Abstract
Intraocular pressure (IOP) is the only treatable risk factor for glaucoma. Yet, current glaucoma management usually relies on single IOP measurements during clinic hours despite the fact that IOP is a dynamic parameter with individual rhythms. Single IOP measurements underpin all major clinical guidelines on treatment of glaucoma. Other potentially informative parameters, such as IOP fluctuations and peak IOP, have been neglected, and effects of IOP-lowering interventions on such measures are largely unknown. The search for continuous 24-hour IOP monitoring started over 50 years ago, but only recent technological advances have provided clinician-researchers with devices for continuous IOP monitoring. Herein, we discuss innovative approaches with permanent and temporary devices for 24-hour IOP monitoring, such as a contact lens sensor. Despite being in their infancy, these devices may soon enable clinicians to use 24-hour IOP data to improve glaucoma management and reduce the glaucoma-related burden of disease.
Keywords: 24-hour, Intraocular Pressure, Glaucoma, Sensimed Triggerfish, Contact Lens Sensor
INTRODUCTION
Glaucoma is the leading cause of irreversible blindness worldwide. While elevated IOP is no longer part of the definition of glaucoma, it is recognized as the only modifiable risk factor for the development and progression of the disease.1 Reducing IOP below a clinically determined target level through either daily application of eye-drops, laser procedures, or surgical interventions is therefore the mainstay of glaucoma therapy.2 Goldmann applanation tonometry (GAT) is the most commonly used tonometric technique and is considered the “gold standard” for measuring IOP.3 This technique was described over half a century ago and largely thanks to its simplicity and elegance has remained unchanged. For GAT, the cornea is flattened over a defined area (3.06 mm in diameter) and the required applanation pressure is used to estimate IOP. It is based on the Imbert-Fick law, which assumes that the eyeball is a perfect sphere and the cornea a perfectly thin, elastic and flexible membrane.4 GAT is influenced by several ocular factors, such as central corneal thickness (CCT), corneal biomechanical properties and scleral rigidity.5,6 These variables can vary widely among individuals. The most significant shortcoming of GAT, however, is the static nature of its measurements, which represent a 1-2 second snapshot of an individual’s IOP, taken in the sitting position.
IOP is a dynamic parameter with a circadian rhythm and spontaneous changes.1 IOP fluctuations of as much as 4-5 mmHg in healthy individuals and substantially higher in some glaucoma patients have been commonly reported.7,8 There is evidence that single IOP measurements in the sitting position during normal office hours neither reflect the true range of an individual’s IOP9 nor peak IOP,10 or variation throughout the day. Studies that measure IOP several times over the entire day find that approximately two-thirds of glaucoma patients had their highest IOPs outside regular clinic hours, most frequently during the nocturnal/sleep period.8,9 It has been suggested that a sub-optimal approach to IOP assessment may account for nearly one-third of treated glaucoma patients showing progressive vision loss.11
Today, the most common method for studying glaucoma patients’ IOP rhythm is through a diurnal tension curve (DTC), which represents multiple IOP readings at different time points during clinic hours and does not account for IOP values during the night. Night-time IOP values can be obtained by hospitalization or within a sleep laboratory, but these are cumbersome, costly and require awakening of patients during the nocturnal/sleep period, potentially introducing stress-related artifacts.12 Consequently, it is estimated that less than 1% of glaucoma patients undergo a DTC or IOP monitoring in a sleep laboratory. Studies performed under the strictly controlled environment of sleep laboratories have shown that IOP in most glaucoma patients is at its highest during the nocturnal/sleep period with the patient in the supine body position.8,13 However, due to the unavailability of IOP-measuring techniques that do not disturb the normal sleep cycle, our understanding of the contribution of nocturnal IOP rhythms to the pathophysiology of glaucomatous optic nerve damage remains insufficient.14
Another contributor to glaucoma development and progression may be the variability of IOP itself. Studies have suggested that fluctuations of IOP are an independent risk factor for glaucoma progression.15,16 In addition to simple IOP reduction, IOP “modulation”, involving a thorough assessment of a patient’s IOP profile and targeted IOP-lowering treatment17, also has been suggested to reduce progression of the disease.
History of 24-Hour IOP Monitoring
The development of ambulatory, frequent, round-the-clock IOP measurement methods has been ongoing for several decades.18-20 These attempts have been pursuing three different strategies: 1) self-tonometry by the patient; 2) permanent IOP monitoring; and 3) temporary IOP monitoring. However, contrary to other fields of medicine, where ambulatory 24-hour monitoring of relevant biological parameters (eg., blood pressure, blood glucose) was introduced into clinical management, the quest for 24-hour IOP data has long been a story of set-backs.
Self-tonometry
Several devices for self-tonometry at home have been introduced in recent years.21-25 Each of them, however, seems to be inaccurate and technically challenging for many older glaucoma patients.26 Although viable self-tonometry does represent an important improvement to the status quo of tonometry and could be a valuable adjunct to office measurements, it does not address the crucial issue of IOP behaviour during undisturbed sleep. Furthermore, there may be safety, regulatory, and ethical concerns when patients measure their IOPs at home and feel empowered to take therapeutic decisions without prior consultation with their healthcare provider.
Permanent IOP monitoring
With permanent IOP monitoring, data are collected over an extended period of months or years. This approach involves the surgical implantation of an IOP sensor. The concept was pioneered half a century ago by Collins27 who proposed the use of a capacitive pressure sensor, consisting of a pair of parallel spiral coils within a gas-filled plastic “pill”. He demonstrated that changes in IOP would produce a compression of the gas bubble and a subsequent change in resonance frequency. This pressure sensor functioned wirelessly by means of a coupled magnetic field. Other investigators modified this approach by placing the pressure sensor in an intraocular lens (IOL), which could replace the natural lens in cataract surgery.28,29 However, it was only recently that advances in bio-engineering and nanotechnology have produced potentially viable approaches. Downs et al30 adapted an existing implantable telemetric pressure transducer system to monitor IOP in non-human primates. They showed that the system was able to provide accurate and continuous IOP monitoring for up to 7 months. However, implantation of the transducer system requires extensive surgical intervention involving the orbital bone and insertion of a tube inside the anterior chamber. Human data are currently not available. It further remains uncertain whether it would be readily accepted by clinicians and patients. Todani et al31, updating an earlier approach by Walter et al,29 were able to measure IOP continuously using a ring-shaped intraocular device placed in the lens capsule of rabbit eyes for up to 25 months. Their results are promising and data from human trials are eagerly awaited. The technology has been developed by a German company (Imlandata AG, Hannover, Germany) and is expected to be commercialized soon. Currently, the main limitation of all approaches to permanent continuous IOP monitoring is the safety associated with surgical implantation. Combining IOP-sensors with IOLs used in routine cataract surgery may facilitate patient acceptance. However, certain risks have to be addressed before these technologies can obtain regulatory approval for clinical use in humans. These include the potential for device failure after implantation, leakage of potentially toxic materials when hermeticity of the intraocular device is breached as well as inaccuracy of measurements due to signal drift over time with the necessity of subsequent intervention for re-calibration. Another drawback is that the group of patients who could benefit from this approach would be restricted to those requiring ocular surgery.
Temporary 24-Hour IOP Monitoring
Temporary IOP monitoring is a non-invasive alternative to the permanent approach and offers three potential advantages: 1) no surgical implantation, 2) easy reversibility and 3) widespread availability to patients. Maurice18 was largely ahead of his time, when he developed an automated recording indentation tonometer more than 50 years ago. The device was a bulky metallic structure fixed to the head of the patient. Due to its obvious impracticability, it was not pursued beyond the prototype. Greene and Gilman were the first to propose the use of a contact lens for IOP monitoring. They proposed embedding 2 strain gauges in a soft contact lens that could measure angular changes at the meridional angle of the corneoscleral junction secondary to IOP variations. The major drawback was that the contact lenses needed to be custom molded for each eye in order to detect small changes occurring at the meridional angle. The costs related to this approach made it unaffordable. In 2009, Twa et al32 proposed integrating the piezoresistive sensor tip of the dynamic contour tonometer (DTC) into a hard contact lens. DTC uses a piezoresistive sensor that provides measurements at a rate of 100 Hz. The sensor is located in a contoured probe, which is assumed to minimize the effect of corneal parameters on measurement errors. They showed that this approach provided reliable IOP measurements in a group of healthy volunteers for up to 100 seconds. Beyond the limited duration of measurements, however, there are other drawbacks of this approach, which include the use of a hard contact lens, patient discomfort, the location of the sensor tip in the center of the contact lens with resulting decrease of vision, and the use of a wire.
Leonardi et al33 updated the concept of soft contact lens embedded strain gauges and developed an approved commercial product. The SENSIMED Triggerfish® (Sensimed AG, Lausanne, Switzerland) disposable contact lens sensor (CLS) monitors IOP continuously. (Figure 1) The device is based on the assumption that small changes in ocular circumference measured at the corneoscleral junction correspond to changes in intraocular pressure and volume.34 The relationship between induced changes in IOP and the output signal of the CLS was previously validated using an in vitro model of cannulated porcine eyes.35 The disposable contact lens exists in 3 different base curves to enable good fit on the ocular surface. A good fit of the CLS is essential for accurate measurements of small changes in the cornea and avoidance of movement artifacts of the strain gauges. The software is designed to plot the 24-hour IOP pattern, whereas each data point can be inspected for ocular pulsation, including systolic and diastolic peaks, ocular pulsation amplitude, and ocular pulsation frequency as IOP fluctuates synchronically with heart rate. The output is an equivalent of the electric voltage (mV) measured due to conformal ocular dimensional changes at the corneoscleral junction that are transmitted to the recorder. The effect of IOP on the corneoscleral junction as measured by the CLS reflects the relationship of the pressure and the volume of the eye under the assumption that ocular fluids cannot be compressed. Despite conformal changes of intraocular structures, the total volume of the eye is not affected. The pressure is exerted equally in all directions of the eye’s external surface and is determined by the resistance to distension of the intraocular volume to these structures (e.g. cornea, sclera, etc). IOP changes are a consequence of volume changes of the eye contents and are partially determined by the resistance offered by the cornea and sclera to distension of the volume.
Figure 1.
The Triggerfish contact lens sensor embeds different intelligent elements, including strain gauges, antenna and a ASIC microchip, in a silicone shell.
Approximately 300 data points are acquired during a 30-second period, every 5 minutes, providing a total of 288 measurements over a 24-hour period. Recorded profiles are visualized graphically on a computer interface (Figure 2). The major value of the device is that it can record IOP fluctuations in an outpatient setting for up to 24 hours including during undisturbed sleep. Data from the device provides potentially useful complimentary information such as the effect of eye blinks and eye movements on IOP, which may serve as surrogate measures of ocular biomechanical parameters (Figure 3). Furthermore, the ocular pulse amplitude can be studied continuously. A major limitation, is the fact that the output signal is not provided in the habitual mmHg units, which underpin glaucoma diagnosis and management. Calibration of the CLS output to mmHg is a challenge as simultaneous use of the CLS and tonometry on the same eye is not feasible. Therefore, simultaneous comparison between the CLS and tonometry is to be done in the contralateral eye, despite the moderate relationship of IOP measurements between eyes.36 A better approach would be to compare IOP monitoring with the CLS and an implantable device in the same eye. This will be the subject of future research.
Figure 2.
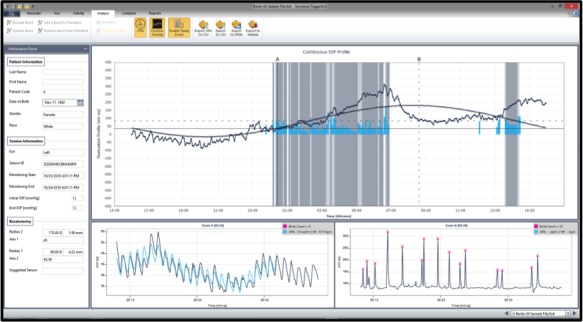
Example of 24-hour intraocular pressure monitoring with the contact lens sensor. Scales on the y-axis correspond to electric voltage changes in mVolts. The software provides automated analysis of acrophase (peak signal) and amplitude using cosinor rhythmometry. The blink detection software can be used to evaluate sleep times.
Figure 3.
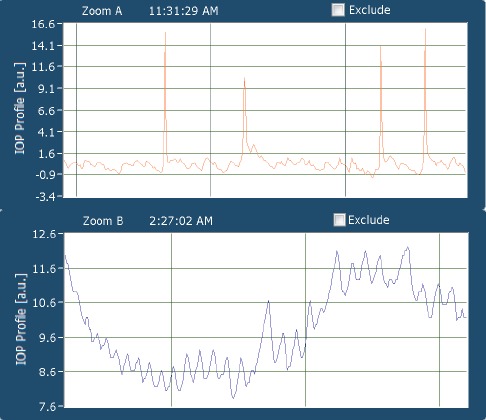
Detailed view of a 30 second window: the patient is awake, the large spikes correspond to effect of eye blinks on contact lens sensor output. Smaller changes correspond to ocular pulsations (top image). The patient is asleep, as evident through an absence of eye blinks. The software automatically adjusts the scale of the y-axis to smaller changes, which correspond to ocular pulsations (bottom image).
Safety and comfort of the CLS
Being integrated in a soft contact lens, this device may be subject to side effects known to occur with standard contact lenses used for vision correction. A recent study evaluated the safety and tolerability of 24-hour IOP recording by using the CLS in patients with suspected glaucoma and with POAG.37 All 40 patients, 21 suspected and 19 established glaucoma, with mean age of 55.5±15.7 years (60% male) were exposed to the CLS wear for 24 hours on the same eye during two sessions (S1 and S2) 7 day apart. The mean exposure duration to CLS wear did not differ between the sessions, 24.0±0.5 h and 24.0±0.3 h, respectively. Of the 149 device-related adverse events (AEs) that occurred in 38 patients (95%), 143 were considered to be mild (96%) in 36 patients (90%). The most common AEs were blurred vision (82%), conjunctival hyperemia 80% and superficial punctate keratitis (15%). Two moderate AEs (1.3%), superficial punctate keratitis and blurred vision, occurred each in 1 patient with POAG during S1. Two other patients with established glaucoma (5.1%) each had a severe AE (2.7%) during both sessions which was ocular hyperemia. The first clinical study that used the CLS in 10 healthy subjects for 24-hours did not report any severe AEs. All device-related AEs resolved within 48 hours.
Freiberg et al,38 studied the effect of overnight wear of the CLS on corneal thickness. They demonstrated a significant mean change from baseline in central corneal thickness (14.3±4.6 mm, P=0.015) but there was no difference between study and contralateral eyes (P=0.075). Hubanova et al,39 in an anterior segment OCT study, found that overnight changes in corneal curvature and thickness were small but significant between CLS-wearing and fellow eyes. The effect of these changes on the CLS output is currently unknown but is assumed to be of small impact overall.
Validation and Reproducibility of IOP Patterns
Whenever a new diagnostic device is introduced, it is essential to investigate its repeatability and reproducibility. Contrary to imaging techniques that measure anatomical parameters, such as the retinal nerve fiber layer thickness, which remain mostly unchanged throughout the circadian period, IOP is a highly dynamic parameter, which is strongly influenced by intrinsic and extrinsic factors in addition to its well-known circadian rhythm. Realini et al have shown that week-to-week repeatability of diurnal IOP measurements using GAT in healthy and glaucomatous individuals is moderate at best.40,41
The CLS has previously been validated ex-vivo in enucleated porcine eyes with good agreement of its output with manometric values.33 For practical reasons, mostly related to obtaining a good fit on the ocular surface, in-vivo manometric studies in animal models and patients are more difficult. Using the CLS, a study by our group found moderate agreement between 24-hour IOP patterns in glaucoma patients and suspects, when monitoring was repeated at a 1-week interval (r=0.59, Pearson correlation).37 Similar results were recently found by Mottet et al, in healthy young eyes.42
Clinical Impact of 24-Hour IOP Data
The availability of continuous 24-hour IOP monitoring signifies a paradigm shift in the field of glaucoma. (Table 1) A major challenge for the clinician is the analysis and interpretation of 24-hour IOP information obtained with the CLS. Generations of ophthalmologists had to rely on single IOP measurements for development of a treatment target and evaluation of treatment response. The translation into practice of the plethora of data provided by these new technologies poses a challenge for the clinician.
Table 1.
Potential benefits of 24-hour intraocular pressure (IOP) monitoring for glaucoma
| Impact | |
|---|---|
| 1. Detection and risk stratification | There may be inherent IOP patterns that predispose to glaucoma development and progression, independent of absolute IOP levels. |
| 2. Individualized management | Not all glaucoma patients respond to the same therapies in the same manner. Identifying the chronobiology of a patient’s IOP may be a useful guide in the selection of treatment as well as timing of application of drops. |
| 3. Improved adherence | Ability to visualize 24-hour IOP patterns and the impact of therapy on these is expected to improve patients’ understanding of glaucoma and adherence with drops. |
| 4. Behavioral changes | Identifying favorable and adverse behavioral and occupational patterns on IOP may have additional beneficial effects. outcomes. |
| 5. Prevention of progression | Personalized choice of glaucoma treatment according to each patient’s 24-hour IOP rhythm may reduce the rate of change in glaucoma and improve treatment |
The challenge is compounded by the fact that output signal is not displayed in mmHg but in mV. We have recently reported on the use of modified cosinor rhythmometry for the analysis of 24-hour IOP patterns obtained with the CLS.43 Applying this modelling to the CLS output simplifies interpretation of data by providing a few key parameters of the circadian IOP rhythm: acrophase and bathyphase (timing of peak and trough IOP) as well as IOP amplitude. We found that 62.9% of glaucoma patients had a repeatable nocturnal acrophase (e.g. occurring during sleep). Mottet et al,42 using a similar methodology, found similar results of repeat 24-hour CLS monitoring in healthy subjects. They found significant intraclass coefficients of the CLS acrophase (0.6 [0-0.9, 95% CI]; P=0.03), with fair to good agreement.
The study of IOP patterns independent of absolute IOP levels may be of prognostic relevance for glaucoma. Grippo et al,44 recently found inherently different IOP patterns between patients with ocular hypertension who subsequently developed glaucoma versus those who did not. If their findings can be reproduced in other populations, it could be the harbinger of personalized management of glaucoma patients.
The Road Ahead
Currently, the CLS is the only commercially available device that provides 24-hour on IOP fluctuations. In near future, other approaches are expected to enter the market and provide different solutions for subsets of glaucoma patients. Before these data can be widely translated into clinical practice, studies will have to elucidate the significance of 24-hour patterns, the role of IOP-lowering medications on IOP patterns, and nocturnal IOP changes on glaucoma development and progression. As IOP patterns are not necessarily conserved from one day to another, even in healthy individuals,41 the frequency at which 24-hour monitoring should be repeated in glaucoma patients is unknown. The fact that the CLS provides its values in an arbitrary unit (instead of mmHg) makes the clinical interpretation challenging. It is hoped that in near future, algorithms will be developed that translate these units into a more clinically relevant form. Another important issue is affordability of the technology. The current commercial price is around 500 Euros per eye per 24-hour period. Although still more economical than the costs associated with a sleep laboratory or hospitalization, it may limit the wide use of this technology. Prospective data are required to show how 24-hour IOP data can improve the long-term prognosis of glaucoma.
Footnotes
Conflicts of Interest
Consultant (Sensimed AG).
REFERENCES
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–1720. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Weinreb RN, Brandt J, Garway-Heath T, Medeiros FA. Intraocular Pressure. Amsterdam: Kugler Publications; 2007. [Google Scholar]
- 3.Goldmann H. [Not Available]. Bull Mem Soc Fr Ophtalmol. 1954;67:474–477. [PubMed] [Google Scholar]
- 4.Whitacre MM, Stein R. Sources of error with use of Goldmann-type tonometers. Surv Ophthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-a. [DOI] [PubMed] [Google Scholar]
- 5.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh) 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Roberts CJ. Influence of corneal biomechanical properties on intraocular pressure measurement: quantitative analysis. J Cataract Refract Surg. 2005;31:146–155. doi: 10.1016/j.jcrs.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Liu JH, Kripke DF, Twa MD, Hoffman RE, Mansberger SL, Rex KM, et al. Twenty-four-hour pattern of intraocular pressure in the aging population. Invest Ophthalmol Vis Sci. 1999;40:2912–2917. [PubMed] [Google Scholar]
- 8.Liu JH, Zhang X, Kripke DF, Weinreb RN. Twenty-four-hour intraocular pressure pattern associated with early glaucomatous changes. Invest Ophthalmol Vis Sci. 2003;44:1586–1590. doi: 10.1167/iovs.02-0666. [DOI] [PubMed] [Google Scholar]
- 9.Barkana Y, Anis S, Liebmann J, Tello C, Ritch R. Clinical utility of intraocular pressure monitoring outside of normal office hours in patients with glaucoma. Arch Ophthalmol. 2006;124:793–797. doi: 10.1001/archopht.124.6.793. [DOI] [PubMed] [Google Scholar]
- 10.Mosaed S, Liu JH, Weinreb RN. Correlation between office and peak nocturnal intraocular pressures in healthy subjects and glaucoma patients. Am J Ophthalmol. 2005;139:320–324. doi: 10.1016/j.ajo.2004.09.062. [DOI] [PubMed] [Google Scholar]
- 11.Hattenhauer MG, Johnson DH, Ing HH, Herman DC, Hodge DO, Yawn BP, et al. The probability of blindness from open-angle glaucoma. Ophthalmology. 1998;105:2099–2104. doi: 10.1016/S0161-6420(98)91133-2. [DOI] [PubMed] [Google Scholar]
- 12.Liu JH, Weinreb RN. Monitoring intraocular pressure for 24 h. Br J Ophthalmol. 2011;95:599–600. doi: 10.1136/bjo.2010.199737. [DOI] [PubMed] [Google Scholar]
- 13.Liu JH, Medeiros FA, Slight JR, Weinreb RN. Diurnal and nocturnal effects of Brimonidine monotherapy on intraocular pressure. Ophthalmology. 2010;117:2075–2079. doi: 10.1016/j.ophtha.2010.03.026. [DOI] [PubMed] [Google Scholar]
- 14.Weinreb RN, Liu JH. Nocturnal rhythms of intraocular pressure. Arch Ophthalmol. 2006;124:269–270. doi: 10.1001/archopht.124.2.269. [DOI] [PubMed] [Google Scholar]
- 15.Mansouri K, Orguel S, Mermoud A, Haefliger I, Flammer J, Ravinet E, et al. Quality of diurnal intraocular pressure control in primary open-angle patients treated with latanoprost compared with surgically treated glaucoma patients: a prospective trial. Br J Ophthalmol. 2008;92:332–336. doi: 10.1136/bjo.2007.123042. [DOI] [PubMed] [Google Scholar]
- 16.Mansouri K, Medeiros FA, Weinreb RN. Letter to the editor: 24-hour versus daytime intraocular pressure phasing in the management of patients with treated glaucoma. Br J Ophthalmol. 2011;95:594–595. doi: 10.1136/bjo.2010.201327. [DOI] [PubMed] [Google Scholar]
- 17.Caprioli J, Varma R. Intraocular pressure: modulation as treatment for glaucoma. Am J Ophthalmol. 2011;152:340–344. doi: 10.1016/j.ajo.2011.05.029. [DOI] [PubMed] [Google Scholar]
- 18.Maurice DM. A recording tonometer. Br J Ophthalmol. 1958;42:321–335. doi: 10.1136/bjo.42.6.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schnell CR, Debon C, Percicot CL. Measurement of intraocular pressure by telemetry in conscious, unrestrained rabbits. Invest Ophthalmol Vis Sci . 1996;37:958–965. [PubMed] [Google Scholar]
- 20.Akaishi T, Ishida N, Shimazaki A, Hara H, Kuwayama Y. Continuous monitoring of circadian variations in intraocular pressure by telemetry system throughout a 12-week treatment with timolol maleate in rabbits. J Ocul Pharmacol Ther. 2005;21:436–444. doi: 10.1089/jop.2005.21.436. [DOI] [PubMed] [Google Scholar]
- 21.Kothy P, Vargha P, Hollo G. Ocuton-S self-tonometry vs. Goldmann tonometry; a diurnal comparison study. Acta Ophthalmol Scand. 2001;79:294–297. doi: 10.1034/j.1600-0420.2001.790317.x. [DOI] [PubMed] [Google Scholar]
- 22.Lam DS, Leung DY, Chiu TY, Fan DS, Cheung EY, Wong TY, et al. Pressure phosphene self-tonometry: a comparison with goldmann tonometry in glaucoma patients. Invest Ophthalmol Vis Sci. 2004;45:3131–3136. doi: 10.1167/iovs.04-0115. [DOI] [PubMed] [Google Scholar]
- 23.Tai MC, Chen PL, Wu JN, Lu DW. Clinical evaluation of the intraocular pressure in patients with glaucoma or ocular hypertension by a self-assessable tonometer. J Ocul Pharmacol Ther. 2005;21:55–61. doi: 10.1089/jop.2005.21.55. [DOI] [PubMed] [Google Scholar]
- 24.Wilensky JT, Gieser DK, Mori MT, Langenberg PW, Zeimer RC. Self-tonometry to manage patients with glaucoma and apparently controlled intraocular pressure. Arch Ophthalmol. 1987;105:1072–1075. doi: 10.1001/archopht.1987.01060080074031. [DOI] [PubMed] [Google Scholar]
- 25.Liang SY, Lee GA, Shields D. Self-tonometry in glaucoma management--past, present and future. Surv Ophthalmol. 2009;54:450–462. doi: 10.1016/j.survophthal.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 26.Tarkkanen A, Ulfves K, Ulfves T. Self-tonometry in glaucoma. Graefes Arch Clin Exp Ophthalmol. 2010;248:1679–1681. doi: 10.1007/s00417-010-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins CC. Miniature passive pressure transensor for implanting in the eye. IEEE Trans Biomed Eng. 1967;14:74–83. doi: 10.1109/tbme.1967.4502474. [DOI] [PubMed] [Google Scholar]
- 28.Svedbergh B, Backlund Y, Hok B, Rosengren L. The IOP-IOL. A probe into the eye. Acta Ophthalmol (Copenh) 1992;70:266–268. doi: 10.1111/j.1755-3768.1992.tb04135.x. [DOI] [PubMed] [Google Scholar]
- 29.Walter P, Schnakenberg U, vom Bögel G, Ruokonen P, Krüger C, Dinslage S, et al. Development of a completely encapsulated intraocular pressure sensor. Ophthalmic Res. 2000;32:278–284. doi: 10.1159/000055626. [DOI] [PubMed] [Google Scholar]
- 30.Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011;52:7365–7375. doi: 10.1167/iovs.11-7955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Todani A, Behlau I, Fava MA, Cade F, Cherfan DG, Zakka FR, et al. Intraocular pressure measurement by radio wave telemetry. Invest Ophthalmol Vis Sci. 2011;52:9573–9580. doi: 10.1167/iovs.11-7878. [DOI] [PubMed] [Google Scholar]
- 32.Twa MD, Roberts CJ, Karol HJ, Mahmoud AM, Weber PA, Small RH. Evaluation of a contact lens-embedded sensor for intraocular pressure measurement. J Glaucoma. 2010;19:382–390. doi: 10.1097/IJG.0b013e3181c4ac3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leonardi M, Pitchon EM, Bertsch A, Renaud P, Mermoud A. Wireless contact lens sensor for intraocular pressure monitoring: assessment on enucleated pig eyes. Acta Ophthalmol. 2009;87:433–437. doi: 10.1111/j.1755-3768.2008.01404.x. [DOI] [PubMed] [Google Scholar]
- 34.Hjortdal JO, Jensen PK. In vitro measurement of corneal strain, thickness, and curvature using digital image processing. Acta Ophthalmol Scand. 1995;73:5–11. doi: 10.1111/j.1600-0420.1995.tb00004.x. [DOI] [PubMed] [Google Scholar]
- 35.Leonardi M, Leuenberger P, Bertrand D, Bertsch A, Renaud P. First steps toward noninvasive intraocular pressure monitoring with a sensing contact lens. Invest Ophthalmol Vis Sci. 2004;45:3113–3117. doi: 10.1167/iovs.04-0015. [DOI] [PubMed] [Google Scholar]
- 36.Sit AJ, Liu JH, Weinreb RN. Asymmetry of right versus left intraocular pressures over 24 hours in glaucoma patients. Ophthalmology. 2006;113:425–430. doi: 10.1016/j.ophtha.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 37.Mansouri K, Medeiros FA, Tafreshi A, Weinreb RN. Continuous 24-hour monitoring of intraocular pressure patterns with a contact lens sensor: safety, tolerability, and reproducibility in patients with glaucoma. Arch Ophthalmol. 2012;130:1534–1539. doi: 10.1001/jamaophthalmol.2013.1350. [DOI] [PubMed] [Google Scholar]
- 38.Freiberg FJ, Lindell J, Thederan LA, Leippi S, Shen Y, Klink T. Corneal thickness after overnight wear of an intraocular pressure fluctuation contact lens sensor. Acta Ophthalmol. 2012;90:e534–539. doi: 10.1111/j.1755-3768.2012.02495.x. [DOI] [PubMed] [Google Scholar]
- 39.Hubanova R, Aptel F, Chiquet C, Mottet B, Romanet JP. Effect of overnight wear of the Triggerfish sensor on corneal thickness measured by Visante anterior segment optical coherence tomography. Acta Ophthalmol. 2013;92:e119–123. doi: 10.1111/aos.12241. [DOI] [PubMed] [Google Scholar]
- 40.Realini T, Weinreb RN, Wisniewski S. Short-term repeatability of diurnal intraocular pressure patterns in glaucomatous individuals. Ophthalmology. 2011;118:47–51. doi: 10.1016/j.ophtha.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 41.Realini T, Weinreb RN, Wisniewski SR. Diurnal intraocular pressure patterns are not repeatable in the short term in healthy individuals. Ophthalmology. 2010;117:1700–1704. doi: 10.1016/j.ophtha.2010.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mottet B, Aptel F, Romanet JP, Hubanova R, Pepin JL, Chiquet C. 24-Hour intraocular pressure rhythm in young healthy subjects evaluated with continuous monitoring using a contact lens Sensor. JAMA Ophthalmol. 2013;131:1507–1516. doi: 10.1001/jamaophthalmol.2013.5297. [DOI] [PubMed] [Google Scholar]
- 43.Mansouri K, Liu JH, Weinreb RN, Tafreshi A, Medeiros FA. Analysis of continuous 24-h intraocular pressure patterns in glaucoma. Invest Ophthalmol Vis Sci. 2012;53:8050–8056. doi: 10.1167/iovs.12-10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grippo TM, Liu JH, Zebardast N, Arnold TB, Moore GH, Weinreb RN. Twenty-four-hour pattern of intraocular pressure in untreated patients with ocular hypertension. Invest Ophthalmol Vis Sci. 2013;54:512–517. doi: 10.1167/iovs.12-10709. [DOI] [PMC free article] [PubMed] [Google Scholar]



