Abstract
This is the first prospective study of deferasirox in adult allogeneic hematopoietic stem cell transplant recipients with transfusional iron overload in hematologic malignancies. Patients at least six months post transplant were treated with deferasirox at a starting dose of 10 mg/kg/day for 52 weeks or until serum ferritin was less than 400 ng/mL on two consecutive occasions. Thirty patients were enrolled and 22 completed the study. A significant reduction from baseline in median serum ferritin and in liver iron concentration at 52 weeks was observed in the overall population: from 1440 to 755.5 ng/mL (P=0.002) and from 14.5 to 4.6 mg Fe/g dw (P=0.0007), respectively. Reduction in serum ferritin in patients who did not discontinue deferasirox therapy was significantly greater than that found in those who prematurely discontinued the treatment (from 1541 to 581 ng/mL vs. from 1416 to 1486 ng/mL; P=0.008). Drug-related adverse events, reported in 17 patients (56.7%), were mostly mild to moderate in severity. There were no drug-related serious adverse events. Twelve patients (40.0%) showed an increase of over 33% in serum creatinine compared to baseline and greater than the upper limit of normal on two consecutive visits. Two patients (6.7%) with active graft-versus-host disease showed an increase in alanine aminotransferase exceeding 10 times upper limit of normal; both resolved. In this prospective study, deferasirox provided a significant reduction in serum ferritin and liver iron concentration over one year of treatment in allogeneic hematopoietic stem cell transplant recipients with iron overload. In addition, the majority of adverse events related to deferasirox were mild or moderate in severity. (clinicaltrials.gov identifier:01335035).
Introduction
Management of iron overload in the setting of post-allogeneic hematopoietic stem cell transplant (allo-HSCT) may be complicated since the use of therapeutic phlebotomies is often not feasible due to ongoing anemia. Limited but compelling data are available regarding the use of deferasirox in allo-HSCT recipients with μ-thalassemia.1–6
Pre-transplant iron overload has been associated with considerable morbidity and increased mortality in patients undergoing HSCT.7–14 Transfusional hemosiderosis and increased non-transferrin bound iron result in generation of free radicals that may eventually cause oxidative organ damage, especially to the liver, heart and endocrine system. Pre-transplant serum ferritin over 1000 ng/mL has been associated with a higher rate of treatment-related complications such as mucositis, acute graft-versus-host disease (GvHD), liver dysfunction, chronic liver disease, exacerbation of chronic GvHD, hepatic sinusoidal obstruction syndrome (SOS), and bacterial, fungal and viral infections. Elevated serum ferritin levels prior to transplant have also been associated with a lower survival rate, and increased overall and treatment-related mortality in patients undergoing HSCT for hematologic malignancies.15 In a study of 112 adults who had undergone allo-HSCT, causes of death within 100 days after transplant among the patients with high serum ferritin were infection (29%), organ failure (29%), GvHD (29%) and SOS (14%).7 Regarding the effects of persistent iron overload on the long-term morbidity of HSCT survivors, Meyer et al. found that, in 290 allo-HSCT recipients, hyperferritinemia had a detrimental effect on post-HSCT survival (0–6 months P<0.001; 6–12 months P<0.001; 1–2 years P=0.02; 2–5 years P=0.002).16 However, because serum ferritin is an imperfect surrogate measure of iron stores, its prognostic role in patients with a history of HSCT and iron overload still has to be determined. Several prospective studies have examined the impact of elevated liver iron concentration (LIC) as measured by magnetic resonance imaging (MRI) on HSCT outcome. There is still no consensus regarding the relevance of LIC in HSCT, mainly due to the use of different LIC thresholds.17–21
Here we report our experience with the prospective use of deferasirox in 30 adults with a variety of hematologic malignancies and transfusional iron overload who survived at least six months after allo-HSCT.
Methods
Study design
This was an open-label, multi-center clinical trial carried out in hematopoietic transplant units in Spain. Eligible subjects were patients over 18 years of age who had undergone an allo-HSCT at least six months prior to enrollment, with transfusional iron overload (serum ferritin ≥1000 ng/mL or transfusional load ≥20 units (~100 mL/kg) of packed red blood cells) and absolute neutrophil count over 1 × 109/L. Exclusion criteria included iron overload not related to transfusion, uncontrolled hypertension, active viral hepatitis, human immunodeficiency virus infection, serum creatinine over 2 times upper limit of normal (ULN) or creatinine clearance less than 50 mL/min, significant proteinuria, serum aspartate aminotransferase over 5 times ULN, active concomitant malignancies and prior use of iron chelators (Online Supplementary Appendix).
The primary objective of the study was to evaluate the median change in serum ferritin after 52 weeks of treatment with deferasirox in patients with iron overload after allo-HSCT. Secondary objectives were to assess safety of deferasirox, and to explore the use of hepatic MRI to assess LIC.
Deferasirox (initial dose 10 mg/kg/day) was administered for 52 weeks or until serum ferritin level was 400 ng/mL or under on two consecutive occasions. Dose reductions based on serum ferritin levels and on safety markers (serum creatinine, urine protein/creatinine ratio, liver enzymes, gastrointestinal events, skin rash, hearing loss, ocular alterations, or change in weight) were allowed. An increase in deferasirox dose to a maximum of 30 mg/kg/day was allowed after three months if serum ferritin increased or failed to decrease by at least 20% from baseline. Additional chelators, aluminum-based antacids and vitamin C over 200 mg/day were not allowed. Assessment of LIC by MRI was performed at selected sites on the basis of availability of equipment. Analysis of MRI images was carried out at a centralized site based on gradient echo sequences.22
Adverse events were reported by the investigators and recorded in the case report forms.
The study protocol was reviewed and approved by local institutional review boards and/or ethics committees. The study was conducted according to the Declaration of Helsinki and its amendment. All patients signed informed consent.
Statistical analysis
Categorical variables were described using frequencies and percentages, and quantitative variables by the mean, number of cases, median, standard deviation and range (minimum and maximum). Since distribution was not normal (because the result of Shapiro Wilk normality test for the principal end point, i.e. serum ferritin, was P<0.05), a non-parametric Wilcoxon test was used for median change in serum ferritin between baseline and after 52 weeks of treatment. Evaluations were performed for the intent-to-treat population
All calculations were performed with SAS for Windows, v.9.1.3 (Cary, NC, USA). Two-sided tests were used; P=0.05 was considered significant. Safety was assessed in all patients who received at least one dose of the study drug. The intent-to-treat population included patients who had received at least one dose of study medication and had at least one base-line and one post-treatment value of the primary study variable (serum ferritin). In case of missing values, the method of ‘last observation carried forward’ (LOCF) was used for the principal variable (serum ferritin).
Results
Patients
Thirty patients were enrolled from December 2008 to April 2010. Eight patients discontinued early, prior to completion of the study, due to: disease progression (n=3), death (n=2; one disease progression (acute myeloid leukemia) and one infection (Staphylococcus auriculanis bacteriemia associated with acute respiratory and multi-organ failure); neither were considered to be related to the study drug, drug-related adverse event (n=1; increased serum creatinine), unsatisfactory therapeutic effect (n=1), and withdrawal of consent (n=1; due to mucositis considered to be related to the study drug). Twenty-two patients completed the study: 14 patients after 52 weeks of deferasirox, and 8 who achieved serum ferritin 400 ng/mL or under (on 2 occasions) before completing 52 weeks of treatment.
The majority of patients in the series were male (66.7%). Median age was 46.7 years. Stem cell transplantation was performed for a range of hematologic malignancies, the most common of which was acute myeloid leukemia (n=17) (Table 1).
Table 1.
Patients’ base-line characteristics (n=30).
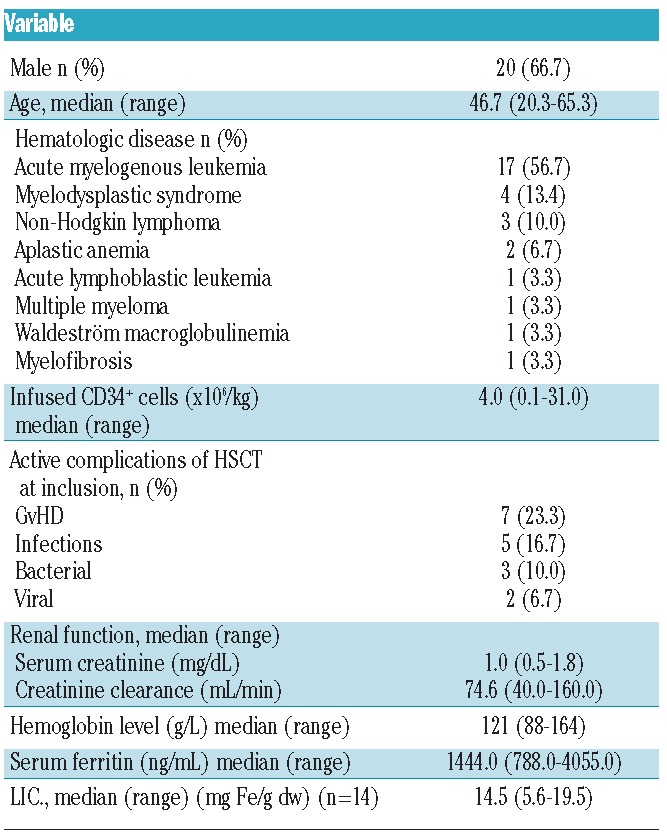
At study enrollment, median time since HSCT was 12.2 months (range 6–39 months). Patients had received a mean 43.5 red blood cell units (range 16–112) before start of study. The conditioning employed included conventional and reduced intensity regimens. Half of the patients had an unrelated donor. Stem cell source was bone marrow or peripheral blood in all patients except 5 for whom cord blood was used. Base-line characteristics are summarized in Table 1.
Drug exposure
Overall, patients received deferasirox for a median of 45.7 (range 7.9–57.9) weeks (34.8 weeks (range 8.7–57.9) and 51.4 weeks (range 7.9–56) in patients with base-line serum ferritin <1500 ng/mL and ≥1500 ng/mL, respectively). Dosage was increased in 5 patients (16.7%) and reduced in 11 (36.7%). Dose reduction due to increase in serum creatinine occurred in 9 patients (30%), and due to increase in hepatic enzymes in 2 patients (6.7%). Treatment was temporarily discontinued in 5 patients due to increased liver enzymes (n=3; 10%), vomiting (n=1; 3.3%), and hearing disturbances (n=1; 3.3%). The median time to the first dose adjustment was 15.9 weeks (range 3–44.6). Concomitant use of cyclosporine did not seem to influence the rate of discontinuation of the study drug. Among the 8 patients who discontinued deferasirox, 2 (14.3%) received concomitant cyclosporine and 6 (37.5%) did not.
Deferasirox dosage under 10 mg/kg/day (median actual dose 7 mg/kg/day) was received by 7 patients, 10 mg/kg/day by 18 patients, and over 10 mg/kg/day (median actual dose 13.4 mg/kg/day) by 5 patients.
Effect of deferasirox on serum ferritin
A significant reduction in median serum ferritin from baseline to 52 weeks was observed (1444 to 755.5 ng/mL; P=0.002) in the intent-to-treat population (LOCF). Significant decreases in median serum ferritin from baseline were also observed in the 8 patients who met the target value of 400 ng/mL or under (−1030.0 ng/mL; P=0.0078), and in the 14 patients who completed 52 weeks of treatment (−661.5 ng/mL; P=0.0134) (Figure 1). Overall, patients without premature discontinuation (n=22) had a significant decrease in median serum ferritin from baseline (−795.5 ng/mL; P<0.0001) (Figure 2). The 8 patients who discontinued deferasirox prematurely had no change in median serum ferritin from baseline (Table 2 and Figure 1).
Figure 1.
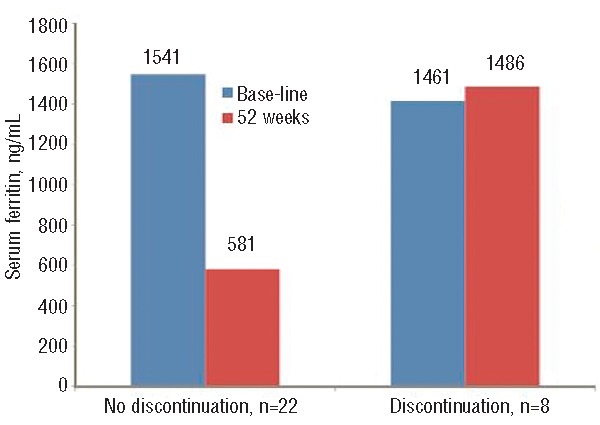
Median serum ferritin based on continuation or not with study drug.
Figure 2.
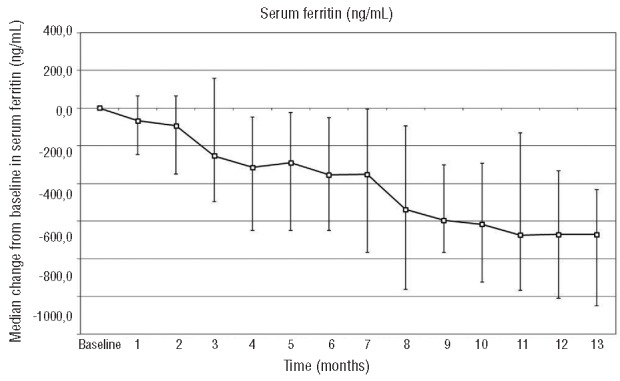
Evolution of change in serum ferritin (+ 25th/75th percentiles)
Table 2.
Change from baseline in median serum ferritin.
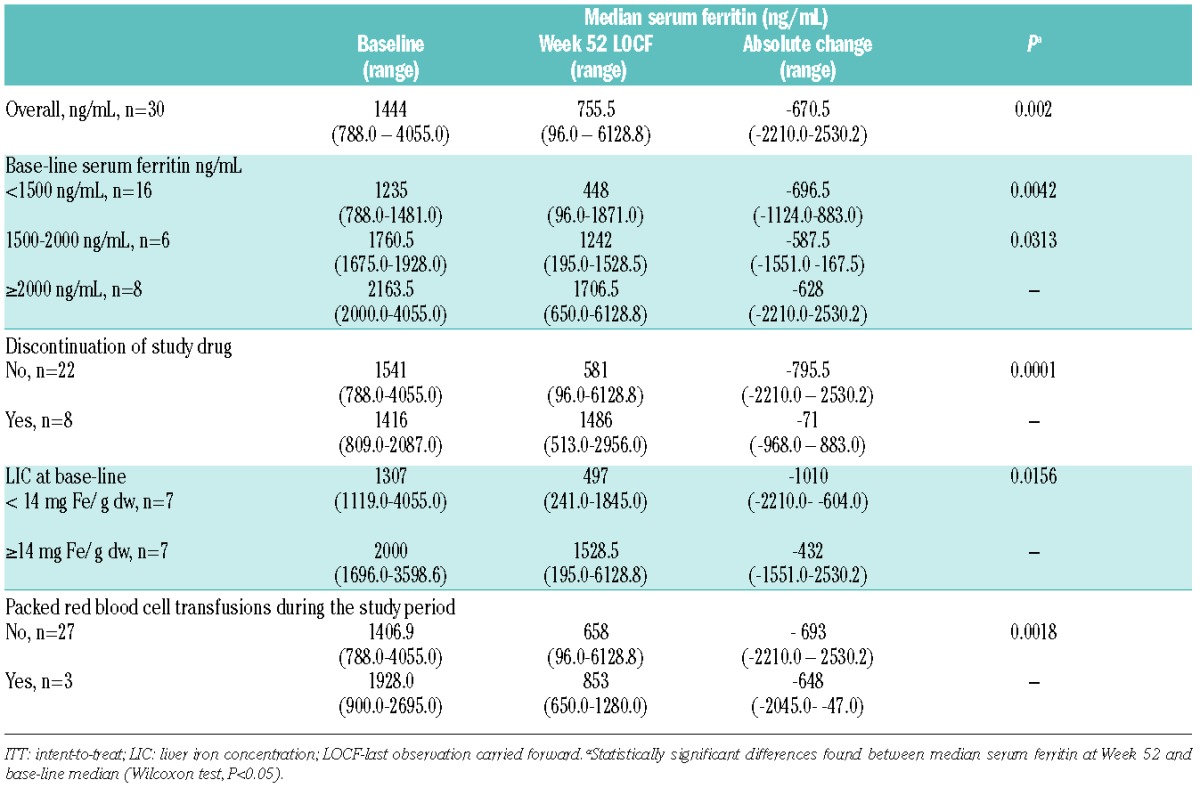
The change in median serum ferritin from baseline to week 52 was not significant in the 3 patients who continued to require red blood cell transfusions during the study (Table 2).
Base-line and final LIC values were available for 7 patients. A significant reduction in median LIC from baseline to 52 weeks was observed (from 14.5 to 4.6 mg Fe/g dw; P=0.007). Among patients with base-line median LIC below 14 mg Fe/g dw, there was a significant decrease from baseline in median serum ferritin at week 52 (from 1307 to 497 ng/mL; P=0.0156). In patients with base-line LIC 14 mg Fe/g dw or over there was a non-statistically significant decrease in serum ferritin (from 2000 to 1528 ng/mL).
Safety
Adverse events (AEs) were reported in 29 patients (96.7%). Drug-related AEs, reported in 17 patients (56.7%), were mostly mild to moderate in severity (Table 3). Gastrointestinal symptoms (diarrhea, constipation, nausea, vomiting, and anorexia) were the most frequent AEs reported.
Table 3.
Drug-related adverse events.
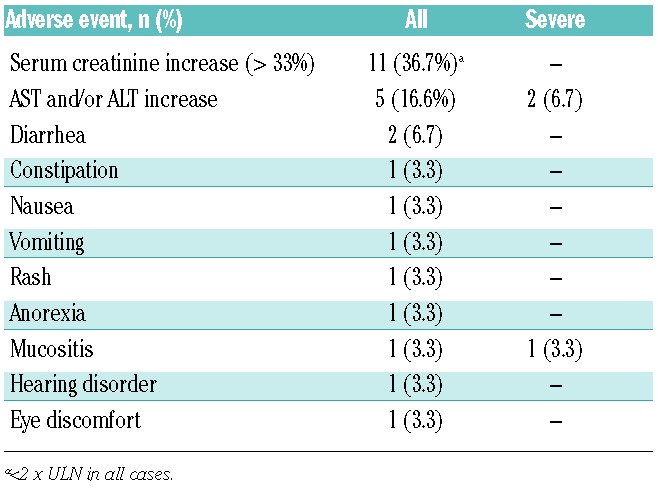
Nine serious AEs were reported in 8 patients (disease progression in 3; herpes zoster, respiratory infection, febrile syndrome, febrile neutropenia, leukocytosis, and acute massive subdural hematoma in one each); none were considered related to the study drug.
Seven patients presented with active GvHD and 5 with infection at study enrollment (Table 1). During the study, 8 patients (26.7%) had new infectious episodes and 6 (20.0%) new evidence of GvHD. None of these events was considered by the investigators to be related to the study drug.
During treatment, 5 patients presented with increased liver transaminases over 4 times ULN; one had active GvHD at inclusion and 2 developed new episodes of GvHD through the trial. Two patients, each of whom had active GvHD at inclusion or presented new episodes of GvHD, had an increase in alanine aminotransferase (ALT) over 10 times ULN on one occasion. Levels returned to normal in one patient following temporary discontinuation of the study drug while the study drug was not modified in the second patient who completed 52 weeks of treatment with ALT values below 2 times ULN.
An over 33% increase in serum creatinine compared to baseline on two consecutive visits and over ULN was reported in 12 patients (40.0%); 4 of these patients had base-line serum creatinine above ULN. The increases in serum creatinine observed in those patients were all below 2 times ULN. Fourteen patients (46.6%) were treated with cyclosporine A during the study. There was no statistically significant difference in the incidence of nephrotoxicity in patients according to concomitant use of cyclosporine [5 of 16 without cyclosporine (31.3%) vs. 7 of 14 patients with cyclosporine (50%)].
Discussion
This is the first report of a prospective study of deferasirox in adult patients with hematologic malignancies who have undergone HSCT. In our study, deferasirox at 10 mg/kg/day administered for 52 weeks post transplant resulted in a significant reduction in median serum ferritin and mean LIC in 22 adult patients who had undergone HSCT at least six months before.
Transfusional iron overload is a relatively common complication in hematologic patients and in allo-HSCT recipients.7,23 Elevated pre-transplant serum ferritin levels have been associated with increased risk of both overall mortality and treatment-related mortality in patients undergoing HSCT, independently of several confounding factors. Elevated serum ferritin has also been associated with increased risk of bacterial infection,7,8 mucositis,9 acute GvHD,10 chronic liver disease,11 and SOS.12,24 However, because serum ferritin is an imperfect measure of total body iron, the exact prognostic role of iron overload in allo-HSCT remains to be determined. Several prospective studies and a multi-center meta-analysis examining the impact of elevated LIC on HSCT outcomes came to different conclusions regarding the prognostic relevance of LIC in HSCT, mainly due to the use of different LIC thresholds.17–21 However, an LIC threshold of over 7 mg Fe/g dw has been associated with a decrease in overall survival and non-relapse mortality.19,20 In the meta-analysis performed by Armand, there was a trend towards increased non-relapse mortality for LIC over 7mg/gdw.21
Data from this trial are based on the analysis of absolute change in serum ferritin from baseline. Although the assessment of serum ferritin has limitations (e.g. levels can be increased in conditions of infection and inflammation), it is simple and inexpensive, and when assessed serially, generally provides a reliable tool to assess total body iron and response to chelation therapy. In addition, the relationship between long-term control of serum ferritin and survival has been clearly demonstrated in cohort studies of chelated patients with thalassemia major.25 MRI is being increasingly used to measure iron burden; however, its use in clinical practice is still limited as the necessary equipment is not widely available. In our study, 7 patients had LIC analysis by MRI at baseline and at the end of the trial.
Initial studies of deferasirox administered following HSCT to patients with transfusional hemosiderosis have proved successful.1–6,26–30 Two studies in patients with thalassemia (one conducted in children) reported no significant difference in post-treatment serum ferritin levels following deferasirox versus phlebotomy.1,4 In children, deferasirox yielded a significant decrease in LIC in patients with baseline serum ferritin of 1000 ng/mL or over compared to phlebotomy (−8.1 mg Fe/g dw vs. −3.5 mg Fe/g dw; P=0.048). Notably, parents of 13 of 14 children randomized to phlebotomy asked for their child to receive deferasirox because of issues concerning pain, anemia, and hospitalizations.1 A small retrospective study of patients with hematologic malignancies who had undergone HSCT showed that while both deferasirox and phlebotomy separately were able to achieve a statistically significant reduction in serum ferritin from baseline (P=0.017 and P=0.025, respectively), their combined use resulted in a faster reduction in serum ferritin.5
In this study, the majority of patients received 10 mg/kg/day. This dosage provided a significant reduction in median serum ferritin from baseline to 52 weeks (from 1444 to 755.5 ng/mL; P=0.002) in the overall population. The starting dose of deferasirox in the majority of the studies in HSCT recipients has been 10 mg/kg/day, i.e. lower than that usually used in the non-transplant setting.1,4,27 However, dose adjustment for poor response resulted in doses up to 20 mg/kg/day being used in some studies.4,27 In one study, the mean dose of deferasirox at the last dose was 11 mg/kg/day in the patients with LIC of 7 mg Fe/g dw or below and 18.1 mg/kg/day in those with LIC over 7 mg Fe/ g dw.1 In our study, the change in median serum ferritin was not significant in 3 patients who continued to require packed red blood cell transfusions during the study, implying that this subgroup of patients needed higher doses of deferasirox or more time under treatment to show a significant decrease in serum ferritin. Also, in patients with base-line LIC of 14 mg Fe/g dw or over there was a non-statistically significant decrease in serum ferritin (from 2000 to 1528 ng/mL). As with the previous subgroup, those patients with higher base-line LIC likely needed a higher dose of deferasirox or a longer duration of treatment.
The safety profile of deferasirox in our study, as in other experiences with HSCT recipients, was similar to that seen in patients with non-malignant transfusional hemosiderosis.1–6,26–30 The most frequent drug-related AEs were increased creatinine levels, increased transaminases, and gastrointestinal symptoms, which were generally mild to moderate in severity.1,4,5,27
In this report, the increases in serum creatinine were all below 2 times ULN. Patients taking cyclosporine did not have a higher incidence of increased serum creatinine compared with patients who did not receive cyclosporine. These results are not consistent with a previously published study in patients with aplastic anemia, where significantly more patients taking cyclosporine had an over 33% increase in serum creatinine above baseline and ULN. However, it should be noted that 56% of patients in that study received deferasirox at 20 mg/kg/day or over.31
In our study, 2 patients had an increase in alanine aminotransferase over 10 times ULN on one occasion. Levels returned to normal in one patient following temporary discontinuation of the study drug and the study drug was not modified in the second patient who completed 52 weeks of treatment with ALT values below 2 times ULN. Although a transient increase in liver enzymes has been reported as an AE with deferasirox, a decrease in median alanine aminotransferase over 12 months of deferasirox treatment has also been reported.4
The impact of iron chelation on long-term outcomes of patients with iron overload undergoing allo-HSCT has not been evaluated in prospective randomized trials. However, recently published studies analyzed the impact of iron chelation with deferasirox on patient outcome.29,30,32 Visani et al. have reported 8 patients with incomplete hematopoietic reconstitution after HSCT who were treated with deferasirox; all patients experienced an increase in hemoglobin levels followed by transfusion independence.29 The mechanisms underlying how deferasirox could induce hematologic improvement have yet to be clarified. Reduction in oxidative stress, a state that has a variety of inhibitory effects on erythroid and hematopoietic function, has been proposed as a possible explanation.33
In the study by Sigvin et al.,80 allo-HSCT patients with iron overload were retrospectively analyzed.30 The patients were divided into two groups: those who did not receive any chelator treatment due to potential side effects or compliance problems and who were treated by phlebotomy (Group 1) and those who received deferasirox treatment (Group 2). In univariate and multivariate analysis, patients in Group 1 showed poorer overall survival compared to those in Group 2 with an increase in risk of death (HR: 3.22, min-max: 1.67–6.23, P=0.001 vs. HR: 3.51, min-max: 1.75–6.99, P<0.001, respectively).
A second retrospective study of 158 adult patients who underwent allo-HSCT reported that after a median follow up of 18 months, the 5-year overall survival probability was significantly higher for patients with serum ferritin below 500 ng/mL at the time of transplant than for those with serum ferritin 500–2500 ng/mL or over 2500 ng/mL (P=0.002) due to higher transplant-related mortality in the patients with serum ferritin over 2500 ng/mL (P=0.04).32 The patients who had received iron chelation had a significantly better overall 5-year survival than non-chelated patients (P=0.008) and experienced significantly less disease relapse (P=0.012).
In conclusion, deferasirox at 10 mg/kg/day administered for 52 weeks post transplant resulted in a significant reduction in median serum ferritin and median LIC in 22 adult patients who had undergone HSCT at least six months before. The majority of AEs related to deferasirox were mild or moderate, and the safety profile of deferasirox was similar to that reported in previous studies of patients with transfusional hemosiderosis.2,3
Additional studies are warranted to determine the exact prognostic role of iron overload in allo-HSCT and the impact of iron chelation with deferasirox on long-term outcomes in patients with hematologic malignancies and iron overload undergoing allo-HSCT.
Acknowledgments
Editorial assistance was provided by Kathryn M. Martin and was supported by Novartis Farmaceutica, Spain. Data from the study was presented at the 38th Annual Meeting of the European Group for Blood and Marrow Transplantation (EBMT) in Geneva, Switzerland, April 1-4, 2012. Members of the Grupo Español de Trasplante Hematopoyético (GETH), Infectious and Non-infectious Complications Subcommittee: Carlos Vallejo, Montserrat Batlle, Lourdes Vázquez, Carlos Solano, Antonia Sampol, Rafael Duarte, Dolores Hernández, Javier López, Montserrat Rovira, Santiago Jiménez, David Valcárcel, Isidro Jarque
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
The study was sponsored by Novartis Farmacéutica S A, Spain.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Inati A, Sbeiti N, Khoriaty E, Cappellini MD, Koussa S, Nasr TA, et al. 1-year results from a prospective randomized trial comparing phlebotomy with deferasirox for the treatment of iron overload in pediatric patients with thalassemia major following curative stem cell transplantation. Blood. 2011;18(21):Abstract 904 [DOI] [PubMed] [Google Scholar]
- 2.Yesilipek MA, Karasu G, Kazik M, Uygun V, Ozturk Z. Posttransplant oral iron-chelating therapy in patients with beta-thalassemia major. Pediatr Hematol Oncol. 2010;27(5):374–9 [DOI] [PubMed] [Google Scholar]
- 3.Majhail NS, Laazarus HM, Burns LJ. A prospective study of iron overload management in allogeneic hematopoietic cell transplantation survivors. Biol Blood Marrow Transplant. 2010;16(6):832–7 [DOI] [PubMed] [Google Scholar]
- 4.Pakakasama S, Sirieung S, Thinkhamrop B, Sirachainan N, Hongeng S. Efficacy of deferasirox on iron chelation in thalassemia patients after hematopoietic stem cell transplantation. Blood. 2011;118(21): Abstract 3136 [Google Scholar]
- 5.Sigvin S, Bahcebasi S, Kaynat L, et al. Efficacy and safety of deferasirox for patients undergoing stem cell transplantation in post-transplant period. In: Proceedings from the European Group for Blood and Marrow Transplant 37th Annual Meeting; 2011; Paris, France: Abstract P705 [Google Scholar]
- 6.Unal S, Kuskonmaz B, Hazirolan T, Eldem G, Aytac S, Cetin M, et al. Deferasirox use after hematopoietic stem cell transplantation in pediatric patients with beta-thalassemia major: preliminary results. Pediatr Hematol Oncol. 2010;27(6):482–9 [DOI] [PubMed] [Google Scholar]
- 7.Kanda J, Mizumoto C, Ichinohe T, Kawabata H, Saito T, Yamashita K, et al. Pretransplant serum ferritin and C-reactive protein as predictive factors for early bacterial infection after allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2011;46(2):208–16 [DOI] [PubMed] [Google Scholar]
- 8.Miceli MH, Doug L, Grazziutti ML, Fassas A, Thertulien R, Van Rhee F, et al. Iron overload is a major risk factor for severe infection after autologus stem cell transplantation: a study of 367 myeloma patients. Bone Marrow Transplant. 2006;37:857–64 [DOI] [PubMed] [Google Scholar]
- 9.Altes A, Remacha AF, Sarda P, Baiget M, Sureda A, Martino R, et al. Early clinical impact of iron overload in stem-cell transplantation. A prospective study. Ann Hematol. 2007;86(6):443–7 [DOI] [PubMed] [Google Scholar]
- 10.Pullarkat V, Blanchard S, Tegtmeier B, Dagis A, Patene K, Ito J, Forman SJ. Iron overload adversely affects outcome of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2008;42(12):799–805 [DOI] [PubMed] [Google Scholar]
- 11.Tomás JF, Pinilla I, Garcia-Buey ML, Garcia A, Giguera A, Gomez-Garcia de Soria VGG, et al. Long-term liver dysfunction after allogeneic bone marrow transplantation: clinical features and course in 61 patients. Bone Marrow Transplant. 2000;26:649–55 [DOI] [PubMed] [Google Scholar]
- 12.Armand P, Kim HT, Cutler CS, Ho VT, Koreth J, Alyea EP, et al. Prognostic impact of elevated pretransplantation serum ferritin in patients undergoing myeloablative stem-cell transplantation. Blood. 2007;109(10):4586–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sivgin S, Eser B. The management of iron overload in allogeneic hematopoietic stem cell transplant (alloHSCT) recipients: where do we stand¿ Ann Hematol. 2009;92:577–86 [DOI] [PubMed] [Google Scholar]
- 14.Kanda J, Kawabata H, Chao NJ. Iron overload and allogeneic hematopoietic stem-cell transplantation. Exp Rev Hematol. 2011;4(1):71–80 [DOI] [PubMed] [Google Scholar]
- 15.Sivgin S, Baldane S, Kaynar L, Kunaz F, Pala C, Ozturk A, et al. Pretransplant serum ferritin level may be a predictive marker for outcomes in patients having undergone allogeneic hematopoietic stem cell transplantation. Neoplasma. 2012;59(2):183–90 [DOI] [PubMed] [Google Scholar]
- 16.Meyer SC, O Meara A, Buser AS, Tichelli A, Passweg JR, Stern M. Prognostic impact of posttransplantation iron overload after allogeneic stem cell transplantation. Biol Blood Marrow Transplant. 2013;19:440–4 [DOI] [PubMed] [Google Scholar]
- 17.Trottier BJ, Burns LJ, DeFor TE, Cooley S, Majhail NS. Association of iron overload with allogeneic hematopoietic cell transplantation outcomes: a prospective cohort study using R2-MRI-measured liver iron content. Blood. 2013;122(9):1678–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armand P, Sainvil M-M, Kim HT, Rhodes J, Cutler C, Ho VT, et al. Does iron overload really matter in stem cell transplantation¿ Am J Hematol. 2012;87(6):569–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitanen JM, Itala-Remes MA, Remes KJ, Vahlberg T, Saunavaara JP, Sinisalo M, et al. Prognostic impact of pretransplant resonance imaging on severe infections in allogeneic stem cell transplantation. Eur J Haematol. 2013;91:85–93 [DOI] [PubMed] [Google Scholar]
- 20.Wermke M, Schmidt A, Middeke JM, Sockel K, von Bonin M, Schonefeldt C, et al. MRI-based liver iron content predicts for nonrelapse mortality in MDS and AML patients undergoing allogeneic stem cell transplantation. Clin Can Res. 2012;18(23):6460–8 [DOI] [PubMed] [Google Scholar]
- 21.Armand P, Kim HT, Virtanen J, Itala-Remes M, Majhail NS, Burns LJ, et al. Iron overload in allogeneic stem cell transplantation outcome: a meta-analysis. Biol Blood Marrow Transplant. 2014;20(S27–S44): Abstract 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomsen C, Wiggers P, Ring-Larsen H, Christiansen E, Dalhoj J, Henriksen O, Christoffersen P. Identification of patients with hereditary haemochromatosis by magnetic resonance imaging and spectroscopic relaxation time measurements. Magn Reson Imaging. 1992;10(6):867–79 [DOI] [PubMed] [Google Scholar]
- 23.Strasser SI, Kowdley KV, Sale GE, McDonald GB. Iron overload in bone marrow transplant recipients. Bone Marrow Transplant. 1998;22:167–73 [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Yoo KH, Sung KW, Koo HH, Kwon YJ, Kwon MM, et al. Hepatic veno-occulusive disease in children after hematopoietic stem-cell transplantation: incidence, risk factors, and outcome. Bone Marrow Transplant. 2010;45(8):1287–93 [DOI] [PubMed] [Google Scholar]
- 25.Olivieri NF, Nathan DG, MacMillian JH, Wayne AS, Liu PP, McGee A, et al. Survival in medically treated patients with homozygous thalassemia. N Eng J Med. 1994;331(9): 574–8 [DOI] [PubMed] [Google Scholar]
- 26.Quarta A, Melpignano A, Quarta G. Oral iron chelator deferasirox in the treatment of secondary hemochromatosis following bone marrow transplantation in a patient with severe aplastic anemia. Acta Haematologica. 2011;125(4):219–21 [DOI] [PubMed] [Google Scholar]
- 27.Al-Ali HK, Jaekel N, Lieder K, Albrecht S, Leismann O, Hubert K, et al. Efficacy and safety of deferasirox in patients with transfusional iron overload after allogeneic hematopoietic cell transplantation: the CICL670ADE02 Trial. Blood. 2012; 120(21):Abstract 485 [Google Scholar]
- 28.Al-Ali HK, Jaekel N, Nass A, Otto O, Bug G, Kroeger N, et al. The oral chelator deferasirox for treatment of transfusional iron overload after allogeneic hematopoietic cell transplantation does not appear to interfere with the calcineurin inhibitor cyclosporine trough serum levels. Blood. 2010;116:Abstract 1826 [Google Scholar]
- 29.Visani G, Guiducci B, Giardini C. Deferasirox improves hematopoiesis after allogeneic hematopoietic SCT. Bone Marrow Transplant. 2014;49(4):585–7 [DOI] [PubMed] [Google Scholar]
- 30.Sivgin S, Baldane S, Akyol G, Keklik M, Kaynar L, Kurnaz F, et al. The oral iron chelator deferasirox might improve survival in allogeneic hematopoietic cell transplant (alloHSCT) recipients with transfusional iron overload. Transf Apheres Sci. 2013;49(2):295–301 [DOI] [PubMed] [Google Scholar]
- 31.Lee JK, Yoon S-S, Shen ZX, Ganser A, Hsu HC, Habr D, et al. Iron chelation therapy with deferasirox in patients with aplastic anemia: a subgroup analysis of 116 patients from the EPIC trial. Blood. 2010;116:2448–54 [DOI] [PubMed] [Google Scholar]
- 32.Michallet M, Sobh M, Morisset S, Labussiere H, Detrait MY, Ducastelle S, et al. Significant impact or iron chelation after allogeneic hematopoietic stem cell transplantation on disease recurrence: potential anti-leukemic activity. Blood. 2013; 122(21):Abstract 180 [Google Scholar]
- 33.Guariglia R, Martorelli MC, Villani O. Positive effects on hematopoiesis in patients with myelodisplastic syndromes receiving deferasirox as oral chelation therapy: a brief review. Leuk Res. 2011;35(5):566–7 [DOI] [PubMed] [Google Scholar]


