Abstract
Purpose
To compare choroidal thickness (CT) between individuals with and without glaucomatous damage and to explore the association of peripapillary and submacular CT with glaucoma severity using spectral domain optical coherence tomography (SD-OCT).
Methods
Ninety-one eyes of 20 normal subjects and 43 glaucoma patients from the UCLA SD-OCT Imaging Study were enrolled. Imaging was performed using Cirrus HD-OCT. Choroidal thickness was measured at four predetermined points in the macular and peripapillary regions, and compared between glaucoma and control groups before and after adjusting for potential confounding variables.
Results
The average (± standard deviation) mean deviation (MD) on visual fields was −0.3 (±2.0) dB in controls and −3.5 (±3.5) dB in glaucoma patients. Age, axial length and their interaction were the most significant factors affecting CT on multivariate analysis. Adjusted average CT (corrected for age, axial length, their interaction, gender and lens status) however, was not different between glaucoma patients and the control group (P=0.083) except in the temporal parafoveal region (P=0.037); nor was choroidal thickness related to glaucoma severity (r=−0.187, P=0.176 for correlation with MD, r=−0.151, P=0.275 for correlation with average nerve fiber layer thickness).
Conclusions
Choroidal thickness of the macular and peripapillary regions is not decreased in glaucoma. Anatomical measurements with SD-OCT do not support the possible influence of the choroid on the pathophysiology of glaucoma.
Keywords: Glaucoma, Spectral-Domain Optical Coherence Tomography, Choroidal Thickness, Peripapillary, Macula
INTRODUCTION
The optic disc and peripapillary area are the primary focus of imaging studies to detect and manage glaucoma, and to better understand its pathophysiology. Limited access to histological samples, especially in early glaucoma, has been an impediment to better understanding of early anatomical changes in glaucoma. After its introduction in the early 1990s, optical coherence tomography (OCT) quickly came into widespread use for quantitative evaluation of the retinal nerve fiber layer (RNFL) and optic nerve head.1 With improving resolution of OCT devices, the technology now provides clinicians with high definition cross-sectional images of the posterior pole comparable to histopathological sections and a unique non-invasive opportunity to detect early microstructural changes in the optic nerve and retina. High definition cross-sectional OCT images are able to depict different retinal layers as they approach the optic nerve along with changes in the shape and position of optic nerve head structures.2
Vascular factors have been implicated in the development and progression of glaucoma, and hemodynamic studies with various technologies have demonstrated alterations in choroidal blood flow in glaucomatous eyes.3-5 The choroid has recently been suggested to contribute to the pathophysiology of angle-closure glaucoma because of the expansile properties of choroidal vascular channels.6 Near the disc, the choroidal circulation joins the short posterior ciliary vessels and branches from the central retinal artery to supply nutrition to the optic nerve. Choroidal peripapillary atrophy is a frequent hallmark of glaucoma.7,8 Postmortem histologic studies have shown decreased density of capillaries and large choroidal vessels, as well as choroidal thinning in advanced glaucoma.9-11 In contrast, Cristini et al12 found that choroidal thickness showed a 20% increase in glaucomatous eyes as compared with normal eyes. Recently published studies with spectral domain OCT (SD-OCT) have failed to find a significant difference in choroidal thickness in glaucoma as compared to normal subjects.13-15
The purpose of this study was to compare peripapillary and macular choroidal thickness in a group of patients with primary open angle glaucoma (POAG) to a control group of glaucoma suspects and normal subjects and define factors affecting choroidal thickness in this group of subjects.
METHODS
Prospectively enrolled participants from the University of California Los Angeles (UCLA) SD-OCT Imaging Study with good quality SD-OCT images (see below) were included in this study. Eligibility criteria for the UCLA SD-OCT Imaging Study include a diagnosis of perimetric glaucoma in at least one eye, visual acuity ≥20/80, prior perimetric experience with mean deviation >−15.0 dB, spherical equivalent refractive error >−8 D and astigmatism <3 D, and no prior glaucoma surgery. Eyes with other significant ocular diseases such as retinopathies or neurological problems were excluded. The Institutional Review Board of UCLA approved this prospective observational study. All participants signed an informed consent and the study protocol adhered to the tenets of the Declaration of Helsinki.
Eligible subjects underwent a comprehensive ophthalmic evaluation, including assessment of visual acuity, autorefractometry, slit lamp biomicroscopy, intraocular pressure (IOP) measurement with Goldmann applanation tonometry, gonioscopy and dilated fundus examination. Visual field examination was performed with either the standard Swedish Interactive Thresholding Algorithm (SITA) or SITA short wavelength automated perimetry (SITA-SWAP) using 24-2 pattern on the Humphrey Field Analyzer. Axial length was measured with IOLMaster (Carl Zeiss Meditec, Dublin, CA, USA). Stereoscopic photographs of the optic disc were taken with a fundus camera (FF3, Carl Zeiss AG, Germany). Patients underwent disc and retinal nerve fiber layer (RNFL) imaging with SD-OCT (Cirrus HD-OCT, Carl Zeiss Meditec, Dublin, CA, USA). The optic disc photographs were separately reviewed by two experienced clinicians and categorized as normal, indeterminate, or glaucomatous. In case of disagreement, the two clinicians reviewed the disc photographs together again to reach an agreement. Glaucoma was diagnosed when a visual field defect consistent with glaucoma was present. A visual field defect was considered to be present if both of the following criteria were met: (1) Glaucoma Hemifield Test outside normal limits; and (2) four abnormal points with p <5% on the pattern deviation plot, both confirmed at least once. These criteria have been found to be highly specific and to have reasonable sensitivity for detection of early visual field loss in glaucoma with both standard achromatic perimetry and SWAP.16 Eyes with questionable or suggestive disc findings with normal or borderline visual fields (i.e., not meeting the criteria for presence of a defect as mentioned above) were excluded. Normal subjects had open angles, no evidence of optic neuropathy or RNFL loss, and normal or borderline visual fields as described above.
Imaging and Image Processing Procedures
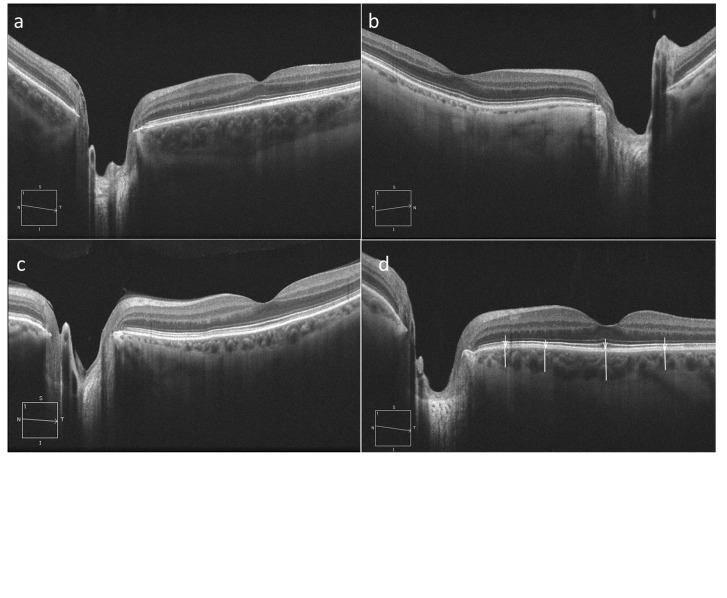
In addition to optic disc/RNFL imaging with the Optic Disc Cube 200x200 algorithm, the HD 5-line raster imaging mode of the Cirrus HD-OCT was used to obtain a high resolution cross-sectional image of the retina and choroid along a 9-mm line passing through the central part of the disc and fovea. The line extended from nasal to the disc to well beyond the fovea. There is no enhanced-depth imaging available on the current version of the Cirrus HD-OCT. However, the single line mode of the HD5 raster scan performs 4,096 consecutive axial scans along the same predetermined line and the measurements are repeated 4 times and averaged to provide the image. The outer border of the choroid can be well visualized on the single line HD5 image in most patients (Figure 1). All images were obtained by two experienced ophthalmic photographers with extensive experience in SD-OCT imaging.
Figure 1.
Examples of high definition 5-line raster images in eyes with varying choroidal thickness: eyes with thick (a), thin (b), and moderate choroidal thickness (c). Choroidal thickness is marked (white lines) at four predetermined locations (white arrows) temporal to the disc (d).
Images with signal strength ≥7 were included in the study. There is no software included in the Cirrus OCT for automatic segmentation of the HD5 images. The HD5 images were exported into ImageJ software (Version 1.43u, National Institutes of Health, USA, http//rsb.info.nih.gov//ij). Two masked reviewers (NN and SM) measured the choroidal thickness at 4 pre-specified locations temporal to the disc marked by one of the investigators (HH). These points included a peripapillary point about 1,000 microns from the clinical disc border, roughly at the same location where the 3.46 mm circumpapillary RNFL measurement circle would cross the linear scan, and three more points under the fovea and 1.5 mm on either side of the fovea. The reviewers were instructed to measure the shortest distance from the outermost border of the retinal pigment epithelium-Bruch’s membrane complex to the outermost boundary of the choroid (sclerochoroidal junction).
The following demographic and clinical variables were extracted from the patient database: age, gender, race, central corneal thickness, automated refraction, axial length and K-readings as measured with IOLMaster, IOP, average and temporal RNFL thickness measurements, and mean deviation along with average loss in the central visual field.
Statistical Analysis
The distribution of various numerical parameters was checked with normal quartile plots and the Wilk-Shapiro test. Intraclass correlation coefficients were used to determine interobserver agreement for choroidal thickness measurements. Bivariate scatter plots of potential factors affecting choroidal thickness vs. choroidal thickness were made and the correlation of such potential variables with choroidal thickness was explored in univariate analyses. Multivariate analyses (backward stepwise) were then performed to determine factors affecting choroidal thickness in the study sample including a dummy variable for diagnosis (glaucoma vs. control). Statistical analyses were performed with Stata software (Version 11.2, Stata Corp., College Station, TX, USA).
RESULTS
One hundred and thirty eyes of 76 patients were available for this study. Seven eyes were excluded because of the following reasons: poorly visible outer choroidal border (choroid-sclera junction, 4 eyes), cystoid macular edema (2 eyes), and retinal vein occlusion (one eye). Thirty-two eyes with suspected glaucoma were also excluded. Table 1 describes the demographic and clinical characteristics of the study groups. Glaucoma patients were older and had longer axial lengths (P<0.001). Fifty-eight eyes were considered to have perimetric glaucoma and 33 eyes belonged to the normal group.
Table 1.
Demographic and clinical characteristics of the study sample
| Variable | Normal subjects | Glaucoma | P value |
|---|---|---|---|
| Number of eyes (Individuals) | 33 (20) | 58 (43) | |
| Age (mean ± SD, year) | 56.6±9.5 | 67.8±8.7 | <0.001 |
| Gender (Female/Male) | 16/4 | 27/16 | 0.025 |
| Visual acuity (mean ± SD, LogMAR) | 0.06±0.11 | 0.08±0.10 | 0.27 |
| Intraocular pressure (mean ± SD, mmHg) | 14.3±2.6 | 13.4±3.5 | 0.176 |
| Axial length (mean ± SD, mm) | 23.7±0.8 | 24.9±1.4 | <0.001 |
| Spherical equivalent (median, range, D) | −0.6±2.2 | −0.9±2.4 | 0.512 |
| Mean deviation (mean ± SD, dB) | −0.3±2.0 | −3.5±3.5 | <0.001 |
| Pattern standard deviation (mean ± SD, dB) | 1.7±0.6 | 5.2±3.7 | <0.001 |
SD, standard deviation; LogMAR, logarithm of minimum angle of resolution; D, diopter; dB, decibel
Intraclass correlation coefficients for the two observers across all 4 locations were 1.00. Measurements by the two reviewers were averaged and the remainder of the results was based on the average measurements.
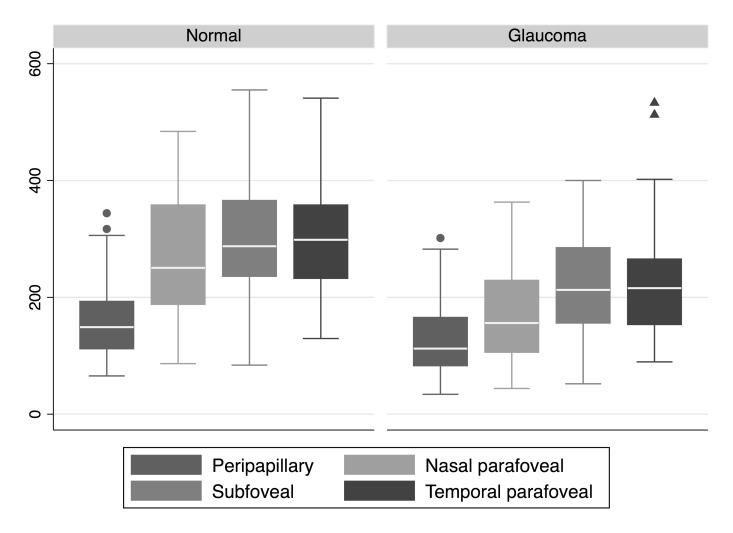
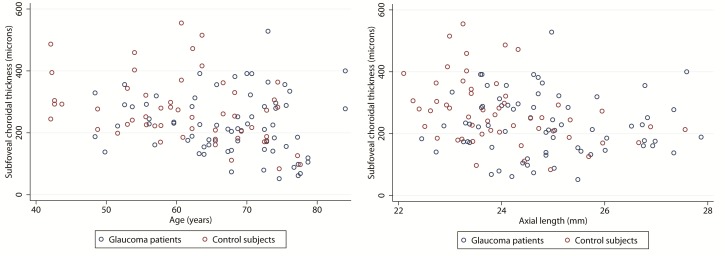
Figure 2 shows the distribution of choroidal thickness measurements at four predetermined locations temporal to the optic disc. Choroidal thickness was highest in the temporal parafoveal region followed by the subfoveal area and thinnest in the peripapillary region. Potential factors influencing choroidal thickness at each of the four locations were subsequently explored in univariate analyses. Age, axial length, and their interaction were consistently found to be strong predictors of choroidal thickness regardless of location (Table 2 and Figure 3). Increasing age and longer axial length were associated with thinner choroidal thickness at all 4 locations (results for only subfoveal location are shown in Figure 3).
Figure 2.
Distribution of choroidal thickness at 4 selected locations on the 9 mm HD5 OCT scan according to diagnosis. Central line in the box = median, borders of the box = interquartile range, and whiskers = 95% confidence intervals, circles or triangles = outliers. HD, high definition; OCT, optical coherence tomography
Table 2.
Univariate relationship of potential clinical and demographic factors with average choroidal thickness at four pre-specified locations temporal to the optic disc
| Average CT |
||
|---|---|---|
| Beta | P value | |
| Age (years) | -2.88 | 0.014 |
| Intraocular pressure (mmHg) | -1.66 | 0.657 |
| Central corneal thickness (μm) | 0.108 | 0.690 |
| Mean deviation (dB) | 3.62 | 0.257 |
| Pattern standard deviation (dB) | -2.86 | 0.249 |
| Axial length (mm) | -15.3 | 0.109 |
| Average RNFLT (μm) | 1.64 | 0.003 |
| Spherical equivalent (D) | -1.85 | 0.697 |
| K-reading (D) | -8.09 | 0.229 |
| Gender (male) | 3.79 | 0.865 |
| Lens status (phakic) | 48.50 | 0.027 |
| Race (White) | 5.80 | 0.194 |
CT, choroidal thickness; dB, decibel; RNFLT, retinal nerve fiber layer thickness; D, diopter
Figure 3.
Bivariate scatter plots of subfoveal choroidal thickness versus age (left) and axial length (right) according to diagnosis.
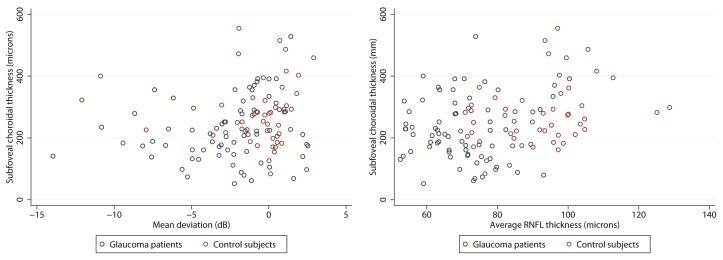
On multivariate analysis, increasing age, longer axial length, and their interaction were the strongest predictors of choroidal thickness at each predefined location. On bivariate scatter plots, no obvious correlation was seen between glaucoma severity (as represented by MD or RNFL thickness) and choroidal thickness (Figure 4). After correcting for potential confounding variables, the severity of glaucoma, as determined by MD, was not predictive of choroidal thickness (P=0.475-0.917 for the four locations; Table 3). Replacing RNFL thickness for MD as a marker of glaucoma severity did not change the results (P>0.3 for all 4 multivariate analyses).
Figure 4.
Bivariate scatter plots of subfoveal choroidal thickness versus visual field mean deviation (left) and retinal nerve fiber layer thickness (RNFL, right) in glaucoma patients. The correlations were not significant for any of the two glaucoma severity variables (r=−0.187, p=0.176 for correlation with mean deviation, r=−0.151, p=0.275 for correlation with average RNFL thickness).
Table 3.
Multivariate regression coefficients for potential predictors of choroidal thickness according to measurement location; numbers in parentheses represent the corresponding P values
| Location | Diagnosis (ref: control) | Age (/year) | Axial length (/mm) | Age * AL interaction | Gender (ref: male) | Lens status (ref: phakic) | MD (/dB) |
|---|---|---|---|---|---|---|---|
| Subfoveal | −21.39 (0.546) | −33.77 (0.066) | −100.04 (0.031) | 1.30 (0.081) | −29.95 (0.173) | 29.34 (0.302) | 0.10 (0.755) |
| Nasal | −9.68 (0.764) | −40.47 (0.005) | −124.67 (0.001) | 1.56 (0.007) | −29.00 (0.207) | 28.18 (0.262) | 1.15 (0.676) |
| Temporal | −70.3 (0.037) | −47.33 (0.041) | −112.14 (0.060) | 1.86 (0.050) | −12.68 (0.606) | 21.44 (0.422) | −3.87 (0.240) |
| Peripapillary | 37.53 (0.083) | −38.57 (0.002) | −115.61 (<0.001) | 1.46 (0.003) | −27.96 (0.148) | 20.88 (0.247) | 0.39 (0.854) |
Ref, reference; AL, axial length; MD, mean deviation
We also compared adjusted mean choroidal thickness between the glaucoma and control groups at the 4 predetermined locations. Mean choroidal thickness was adjusted for age, axial length, their interaction, gender, and lens status based on the multivariate equations. There was no significant difference between the two groups at any of the 4 locations (Table 4).
Table 4.
Adjusted (marginal) means for choroidal thickness (CT) in the study groups measured at four locations temporal to the optic disc
| CT (mean ± SE, μm) | Controls | Glaucoma | P value |
|---|---|---|---|
| Subfoveal | 264.3 (± 27.8) | 242.9 (±14.6) | 0.546 |
| Temporal | 295.7 (± 27.3) | 225.4(± 13.9) | 0.037 |
| Nasal | 211.6 (± 22.3) | 201.9 (± 15.8) | 0.764 |
| Peripapillary | 116.6 (± 13.1) | 154.1 (±12.3) | 0.083 |
The measurements have been adjusted for axial length, age, the interaction between age and axial length, gender, and lens status.
SE, standard error
DISCUSSION
In a group of open-angle glaucoma patients and normal subjects, we found that choroidal thickness could be measured with acceptable reproducibility on Cirrus HD-OCT’s cross-sectional HD 5-line raster images. Although the Cirrus HD-OCT lacks a module to perform enhanced-depth imaging (EDI), the very high sampling density and the high repetition rate used with the HD 5 algorithm has been demonstrated to perform as well as SD-OCT machines using the EDI algorithm.17 We recruited normal eyes and eyes with established perimetric damage in this study. Choroidal thickness was lowest in the peripapillary area and highest in the temporal parafoveal and subfoveal locations. The main factors affecting choroidal thickness in this group of eyes (in negative direction) were age, axial length, and their interaction. Although unadjusted average choroidal thickness measurements were thinner in the glaucoma group (Figure 2), mean choroidal thickness after adjusting for the above-mentioned confounding factors was similar in the two groups at all predetermined measurement locations except for the temporal parafoveal location. Glaucoma severity as defined by functional or structural measures (MD or RNFL thickness, respectively) was not associated with choroidal thickness.
Histological studies have reported varying results regarding choroidal thickness in glaucoma, likely as a result of the fact that the choroid is a very dynamic tissue and therefore post-mortem measurements may not provide relevant information.9 With the advent of SD-OCT devices, for the first time, we have the opportunity to image the choroid in vivo. Margolis et al18 evaluated choroidal thickness in normal eyes and reported that the choroid was thickest underneath the fovea and that thickness decreased rapidly in the nasal direction. Increasing age was associated with less choroidal thickness. Subfoveal choroidal thickness decreased by 15.6 μm for each decade of older age. Ho et al19 measured peripapillary choroidal thickness in a group of normal subjects with Cirrus HD-OCT. The inferior peripapillary choroid was significantly thinner compared to other quadrants at all measured distances from the optic nerve. Neither RNFL thickness nor age was significantly correlated with choroidal thickness.
With renewed interest in the potential role of the choroid in the pathophysiology of various ocular diseases including glaucoma, some recent studies have explored choroidal thickness in glaucoma. Mwanza et al15 reported their experience with in vivo imaging of the choroid with Spectralis SD-OCT in primary open-angle and normal-tension glaucoma and normal control subjects. They confirmed that choroidal thickness was mainly influenced by age and axial length (thinner choroid with older age and longer axial length). However, the authors did not find any significant difference among the three groups with respect to choroidal thickness under the foveal center or up to 3 mm nasal or temporal to the fovea. In another study on patients with unilateral glaucoma, the same findings were confirmed.20 Maul et al14 measured choroidal thickness with enhanced depth SD-OCT imaging in a group of glaucoma suspects and glaucoma patients. Older age, longer axial length, thicker central cornea, and lower diastolic ocular perfusion pressure were significantly associated with thinner choroid. However, the severity of glaucomatous damage was not found to be associated with choroidal thickness. Ehlrich et al13 also assessed circumpapillary choroidal thickness with Spectralis SD-OCT in a retrospective study and concluded that eyes with primary open angle glaucoma did not demonstrate reduced choroidal thickness nor was there a correlation between RNFL thickness and choroidal thickness in corresponding quadrants. However, choroidal thickness measurements were not corrected for axial length. The above findings have been corroborated in another small study.21
Our results indicate that choroidal thickness is not altered in early glaucoma. The only significant difference was found at the temporal parafoveal location between the two groups which is of uncertain significance. However, the choroid is a highly dynamic vascular tissue and purely anatomic measurements, such as choroidal thickness, may not adequately describe altered hemodynamic physiology in various ocular diseases. Specifically, flow patterns in the peripapillary choroid would be of great interest in glaucoma. As previously reported, age and axial length were the main factors affecting choroidal thickness in this group of patients. Additionally, we found that older age and longer axial length had a synergistic effect on the attenuation of the choroid as detected by the significant interaction between the two covariates. The synergistic effect of age and axial length on CT has not been reported previously and likely explains the severe progressive atrophy of the retinal pigment epithelium and choroid with aging in highly myopic eyes. There is recent evidence suggesting that choroidal thickness may be altered in eyes with senile sclerotic glaucomatous damage.22 The findings, however, need confirmation in additional studies.
The limitations of our study need to be considered. The HD5 raster images have a very high resolution and therefore can be performed once at a time. Only one 9-mm linear cross-sectional image was available for our study extending from the nasal peripapillary area to the area temporal to the fovea. It might be argued that choroidal or peripapillary changes in glaucomatous eyes may be missed or may not be prominent if choroidal thickness is not measured in the superior and inferior peripapillary areas. However, none of the recent studies mentioned above found a difference in choroidal thickness in any location in the peripapillary area. Despite the very high resolution of the linear HD5 images, the choroid-sclera junction could not be delineated with absolute certainty in some patients. Four eyes were excluded because one or both observers were not able to mark the outer boundary of the choroid with certainty. This occurred more commonly in eyes with thicker choroid; however, there is no a priori reason to believe that this would have introduced a systematic bias in detection of the relationship between glaucoma severity and choroidal thickness. Since the original sample size calculations were not based on the ability to detect a difference in choroidal thickness between the two groups, the power of the current study was fairly limited.
In summary, we found that choroidal thickness in the macular and peripapillary areas did not vary as a function of glaucomatous damage. Age and axial length were the most significant factors affecting choroidal thickness. Based on anatomical measurements, the choroid does not seem to be significantly altered in POAG patients. Future studies investigating the choroidal vascular flow are indicated to better explore the role of the choroid in the pathophysiology of open-angle glaucoma.
Acknowledgments
The authors would like to thank Jeffrey Gornbein, DrPH, for assistance with statistical analyses.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Schuman JS, Hee MR, Arya AV, Pedut-Kloizman T, Puliafito CA, Fujimoto JG, et al. Optical coherence tomography: a new tool for glaucoma diagnosis. Curr Opin Ophthalmol. 1995;6:89–95. doi: 10.1097/00055735-199504000-00014. [DOI] [PubMed] [Google Scholar]
- 2.Lee KY, Tomidokoro A, Sakata R, Konno S, Mayama C, Saito H, et al. Cross-sectional anatomic configurations of peripapillary atrophy evaluated with spectral domain-optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51:666–671. doi: 10.1167/iovs.09-3663. [DOI] [PubMed] [Google Scholar]
- 3.Deokule S, Vizzeri G, Boehm AG, Bowd C, Medeiros FA, Weinreb RN. Correlation among choroidal, parapapillary, and retrobulbar vascular parameters in glaucoma. Am J Ophthalmol. 2009;147:736–743. doi: 10.1016/j.ajo.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 4.Leske MC, Heijl A, Hyman L, Bengtsson B, Dong LM, Yang Z. Predictors of long-term progression in the early manifest glaucoma trial. Ophthalmology. 2007;114:1965–1972. doi: 10.1016/j.ophtha.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Leske MC, Wu SY, Hennis A, Honkanen R, Nemesure B. Risk factors for incident open-angle glaucoma: The Barbados Eye Studies. Ophthalmology. 2008;115:85–93. doi: 10.1016/j.ophtha.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Quigley HA. What's the choroid got to do with angle closure? Arch Ophthalmol. 2009;127:693–694. doi: 10.1001/archophthalmol.2009.80. [DOI] [PubMed] [Google Scholar]
- 7.Uchida H, Ugurlu S, Caprioli J. Increasing peripapillary atrophy is associated with progressive glaucoma. Ophthalmology. 1998;105:1541–1545. doi: 10.1016/S0161-6420(98)98044-7. [DOI] [PubMed] [Google Scholar]
- 8.Wilensky JT, Kolker AE. Peripapillary changes in glaucoma. Am J Ophthalmol. 1976;81:341–345. doi: 10.1016/0002-9394(76)90251-8. [DOI] [PubMed] [Google Scholar]
- 9.Kubota T, Jonas JB, Naumann GO. Decreased choroidal thickness in eyes with secondary angle closure glaucoma. An aetiological factor for deep retinal changes in glaucoma? Br J Ophthalmol. 1993;77:430–432. doi: 10.1136/bjo.77.7.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spraul CW, Lang GE, Lang GK, Grossniklaus HE. Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vision Res. 2002;42:923–932. doi: 10.1016/s0042-6989(02)00022-6. [DOI] [PubMed] [Google Scholar]
- 11.Yin ZQ, Vaegan A, Millar TJ, Beaumont P, Sarks S. Widespread choroidal insufficiency in primary open-angle glaucoma. J Glaucoma. 1997;6:23–32. [PubMed] [Google Scholar]
- 12.Cristini G, Cennamo G, Daponte P. Choroidal thickness in primary glaucoma. Ophthalmologica. 1991;202:81–85. doi: 10.1159/000310179. [DOI] [PubMed] [Google Scholar]
- 13.Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S, Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011;92:189–194. doi: 10.1016/j.exer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 14.Maul EA, Friedman DS, Chang DS, Boland MV, Ramulu PY, Jampel HD, et al. Choroidal thickness measured by spectral domain optical coherence tomography: Factors affecting thickness in glaucoma patients. Ophthalmology. 2011;118:1571–1579. doi: 10.1016/j.ophtha.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwanza JC, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:3430–3435. doi: 10.1167/iovs.10-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson CA, Sample PA, Cioffi GA, Liebmann JR, Weinreb RN. Structure and function evaluation (SAFE): I. criteria for glaucomatous visual field loss using standard automated perimetry (SAP) and short wavelength automated perimetry (SWAP). Am J Ophthalmol. 2002;134:177–185. doi: 10.1016/s0002-9394(02)01577-5. [DOI] [PubMed] [Google Scholar]
- 17.Regatieri CV, Branchini L, Fujimoto JG, Duker JS. Choroidal imaging using spectral-domain optical coherence tomography. Retina. 2012;32:865–876. doi: 10.1097/IAE.0b013e318251a3a8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Margolis R, Spaide RF. A pilot study of enhanced depth imaging optical coherence tomography of the choroid in normal eyes. Am J Ophthalmol. 2009;147:811–815. doi: 10.1016/j.ajo.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Ho J, Branchini L, Regatieri C, Krishnan C, Fujimoto JG, Duker JS. Analysis of normal peripapillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011;118:2001–2007. doi: 10.1016/j.ophtha.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mwanza JC, Sayyad FE, Budenz DL. Choroidal thickness in unilateral advanced glaucoma. Invest Ophthalmol Vis Sci. 2012;53:6695–6701. doi: 10.1167/iovs.12-10388. [DOI] [PubMed] [Google Scholar]
- 21.Fénolland J, Giraud JM, Maÿ F, Mouinga A, Seck S, Renard JP. Enhanced depth imaging of the choroid in open-angle glaucoma: a preliminary study. J Fr Ophtalmol. 2011;34:313–317. doi: 10.1016/j.jfo.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Roberts KF, Artes PH, O'Leary N, Reis AS, Sharpe GP, Hutchison DM, et al. Peripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Arch Ophthalmol. 2012;130:980–986. doi: 10.1001/archophthalmol.2012.371. [DOI] [PubMed] [Google Scholar]