The demethylating agent azacitidine (AZA) is currently the standard of care for patients with higher-risk myelodysplastic syndromes (MDS) not eligible for allogeneic stem cell transplant (HSCT). Although approximately 50% of patients show a response to AZA it is not currently possible to accurately predict which patients will respond. In addition, higher-risk MDS frequently progress to secondary acute myeloid leukemia (sAML) within months, even in the presence of continuous therapy with AZA. Somatic gene mutations affecting prognosis have recently been identified in MDS.1–4 Among these, mutations in TP53, EZH2, ETV6, RUNX1 and ASXL1 are associated with poor overall survival.1–4 TP53 mutations are mainly seen in high-risk MDS with abnormal chromosome 5 or complex karyotype.5,6 In particular, TP53 mutations have been shown to confer resistance to lenalidomide in MDS with del(5q).7 In a recent publication by Saft et al., p53 expression as determined by immunohistochemistry (IHC) was shown to predict for lower cytogenetic response rate, higher risk for transformation to sAML and shorter overall survival (OS) in lenalidomide-treated MDS patients with del(5q).8 However, whether p53 expression also influences response to AZA is unclear. To answer this question, we analyzed the prevalence of p53 expression in a cohort of 100 patients with higher-risk MDS (IPSS intermediate-2 or high), sAML or MDS/MPN treated with at least one complete cycle of AZA (75 mg/m2/day for 7 days) and correlated this to outcome.
Bone marrow (BM) trephine biopsies were obtained from all patients before the start of AZA. Sections (2 μm) on SuperFrost microscope slides were de-paraffinized and pre-treated at 95°C for 7 min in citrate buffer (pH 6). For quantification and assessment of distribution of progenitor cells, samples were stained for CD34 (Cellmarque Rocklin, CA, USA). The DO-7 antibody (DakoCytomation, Denmark), which labels both wild-type and mutant-type p53 proteins, was used to detect p53 protein expression.6 The entire trephine section was assessed for p53 protein nuclear staining in hematopoietic progenitor cells, as previously described.6–9 In order to minimize the possibility of false positive results, p53 protein expression was considered positive only if strong nuclear staining (score 3+) was present in at least 5% of hematopoietic cells in the entire BM.6,9 To ensure correct staining, a positive control (urothelial carcinoma) was included on each slide. Molecular TP53 mutation analysis was performed by deep sequencing, as described previously.2
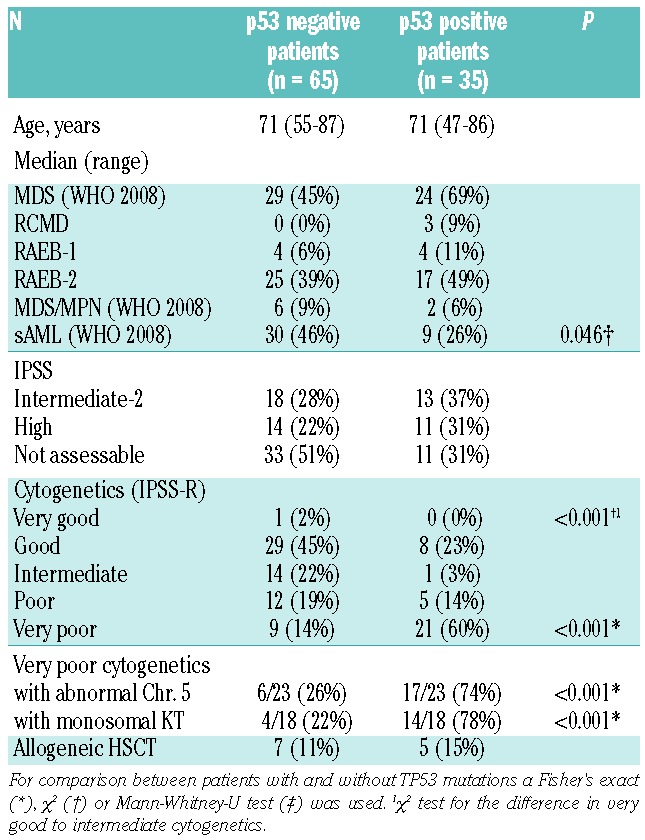
Patients’ demographic and clinical characteristics are detailed in Table 1. Thirty-five patients were positive for p53 expression, 69% of whom had higher-risk MDS (Table 1). In sAML, patients lacking p53 expression were significantly more frequent (46%) than p53-positive patients (26%) (P=0.046). Cytogenetics were scored according to IPSS-R.10 P53 positive patients were predominantly found in the very poor risk group (60% p53-positive vs. 14% p53-negative; P<0.001) (Table 1) and 74% had involvement of chromosome 5 aberrations. Five patients showed an isolated del(5q) aberration and TP53 mutations were found in 2 of these (40%). The majority of patients with monosomal karyotypes were also p53-positive (78%; P<0.001).11 The chance of exhibiting strong p53 expression was significantly increased in patients with very poor risk cytogenetics (Odds Ratio (OR): 9.333; 95%CI: 3.517; 24.771; P<0.001) and with chromosome 5 aberrations (OR: 7.364; 95%CI: 2.886; 18.791; P<0.001) as assessed by univariate binary logistic regression. In multivariable logistic regression analysis, a significantly increased chance of p53 expression was seen only for patients with very poor risk cytogenetics (OR: 1.488; 95%CI: 1.115; 1.986; P=0.007).
Table 1.
Patients’ demographic and clinical characteristics.
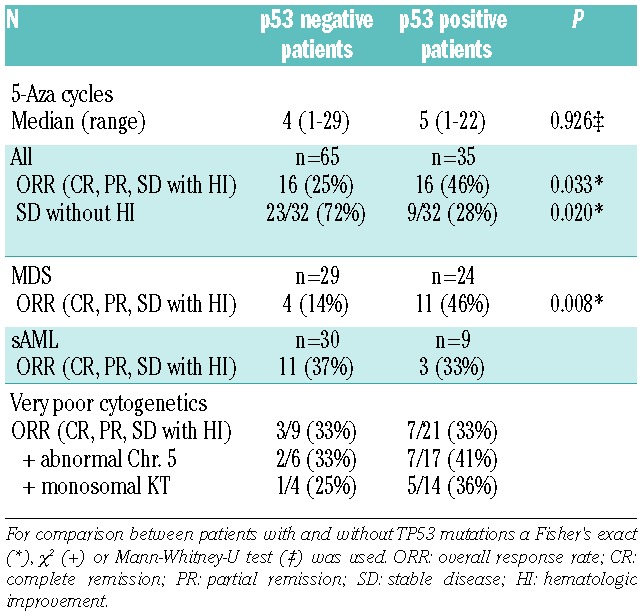
The overall response rate (ORR) to AZA was assessed according to IWG 2006 criteria.12 Complete remission in the bone marrow (BM-CR) without improvement of peripheral blood values and stable disease without hematologic improvement (HI) were not included in the calculation of ORR. The ORR for the total cohort was 46% for p53-positive patients and 25% for p53-negative patients (P=0.033) (Table 2). The number of AZA cycles administered was similar in both cohorts. Of note, MDS patients with strong p53 expression showed a significantly higher ORR than p53 negative patients (46% vs. 14%; P=0.008). Interestingly, there was no significant difference in ORR to AZA in sAML patients according to p53 status (33% ORR for p53-positive and 37% ORR for p53-negative patients). In terms of cytogenetic risk, the subgroup of patients with very poor risk cytogenetics and strong p53 expression responded as well as patients without p53 expression to treatment with AZA (33% vs. 33%, respectively). There appeared to be a better ORR in patients with very poor risk cytogenetics, chromosome 5 aberation and p53 expression than in similar patients without p53 expression (ORR 41% vs. 33%, respectively). A potentially greater ORR also appeared to be seen in patients with very poor risk cytogenetics, monosomal karyotype and p53 expression than in similar patients without p53 expression (ORR 36% vs. 25%, respectively). However, both subgroups were too small to reach statistical significance. Interestingly, the considerable subgroup of patients achieving stable disease without HI (n=32) consisted mainly of p53-negative patients (72% vs. 28%; P=0.020). Given the retrospective nature of the analysis, and the fact that patients were not part of a clinical trial, there was no regular response assessment during follow up.
Table 2.
Response to azacitidine.
Overall survival was measured beginning from start of AZA. Patients who underwent allogeneic HSCT were censored at the day of transplantation. The median OS of our total cohort was 380 days (95%CI: 217; 543), with 246 days (95%CI: 62; 430) for p53-positive patients and 410 days (95%CI: 272; 548) for p53-negative patients (P=NS), confirming the very poor survival for p53-positive patients despite response to AZA.1,5–7 A limitation of our study is that we were not able to evaluate response duration. Of note, compared to the randomized trial of higher-risk MDS patients treated with AZA, the ORR in our cohort was lower.13 This is most likely due to the fact that our patients were older and included a higher percentage of complex karyotypes (33% poor or very poor karyotype in our p53-negative group, 74% poor or very poor karyotype in our p53-positive group compared to 28% poor risk karyotype in the AZA-001 trial). Although patients in our cohort with p53 positivity responded to AZA, response duration may be shorter than for p53-negative patients; one probable reason for the observed poor overall survival.
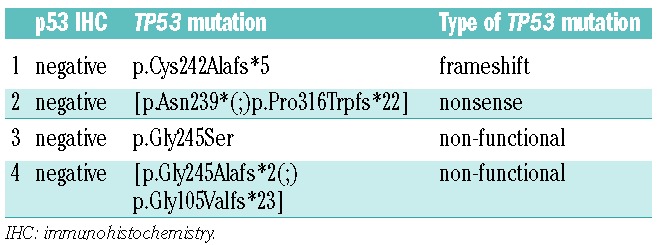
To validate the quality of p53 IHC to detect TP53 mutations, we performed TP53 sequencing of 37 randomly selected BM samples. TP53 sequencing revealed mutations in 14 of 37 samples and p53 IHC was positive in 10 of 37 samples. Substantial agreement (Cohens Kappa=0.645; P<0.001) was seen between both methods. Analysis of the four TP53 mutations not detected by p53 IHC showed two non-functional mutations: one frameshift and one nonsense mutation (Table 3). Frameshift and nonsense mutations represent approximately 6–10% and 3–8% of TP53 mutations, respectively.6,14 Regarding the value of TP53 sequencing in relation to IHC for p53 expression, it is worth noting that sequencing data do not always directly reflect p53 function since not all TP53 mutations lead to loss of p53 function. In contrast, strong p53 expression is an indicator of loss of p53 function due to impairment of the auto-regulatory feedback loop inducing p53 degradation. Finally, IHC for p53 expression has recently been extensively validated for MDS and can thus be considered a reliable method to assess for dysfunctional TP53.6,7,8
Table 3.
TP53 mutations causing false negative p53 IHC.
In summary, we show in a large cohort of 100 patients with higher-risk MDS and sAML treated with AZA that p53 expression as a surrogate for the presence of TP53 mutations does not negatively impact on treatment response. Accordingly, the combination of AZA and lenalidomide may confer a benefit in higher-risk MDS patients with del(5q) harboring TP53 mutations and should be assessed in future trials.15
Footnotes
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Bejar R, Stevenson K, Abdel-Wahab O, Galili N, Nilsson B, Garcia-Manero G, et al. Clinical Effect of Point Mutations in Myelodysplastic Syndromes. N Engl J Med. 2011;364(26):2496–506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Haferlach T, Nagata Y, Grossmann V, Okuno Y, Bacher U, Nagae G, et al. Landscape of genetic lesions in 944 patients with myelodysplastic syndromes. Leukemia. 2014;28(2)241–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papaemmanuil E, Gerstung M, Malcovati L, Tauro S, Gundem G, Van Loo P, et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood. 2013;122(22):3616–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thol F, Friesen I, Damm F, Yun H, Weissinger EM, Krauter J, et al. Prognostic Significance of ASXL1 Mutations in Patients With Myelodysplastic Syndromes. J Clin Oncol. 2011;29(18):2499–506 [DOI] [PubMed] [Google Scholar]
- 5.Kita-Sasai Y, Horiike S, Misawa S, Kaneko H, Kobayashi M, Nakao M, et al. International prognostic scoring system and TP53 mutations are independent prognostic indicators for patients with myelodysplastic syndrome. Br J Haematol. 2001;115(2):309–12 [DOI] [PubMed] [Google Scholar]
- 6.Kulasekararaj AG, Smith AE, Mian SA, Mohamedali AM, Krishnamurthy P, Lea NC, et al. TP53 mutations in myelodysplastic syndrome are strongly correlated with aberrations of chromosome 5, and correlate with adverse prognosis. Br J Haematol. 2013;160(5):660–72 [DOI] [PubMed] [Google Scholar]
- 7.Jädersten M, Saft L, Smith A, Kulasekararaj A, Pomplun S, Göhring G, et al. TP53 Mutations in Low-Risk Myelodysplastic Syndromes With del(5q) Predict Disease Progression. J Clin Oncol. 2011;29(15):1971–9 [DOI] [PubMed] [Google Scholar]
- 8.Saft L, Karimi M, Ghaderi M, Matolcsy A, Mufti GJ, Kulasekararaj A, et al. P53 protein expression independently predicts outcome in patients with lower-risk myelodysplastic syndromes with del(5q). Haematologica. 2014;99(6):1041–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iwasaki T, Murakami M, Sugisaki C, Sobue S, Ohashi H, Asano H, et al. Characterization of myelodysplastic syndrome and aplastic anemia by immunostaining of p53 and hemoglobin F and karyotype analysis: Differential diagnosis between refractory anemia and aplastic anemia. Pathol Int. 2008;58(6):353–60 [DOI] [PubMed] [Google Scholar]
- 10.Schanz J, Tüchler H, Solé F, Mallo M, Luno E, Cervera J, et al. New Comprehensive Cytogenetic Scoring System for Primary Myelodysplastic Syndromes (MDS) and Oligoblastic Acute Myeloid Leukemia After MDS Derived From an International Database Merge. J Clin Oncol. 2012;30(8):820–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breems DA, Van Putten WLJ, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KBJ, Mellink CHM, et al. Monosomal Karyotype in Acute Myeloid Leukemia: A Better Indicator of Poor Prognosis Than a Complex Karyotype. J Clin Oncol. 2008;26(29):4791–7 [DOI] [PubMed] [Google Scholar]
- 12.Cheson BD, Greenberg PL, Bennett JM, Lowenberg B, Wijermans PW, Nimer SD, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108(2):419–25 [DOI] [PubMed] [Google Scholar]
- 13.Fenaux P, Mufti GJ, Hellström-Lindberg E, Santini V, Finelli C, Giagounidis A, et al. Efficacy of azacitidine compared with that of conventional care regimens in the treatment of higher-risk myelodysplastic syndromes: A randomized, open-label phase III study. Lancet Oncol. 2009;10:223–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petitjean A, Mathe E, Kato S, Ishioka C, Tavtigian SV, Hainaut P, et al. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: lessons from recent developments in the IARC TP53 database. Hum Mutat. 2007;28(6):622–9 [DOI] [PubMed] [Google Scholar]
- 15.Platzbecker U, Braulke F, Kündgen A, Götze K, Bug G, Schonefeldt C, et al. Sequential combination of azacitidine and lenalidomide in del(5q) higher-risk myelodysplastic syndromes or acute myeloid leukemia: a phase I study. Leukemia. 2013;27(6):1403–7 [DOI] [PMC free article] [PubMed] [Google Scholar]


