Abstract
We investigated the clinico-biological features, outcomes, and prognosis of 949 patients with chronic lymphocytic leukemia according to age. No biological differences (cytogenetics by fluorescent in situ hybridization, IGHV, ZAP-70, CD38, NOTCH1, SF3B1) were found across age groups. Elderly patients (>70 years; n=367) presented more frequently with advanced disease (Binet C/Rai III-IV: 10/12% versus 5/5%; P<0.001), were treated less frequently (23.8% versus 41.9% at 3 years; P<0.001) and in most cases did not receive highly effective regimens and thus had a lower overall response rate (49% with 14% having complete responses versus 69% with 31% having complete responses; P<0.001). The elderly patients also had a shorter overall survival (6.6 versus 13.3 years; P<0.001) and higher disease-unrelated mortality (34.9% versus 6.9% at 10 years; P<0.001). However, disease-attributable mortality was not significantly different between younger and older patients. A combination of Binet stage, ZAP-70 level, β2-microglobulin concentration and comorbidity identified two risk groups (low-risk: 0–1 parameters; high-risk: 2–4 parameters) with different overall survivals (median: 6.8 versus 11.4 years, P<0.001). In patients requiring treatment, comorbidity at treatment (Cumulative Illness Rating Scale-T>4; hazard ratio 2.2, P<0.001) and response (treatment failure versus response: hazard ratio 1.60, P<0.04) were the most important prognostic factors for overall survival. In conclusion, in our series, elderly patients with chronic lymphocytic leukemia did not present with any biological features distinct from those of younger patients, but did have a poorer clinical outcome. This study highlights the importance of comprehensive medical care, achieving response to therapy, and specific management strategies for elderly patients with chronic lymphocytic leukemia.
Introduction
Chronic lymphocytic leukemia (CLL), which is the most frequent form of leukemia in Western countries, predominantly affects the elderly.1,2 Chemoimmunotherapy is considered the standard therapy for CLL.3,4 Unfortunately, most elderly patients do not tolerate intensive therapy, thus leaving them without effective treatment.5 In this context, new agents which target specific CLL pathogenic pathways (e.g., BCR-signal inhibitors) and have a good safety profile could represent important progress in the treatment of CLL in the elderly.6–8 A better understanding of the disease characteristics and prognostic factors in elderly patients is also important for improving the outcome of this group.
Here we report the clinico-biological characteristics of a large, single-institution series of subjects with CLL according to age-groups and focus on the outcomes and prognosis of elderly subjects (≥70 years) with CLL.
Methods
Patients and study design
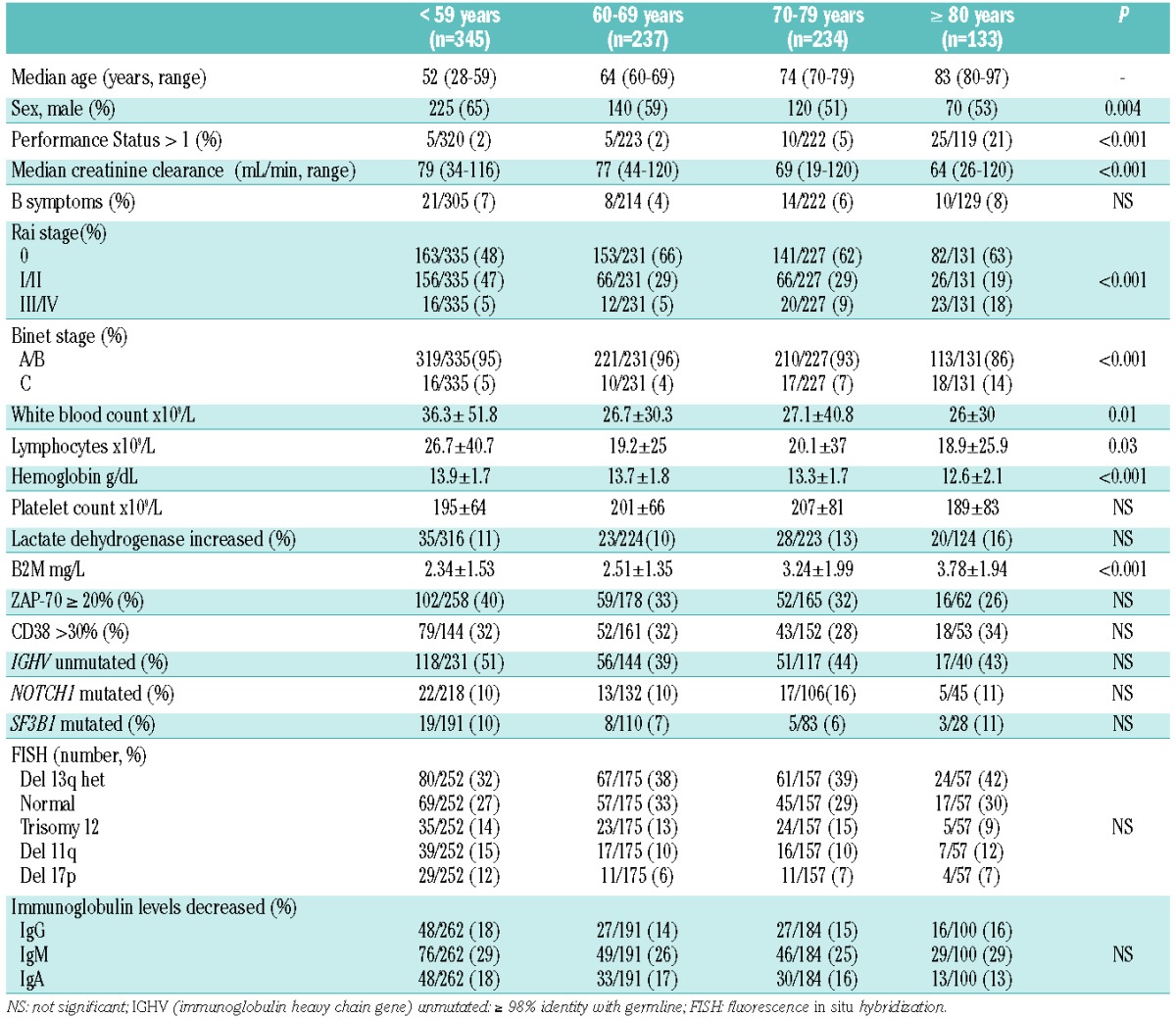
In total, 949 patients diagnosed with CLL from January 1990 to December 2012 at the Hospital Clínic of Barcelona were evaluated. The study was approved by the local Institutional Review Board and was performed in accordance with the Declaration of Helsinki. Four groups of patients were distinguished: <59 years old (n=345), 60–69 years old (n=237), 70–79 years old (n=234) and ≥80 years old (n=133). Data collected at diagnosis included age, sex, functional status, Binet and Rai stage, absolute lymphocyte count, immunoglobulin levels, cytogenetic abnormalities determined by fluorescent in situ hybridization (FISH), β2-microglobulin (B2M) and lactate dehydrogenase levels, ZAP-70 and CD38 expression, IGHV mutational status and NOTCH1 and SF3B1 mutations, using previously described methods.9–11 Due to the retrospective nature of the study not all variables were available for all patients.
Treatment modalities varied over the years. Renal function at diagnosis was estimated by calculation of the creatinine clearance using the Modification of Diet in Renal Disease (MDRD) formula. For patients aged ≥70 years, the Cumulative Illness Rating Scale (CIRS) was retrospectively assessed at the time of diagnosis (CIRS-D) and first-line treatment (CIRS-T).12,13 The assessment of the CIRS is detailed in the Online Supplementary Appendix.
Statistical methods
Baseline characteristics of patients of different age groups were compared using the χ2 or ANOVA test as appropriate. Cut-off levels for CIRS-D and CIRS-T were determined using maximally selected rank statistics. Time to first treatment was defined as time from diagnosis to date of initiation of first treatment or last follow-up. Overall survival was defined as the time between diagnosis and the date of death or last follow-up using the Kaplan-Meier method. Comparisons between different age groups and other covariates were performed by means of the log-rank test. Relative survival was calculated comparing the actuarial survival with that of the Spanish population matched by age, sex and calendar year of diagnosis. In patients aged 70 or older, multivariate analysis of prognostic factors for time to first treatment, overall survival and survival from first treatment was performed with Cox regression models after multiple imputation of missing data.14 IGHV, NOTCH1 and SF3B1 mutations were not included in the multivariate analysis because of the high number of missing data (>50%).
To avoid a bias in favor of responders, the impact of response on the overall survival was determined by a landmark analysis (9 months after treatment initiation).15 Unadjusted P-values <0.05 were considered statistically significant. Statistical methods are detailed in the Online Supplementary Appendix.
Results
Patients’ characteristics
The median age at diagnosis of the whole series of 949 patients was 65 years (range, 28–97), with 367/949 (39%) being 70 years of age or older. The median follow-up was 7.8 years. The main characteristics according to age groups are shown in Table 1. In summary, older patients (>70 years) presented with a poorer performance status, worse renal function and a more advanced stage of disease than did younger ones.
Table 1.
Characteristics of 949 patients with CLL according to age.
Treatment
The cumulative proportion of patients receiving therapy was significantly lower for elderly patients [23.8% (19.5–28.3%) versus 41.9% (37.7–46%) at 3 years and 42.7 (37.1–48.3%) versus 63.3% (58.7–67.7%) at 10 years, P<0.001].
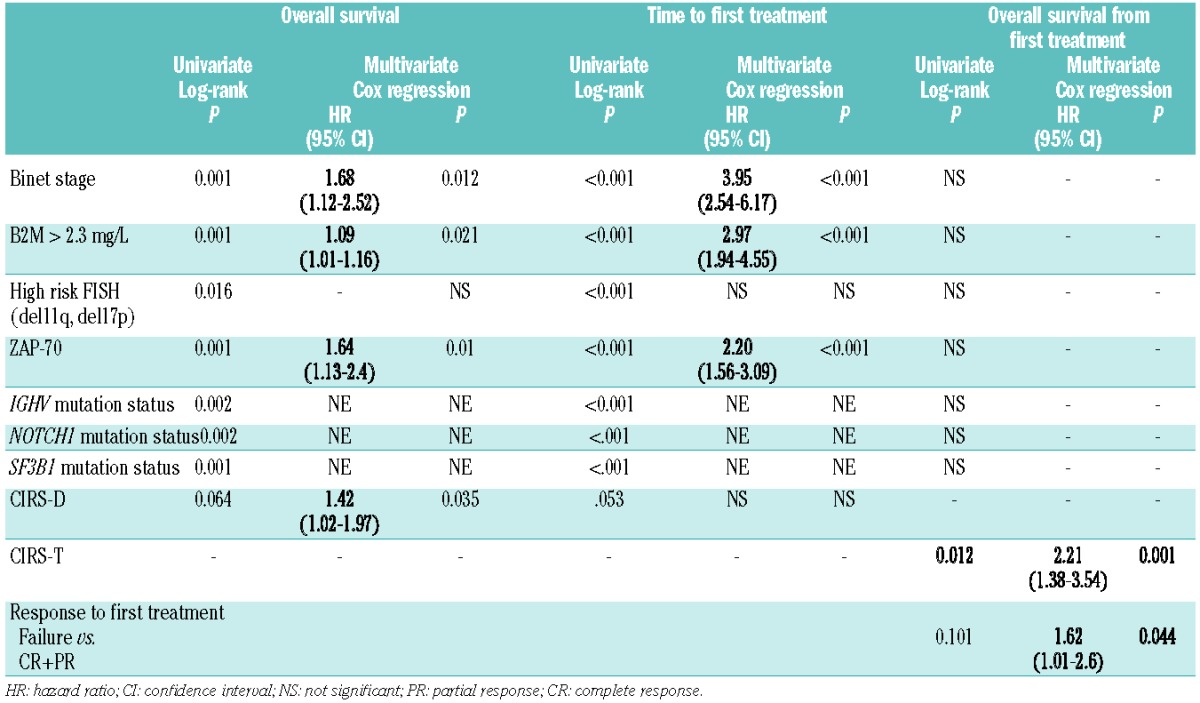
In the univariate analysis, parameters correlated with a shorter time to first treatment were advanced clinical stage (P<0.001), increased B2M (P<0.001), high-risk FISH cytogenetics as defined by the presence of 11q- or 17p deletion (P<0.001), increased ZAP-70 (P<0.001), unmutated IGHV (P<0.001), and NOTCH1 (P<0.001) and SF3B1 (P<0.001) mutations. Patients with a CIRS higher than 6 (CIRS-D>6) tended to have a longer time to first treatment (P=0.053). In the multivariate analysis clinical stage, B2M, and ZAP-70 retained prognostic significance (Table 2).
Table 2.
Hazard ratios and 95% confidence intervals for overall survival, time to first treatment and overall survival from first treatment according to clinico-biological features in patients with CLL aged 70 years or older.
Elderly patients were more likely to initially receive alkylating agents (70% versus 36%) than purine analogs (10% versus 32%) or chemoimmunotherapy (5% versus 25%) (P<0.001 for all comparisons) and their response to therapy was lower (overall response rate 49% versus 69%; P<0.001; complete response 14% versus 31%; P<0.001). Moreover, a higher proportion of elderly patients failed to benefit from therapy (24% versus 16%; P<0.001).
Overall survival
The median overall survival was significantly shorter in elderly patients (6.6 versus 13.3 years; P<0.001, Figure 1). However, when adjusted for age, sex and year of diagnosis, CLL-attributable mortality was similar in both cohorts: 3.7% versus 5.2% at 3 years, and 28.8% versus 30.2% at 10 years. CLL-unrelated mortality was significantly higher in elderly patients [12% (8.6–15.4%) versus 1.5% (.5–2.5%) at 3 years; 35% (29.5–40.5%) versus 7% (4.6–9.4%) at 10 years; P<0.001].
Figure 1.
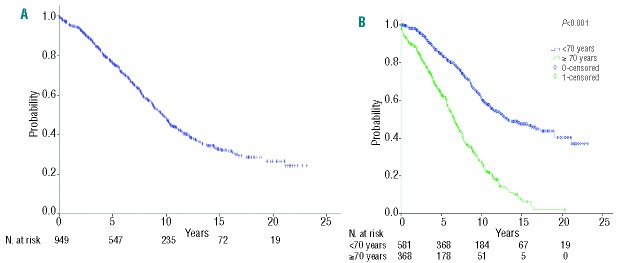
Overall survival of the (A) whole series (median 9.7 years), and (B) according to age groups (<70 vs. ≥70 years) in patients with CLL (median 6.6 vs. 13.3 years; P<0.001).
Variables associated with overall survival in elderly patients are shown in Table 2 and Figure 2A–D. In this cohort, beside standard prognostic variables, comorbidity was also evaluated. The median comorbidity burden at diagnosis (CIRS-D) was 5 (range, 0–17). Elderly patients with a CIRS-D >6 tended to have a shorter overall survival (univariate analysis, P=0.064) (Figure 2D). The multivariate analysis showed independent prognostic value for clinical stage, B2M, ZAP-70, and comorbidity. High-risk FISH cytogenetics did not enter into the prognostic model.
Figure 2.
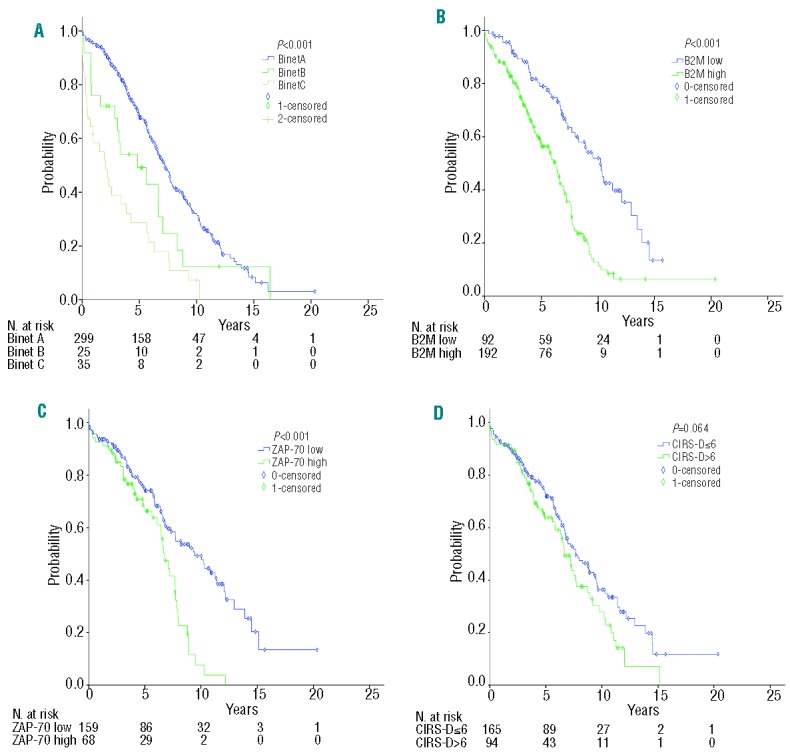
Estimates of overall survival since diagnosis for patients with CLL >70 years based on (A) Binet clinical stage (A, B, C) (median 7.2 vs. 4.9 vs. 2.1 years; P<0.001), (B) serum levels of β2-microglobulin (B2M) (median 10.2 vs. 6.2 years; P<0.001), (C) ZAP-70 expression (median 9.4 vs. 7.7 years; P<0.001), and (D) comorbidity assessed by CIRS at diagnosis (CIRS-D) (median 7.7 vs. 6.7 years; P=0.064). Univariate analysis was performed using the Kaplan-Meier method and log-rank test; P<0.05.
Based on the results of the multivariate analysis we tentatively designed a prognostic model including clinical stage (Binet A versus B/C), B2M (<2.3 mg/dL versus ≥2.3 mg/dL), ZAP-70 (<20% versus ≥20%), and comorbidity (CIRS-D ≤6 versus >6). This model identified two prognostic groups with significantly different survival [low risk (0–1 parameters), median overall survival 11.4 years versus high risk (2–4 parameters), median overall survival 6.8 years; P<0.001] (Figure 3).
Figure 3.
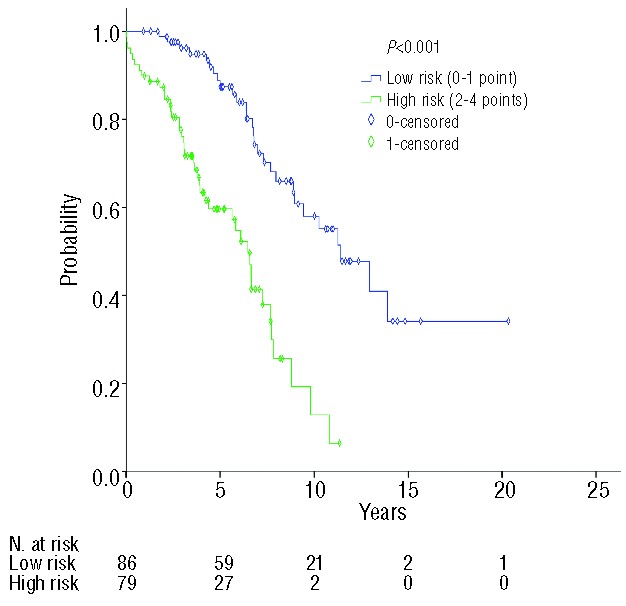
Two different prognostic groups (low vs. high risk with median survivals of 11.4 vs. 6.8 years; P<0.001) of elderly patients with CLL based on four parameters of independent prognostic value for overall survival by multivariate analysis (advanced Binet clinical stage, increased levels of B2M, high expression of ZAP-70 and CIRS-D >6).
Finally, we evaluated the prognosis of patients aged ≥70 years requiring treatment using a landmark analysis (n=115). To this end we reassessed CIRS at treatment (CIRS-T) which was comparable to that at diagnosis (median CIRS-D: 5; range, 0–17; median CIRS-T: 5; range, 0–15). There was a correlation between the overall response rate and CIRS-T (45% for patients with CIRS-T >4 versus 55% for patients with CIRS-T ≤4; P=0.049). The univariate analysis revealed a significant correlation between overall survival and comorbidity at treatment (CIRS-T >4; P=0.012) while there was a tendency towards a correlation between response to treatment and overall survival (P<0.101). In the univariate analysis, clinical stage and other variables such as B2M, high-risk FISH cytogenetics, ZAP-70, CD38, mutational status of IGHV and NOTCH1 and SF3B1 mutations did not show significant correlations with survival after therapy. In adjusted analysis, only response to therapy and comorbidity showed independent prognostic value for overall survival (CIRS-T >4; hazard ratio 2.21; P=0.001 and treatment failure versus response to treatment; hazard ratio 1.62; P=0.044) (Table 2).
Discussion
The definition of elderly subjects is arbitrary and, as a result of the increasing life-expectancy of the general population, submitted to continuous revision. In our study, we defined elderly CLL patients as those ≥70 years of age, a cut-off that separated patients with marked differences in overall survival. Patients ≥70 years of age accounted for 39% of the series, a proportion which has been maintained constant over the years (data not shown). This percentage is similar to that found in other studies16 but smaller than that reported by the SEER (54%),1 which most likely reflects differences between data from academic centers and population-based data. While the well-known male predominance in patients with CLL was corroborated in the overall series (58%), it was not observed in subjects ≥70 years of age (52%). The reasons for this disparity could include differences in life expectancy (longer in women) and differences in access to medical care. In those patients for whom information was available, biological parameters such as ZAP-70, CD38, FISH cytogenetics, and IGHV mutational status or NOTCH1 and SF3B1 mutations (Table 1) were not differently distributed across age groups. Not surprisingly, elderly CLL patients presented with a poorer performance status and worse renal function than younger ones, which limits treatment with intensive regimens.
There are few studies investigating the prognostic impact of comorbidity in CLL.17–19 In a pooled analysis comprising 555 patients comorbidity had an independent impact on overall survival (71.7 versus 90.2 months; P<0.001) due to higher therapy-related, CLL-unrelated, and, particularly, CLL-related deaths.19 In the present study we assessed comorbidity by CIRS12,13 at diagnosis (CIRS-D) and prior to first treatment (CIRS-T). No correlation was found between comorbidity and performance status, which is in agreement with other studies.13 However, we detected a relationship between overall response rate and comorbidity at time of treatment (CIRS-T), which is in line with other studies.18,19 A moderate to severe comorbidity burden (CIRS-D >6 and CIRS-T >4) was strongly associated with shorter overall survival. Of note, the cutoff that better correlated with overall survival (CIRS-T >4) was different from that in other studies.20 This indicates that the significant comorbidity index regarding prognosis can vary depending on the population and emphasizes the need to standardize comorbidity assessment in patients with CLL.
Clinical stage, B2M level and ZAP-70 expression were identified as predictors for time to first treatment while other parameters significant in univariate analysis, including FISH cytogenetics, did not retain prognostic significance. In agreement with other reports,16,21 elderly patients were treated less frequently than younger ones. There are various potential reasons for this, including less aggressive disease21 and a more, not pre-determined conservative approach to the management of older subjects. Also, most elderly patients were given no effective therapies, which resulted in a lower overall response rate. Although age by itself has been associated with a lower response rate,3,4,22–24 the small number of elderly patients treated with fludarabine-based therapy (15%) precludes a meaningful analysis of treatment effectiveness according to age. As shown by others,16,25,26 biomarkers were not correlated with response. This is not surprising because predictive biomarkers have been mainly identified in younger patients treated with effective therapies.3,4 It should not, however, be concluded that predictive markers are unnecessary in elderly subjects. On the contrary, predictive markers need to be studied prospectively, as done in recent studies.20,27 One point to be taken into account is that, because of the differences in the mechanisms of action of antileukemic drugs, predictive markers for patients treated with new agents could be different from those identified for patients treated with fludarabine-based therapy.
The advent of new agents (e.g., ibrutinib and idelalisib) and monoclonal antibodies (e.g., obinutuzumab) for CLL therapy is likely to change treatment paradigms in this form of leukemia, particularly in elderly subjects.6–8,20,28,29 Several trials investigating treatment with oral kinase inhibitors as single agents6,8 and in combination regimens7,28 have shown that these drugs can provide long-lasting disease control. Moreover, new monoclonal anti bodies in combination with chlorambucil produced a substantial proportion of deep responses with an acceptable safety profile.20,29 Further clinical trials in well stratified patients are warranted. To that end, we devised a prognostic model comprising information on clinical stage, comorbidity, B2M, and ZAP-70 which discriminated elderly patients with CLL into two risk groups with significant differences in survival (Figure 3). This prognostic model should be validated in other independent series and the relative merits of different biomarkers elucidated.30 Furthermore, the possibility that new compounds overcome or mitigate the negative influence of some parameters should be taken into account, and outcome predictors refined based upon the discovery of biomarkers and progresses in treatment.
Footnotes
Presented in part at the 55th Annual Meeting of the American Society of Hematology, December 7-10, 2013, New Orleans, LA, USA.
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by the Red Temática de Investigación Cooperativa en Cáncer RT 06/0020/002051 and RD12/0036/0023 grants and by research funding from the Spanish Ministry of Science and Innovation (MICINN) through the Instituto de Salud Carlos III (ISCIII) FISS PI080304 and ICGC-CLL Genome Project, Generalitat de Catalunya 2009SGR1008, and CLL Global Foundation. TB was supported by an “Emili Letang” grant; EM is a recipient of the “August Pi i Sunyer” award.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.National Cancer Institute: Surveillance, Epidemiology, and End Results: Populations (1969–2010). http://seer.cancer.gov/
- 2.Sant M, Allemani C, Tereanu C, De Angelis R, Capocaccia R, Visser O, et al. Incidence of hematologic malignancies in Europe by morphologic subtype: results of the HAEMACARE project. Blood. 2010;116(19):3724–34 [DOI] [PubMed] [Google Scholar]
- 3.Tam CS, O’Brien S, Wierda W, Kantarjian H, Wen S, Do K-A, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112(4):975–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74 [DOI] [PubMed] [Google Scholar]
- 5.Eichhorst B, Goede V, Hallek M. Treatment of elderly patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2009;50(2):171–8 [DOI] [PubMed] [Google Scholar]
- 6.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369(1):32–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370(11):997–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: an open-label, multicentre, phase 1b/2 trial. Lancet Oncol. 2014;15(1):48–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. N Engl J Med. 2003;348(18):1764–75 [DOI] [PubMed] [Google Scholar]
- 10.Puente XS, Pinyol M, Quesada V, Conde L, Ordóñez GR, Villamor N, et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature. 2011;475(7354):101–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quesada V, Conde L, Villamor N, Ordóñez GR, Jares P, Bassaganyas L, et al. Exome sequencing identifies recurrent mutations of the splicing factor SF3B1 gene in chronic lymphocytic leukemia. Nat Genet. 2012;44(1):47–52 [DOI] [PubMed] [Google Scholar]
- 12.Linn BS, Linn MW, Gurel L. Cumulative illness rating scale. J Am Geriatr Soc. 1968;16(5):622–6 [DOI] [PubMed] [Google Scholar]
- 13.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16(4):1582–7 [DOI] [PubMed] [Google Scholar]
- 14.Ibrahim JG, Chu H, Chen M-H. Missing data in clinical studies: issues and methods. J Clin Oncol. 2012;30(26):3297–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–9 [DOI] [PubMed] [Google Scholar]
- 16.Shanafelt TD, Rabe KG, Kay NE, Zent CS, Jelinek DF, Reinalda MS, et al. Age at diagnosis and the utility of prognostic testing in patients with chronic lymphocytic leukemia. Cancer. 2010;116(20):4777–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurmes P, Call T, Slager S, Zent C, Jenkins G, Schwager S, et al. Comorbid conditions and survival in unselected, newly diagnosed patients with chronic lymphocytic leukemia. Leuk Lymphoma. 2008;49(1):49–56 [DOI] [PubMed] [Google Scholar]
- 18.Eichhorst BF, Busch R, Stilgenbauer S, Stauch M, Bergmann MA, Ritgen M, et al. First-line therapy with fludarabine compared with chlorambucil does not result in a major benefit for elderly patients with advanced chronic lymphocytic leukemia. Blood. 2009;114(16):3382–91 [DOI] [PubMed] [Google Scholar]
- 19.Goede V, Cramer P, Busch R, Bergmann M, Stauch M, Hopfinger G, et al. Interactions between comorbidity and treatment of chronic lymphocytic leukemia: results of German CLL Study Group trials. Haematologica. 2014;99(6):1095–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goede V, Fischer K, Busch R, Engelke A, Eichhorst B, Wendtner CM, et al. Obinutuzumab plus chlorambucil in patients with CLL and coexisting conditions. N Engl J Med. 2014;370(12):1101–10 [DOI] [PubMed] [Google Scholar]
- 21.Parikh SA, Rabe KG, Kay NE, Call TG, Ding W, Schwager S, et al. Chronic lymphocytic leukemia in young (less than 55 years) patients: a comprehensive analysis of prognostic factors and outcomes. Haematologica. 2014;99(1):140–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woyach JA, Ruppert AS, Rai K, Lin TS, Geyer S, Kolitz J, et al. Impact of age on outcomes after initial therapy with chemotherapy and different chemoimmunotherapy regimens in patients with chronic lymphocytic leukemia: results of sequential Cancer and Leukemia Group B studies. J Clin Oncol. 2013;31(4):440–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Badoux XC, Keating MJ, Wang X, O’Brien SM, Ferrajoli A, Faderl S, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117(11):3016–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer K, Cramer P, Busch R, Böttcher S, Bahlo J, Schubert J, et al. Bendamustine in combination with rituximab for previously untreated patients with chronic lymphocytic leukemia: a multicenter phase II trial of the German Chronic Lymphocytic Leukemia Study Group. J Clin Oncol. 2012;30(26):3209–16 [DOI] [PubMed] [Google Scholar]
- 25.Josefsson P, Geisler CH, Leffers H, Petersen JH, Andersen MK, Jurlander J, et al. CLLU1 expression analysis adds prognostic information to risk prediction in chronic lymphocytic leukemia. Blood. 2007;109(11):4973–9 [DOI] [PubMed] [Google Scholar]
- 26.Döhner H, Stilgenbauer S, James MR, Benner A, Weilguni T, Bentz M, et al. 11q deletions identify a new subset of B-cell chronic lymphocytic leukemia characterized by extensive nodal involvement and inferior prognosis. Blood. 1997;89(7):2516–22 [PubMed] [Google Scholar]
- 27.Hillmen P, Gribben JG, Follows GA, Milligan D, Sayala HA, Moreton P, et al. Rituximab plus chlorambucil as first-line treatment for chronic lymphocytic leukemia: final analysis of an open-label phase II study. J Clin Oncol. 2014;32(12):1236–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O’Brien SM, Lamanna N, Kipps TJ, et al. A phase 2 study of the selective phosphatidylinositol 3-kinase delta (PI3Kdelta) inhibitor idelalisib (GS-1101) in combination with rituximab in treatment-naïve patients ≥65 years with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL). J Clin Oncol. 2013;31(suppl.): abstr.7005 [Google Scholar]
- 29.Hillmen P, Robak T, Janssens A, Govindbabu K, Grosicki S, Mayer J, et al. Ofatumumab + chlorambucil versus chlorambucil alone in patients with untreated chronic lymphocytic leukemia (CLL): results of the phase III study complement 1 (OMB110911). Blood (ASH Annual Meeting Abstracts). 2013;122:528 [Google Scholar]
- 30.Mertens D, Stilgenbauer S. Prognostic and predictive factors in patients with chronic lymphocytic leukemia: relevant in the era of novel treatment approaches? J Clin Oncol. 2014;32(9):869–72 [DOI] [PubMed] [Google Scholar]


