Abstract
Purpose
To evaluate the short-term effects of a single subconjunctival injection of triamcinolone acetonide as an adjunct to subconjunctival bevacizumab for prevention of corneal neovascularization in rats.
Methods
Chemical cauterization was performed in the central cornea of the right eye in 48 male Sprague-Dawley rats (4 eyes were excluded due to perforation and/or infection). Immediately after the injury, the rats were randomly assigned to four treatment groups: controls (n=10), received subconjunctival injection of 0.02 mL balanced salt solution; group 1 (n=12), received 0.02 mL bevacizumab (25 mg/mL); group 2 (n=11), were treated with 0.02 mL triamcinolone acetonide (40 mg/mL); and group 3 (n=11), received both bevacizumab and triamcinolone acetonide. On days 7 and 14 after cauterization, digital photographs of the corneas were taken and the area of neovascularization was calculated and compared among the study groups.
Results
The area of corneal neovascularization in all three treatment groups was less than the controls (P<0.05 for all comparisons). On day 7, the corneal avascular area was largest in group 3 (63%). On day 14, the area of corneal neovascularization in groups 2 and 3 was smaller than that in group 1 (P=0.031 and 0.011, respectively), but the difference between groups 2 and 3 was not statistically significant (P=0.552). Microscopic evaluation of the cornea was compatible with gross findings; inflammation and the number of new vessels was the least in group 3.
Conclusion
Triamcinolone acetonide was more effective than bevacizumab in inhibiting corneal neovascularization. Its adjunctive administration to bevacizumab resulted in even better prevention of corneal neovascularization. However, the produced combined effect was less than the sum of their separate effects and did not match additive or synergistic interactions.
Keywords: Corneal Neovascularization, Triamcinolone Acetonide, Subconjunctival Bevacizumab Adjunctive Therapy, Neovascularization Inhibition
INTRODUCTION
Corneal neovascularization occurs when the balance between corneal angiogenic and anti-angiogenic factors is tipped toward angiogenesis.1 The condition may result in corneal blindness and graft rejection by disturbing the corneal ‘angiogenic privilege’. Various inflammatory, infectious, degenerative and traumatic insults affecting the cornea may induce neovascularization, scarring and lipid deposition, eventually leading to diminished visual acuity.2,3
A variety of agents have been proposed as inhibitors of corneal neovascularization, among which corticosteroids have been the treatment of choice in practice.4 Triamcinolone acetonide (TA) possesses pronounced anti-inflammatory properties with action lasting for several days to weeks.5 It had been widely used for treatment of various ocular disorders including diabetic macular edema, retinal vein occlusion, and choroidal and corneal neovascularization.5-7 The anti-angiogenic properties of TA is believed to be the result of its direct inhibitory effect on vascular endothelial cells. Furthermore, it decreases RNA stability of the vascular endothelial growth factor (VEGF) gene, in addition to blocking downstream VEGF pathways and its stimulating agents such as interleukin-6 (IL-6) receptors.8,9
Bevacizumab (Avastin, Genentech Inc., San Francisco, CA, USA) is a full-length humanized murine monoclonal antibody against VEGF (anti-VEGF).10,11 Regarding the major role of VEGF in angiogenesis pathways, experimental and clinical studies have been performed to assess its efficiency for prevention and treatment of corneal neovascularization.12 These studies showed significant inhibition or regression of corneal neovascularization, although all of them failed to show complete regression of neovascularization. Moreover, it has been shown that neovascularization increases 7-14 days after bevacizumab injection.13 Theoretically, VEGF inhibition in conjunction with blocking its downstream pathways and suppression of vascular endothelial cells could be more effective than targeting the VEGF system alone.14,15
This study aimed to evaluate the short term effects of a single subconjunctival injection of TA, as an adjunct to bevacizumab, for prevention of experimentally induced corneal neovascularization in a rat model.
METHODS
Animals
This experimental study was conducted on 48 male Sprague-Dawley rats weighing 200-250g. The animals were kept at room temperature of 24±1 °C on a 12 hour light/12 hour dark cycle with access to appropriate food and water. All procedures on the animals were conducted in accordance with the Association for Research in Vision and Ophthalmology (ARVO) Statement for the Use of Animals in Ophthalmic and Visual Research. The Institutional Animal Care and Use Committee at Tehran University of Medical Sciences approved the study.
Corneal Cauterization
All procedures were performed on rats under general anesthesia induced by intraperitoneal injection of 10% ketamine hydrochloride (50 mg/kg) and xylazine hydrochloride (5 mg/kg). A single drop of topical 1% tetracaine hydrochloride (Alcaine, Alcon, Fort Worth, TX, USA) was then used to provoke topical anesthesia for further blockage of the corneal reflex. Prior to cauterization, corneas of each animal were checked for the presence of injury or new vessels. Only the right eye of each rat was used for the study.
Chemical cauterization of the central cornea was done by pressing a 1.8 mm diameter applicator coated with 75% silver nitrate /25% potassium nitrate (Arzol, Keene, NH, USA) for 10 seconds.16 Eyes were rinsed afterwards with 5 mL of balanced salt solution (BSS) and blotted gently with a cellulose sponge (Beaver-Visitec International Inc., MA, USA) to remove excess silver nitrate. The animals were then randomly assigned to 4 different treatment groups. To increase the reproducibility of the alkali burn and the consequent scar formation, the whole process was performed by the same investigator (RG) on all animals.
Treatment Groups
Immediately after corneal cauterization, subconjunctival injection was done for each of the eyes at two sites, i.e. 2 mm from the superior and inferior limbus. To keep the investigator (RG) masked to the treatment groups, all syringes were covered to hide their contents (TA has a milky appearance). Injections were performed with 30-gauge needles under a surgical microscope.
The control group (n=10) received a sham injection of 0.02 mL BSS at both sites. Eyes in group 1 (bevacizumab; TB; n=12) were treated with a subconjunctival injections of 0.02 mL bevacizumab 25 mg/mL at one site and 0.02 mL BSS at the other. Group 2 (triamcinolone acetonide; TT; n=11) received 0.02 mL (0.8 mg) of TA (40 mg/mL) at one site and 0.02 mL of BSS at the other. Group 3 (bevacizumab and triamcinolone acetonide; TB+T; n=11) were treated with 0.02 mL of bevacizumab 25 mg/mL at one site and 0.02 mL of TA 40 mg/mL at the other site.
Evaluation of Corneal Neovascularization
Evaluation of corneal neovascularization was performed on days 7 and 14 after corneal cauterization. Each animal was anesthetized as described above. Using a slit lamp biomicroscope, we scored the burns according to a grading scale introduced by Manzano et al12: 0 (no blister, not raised above the corneal surface), 1 (small blister, raised slightly above the corneal surface), 2 (medium blister, raised moderately above the corneal surface), or 3 (large blister). Eyes with a burn score of less than 2, poor burn extent, infection or perforation were excluded from the study.
A drop of a mydriatic agent (tropicamide 0.5%) was instilled to facilitate discrimination of corneal neovascularization from iris vessels. Corneal digital photographs were taken from the rats’ eyes with 16× and 25× magnification using a 12 megapixel Canon digital camera attached to the slit lamp. Using the images, an investigator masked to the treatment groups (HZM), measured the extent of the chemical injury (scar tissue) in terms of percentage of the corneal surface that was opaque and scarred. Image analysis and processing was done using the software program Image J1.31v (Wayne Rasband, Research Services Branch, National Institute of Mental Health, Bethesda, Maryland, USA). Likewise, the extent of avascular cornea in terms of percentage of the corneal surface that was not covered by neovascularization tissue was measured. The efficacy of each anti-neovascular treatment was evaluated based on the area of avascular cornea and prevention of total corneal neovascularization on day 14.
In addition to the above mentioned quantitative assessment of corneal neovascularization, we defined the index “Total corneal neovascularization (NV Total)”as follows:
Formula 1
Histopathologic Evaluation
The injured eyes were enucleated after the animals were euthanized by inhalation of carbon dioxide. Eyes were sutured at the superior limbus to provide a reference point for further histopathologic studies. Then, the enucleated globes were penetrated using a 27-gauge needle to allow the fixative substance (formaldehyde 10%) to fill the eyes rapidly. After fixation for 24 hours, corneas were separated and stained with hematoxylin and eosin for light microscopy. Corneas were sagittally divided into two halves through the center of the lesion. On each half, four sections were evaluated for neovascularization intensity. Measurment of new vessels was done in three areas with highest intensity of neovascularization under ×250 magnification. An examiner who was masked to the treatment groups performed light microscopic examination (MM).
Statistical Analysis
Data analysis was performed using SPSS software version 17 (SPSS Inc., Chicago, Illinois, USA). Statistical significant was set at 0.05. The one-sample Kolmogorov-Smirnov test was used to check normality of the distribution of the data.
As the data fitted the normality curve, parametric tests were used. Treatment groups were compared in terms of corneal avascular area and scar tissue at specific time points, using the analysis of variance (ANOVA) test. Equality of the variances was compared using Levene’s test. Once the variances were equal or unequal, pairwise multiple comparisons were tested using the Tukey or Tamhane tests, respectively. We used the Chi-square test to compare categorical variables.
RESULTS
Burn Score and Scar Formation
On day 7 after cauterization, all 48 eyes had reasonable corneal neovascularization and corneal scar corresponding to the site of injury. Four eyes were omitted from the study including two eyes from the control group which were excluded due to perforation and one eye each from the TT and TB+T group because of infection. Treatment groups were comparable in terms of the burn score (all P values >0.05). The percentage of the scarred area in each treatment group is detailed in Table 1.
Table 1.
Percentage ± standard deviation of corneal avascular and scar areas 7 and 14 days after cauterization
| Day 7 | Day 14 | |||
|---|---|---|---|---|
| Avascular area | Scar area | Avascular area | Scar area | |
| Controls (n = 10) | 24.48 ± 8.69 | 10.9 ± 3.3 | 3.33 ± 9.89 | 10.4 ± 2.8 |
| Group 1 (n = 12) | 36.71 ± 13.53 | 11.2 ± 3.1 | 23.47 ± 16.43 | 10.7 ± 3.4 |
| Group 2 (n = 11) | 50.23 ± 11.74 | 10.4 ± 4.5 | 42.73 ± 17.04 | 11.1 ± 3.6 |
| Group 3 (n = 11) | 63.11 ± 12.95 | 9.8 ± 3.7 | 50.69 ± 18.21 | 10.3 ± 3.9 |
Mean percentage of corneal avascular area was significantly higher in all 3 treatment groups as compared to the control group on days 7 and 14 after corneal cauterization (ANOVA; all P values less than 0.05). A statistically significant difference was found between groups 2 and 3 as compared to group 1 on days 7 and 14 (P=0.028 and P<0.001 on day 7, P=0.031 and P=0.011 on day 14). Although the area of avascular cornea was found to be significantly higher in group 3 than that in group 2 on day 7, the difference was not statistically significant on day 14 (P=0.047 and 0.556 respectively). There was no significant difference between the groups in terms of corneal scar area.
Group 1, bevacizumab-treated eyes; Group 2, triamcinolone-treated eyes; Group 3, combined treatment with bevacizumab and triamcinolone
Corneal Neovascularization
Findings at each of the two examination points corresponded to each other (Table 1) as corneal neovascularization progressed between the two time points. Generally, the control group had significantly less avascular areas than all treatment groups (Figure 1). In the control group, mean area of cornea covered by new vessels was 75.5% on day 7 after cauterization which progressed to 96.7% on day 14 equal to NVTotal of 90%.
Figure 1.
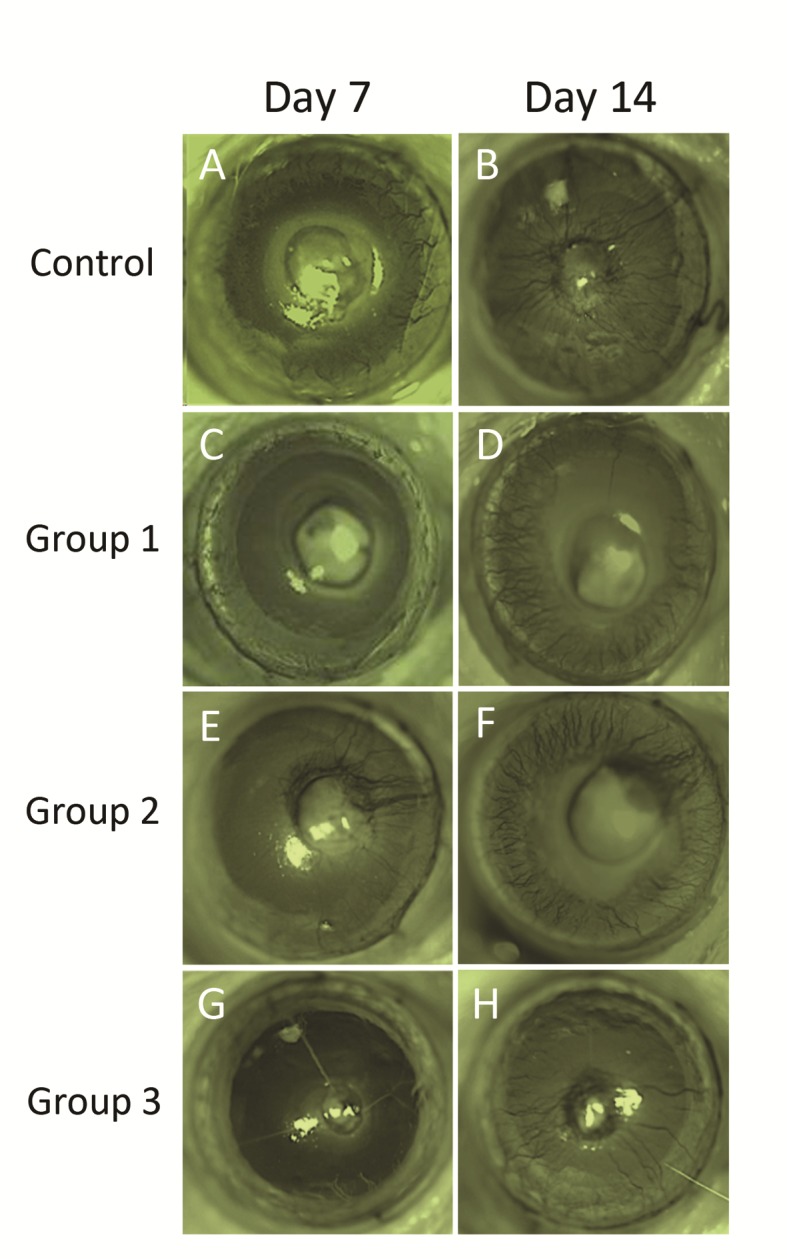
Representative photographs of the study groups demonstrating extension of neovascularization 7 and 14 days after corneal chemical burn. Note the progression of corneal neovascularization in all study groups between the two examination points. A and B: an eye in the control group treated with balanced salt solution. C and D: an eye in group 1 received a single subconjunctival injection of bevacizumab. E and F: an eye in group 2 treated with a single subconjunctival injection of triamcinolone acetonide. G and H: an eye in group 3 treated with both triamcinolone acetonide and bevacizumab.
In group TB, subconjunctival injection of bevacizumab significantly inhibited corneal neovascularization as compared to the control group throughout the follow-up period (Table 1, P=0.031 on day 7 and P=0.048 on day 14). Between the two consecutive measurements, neovascularization extended by about 13.2%. By day 14, half of the eyes were totally neovascularized (NVTotal=50%).
In group TT, corneal neovascularization was significantly less than both the control and TB groups (P<0.001 and 0.028 on day 7, and P<0.001 and 0.031 on day 14, respectively). Between the two measurements, neovascularization extended over the cornea by about 7.5% in eyes treated with TA which was less than other treatment groups.
In the TB+T group, a mean area of 63.1% of the corneas was clear of neovascularization on day 7 which decreased to 50.7% on day 14. When compared to the control and TB groups, combined injection of bevacizumab and TA inhibited corneal neovascularization more effectively at both measurement points (P <0.001 and <0.001 on day 7 and P<0.001 and 0.011 on day 14, respectively). However a statistically significant difference was only observed between the TT and TB+T groups on day 7 (P= 0.047 and 0.556 on days 7 and 14, respectively). None of the injured eyes treated with TA (groups TT and TB+T) were totally neovascularized on day 14 which was significantly better than the outcome of the other study groups (Chi-square test; P< 0.001).
Histopathologic evaluation of the corneas was in accordance with clinical findings on slit lamp examination (Figure 2). As illustrated in Table 2, the number of new vessels was highest in the control group and smallest in the TB+T group.
Figure 2.
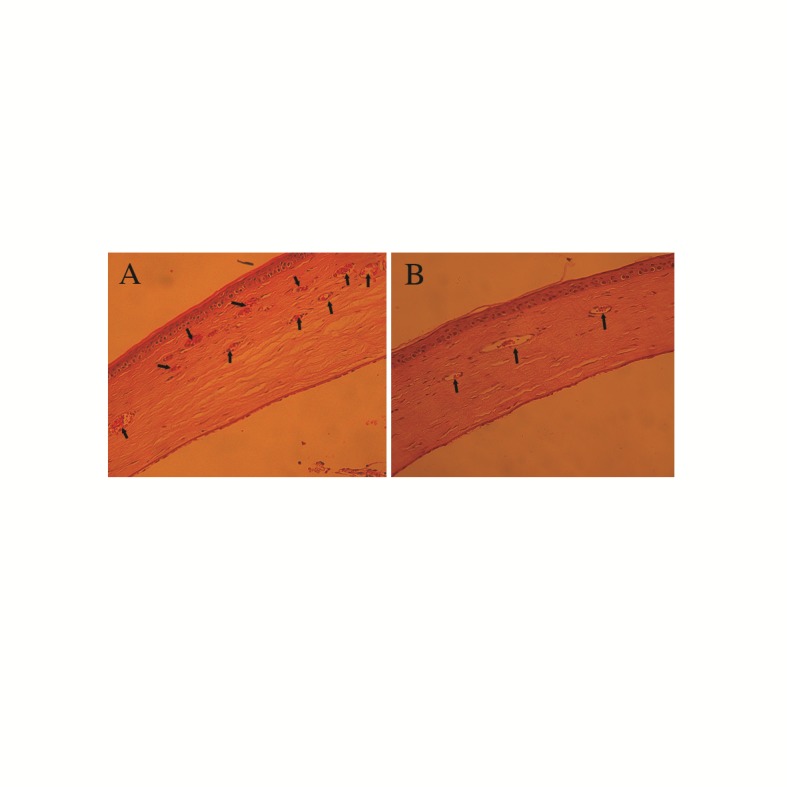
Photomicrographs of corneal neovascularization 14 days post cauterization (hematoxylin and eosin stain; magnification, ×300) in (A) a control eye receiving sham and (B) an eye treated with both triamcinolone acetonide and bevacizumab. Note the extensive stromal neovascularization (arrows) in figure A versus B.
Table 2.
Number of new vessels on histopathologic evaluation of corneal sections in each group (mean ± standard deviation)
| Study Group† | Number of new vessels* |
|---|---|
| Controls (n = 10) | 18.8 ± 5.8 |
| Group 1 (n = 12) | 11.4 ± 3.7 |
| Group 2 (n = 11) | 10.5 ± 4.3 |
| Group 3 (n = 11) | 8.8 ± 3.6 |
Mean number of new vessels was significantly higher in the control group as compared to groups 1, 2 and 3 (P=0.007, 0.003 and <0.001, respectively).
Group 1, bevacizumab-treated eyes; Group 2, triamcinolone treated and triamcinolone
DISCUSSION
Maintaining corneal clarity requires a delicate balance between angiogenic and anti-angiogenic factors, which has been termed corneal “avascular privilege”. Any insult interfering with this balance may result in invasion of vessels into the clear cornea. Different therapeutic agents such as steroids,4,17 heparin,18 methotrexate,19 indomethacin,20 anti-VEGFs and thalidomide21 have been shown to effectively block corneal neovascularization pathways. But, all of them had partial efficiency for treatment and inhibition of corneal neovascularization, which highlights the need for broader blockage of pathways involved in corneal neovascularization.22,23
In our study, all three treatment groups had significantly less corneal neovascularization as compared to the control group throughout 14 days of follow-up. Combination therapy with bevacizumab and TA was found to be more effective than their usage alone. On day 7, corneal neovascularization was significantly less in Group 3 as compared to groups 1 and 2. But, on day 14, the difference was not significant between groups 3 and 2. This finding may be due to a decrease in the bioactivity of bevacizumab. Peters et al13 have demonstrated that endothelial fenestrations of the choriocapillaris are reduced on day 4 but increased on days 7-14 after intravitreal bevacizumab.
TA has been shown to inhibit new vessel formation indirectly by preventing migration and activation of inflammatory cells and by blocking interleukin-6, VEGF downstream pathways and other cytokines associated with neovascularization. Also, steroids (including TA) directly inhibit neovascularization by suppressing vascular extracellular matrix turnover and altering endothelial cell function. In our study, administration of 0.08 mg TA had significantly better efficacy on corneal neovascularization compared to 0.02 mL bevacizumab (25 mg/mL).
In a recent study, Kang et al22 evaluated the effect of bevacizumab and TA combination therapy on regression of stabilized new vessels in a rabbit model of corneal neovascularization. Their results showed that TA was less effective than bevacizumab for treatment of corneal neovascularization. These findings are not in line with ours, but it should be noted that we treated the eyes immediately after injury and before initiation of the inflammatory response. Moreover, in the current study we used chemical cauterization to induce corneal neovascularization, which is associated with a more severe inflammatory response than suturing the cornea.24 Therefore triamcinolone acetonide may be more effective in the early inflammatory phase of corneal neovascularization, when it can act both directly and indirectly. Thus, TA may be more effective for inhibition corneal new vessels rather than involution of stabilized neovascularization.
At first it might be assumed that adjunctive administration of TA to bevacizumab should produce a combined effect equal or greater than the sum of their separate effects (i.e. additive or synergistic effect). But, we expected inaccuracy of such an assumption because TA and bevacizumab had been found to act on different levels of a common pathway. In fact, the strategy of combination therapy is best effective when different pathways are targeted and/or when this approach provides the chance to use lower doses of each component. As an example, the efficacy of combination therapy with topical Sunitinib, a tyrosine kinase inhibitor which selectively inhibits VEGF receptor 2 and platelet derived growth factor receptor, and topical bevacizumab on inhibiting corneal neovascularization have been evaluated recently. The study revealed that such combination therapy resulted in about 3-fold greater inhibition of corneal neovascularization than that achieved by topical bevacizumab alone.25
Triamcinolone acetonide is a long acting steroid and Murata et al26 showed that two weeks after subconjunctival injection of TA, its therapeutic levels are maintained. As a result, TA has been widely used in clinic settings for treating ocular pathologies accompanied with neovascularization. But, use of steroids such as TA is associated with several complications such as cataracts, glaucoma and infection. Combination therapy may allow one to decrease the therapeutic dose of such agents, which can lead to less adverse effects. Future studies are required to evaluate the long-term efficacy of adjunctive administration of TA to bevacizumab, with regard to their molecular pathways i.e. IL-6 and VEGF, and their possible side effects.
In summary, in our study a single subconjunctival injection of triamcinolone acetonide and/or bevacizumab significantly inhibited corneal angiogenesis 14 days after induced corneal chemical injury in rats. TA was more effective than bevacizumab in inhibiting corneal neovascularization. Although, its adjunctive administration to bevacizumab resulted in even better prevention of neovascularization, our findings did not support their additive or synergistic effect on preventing corneal neovascularization. When applied immediately after corneal injury, triamcinolone acetonide alone showed a stronger anti-neovascularization effect than bevacizumab alone. This could be attributed to its broader anti-inflammatory effects. Concomitant administration of triamcinolone acetonide and bevacizumab may have potential as a novel approach for restoration of corneal immune privilege.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Qazi Y, Maddula S, Ambati BK. Mediators of ocular angiogenesis. J Genet. 2009;88:495–515. doi: 10.1007/s12041-009-0068-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cursiefen C, Kuchle M, Naumann GO. Angiogenesis in corneal diseases: histopathologic evaluation of 254 human corneal buttons with neovascularization. Cornea. 1998;17:611–613. doi: 10.1097/00003226-199811000-00008. [DOI] [PubMed] [Google Scholar]
- 3.Maddula S, Davis DK, Burrow MK, Ambati BK. Horizons in therapy for corneal angiogenesis. Ophthalmology. 2011;118:591–599. doi: 10.1016/j.ophtha.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein RJ, Stulting RD, Hendricks RL, Harris DM. Corneal neovascularization Pathogenesis and inhibition. Cornea. 1987;6:250–257. doi: 10.1097/00003226-198706040-00004. [DOI] [PubMed] [Google Scholar]
- 5.Jermak CM, Dellacroce JT, Heffez J, Peyman GA. Triamcinolone acetonide in ocular therapeutics. Surv Ophthalmol. 2007;52:503–522. doi: 10.1016/j.survophthal.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Jonas JB. Intravitreal triamcinolone acetonide: a change in a paradigm. Ophthalmic Res. 2006;38:218–245. doi: 10.1159/000093796. [DOI] [PubMed] [Google Scholar]
- 7.Jonas JB, Kreissig I, Degenring R. Intravitreal triamcinolone acetonide for treatment of intraocular proliferative, exudative, and neovascular diseases. Prog Retin Eye Res. 2005;24:587–611. doi: 10.1016/j.preteyeres.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Ebrahem Q, Minamoto A, Hoppe G, Anand-Apte B, Sears JE. Triamcinolone acetonide inhibits IL-6- and VEGF-induced angiogenesis downstream of the IL-6 and VEGF receptors. Invest Ophthalmol Vis Sci. 2006;47:4935–4941. doi: 10.1167/iovs.05-1651. [DOI] [PubMed] [Google Scholar]
- 9.Sears JE, Hoppe G. Triamcinolone acetonide destabilizes VEGF mRNA in Muller cells under continuous cobalt stimulation. Invest Ophthalmol Vis Sci. 2005;46:4336–4341. doi: 10.1167/iovs.05-0565. [DOI] [PubMed] [Google Scholar]
- 10.Wang Y, Fei D, Vanderlaan M, Song A. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7:335–345. doi: 10.1007/s10456-004-8272-2. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 12.Manzano RP, Peyman GA, Khan P, Carvounis PE, Kivilcim M, Ren M, et al. Inhibition of experimental corneal neovascularisation by bevacizumab (Avastin). Br J Ophthalmol. 2007;91:804–807. doi: 10.1136/bjo.2006.107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peters S, Heiduschka P, Julien S, Ziemssen F, Fietz H, Bartz-Schmidt KU, Schraermeyer U. Ultrastructural findings in the primate eye after intravitreal injection of bevacizumab. Am J Ophthalmol. 2007;143:995–1002. doi: 10.1016/j.ajo.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Bradley J, Ju M, Robinson GS. Combination therapy for the treatment of ocular neovascularization. Angiogenesis. 2007;10:141–148. doi: 10.1007/s10456-007-9069-x. [DOI] [PubMed] [Google Scholar]
- 15.Spaide RF. Rationale for combination therapies for choroidal neovascularization. Am J Ophthalmol. 2006;141:149–156. doi: 10.1016/j.ajo.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Mahoney JM, Waterbury LD. Drug effects on the neovascularization response to silver nitrate cauterization of the rat cornea. Curr Eye Res. 1985;4:531–535. doi: 10.3109/02713688508999984. [DOI] [PubMed] [Google Scholar]
- 17.Crum R, Szabo S, Folkman J. A new class of steroids inhibits angiogenesis in the presence of heparin or a heparin fragment. Science. 1985;230:1375–1378. doi: 10.1126/science.2416056. [DOI] [PubMed] [Google Scholar]
- 18.Benelli U, Bocci G, Danesi R, Lepri A, Bernardini N, Bianchi F, et al. The heparan sulfate suleparoide inhibits rat corneal angiogenesis and in vitro neovascularization. Exp Eye Res. 1998;67:133–142. doi: 10.1006/exer.1998.0512. [DOI] [PubMed] [Google Scholar]
- 19.Joussen AM, Kruse FE, Volcker HE, Kirchhof B. Topical application of methotrexate for inhibition of corneal angiogenesis. Graefes Arch Clin Exp Ophthalmol. 1999;237:920–927. doi: 10.1007/s004170050387. [DOI] [PubMed] [Google Scholar]
- 20.Deutsch TA, Hughes WF. Suppressive effects of indomethacin on thermally induced neovascularization of rabbit corneas. Am J Ophthalmol. 1979;87:536–540. doi: 10.1016/0002-9394(79)90245-9. [DOI] [PubMed] [Google Scholar]
- 21.Kenyon BM, Browne F, D'Amato RJ. Effects of thalidomide and related metabolites in a mouse corneal model of neovascularization. Exp Eye Res. 1997;64:971–978. doi: 10.1006/exer.1997.0292. [DOI] [PubMed] [Google Scholar]
- 22.Kang S, Chung SK. The effect of subconjuctival combined treatment of bevacizumab and triamcinolone acetonide on corneal neovascularization in rabbits. Cornea. 2011;29:192–196. doi: 10.1097/ICO.0b013e3181b1c82f. [DOI] [PubMed] [Google Scholar]
- 23.Hashemian MN, Z-Mehrjardi H, Moghimi S, Tahvildari M, Mojazi-Amiri H. Prevention of corneal neovascularization: comparison of different doses of subconjunctival bevacizumab with its topical form in experimental rats. Ophthalmic Res. 2011;46:50–54. doi: 10.1159/000322061. [DOI] [PubMed] [Google Scholar]
- 24.Shi W, Ming C, Liu J, Wang T, Gao H. Features of corneal neovascularization and lymphangiogenesis induced by different etiological factors in mice. Graefes Arch Clin Exp Ophthalmol. 2011;249:55–67. doi: 10.1007/s00417-010-1442-6. [DOI] [PubMed] [Google Scholar]
- 25.Pérez-Santonja JJ, Campos-Mollo E, Lledó-Riquelme M, Javaloy J, Alió JL. Inhibition of corneal neovascularization by topical bevacizumab (Anti-VEGF) and Sunitinib (Anti-VEGF and Anti-PDGF) in an animal model. Am J Ophthalmol. 2010;150:519–528. doi: 10.1016/j.ajo.2010.04.024. [DOI] [PubMed] [Google Scholar]
- 26.Murata M, Shimizu S, Horiuchi S, Taira M. Inhibitory effect of triamcinolone acetonide on corneal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2006;244:205–209. doi: 10.1007/s00417-005-0036-1. [DOI] [PubMed] [Google Scholar]


