Abstract
Conflicting data have been reported about the frequency and function of regulatory T cells in multiple myeloma. Most studies have investigated peripheral blood rather than bone marrow Tregs and side-by-side comparisons with bone marrow from healthy donors have still not been made. In this study, we show that regulatory T-cells total count, subset distribution, and expression of chemokine receptors are similar in the bone marrow of myeloma patients and healthy donors. Regulatory T cells are not recruited by myeloma cells in the bone marrow and their counts are unaffected by the tumor burden and the disease status. The diversity of T-cell receptor repertoire is highly preserved ensuring broad reactivity and effective suppressor function. Our results indicate that regulatory T cells may not be the main players of immunological tolerance to myeloma cells under base-line conditions, but their fully preserved immune competence may promote their inadvertent activation and blunt T-cell driven anti-myeloma immune interventions even after myeloma cells have successfully been cleared by chemotherapy.
Introduction
Multiple myeloma (MM) is a B-cell malignancy that remains incurable with conventional or high-dose chemotherapy. The in vitro and in vivo susceptibility of MM cells to recognition and elimination by immune effector cells have fostered the investigation and development of immune-based interventions. The unique availability of a highly specific tumor antigen such as idiotype has paved the way to the development of vaccination trials aimed at generating tumor-specific immune responses to clear the minimal residual disease and provide long-term protection against disease relapse.1 However, these approaches have fallen short of clinical expectations probably because, among other biases, they have neglected the antagonistic role played by immune suppressor subsets such as regulatory T cells (Tregs). It is now well established that the increase in Tregs in the peripheral blood (PB) or their accumulation at the tumor site are negative prognosticators in solid tumors.2 By contrast, the role of Tregs in patients with B-cell malignancies is much more strongly debated,3,4 especially in MM where controversy remains with regard to their frequency and function.5–14 The lack of conclusive data is probably due to the fact that Tregs have usually been investigated in the PB rather than the bone marrow (BM), and their identification based on different phenotypes. These biases have been exacerbated by the difficulty in obtaining total counts from BM samples and the lack of BM from healthy donors for comparative analysis. Zhao et al. have reported that the BM is a privileged site of Tregs accumulation in healthy donors under physiological conditions,15 and, therefore, a direct comparison with BM from healthy donors is a required condition in order to interpret the real size and function of the Tregs pool in MM.
In this study, we performed a comprehensive analysis of BM Tregs in a large series of MM patients at diagnosis (MM-dia), in individuals with monoclonal gammopathy of undetermined significance (MGUS), in MM patients in remission (MM-rem), and in MM patients in relapse (MM-rel), and compared, for the first time, these findings with BM from healthy donors (CTRL). Our results indicate that total counts, phenotypes, function, and T-cell receptor (TCR) diversity of BM Tregs are similar in MM and healthy donors and are not influenced by the disease status.
Methods
Multiple myeloma and healthy donors’ characteristics
One hundred and eight MM patients and 12 subjects with MGUS entered the study. The MM series included 71 MM-dia, 23 MM-rem, and 14 MM-rel. BM and PB were investigated side-by-side in 14 MM-dia.
CTRL included PB samples from 41 healthy blood donors kindly provided by the local Blood Bank and Transfusion Service, and 13 BM samples from individuals undergoing post-degenerative or post-traumatic hip prosthesis implantation at the local Traumatological and Orthopedic Center. Samples were collected after informed consent and approval by the local Institutional Review Board (DN 2012/388). In addition, 7 frozen human normal BM samples consisting of bone marrow mononuclear cell (BMMC) samples were purchased from Stem Cells Technologies (Vancouver, Canada).
Flow cytometry
Multicolor flow cytometry was used to determine the frequency and total counts of CD4+CD25hiFoxp3+ and CD4+CD25+CD127loFoxp3+ T cells in BM and PB samples from MM patients and CTRL.
More details about the phenotypic and functional characterization of Tregs are available in the Online Supplementary Methods.
BM Tregs total counts
Automated complete cell counts per µL were obtained in BM samples with a Coulter AcT Diff Hematology Analyzer (Beckman Coulter, Brea, CA, USA). One million BM cells were then stained with anti-human CD45 APC-Cy7 (Becton Dickinson, Mountain View, CA, USA) and the percentage of lymphocytes calculated by flow cytometry based on the scatter profile and CD45hi expression. Total counts of lymphocytes were calculated by multiplying percentages by complete BM cell counts. Tregs total counts were obtained by multiplying the percentages of CD4+CD25hiFoxp3+ and CD4+CD25+CD127loFoxp3+ by absolute lymphocyte counts.
Immunohistochemical staining of BM Foxp3+ cells
Five micron thin sections were cut from paraffin blocks of BM specimens, coated on electrically charged slides, de-waxed, rehydrated and submitted to antigen retrieval by micro-waving in 1 mM EDTA (pH 8.0) at 900 W for 10 min. Slides were then incubated with 1:50 diluted purified-anti-Foxp3 (eBioscience, San Diego, CA, USA) for 30 min at room temperature. Antibody binding was detected using the peroxidase-based En Vision++ system as previously described.16 Sections were then counterstained with hematoxilin and the immunohistochemical tests were carried out on an automatic stainer device (Dakoautostainer Dakocytomation Glastrup, Denmark).
TCRBV repertoire analysis
TCR diversity of Tregs was determined by estimating the length distribution of the complementarity-determining regions 3 (CDR3) of β variable (BV) gene segments with a two-step multiplex PCR assay developed in our laboratory.17
More details are available in the Online Supplementary Methods.
Tregs isolation and suppression assay
Tregs suppressor function was assessed using irradiated allogeneic PBMC as accessory cells, purified BM or PB CD4+CD25+ or CD4+CD127loCD25+ cells as Tregs, autologous CD4+CD25−as responder cells and soluble anti-CD3 as polyclonal activator, as previously reported.18
More details are available in the Online Supplementary Methods.
Statistical analysis
The results are expressed as median and range or mean ± SE as indicated. Between-group differences were evaluated with the Wilcoxon-Mann-Whitney non-parametric test for paired or unpaired samples as appropriate and considered statistically significant for P<0.05. Correlation analyses were performed with the non-parametric Spearman rank order test with a cut off of P<0.05. The SigmaStat software (Systat Software Inc., Richmond, USA) was used for these analyses.
Results
Frequencies and total counts of Tregs are similar in the BM and PB of MM and CTRL
Tregs were identified based on the CD4+CD25hiFoxp3+ and CD4+CD25+CD127loFoxp3+ phenotypes (Online Supplementary Figure S1). Frequencies (Figure 1A) and total counts (Figure 1B) of BM Tregs were similar in CTRL, MGUS, and MM patients independently of the phenotype used for their identification and the disease status (MM-dia, MM-rem, MM-rel) (P always >0.05). Medians and ranges of BM values in CTRL, MGUS, MM-dia, MM-rem, and MM-rel and of PB values in CTRL and MM-dia are listed in Online Supplementary Tables S1 and S2, respectively. The results indicate that the pool of Tregs has not increased and is not influenced by the disease status.
Figure 1.
Frequencies and total counts of Tregs in the BM of MM and healthy donors. Tregs were identified as CD4+CD25hiFoxp3+ T or CD4+CD25+CD127loFoxp3+ T cells by using a multigating strategy as shown in Online Supplementary Figure S1. (A) Percentages and (B) total counts of Tregs in the BM of CTRL, MGUS, MM-dia, MM-rem, and MM-rel. Results are shown as dot density plots and median lines. Differences between medians are not statistically significant (P> 0.05). See also Online Supplementary Table S1 for medians and ranges values.
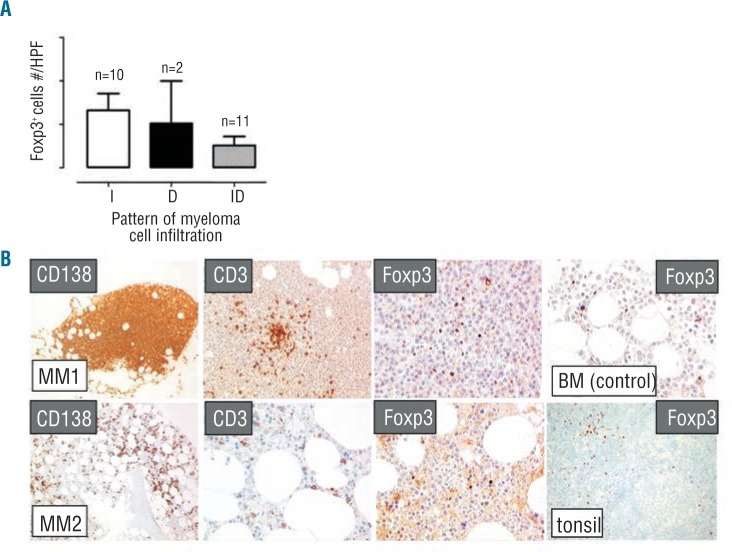
Tregs are infrequent in the tumor microenvironment and not correlated with the myeloma cell burden and infiltration pattern
To further investigate the relationship between Tregs and the tumor burden, the frequency of Tregs and myeloma cells were determined by immunohistochemical stain in BM trephine biopsies from 23 MM-dia (Figure 2). No correlation was observed between the frequency of Tregs and the pattern of myeloma cell infiltration (i.e. interstitial, diffuse, interstitial/diffuse) (Figure 2A). Representative immunohistochemical stains of myeloma cell (CD138+), T cells (CD3+), and Tregs (Foxp3+) in 2 MM BM samples are shown in Figure 2B. Foxp3 stain of Tregs in the BM and tonsils from CTRL are also shown in Figure 2B.
Figure 2.
The frequency of BM Tregs is not correlated with the myeloma cell burden and infiltration pattern. Tregs and myeloma cells were both identified in BM trephine biopsies from 23 MM-dia by immunohistochemical Foxp3 and CD138 stains. (A) The frequency of Tregs was reported as number of Foxp3+ cells per high power field (HPF) after patients’ subcategorization based on the pattern of BM myeloma cell infiltration (I: interstitial; D: diffuse; ID: interstitial/diffuse). Bars represent mean ± SE. Differences are not statistically significant (Wilcoxon test, P>0.05). (B) Immunohistochemical stains of myeloma cell (CD138+)(×100 magnification), T cells (CD3+)(×200), and Tregs (Foxp3+) (×400) in BM samples from 2 representative MM patients with diffuse (MM1) and interstitial (MM2) myeloma cell infiltration. Foxp3 stain of Tregs in the BM (×400) and reactive tonsils (×200) from CTRL are also shown as controls.
To further assess the recruitment susceptibility at the tumor site, we evaluated the surface expression of CCR4 that is known to attract Tregs in response to positive CCL22/CCL17 gradients and the activation status using the combined expression of CD45RA and CD27 which regulates Tregs homing capacity at the tumor site. The great majority of BM Tregs expressed CCR4 on their surface at high levels and showed a predominant CM phenotype in all MM patients irrespective of the disease status, but there was no difference between these values and those observed in CTRL and MGUS (P>0.05) (Online Supplementary Figure S2).
TCR diversity of BM MM Tregs
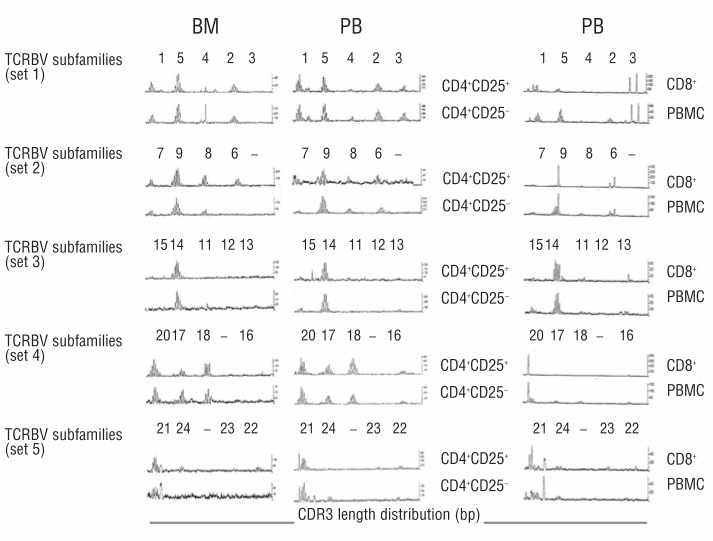
The TCRBV repertoire of BM and PB Tregs and CD4+CD25− cells was investigated in 6 MM-dia and 2 CTRL. The CD3 length distribution profile of BV subfamilies was prevalently polyclonal in BM (n=1) and PB (n=6) MM Tregs and identical to that of CD4+CD25− cells (Figure 3). Only 5 of 66 successfully amplified BV subfamilies were found oligoclonal in 3 of 6 PB MM Tregs, which was equivalent to 7.5±3.0% of their total TCRBV repertoire. Parallel analysis of CD8+ cells from 2 MM patients showed that 20 of 27 successfully amplified BV subfamilies were oligoclonal (75±5% of their TCRBV repertoire) as previously reported19 (Figure 3).
Figure 3.
TCR diversity is highly preserved in the BM and PB Tregs of MM patients. The TCRBV repertoires of purified BM and PB Tregs (CD4+CD25+) and CD4+CD25− cells from a representative MM-dia are shown. The CDR3 length distribution profiles at the level of BV-BC transcripts from 22 BV subfamilies are grouped in 5 sets. In the successfully amplified BV subfamilies, the polyclonal profile is distinguished by a bell-shaped distribution of BV-BC transcripts or by one or more peaks slightly above the normal bell-shaped background. The loss of TCR diversity characterized by the loss of BV subfamilies and the emergence of oligoclonal profiles in autologous CD8+ PB cells and PBMC is shown as control. Results are from 6 MM-dia and 2 CTRL experiments.
Both BM and PB MM Tregs are endowed with suppressor activity
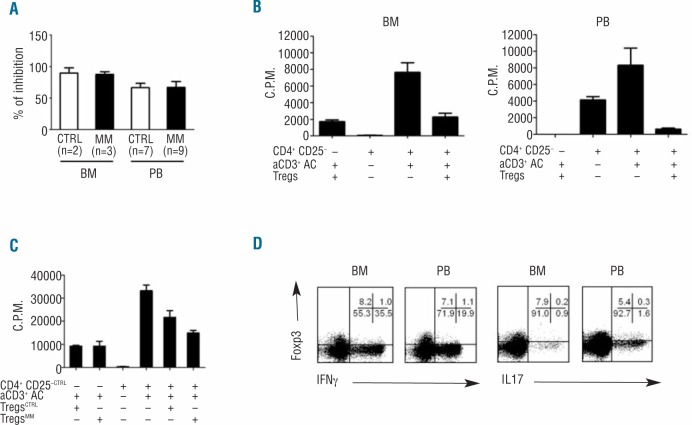
To assess the regulatory function of MM Tregs, we performed a standard in vitro proliferation inhibition assay. The percent inhibition was calculated as reported in the Methods (Online Supplementary Appendix). Purified BM and PB MM CD4+CD25+ Tregs inhibited the proliferation of autologous CD4+CD25− cells by 88±7% and 67±26%, respectively (P>0.05). Similar inhibition was exerted by purified BM and PB CTRL on autologous CD4+CD25− cells (89±12% and 66±18%, respectively) (P>0.05) (Figure 4A). Representative experiments of MM BM and PB Tregs suppressor activity are shown in Figure 4B. Purified PB CD4+CD127loCD25+ T cells were also used as an alternative source of Tregs in 3 experiments and shown to have similar suppressor function in both CTRL and MM-dia (data not shown).
Figure 4.
The suppressor function of BM and PB Tregs is highly preserved in MM. (A–C) Tregs suppressor function was assessed using irradiated allogeneic PBMC as accessory cells (AC), purified CD4+CD25+ cells as Tregs, and autologous CD4+CD25− as responder cells, and soluble anti-CD3 (aCD3) as previously reported18. (A) Purified BM and PB MM Tregs inhibited the proliferation of autologous CD4+CD25− cells by 88 ± 7% and 67 ± 26%, respectively. Similar inhibition was exerted by purified BM and PB CTRL on autologous CD4+CD25−cells (89 ± 12% and 66 ± 18% respectively). Bars represent the mean value ± SE from 2–9 experiments, each performed in triplicates. Differences are not statistically significant (P>0.05). (B) Representative suppressor activity exerted by MM BM and PB Tregs on autologous CD4+CD25−cells. (C) MM Tregs suppress the proliferation of CTRL CD4+CD25−cells with the same efficiency of CTRL Tregs. (D) Intracellular cytokine assay was performed in combination with Foxp3 staining to confirm Tregs suppressive function. IFN-γ and IL-17 production in BM and PB CD4+Foxp3+ cells after PMA + ionomycin stimulation in a representative MM-dia. Data are from one of 7 BMMC and 6 PBMC experiments. Percentages are backgated on CD4+ cells.
A cross-over experiment was also performed in which purified MM Tregs and CTRL Tregs were used side-by-side to suppress autologous CTRL CD4+CD25− cells whose proliferation to anti-CD3 mAb was occasionally found to be more vigorous than that of MM CD4+CD25− cells. Also in this setting, MM PB Tregs suppressed the proliferation of CTRL CD4+CD25−cells with the same efficiency of Tregs purified from healthy donors (Figure 4C).
The authentic regulatory function of BM and PB MM Tregs was confirmed by the production of effector cytokines such as IFN-γ and IL-17 which was restricted to Foxp3− after PMA + ionomycin stimulation (Figure 4D).
Discussion
The contribution of Tregs to the immune dysregulation of MM patients is still controversial. The current view that BM Tregs are increased in MM comes from indirect comparisons with PB of CTRL or MGUS individuals.7–13 So far, only one study has compared Tregs BM percentages in MM-dia and CTRL without detecting any difference.14 Our results clearly indicate that the pool of BM Tregs is equivalent in MM and CTRL irrespectively of the phenotype used for their identification, and the same holds true in the PB.
Previous reports have provided contradictory results about possible correlations between Tregs and the tumor burden or the disease status.7,11,13,14 Our findings indicate that there is no specific Treg recruitment at the tumor site driven by myeloma cells because frequencies and total counts were similar in MGUS, MM-dia, MM-rem, and MM-rel. Moreover, no correlation was found in BM trephine biopsies between the topographic distribution of Tregs, which is an important prognosticator in other B-cell malignancies, and the pattern of BM myeloma cell infiltration (i.e. diffuse vs. interstitial vs. diffuse/interstitial).
CCR4 expression was also evaluated to assess the recruitment susceptibility of Tregs at the tumor site, but no differences were observed between CTRL, MGUS, MM-dia, MM-rem, and MM-rel. These data further support the conclusion that the pool of Tregs in the BM of MM patients mainly consists of resident cells as in normal BM, and that there is no major recruitment operated by the tumor burden and disease activity via the CCR4/CCL22 axis.
Recent findings in various tumor models indicate that the influence of Tregs on tumor progression also depends on their functional status, which can be predicted on the basis of their naïve/memory phenotype and concurrent CCR4 expression.20–23 Only activated/memory, but not naïve Tregs, have been shown to home at the tumor site, since antigen-priming is necessary to gain the appropriate trafficking receptors.22,23
Here, we have characterized the activation status of BM Tregs in MM and CTRL by using the combined expression of CD45RA and CD27 that allows their further classification into the naïve, CM, EM, and TEMRA subsets. Activated/memory Tregs have been reported to be mainly represented in the CM (CD45RA− CD27+) and EM (CD45RA− CD27−) subsets.7,20 CM was the most represented subset in the BM but Tregs subset distribution was similar in CTRL, MGUS, MM-dia, MM-rem, and MM-rel.
Activated/memory Tregs are more inclined than naïve Tregs to acquire suppressor functions upon the TCR-dependent recognition of the self-antigens expressed by tumor cells.21–23 Thus, an extended TCR repertoire is a good match to keep the reactivity of Tregs broadly-based, and to ensure their optimal function and homeostasis. In previous studies, Tregs from CTRL PB showed a polyclonal TCR repertoire similar to that of CD4+CD25− cells.17,24–26 Here, we show that the TCR repertoire of BM Tregs from MM-dia is also polyclonal, and identical to that of CD4+CD25− cells. Side-by-side BM and PB comparison in the same patient showed that the TCR repertoire was not skewed at the tumor site and there was no preferential accumulation of oligoclonal Tregs driven by myeloma cells. This is very important considering the unique ability of these cells, once activated via the TCR, to indifferently inhibit the proliferation of naïve and memory CD4+ and CD8+ lymphocytes as well as innate immune effectors in a non-antigen-specific manner.
The very high degree of TCR diversity in Tregs was in marked contrast with that of CD8+ cells whose TCR repertoire was highly disrupted in both the BM and PB and characterized by the emergence of oligoclonal expansions and a significant loss of TCR diversity as previously reported.19
A shaped TCR repertoire reflects the imprinting operated by the long-term interplay with tumor cells. Our results indicate that CD8+ cells are more perturbed by their challenge to hold in check myeloma cells than Tregs to exert their regulatory function. Functional analysis showed that neither BM nor PB MM Tregs responded to anti-CD3 stimulation and both inhibited the proliferation of autologous CD4+CD25− cells with the same efficiency as CTRL BM and PB Tregs. The authentic suppressor function of Tregs was confirmed by determining the production of IFN-γ and IL-17 at the single-cell level. As expected, and in contrast with conventional non-regulatory CD4+Foxp3− T cells, neither BM nor PB MM Tregs produced IFN-γ and IL-17 even after polyclonal stimulation with PMA + ionomycin.
In conclusion, our data indicate that the suppressor function of BM MM Tregs is highly preserved and similar to that of BM CTRL Tregs although the latter have never been exposed to myeloma cells and bystander cells. The unchanged permanence of BM Tregs during the disease progression may reflect the local production of increasing amounts of TGF-β and IL-6 that synergistically promote Foxp3 degradation27 and the preferential differentiation of CD4+ cells into Th17+ cells at disadvantage of Tregs.28 Moreover, cytokine production is not normalized when MM patients enter remission29 and this may explain why BM Tregs stay unchanged in MGUS, MM-dia, MM-rem, and MM-rel.
The presence of a functional Tregs pool steadily rooted in the BM of MM patients irrespectively of the disease status indicates that Tregs are unlikely to influence the natural disease evolution but could inadvertently be activated by immune-based interventions. Indeed, idiotype vaccination combined with IL-12 or IL-12 and GM-CSF30 and the injection of cytokine-matured dendritic cells31 have been reported to increase Tregs frequency in MM patients, providing further explanation as to why these strategies have fallen short of clinical expectations. More recently, thalidomide and lenalidomide have also been shown to increase the frequency and function of Tregs in MM, and lenalidomide, in association with dexamethasone, has been reported to increase Tregs and concurrently decrease the effector functions of T and NK cells.32
Finally, our data indicate that Tregs neutralization should be considered to maximize the therapeutic effects of immune-based interventions in MM and different strategies are now available to achieve this goal.33,34
Acknowledgments
We are indebted to the Blood Bank of the “Azienda Ospedaliera-Universitaria Citta’ della Salute e della Scienza di Torino”, Torino, Italy, for providing normal samples from healthy donors.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work was supported by PRIN 2010NECHBX_002 (MM) and AIRC IG 13119 (MM). MF and BC are research fellows supported by AIRC (MF) and PRIN (BC).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Coscia M, Mariani S, Battaglio S, Di Bello C, Fiore F, Foglietta M, et al. Long-term follow-up of idiotype vaccination in human myeloma as a maintenance therapy after high-dose chemotherapy. Leukemia. 2004; 18(1):139–45 [DOI] [PubMed] [Google Scholar]
- 2.Wilke CM, Wu K, Zhao E, Wang G, Zou W. Prognostic significance of regulatory T cells in tumor. Int J Cancer. 2010;127(4):748–58 [DOI] [PubMed] [Google Scholar]
- 3.Lindqvist CA, Loskog AS. T regulatory cells in B-cell malignancy tumour support or kiss of death¿ Immunology. 2012;135(4):255–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farinha P, Al-Tourah A, Gill K, Klasa R, Connors JM, Gascoyne RD. The architectural pattern of FOXP3-positive T cells in follicular lymphoma is an independent predictor of survival and histologic transformation. Blood. 2010;115:289–295 [DOI] [PubMed] [Google Scholar]
- 5.Prabhala RH, Neri P, Bae JE, Tassone P, Shammas MA, Allam CK, et al. Dysfunctional T regulatory cells in multiple myeloma. Blood. 2006;107(1):301–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta R, Ganeshan P, Hakim M, Verma R, Sharma A, Kumar L. Significantly reduced regulatory T cell population in patients with untreated multiple myeloma. Leuk Res. 2011;35(7):874–78 [DOI] [PubMed] [Google Scholar]
- 7.Beyer M, Kochanek M, Giese T, Endl E, Weihrauch MR, Knolle PA, et al. In vivo peripheral expansion of naive CD4+CD25high FoxP3+ regulatory T cells in patients with multiple myeloma. Blood. 2006;107(10):3940–9 [DOI] [PubMed] [Google Scholar]
- 8.Giannopoulos K, Kaminska W, Hus I, Dmoszynska A. The frequency of T regulatory cells modulates the survival of multiple myeloma patients: detailed characterisation of immune status in multiple myeloma. Br J Cancer. 2012;106(3):546–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bryant C, Suen H, Brown R, Yang S, Favaloro J, Aklilu E, et al. Long-term survival in multiple myeloma is associated with a distinct immunological profile, which includes proliferative cytotoxic T-cell clones and a favourable Treg/Th17 balance. Blood Cancer J. 2013;3:e148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brimnes MK, Vangsted AJ, Knudsen LM, Gimsing P, Gang AO, Johnsen HE, et al. Increased level of both CD4+FOXP3+ regulatory T cells and CD14+HLA-DR-/low myeloid-derived suppressor cells and decreased level of dendritic cells in patients with multiple myeloma. Scand J Immunol. 2010;72(6):540–7 [DOI] [PubMed] [Google Scholar]
- 11.Feyler S, von Lilienfeld-Toal M, Jarmin S, Marles L, Rawstron A, Ashcroft AJ, et al. CD4(+)CD25(+)FoxP3(+) regulatory T cells are increased whilst CD3(+)CD4(−)CD8(−) alphabetaTCR(+) Double Negative T cells are decreased in the peripheral blood of patients with multiple myeloma which correlates with disease burden. Br J Haematol. 2009;144(5):686–95 [DOI] [PubMed] [Google Scholar]
- 12.Ostad M, Andersson M, Gruber A, Sundblad A. Expansion of immunoglobulin autoreactive T-helper cells in multiple myeloma. Blood. 2008;111(5):2725–32 [DOI] [PubMed] [Google Scholar]
- 13.Muthu Raja KR, Rihova L, Zahradova L, Klincova M, Penka M, Hajek R. Increased T. regulatory cells are associated with adverse clinical features and predict progression in multiple myeloma. PLoS One. 2012;7(10):e47077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atanackovic D, Cao Y, Luetkens T, Panse J, Faltz C, Arfsten J, et al. CD4+CD25+FOXP3+ T regulatory cells reconstitute and accumulate in the bone marrow of patients with multiple myeloma following allogeneic stem cell transplantation. Haematologica. 2008;93(3):423–30 [DOI] [PubMed] [Google Scholar]
- 15.Zhao E, Xu H, Wang L, Kryczek I, Wu K, Hu Y, et al. Bone marrow and the control of immunity. Cell Mol Immunol. 2012; 9(1):11–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabattini E, Bisgaard K, Ascani S, Poggi S, Piccioli M, Ceccarelli C, et al. The EnVisionTM+ system: a new immunohistochemical method for diagnostics and research. Critical comparison with the APAAP, ChemMateTM, CSA, LABC, and SABC technique. J Clin Pathol. 1998; 51:506–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mariani S, Hwang SY, Foglietta M, Bonello L, Vitale C, Coscia M, et al. Comprehensive assessment of the TCRBV repertoire in small T-cell samples by means of an improved and convenient multiplex PCR method. Exp Hematol. 2009; 37(6):728–38 [DOI] [PubMed] [Google Scholar]
- 18.Castella B, Riganti C, Fiore F, Pantaleoni F, Canepari ME, Peola S, et al. Immune modulation by zoledronic acid in human myeloma: an advantageous cross-talk between Vγ9Vδ2 T cells, αβCD8+ T cells, regulatory T cells, and dendritic cells. J Immunol. 2011;187(4):1578–90 [DOI] [PubMed] [Google Scholar]
- 19.Mariani S, Coscia M, Even J, Peola S, Foglietta M, Boccadoro M, et al. Severe and long-lasting disruption of T-cell receptor diversity in human myeloma after high-dose chemotherapy and autologous peripheral blood progenitor cell infusion. Br J Haematol. 2001;113(4):1051–9 [DOI] [PubMed] [Google Scholar]
- 20.Beyer M, Schultze JL. CD4+CD25highFOXP3+ regulatory T cells in peripheral blood are primarily of effector memory phenotype. J Clin Oncol. 2007; 25(18):2628–30; author reply 2630–2. Erratum in: J Clin Oncol. 2012;30(31):3903 [DOI] [PubMed] [Google Scholar]
- 21.Huehn J, Siegmund K, Lehmann JC, Siewert C, Haubold U, Feuerer M, et al. Developmental stage, phenotype, and migration distinguish naive- and effector/memory-like CD4+ regulatory T cells. J Exp Med. 2004;199(3):303–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Darrasse-Jèze G, Podsypanina K. How Numbers, Nature, and Immune Status of Foxp3+ Regulatory T-Cells Shape the Early Immunological Events in Tumor Development. Front Immunol. 2013;4:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sugiyama D, Nishikawa H, Maeda Y, Nishioka M, Tanemura A, Katayama I, et al. Anti-CCR4 mAb selectively depletes effector-type FoxP3+CD4+ regulatory T cells, evoking antitumor immune responses in humans. Proc Natl Acad Sci USA. 2013; 110(44):17945–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Föhse L, Suffner J, Suhre K, Wahl B, Lindner C, Lee CW, et al. High TCR diversity ensures optimal function and homeostasis of Foxp3+ regulatory T cells. Eur J Immunol. 2011;41(11):3101–13 [DOI] [PubMed] [Google Scholar]
- 25.Pacholczyk R, Kern J. The T-cell receptor repertoire of regulatory T cells. Immunology. 2008;125(4):450–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazilleau N, Bachelez H, Gougeon ML, Viguier M. Cutting edge: size and diversity of CD4+CD25high Foxp3+ regulatory T cell repertoire in humans: evidence for similarities and partial overlapping with CD4+CD25− T cells. J Immunol. 2007; 179(6):3412–6 [DOI] [PubMed] [Google Scholar]
- 27.Gao Z, Gao Y, Li Z, Chen Z, Lu D, Tsun A, et al. Synergy between IL-6 and TGF-β signaling promotes FOXP3 degradation. Int J Clin Exp Pathol. 2012;5(7):626–33 [PMC free article] [PubMed] [Google Scholar]
- 28.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, et al. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science. 2007; 317(5835):256–60 [DOI] [PubMed] [Google Scholar]
- 29.Zheng MM, Zhang Z, Bemis K, Belch AR, Pilarski LM, Shively JE, et al. The systemic cytokine environment is permanently altered in multiple myeloma. PLoS One. 2013;8(3):e58504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hansson L, Abdalla AO, Moshfegh A, Choudhury A, Rabbani H, Nilsson B, et al. Long-term idiotype vaccination combined with interleukin-12 (IL-12), or IL-12 and granulocyte macrophage colony-stimulating factor, in early-stage multiple myeloma patients. Clin Cancer Res. 2007;13(5):1503–10 [DOI] [PubMed] [Google Scholar]
- 31.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokine-matured DCs in myeloma patients. Blood. 2006;108(8):2655–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muthu Raja KR, Kovarova L, Hajek R. Induction by lenalidomide and dexamethasone combination increases regulatory cells of patients with previously untreated multiple myeloma. Leuk Lymphoma. 2012; 53(7):1406–8 [DOI] [PubMed] [Google Scholar]
- 33.Byrne WL, Mills KH, Lederer JA, O’Sullivan GC. Targeting regulatory T cells in cancer. Cancer Res. 2011;71(22):6915–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le DT, Jaffee EM. Regulatory T-cell modulation using cyclophosphamide in vaccine approaches: a current perspective. Cancer Res. 2012;72(14):3439–4 [DOI] [PMC free article] [PubMed] [Google Scholar]