10,11] However, the deficits may not be as marked in the unaffected siblings as in patients.[9,10] In terms of the strength of the findings, meta-analysis suggest that compared with the healthy controls, the neurocognitive deficits in the first degree relatives have a small effect size for executive functions,[5,7] verbal memory and sustained attention.[5] Medium effect size is reported for response inhibition.[7] However, data on neurocognitive deficits in unaffected siblings of BD is limited and there is a need to expand this data.
For a trait to be recognized as an endophenotype, there is a need for establishing the same across different ethnic groups. Occasional studies involving healthy siblings of patients with BD have been conducted in India;[12,13] however, these studies have compared the relatives of patients with BD with healthy controls only. In this background, this study aimed to compare the cognitive function of unaffected siblings of patients of BD with that of patients of BD and a group of healthy controls.
MATERIALS AND METHODS
Setting of the study and participants
This study was conducted at the outpatient clinic of Department of Psychiatry of Postgraduate Institute of Medical Education and Research, Chandigarh, a tertiary care hospital in North India. The study was approved by the Institutional Ethics Committee and all the participants were enrolled about obtaining written informed consent.
The study included three groups: The unaffected siblings of patients with BD, patients of BD and a healthy control group. All the groups comprised of 20 participants. The inclusion criteria for the participants in all the three groups were age between 18 and 60 years, being able to read and write Hindi and English, and being free from any significant medical comorbidity (involving the brain, raised blood pressure etc.) and auditory/visual impairment. The “unaffected siblings” group included subjects with a diagnosis of BD Type I in their first degree relative but themselves were never diagnosed to have a psychiatric disorder. The psychiatric disorders (except for nicotine dependence) in the siblings were ruled out on the basis of the history provided by the participants, patients and other family members were ever available. The “patient” group included those with a diagnosis of BD Type I according to DSM-IV criteria, and currently in euthymic phase (Hamilton Depression Rating Scale [HDRS] score <8 and Young Mania Rating Scale [YMRS] score <6), were not taking benzodiazepines during the day time and free from any other comorbid psychiatric disorder apart from nicotine dependence. The “healthy controls” comprised of those who did not have any first or second degree relative suffering from any psychiatric disorder and they were not suffering from any psychiatric disorder apart from nicotine dependence. This was established on the basis of the history provided by the participants and their relatives.
Instruments
Hamilton Depression Rating Scale[14] is a widely used scale for assessment of depression. This observer rated scale, consists of 17 items and scores can range from 0 to 50. Depression is considered to be absent if scores are below 8.
Young Mania Rating Scale[15] is a scale used to measure the severity of mania. The scores can range from 0 to 60 and a total score <6 is considered to be indicative of euthymic range.
The neurocognitive battery included the following tests Wisconsin card sorting test[16]
This test is a measure of executive function that requires planning, use of feedback and cognitive set shifting. The computerized version-4 of the test was used for the study. In this test, subjects are required to match a card that appears on the bottom part of the computer screen to one of four stimulus cards that are present on the upper part of the computer screen. The stimulus cards have four different designs, that is, the first has a red circle, the second has two green stars, the third has three yellow crosses, the forth has four blue circles. The cards that subject are required to match to one of four stimulus cards vary in color, geometric form and number. Subjects receive feedback each time on correct or incorrect performances. There is no time limit for this test.
Brief visuospatial memory test-revised[17]
In this test, a subject is presented with 8½ inches by 11 inches card with six simple design in a 2 by 3 matrix. The stimulus is shown for 10 s after which the subject is asked to reproduce as many designs in an accurate and correct location in a blank sheet of paper. The subjects are asked to reproduce the design on three trials and after a 25 min delay. This is followed by a recognition trial in which 12 designs are shown again, one at a time (6 from the original design) and the subject is asked whether the design was present in the original matrix. The test measures immediate recall, learning rate, delayed recall as well as recognition for visuospatial information.
Hopkins verbal learning test-revised[18]
This test assesses the short-term verbal learning and memory performance. The test includes three learning trials for a set of words each followed by a recall and then followed by a delayed recall after 20 min delay. This is followed by a “yes” or “no” recognition trial, in which the subject is asked to identify the words from a list of words which also include the initially read out words. The list in this recognition trial is also read out to the subject.
Wechsler adult intelligence scale digit symbol test[19]
This test measures psychomotor speed, concentration and graphomotor abilities. This test requires the subject to match symbols to numbers as quickly as possible using a visual reference. The score is based upon the number of symbols drawn in 120 s duration.
Procedure
The siblings of patients with BD-I were recruited among the caregivers who were attending the psychiatry outpatient clinic of the hospital and came along with patients of BD-I. Similarly, the patients with BD-I were recruited from the patients attending the psychiatry outpatients. The control group was recruited from the nongenetically related caregivers of the patients with BD and hospital staffs. All the three groups were recruited by purposive sampling. Initially the participants were evaluated on the selection criteria and those who fulfilled the selection criteria were enrolled. The three groups were matched on age and level of education. The psychiatric evaluations were done by a qualified psychiatrist. The neurocognitive battery was administered by a clinical psychologists (RN, SS, AS), who have sufficient experience of using the tests. The neurocognitive testing was done between 10 am and 2 pm over 1-2 sitting, not more than 1 h apart. For the patients receiving benzodiazepines, it was ensured that they did not receive the same during the 12 h prior to the assessment on neurocognitive testing.
Statistical analysis
Statistical analysis was carried out using SPSS software version 14 (SPSS, Chicago [IL], US). Descriptive statistics in the form of frequencies, percentages, means, and standard deviations were used for studying the demographic, clinical and neurocognitive variables. The three groups were compared by using the Chi-square test for the categorical variables and analysis of variance for continuous variables. When the frequency of the number of cases for a particular variable was <5 in a cell, weighted cases was used to calculate the Chi-square value. To account for the confounders, analysis of covariance was used for computation of corrected F scores. Post-hoc test of Bonferroni was used for comparison of individual groups. Effect sizes of the differences in neurocognitive performance were calculated using partial η2 scores.
RESULTS
Sociodemographic and clinical profile
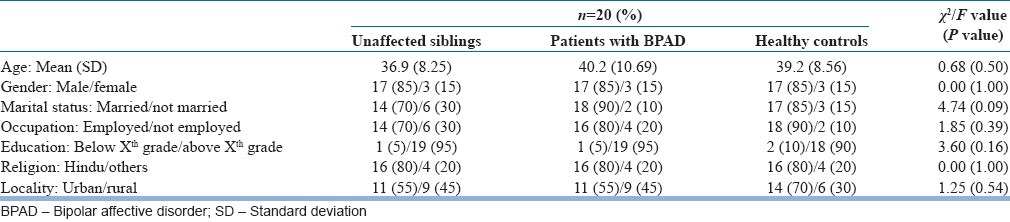
As the number of cases in some of the cells was <5, for the variables of gender, marital status, education, occupation and religion, weighted cases were taken to carry out the Chi-square test. As shown in Table 1, there was no significant difference among the three study groups with respect to various sociodemographic variables such as age, marital status, employment status, education level, religion and locality of living.
Table 1.
Demographic profile of participants
Clinical profile of the patient group
The mean duration of illness in patients with BD was 102 (standard deviation [SD]-70.8) months and the mean duration of treatment was 89.4 (SD-67.3) months. The mean HDRS score of the patient group was 2.4 (SD-1.90) with a median of 2. Similarly, the mean YMRS score was 1.00 (SD-1.12) with a median of 0.5. All patients of the BD were on medications, of them 35% were receiving valproate, 55% were receiving lithium carbonate and 10% were not on any mood stabilizers. Ten patients (50%) were receiving antipsychotics, 2 (10%) patients were on antidepressants and 4 (20%) patients were receiving benzodiazepines. Prior to neuropsychological assessment for the current study, the mean duration of euthymia prior to assessment was 16.5 (SD-19.25) months, the mean number of lifetime manic episodes were 2.85 (SD-1.38), mean number of lifetime depressive episodes were 1.50 (SD 1.23) and the mean number of mixed episodes were 0.50 (SD-0.22). Total number of lifetime episodes prior to assessment for the current study was 4.40 (SD-2.8).
Comparison of cognitive functioning of the three groups
As shown in Table 2, on Wisconsin Card Sorting Test (WCST), relatives of patients with BD performed significantly better than the patients in terms of WCST total score, WCST total number of errors, WCST nonperseverative errors and WCST conceptual level responses.
Table 2.
Comparison of findings of cognitive functioning
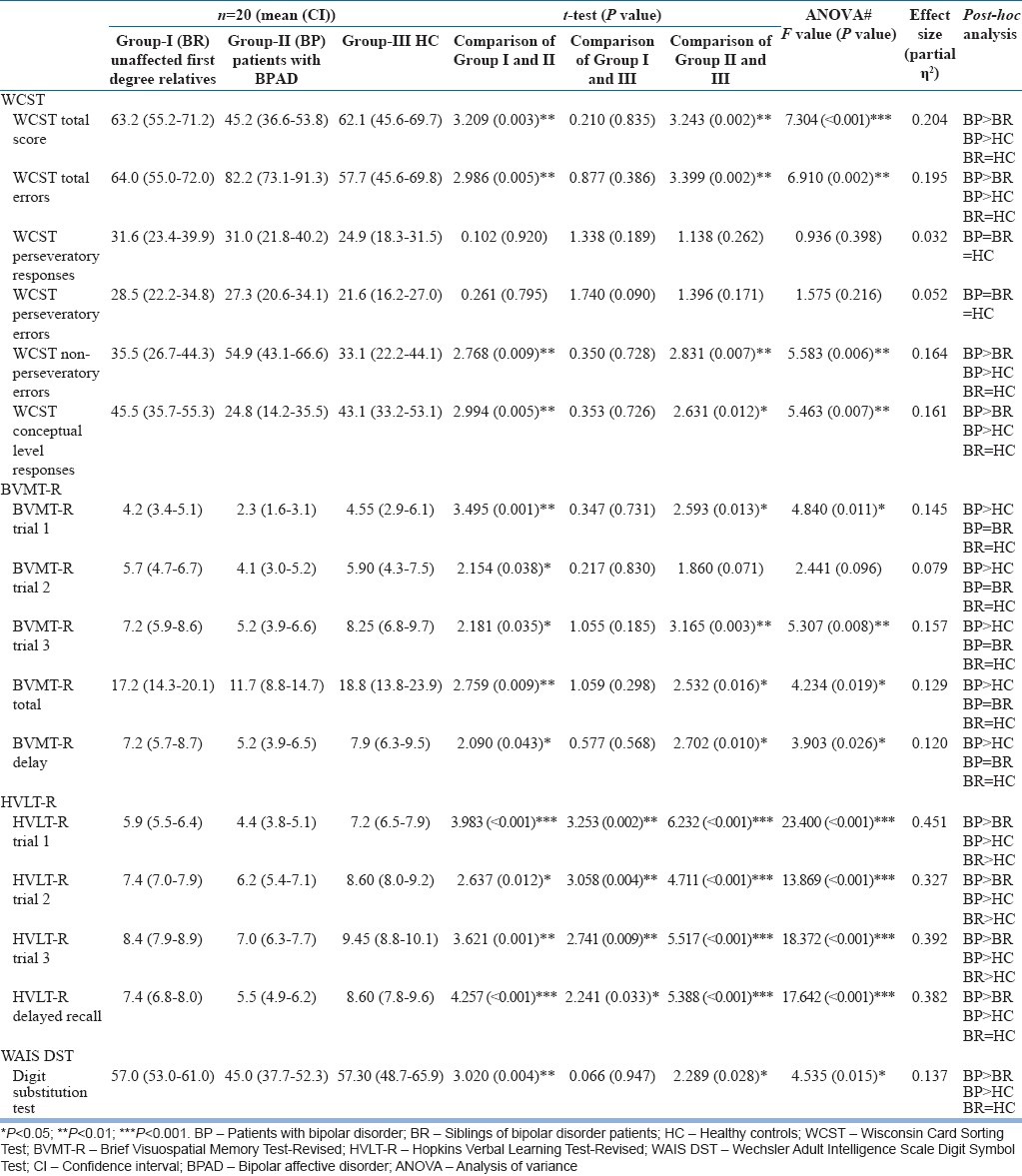
As is evident from Table 2, the performance of patients with BD was significantly poorer than the unaffected siblings and healthy controls in terms of WCST total scores, WCST total errors, WCST nonperseveratory errors and WCST concept responses. Similar differences were also seen between patients of BD and the healthy controls. However, the siblings of patients with BD did not differ significantly from the healthy controls on any of the WCST measures.
On Brief Visuospatial Memory Test (BVMT) and Wechsler Adult Intelligence Scale Digit Symbol Test (WAIS DST), patients with BD performed poorly than the unaffected siblings and healthy controls on all the measures. However, no significant differences were seen between the unaffected siblings and the healthy controls on BVMT and WAIS DST.
On Hopkins Verbal Learning Test too, there was significant difference between the patients with BD and the unaffected siblings on all the measures. In addition, the performance of unaffected siblings was significantly poorer compared to healthy controls.
DISCUSSION
Most of the previous studies which have evaluated patients with BD have compared them with healthy controls. Studies which have evaluated the first degree relatives or siblings have compared them with a control group. In contrast, this study included siblings, patients and a healthy control group. This design can be considered to provide better information about the existent of endophenotypes associated with a disorder.
Healthy first degree relatives of patients with BD are considered to be an important group to study the endophenotypes. This study attempted to compare the neurocognitive functioning of unaffected siblings of patients with BD, patients with BD and the healthy controls. All the three groups were matched on the sociodemographic variables, suggesting that the differences in the neuropsychological domains seen in the present study cannot be attributed to these variables.
For this study, we selected the tests for the cognitive domains of executive functioning, visual memory, verbal memory and learning, psychomotor speed, concentration and graphomotor abilities because these are among the most important cognitive domains for daily functioning[12] and have been reported to be commonly involved in the siblings of patients with BD.[5] The finding of present study, that is, compared with the healthy controls, euthymic patients of BD have cognitive deficits in all the neurocognitive domains studied is in accordance to the existing literature. Meta-analytic studies suggest that patient with BD have neuropsychological impairments in the domains of attention and processing speed, verbal learning, memory and executive functioning etc.[2,3,4]
Endophenotypes are considered to be intermediate phenotypes which arise due to genetic vulnerability associated with a particular disorder. Considering this fact finding any neurocognitive endophenotype associated with BD can help in understanding this disorder better. Three meta-analysis have evaluated the level of neurocognitive dysfunction in relatives/siblings of patients with BD and have reported consistently that the relatives of BD have deficit in the domain of verbal memory, although there are some differences with regards to the subdomains of verbal memory. Further these meta-analyses concur mostly about other neurocognitive deficits.[5,7,20] One meta-analysis included 17 studies comprising of 443 relatives of patients with BD and 797 healthy controls and suggested that relatives of patients with BD have impairments in the trail making test-B, WCST perseveration, continuous performance test omission, verbal learning and immediate recall. However, the effects were small. The second meta-analysis which included 532 relatives and 854 healthy controls suggested that 6 out of the 11 studies included reported impairment in the broad domain of verbal memory and learning in relatives compared to the healthy controls.[20] When the effect size was specifically evaluated for the twin studies, unaffected co-twins had impairment in the domain of verbal declarative memory, but the effect size was not as compelling as seen with mixed group of relatives. Among the various domains of verbal memory, long delayed recall was reported to be more specifically impaired in the list learning task, but not in the story recall task.[20] Further, the meta-analysis concluded that in relatives of patients with BD visualspatial learning and memory, alternating attention, psychomotor speed, abstraction/cognitive flexibility, sustained attention and selective attention are mostly preserved. The third meta-analysis reported large effect sizes for impairment of verbal memory and executive function (working memory, executive control, fluency) in relatives of patients with BD compared with healthy controls.[7] The same meta-analysis also reported that compared with healthy controls relatives of BD also have impairments in the domain of executive function (concept shifting, executive control), mental speed, visual memory, and sustained attention, but the effect sizes are of medium sizes (0.5-0.8).
When we compare the findings of our study with these meta-analysis, our results concur with regards to the impairment in the domain of verbal memory with all the meta-analyses[5,7,20] and preserved functioning in the domains of executive functioning, visual memory, psychomotor speed, concentration and graphomotor abilities, with 2 of the 3 meta-analyses.[5,20] Our findings also concur with that reported in a previous study from India which reported impairment in the verbal memory.[21] However, our findings differ from some of the studies done from India, which have reported impairment in some of the domains of executive functions.[12,13] However, it must be remembered that one of these study included only 10 siblings of BD patients[12] and other included unaffected first degree relatives rather than siblings only.[13] It is quite possible that the differences in the findings may be influenced by the sample size and genetic loading for bipolarity. Lack of difference on most of the neuropsychological tests between healthy controls and siblings of patients with BD suggests that these may not be endophenotypes associated with BD. The consistent finding from the existing literature and our study suggest that verbal memory is an important endophenotype of BD.
Some limitations of the present study should be considered, while interpreting the findings. All the study groups were recruited through purposive sampling and the study included only 20 participants in each group. The study was limited to a clinic population and generalization to community sample should be done with caution. We did not assess the general intelligence and social cognition of the participants. Our sample did not include twin-pairs and the sociodemographic data was group matched rather than case to case matched.
CONCLUSION
To conclude, the findings of the present study suggest that some cognitive markers can distinguish unaffected siblings of BD from healthy controls. This can be useful in clinical practice to ascertain the genetic liability in an index case for BD. Furthermore, it may provide quantitative deficits of neurocognitive functioning which can help in implementing remedial measures. The present evidence base for such neurocognitive markers has not consolidated to the point of promulgation of specific tests or domains as specific replicable discriminating endophenotypes. Further research is warranted to find reliable neurocognitive endophenotypes for BD.
ACKNOWLEDGMENTS
This work was funded by the Research Grant of the Institute in which this study was conducted.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Gottesman II, Gould TD. The endophenotype concept in psychiatry: Etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 2.Mann-Wrobel MC, Carreno JT, Dickinson D. Meta-analysis of neuropsychological functioning in euthymic bipolar disorder: An update and investigation of moderator variables. Bipolar Disord. 2011;13:334–42. doi: 10.1111/j.1399-5618.2011.00935.x. [DOI] [PubMed] [Google Scholar]
- 3.Robinson LJ, Thompson JM, Gallagher P, Goswami U, Young AH, Ferrier IN, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93:105–15. doi: 10.1016/j.jad.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Torres IJ, Boudreau VG, Yatham LN. Neuropsychological functioning in euthymic bipolar disorder: A meta-analysis. Acta Psychiatr Scand Suppl. 2007;434:17–26. doi: 10.1111/j.1600-0447.2007.01055.x. [DOI] [PubMed] [Google Scholar]
- 5.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: A meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113:1–20. doi: 10.1016/j.jad.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Glahn DC, Bearden CE, Niendam TA, Escamilla MA. The feasibility of neuropsychological endophenotypes in the search for genes associated with bipolar affective disorder. Bipolar Disord. 2004;6:171–82. doi: 10.1111/j.1399-5618.2004.00113.x. [DOI] [PubMed] [Google Scholar]
- 7.Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38:771–85. doi: 10.1017/S0033291707001675. [DOI] [PubMed] [Google Scholar]
- 8.Clark L, Kempton MJ, Scarnà A, Grasby PM, Goodwin GM. Sustained attention-deficit confirmed in euthymic bipolar disorder but not in first-degree relatives of bipolar patients or euthymic unipolar depression. Biol Psychiatry. 2005;57:183–7. doi: 10.1016/j.biopsych.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 9.Doyle AE, Wozniak J, Wilens TE, Henin A, Seidman LJ, Petty C, et al. Neurocognitive impairment in unaffected siblings of youth with bipolar disorder. Psychol Med. 2009;39:1253–63. doi: 10.1017/S0033291708004832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frantom LV, Allen DN, Cross CL. Neurocognitive endophenotypes for bipolar disorder. Bipolar Disord. 2008;10:387–99. doi: 10.1111/j.1399-5618.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 11.Szöke A, Schürhoff F, Golmard JL, Alter C, Roy I, Méary A, et al. Familial resemblance for executive functions in families of schizophrenic and bipolar patients. Psychiatry Res. 2006;144:131–8. doi: 10.1016/j.psychres.2005.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trivedi JK, Goel D, Dhyani M, Sharma S, Singh AP, Sinha PK, et al. Neurocognition in first-degree healthy relatives (siblings) of bipolar affective disorder patients. Psychiatry Clin Neurosci. 2008;62:190–6. doi: 10.1111/j.1440-1819.2008.01754.x. [DOI] [PubMed] [Google Scholar]
- 13.Pattanayak RD, Sagar R, Mehta M. Neurocognition in unaffected first-degree relatives of patients with bipolar disorder type I from India a potential vulnerability marker? Sage Open. 2012:2. [Google Scholar]
- 14.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–35. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- 16.Heaton RK. Florida: Psychological Assessment Resources; 2003. Wisconsin Card Sorting Test: Computerized Version 4 (WCST: CV4) [Google Scholar]
- 17.Benedict RH. Odessa, Florida: Psychological Assessment Resources; 1997. Brief Visuospatial Memory Test-Revised. [Google Scholar]
- 18.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins verbal learning test – Revised: Normative data and analysis of inter-form and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 19.Psychological Corporation. 3rd ed. San Antonio, TX: Psychological Corporation; 1998. Wechsler Adult Intelligence Scale. [Google Scholar]
- 20.Balanzá-Martínez V, Rubio C, Selva-Vera G, Martinez-Aran A, Sánchez-Moreno J, Salazar-Fraile J, et al. Neurocognitive endophenotypes (endophenocognitypes) from studies of relatives of bipolar disorder subjects: A systematic review. Neurosci Biobehav Rev. 2008;32:1426–38. doi: 10.1016/j.neubiorev.2008.05.019. [DOI] [PubMed] [Google Scholar]
- 21.Kulkarni S, Jain S, Janardhan Reddy YC, Kumar KJ, Kandavel T. Impairment of verbal learning and memory and executive function in unaffected siblings of probands with bipolar disorder. Bipolar Disord. 2010;12:647–56. doi: 10.1111/j.1399-5618.2010.00857.x. [DOI] [PubMed] [Google Scholar]


