Abstract
Acute right heart syndrome is a sudden deterioration in right ventricular performance, resulting in right ventricular failure and confers significant in-hospital morbidity and mortality. In critically ill patients, the syndrome is often undiagnosed and untreated, as these patients do not usually exhibit the common clinical manifestations of the condition, making the diagnosis challenging for the intensivist. In this narrative review we focus on the pathophysiology of acute right heart syndrome, in critical illness, diagnostic modalities used to assess right ventricular function and management of acute right heart syndrome, including mechanical ventilation strategies and circulatory support.
Keywords: critical illness, right heart failure, pulmonary artery pressure, mechanical ventilation, echocardiography
Introduction
Acute right heart syndrome (ARHS) may be defined as sudden deterioration in the right ventricular (RV) function and failure of the RV of the heart to deliver adequate blood flow to the pulmonary circulation, resulting in systemic hypoperfusion [1]. In the context of critical illness, ARHS is associated with poor outcomes and increased mortality [2]. Evidence of central venous pressure (CVP) overload in conjunction with RV contractile dysfunction, is usually present in ARHS [1].
We searched PubMed, EMBASE, Cochrane library and Google Scholar, for articles reporting on RV dysfunction and failure. The relevant papers were extracted in full and references from extracted papers were checked for any additional relevant articles. An overview of the ARHS pathophysiology, diagnostic tools for the assessment of the acutely failing RV in critical illness and measures including vasoactive agents, ventilatory strategies and mechanical support is provided in the current paper.
THE RV IN HEALTH
The main functions of the RV are:
a) maintenance of adequate pulmonary perfusion pressure in order to deliver desaturated mixed venous blood to the respiratory membrane;
b) maintenance of low systemic venous pressure in order to prevent organ congestion.
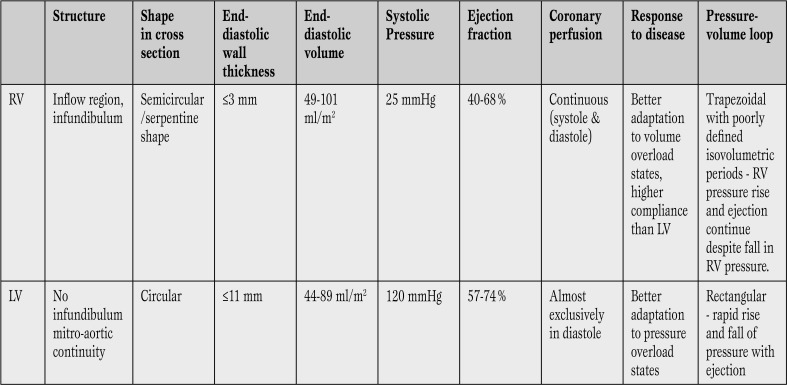
The RV is anatomically adapted for the generation of low-pressure perfusion and it is very sensitive to changes in afterload. [3]. The differences between the structure and function of the RV compared to the left ventricle (LV) are outlined in Table 1 and Figure 1 compares the pressure-volume (P-V) loop of the RV with that of the LV [3,4,5].
Table 1.
Differences between RV and LV (3-9, 25).
RV = right ventricle; LV = left ventricle.
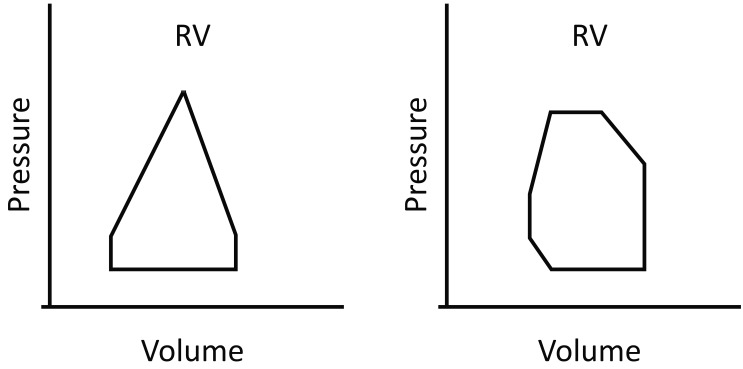
Figure 1.
Pressure-volume (P-V) loops for RV and LV. Once RV pressure reaches the PA pressure, the pulmonary valve opens. Little time is spent in isovolumetric contraction, giving a triangular-shaped RV P-V loop, in contrast to the almost square loop of the LV (25). (Adopted from: Kevin LG, Barnard M. Right ventricular failure. Contin Educ Anaesth Crit Care Pain 2007; 7: 89-94). Permission to reproduce granted under Oxford university press’s general terms.
RV = right ventricle; LV = left ventricle.
THE RV IN CRITICAL ILLNESS
ARHS is not necessarily associated with an increase in pulmonary vascular resistance (PVR) and pulmonary arterial hypertension (PAH) [6]. The syndrome can be due to RV pressure/volume overload or RV contractile dysfunction [1]. Consequence is low cardiac output (CO) with low mean arterial pressure (MAP), exacerbating RV dysfunction.
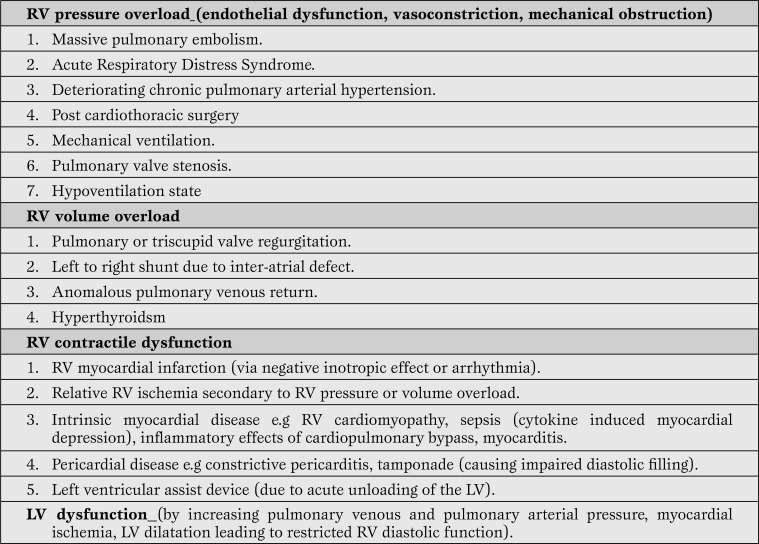
Thus, “RV failure begets RV failure” leading to a progressive downward spiral of worsening ischemia, myocardial dysfunction and shock. In mechanically ventilated patients with ARHS, low CO is multifactorial and could be due to RV systolic dysfunction, tricuspid regurgitation, ventricular interdependence (dilatation of the RV shifting the interventricular septum toward the left and decreasing the LV distensibility and preload), arrhythmias or suboptimal preload [6]. RV diastolic dysfunction causes impaired RV filling and high diastolic RV and right atrial (RA) pressures leading to organ congestion [6]. The causes and precipitating events of ARHS are summarized in Table 2 [7,8,9,10,11,12,13,14,15].
Table 2.
Precipitating events / causes of ARHS in the ICU (7-15).
ARHS = acute right heart syndrome; ICU = intensive care unit; RV = right ventricular; LV = left ventricular.
ARHS in Acute Respiratory Distress Syndrome (ARDS)
ARDS is one of the most common causes of ARHS secondary to RV pressure overload (acute cor pulmonale). In critically ill ventilated patients with ARDS, ARHS occurs in 61% of patients submitted to conventional tidal volume mechanical ventilation (MV) and 25% of those receiving lung protective MV using low tidal volumes [15, 16]. Apart from MV, the pathologic features of the syndrome per se, contribute to increased pulmonary vascular tone, acute pulmonary arterial hypertension (PAH) and cor pulmonale. Contributors to elevated pulmonary vascular resistance (PVR) in ARDS include: vasoconstrictor: vasodilator imbalance (excess ET-1, 5HT, PDE, reduced NO and prostanoids), endothelial injury, hypoxic pulmonary vasoconstriction (80% arteriolar), hypercapnia (including permissive hypercapnia), acidemia, in situ thrombosis and pulmonary vascular remodelling (muscularization of non-muscularized arteries) [8, 16, 17].
ARHS in the setting of massive pulmonary embolism (PE)
Critically ill patients are at risk of PE despite thromboprophylaxis [3, 18]. In a ten-year retrospective study, Vieillard-Baron et al. [19] showed that ARHS was present in 61% of medical intensive care unit (ICU) patients with massive PE and carried a 23% mortality.
The normal RV can generate a mean pulmonary artery pressure up to 40 mmHg, requiring 50-75% of the pulmonary vasculature to be occluded by emboli before acute RV failure occurs [19].
Hypoxemia induced by the emboli results in pulmonary vasoconstriction and the physiological response to platelet activation leading to release of vasoactive agents such as serotonin, thromboxane and histamine, causes further increase in PVR and RV pressure overload [19, 20].
ARHS in sepsis
In severe sepsis and septic shock the RV function might be impaired. RV systolic dysfunction in sepsis is directly associated with markers of endothelial dysfunction (endothelin 1, vascular cellular adhesion molecule 1) and directly related to the severity of sepsis [21].
A proposed mechanism for ARHS in sepsis is increased PVR secondary to sepsis-induced endothelial cell injury and altered vaso-reactivity, despite concomitant decrease in systemic vascular resistance (SVR). Substantial increases in PVR also occur when the left ventricle needs to considerably increase the cardiac output in order to compensate for the fall in the SVR, causing further increase in RV afterload [21, 22].
DIAGNOSIS OF ARHS IN THE CRITICALLY ILL
CLINICAL FEATURES
The clinical features of ARHS, including acute onset shortness of breath, orthopnea and bilateral lower extremity edema, are non-specific and difficult to identify in the sedated critically ill patient [6]. Increased oxygen requirements or sudden cardiovascular collapse might be the chief clinical manifestations of ARHS in a mechanically ventilated patient [23].
Other prominent clinical signs include atrial or ventricular arrhythmias, raised jugular venous pressure and gallop rhythm at the left sternal edge, systolic murmur of tricuspid regurgitation, organomegaly, signs of deep venous thrombosis (in the context of venous thromboembolism) [6].
It is important to consider ARHS in persistent respiratory weaning failure (RV dysfunction leads to an imbalance between ventilator needs and cardiorespiratory capacity), especially in patients with LV systolic dysfunction [6, 9, 24, 25]. A high index of suspicion is needed in high risk patients such as those with pre-existing PAH and recent deep venous thrombosis [25].
BEDSIDE STUDIES
Available bedside studies include: chest X-ray (CXR), electrocardiography (EKG), arterial blood gas (ABG) analysis, hemodynamic and echocardiographic diagnostic tools.
Chest X-ray
Enlargement of the main pulmonary artery and regional oligemia are seen in massive PE. However, CXR cannot be utilized to confirm the diagnosis of ARHS and should only contribute to the diagnostic approach by ruling out conditions that mimic ARHS in the ICU, such as atelectasis, pleural effusions, pulmonary edema and pneumothorax [6].
Electrocardiography
Kucher et al. showed that Qr in V1 is a strong predictor of RV dysfunction, and it is highly associated with troponin leakage and myocardial shear stress [26]. It has also been demonstrated that in patients with right bundle branch block, R duration in lead V1>100 ms is predictive of RV systolic dysfunction [43].
Other EKG findings suggestive of RV strain include inversion of T waves in leads V1-V4 and the classic S1Q3T3 pattern. Acute anterior Q-wave pattern in leads V1-V3, as well as a right-sided Q pattern in leads V3R-V6R, might suggest RV infarction [12]. EKG, although specific, lacks sensitivity [11].
Arterial blood gas analysis
ABG analysis may reveal grossly impaired gas exchange and low cardiac output might result in acidemia with lactic acidosis due to tissue hypoperfusion [27].
Hemodynamic bedside diagnostic modalities
Central venous catheters and central venous pressure
An accurately placed central venous catheter (in the superior vena cava), can provide information on CVP and used as a surrogate for RV end-diastolic volume (RVEDV) and RV end-diastolic pressure (RVEDP) [25, 28]. In severe tricuspid regurgitation causing ARHS, a broad, tall systolic c-v wave is seen due to abnormal systolic filling of the right atrium (RA) and the CVP trace is said to be ventricularized because it resembles right ventricular pressure [25, 28]. RVEDP reflects RVEDV (which is proportional to preload) only when ventricular compliance is normal.
Therefore, in conditions such as PAH, tamponade and myocardial ischemia, where RV compliance is decreased, CVP is likely to be raised and cannot be accurately assessed [28, 29].
Right heart catheterization
Right heart catheterization using a pulmonary artery catheter (PAC) is frequently required when ARHS is clinically suspected and interpretation of imaging studies is difficult or inconclusive. Hemodynamic data obtained from an accurately placed PAC, by thermodilution, may provide diagnostic clues and guide therapy. PAC allows direct simultaneous measurement of RA, RV, PA and pulmonary artery wedge pressures and indirect measurement of cardiac output, cardiac index (CI), RV stroke work index, mixed venous oxygen saturation, PVR and SVR [29, 30].
Hemodynamically, ARHS is suspected if RA pressure >8-10 mmHg, or RA pressure to pulmonary capillary wedge pressure ≥0.8 (isolated RV failure) and CI is low. In the presence of RV-PA gradient >25 mmHg, RV outflow tract obstruction should be excluded by echocardiography [31].
In the context of PAH and suspected ARHS, right heart catheterization allows assessment of left-sided heart disease and its contribution to PAH. Besides, calculation of PVR and SVR help decide whether pulmonary or systemic vasodilators/pressors are needed and monitor response to therapy [29]. In patients with pre-existing PAH, a decrease in PA pressure might reflect low RV ejection fraction and worsening RV dysfunction [12].
Arterial pulse contour analysis
Arterial pulse contour analysis enables calculation of CO, pulse pressure variation (PPV), stroke volume variation (SVV) and SVR, from the arterial pulse pressure waveform, in mechanically ventilated patients. Dynamic indices (SVV, PPV) have been used to predict preload responsiveness and monitor the hemodynamic effect of volume expansion in critically ill patients [32].
Wyler Von Ballmoos et al. reported that PPV is not accurate predictor of fluid responsiveness in mechanically ventilated patients with acute PAH (at risk of ARHS), early after cardiac surgery and in septic shock [33]. In the context of a pressure overloaded RV, increased PPV values are related to an increase in the RV afterload and not to a decrease in RV preload and therefore, further volume expansion could potentially be harmful [33, 34]. However, it could be reasonably contended that lack of response to a fluid challenge, while PPV or SVV is high, could be seen as an indicator of RV dysfunction necessitating further investigations [35].
Bedside imaging modalities
Echocardiography
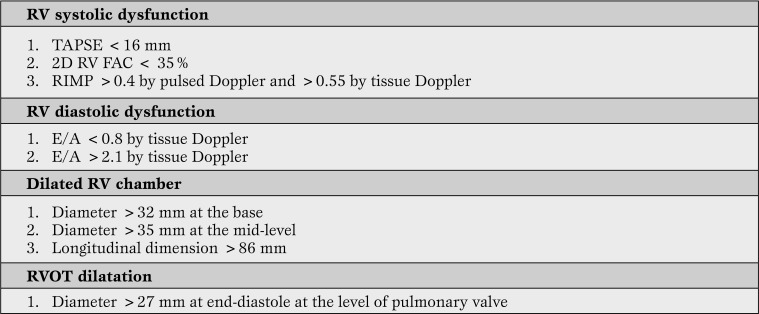
Transthoracic (TTE) and transesophageal echocardiography (TEE) are bedside diagnostic tools which also provide rapid risk stratification and could potentially direct therapeutic strategies. In experienced hands, echocardiography allows assessment of the RV performance and loading conditions. Useful echocardiography-derived measures of RV function, when ARHS is clinically suspected are outlined in Table 3 [36].
Table 3.
Echocardiographic quantitative parameters pointing towards ARHS (36-38)
RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion; 2DRVFAC = two-dimensional right ventricular fractional area change; RIMP = right ventricular index of myocardial performance; E/A = early (E) to late (A) ventricular filling velocities ratio; RVOT = right ventricular outflow tract.
TTE is an easy and non-invasive way to assess the size and kinetics of the RV. The diagnosis of ARHS due to RV pressure overload, with TTE, has good positive predictive value for indirect diagnosis of massive PE [37]. Main limitations of TTE in critically ill patients ventilated with high level of positive end-expiratory pressure (PEEP) include: inadequate imaging due to interposition of the inflated lung between the heart and the chest wall, low diagnostic accuracy in the patients with pre-existing cardiopulmonary disease, the operator dependent nature of TTE [37].
RV function, size and shape, are more accurately assessed with TEE.
It has been suggested that in the presence of significant and otherwise unexplained RV strain without clots present on TTE, TEE should rapidly follow at the bedside, providing there is local availability and expertise [38]. TEE is a semi-invasive procedure and commonly reported complications associated with TEE in critically ill patients receiving MV, include: hypo- or hypertension, dysrrhythmias, trauma to the gastrointestinal tract, hypoxemia and dislodgment of endotracheal or nasogastric tubes. The over-all complication rate associated with TEE use is low and it is estimated to be approximately 2.6% [38].
Additional imaging modalities
Computed tomography (CT)
CT pulmonary angiography (CTPA) is being used increasingly as a diagnostic tool in PE, with documented sensitivities of 50-100% and specificities of 81-100% [39]. CTPA has become the preferred diagnostic modality for suspected ARHS due to PE, in hemodynamically stable ICU patients [66]. Chest CT signs suggestive of ARHS include: flattening or displacement of the intraventricular septum toward the LV, reflux of contrast into the inferior vena cava, RV diameter (RVD) to LV diameter (LVD) ratio on axial sections greater than 1.0 (RVDaxial/LVDaxial >1) [39].
Cardiovascular Magnetic Resonance (CMR)
CMR is the most sensitive method to assess the RV size and function. Imaging quality is not influenced by acoustic windows or pre-existing cardiopulmonary disease [40]. However, CMR is rarely used for ICU patients receiving MV, as the MR environment carries significant risks to patients during transportation and prolonged periods in the MR scanner.
LABORATORY TESTS
The usefulness of laboratory tests such as D-dimmer, troponins and B-type natriuretic peptide levels, as diagnostic tests in ICU patients with suspected RV failure, is limited, as they are non-specific and confounded in the context of critical illness [41, 42].
In summary, in critically ill patients with clinically suspected ARHS, echocardiography (TTE and/or TEE) and right heart catheterization are the preferred diagnostic modalities. If PE is the most likely cause of ARHS, then CTPA is necessary to confirm the diagnosis, provided the patient is suitable for transfer to radiology.
In any given scenario, the diagnostic approach will depend upon the expertise and availability of the different diagnostic modalities.
TREATMENT
The principles and key components of ARHS management include reversal of precipitating events and control of contributing factors (hypoxemia, hypercapnia, anemia, acidemia, sepsis, dysrrhythmias), fluid volume optimization, maintenance of perfusion pressure, positive inotropy, use of pulmonary vasodilators and protective MV [12, 43]. The management principles and strategies will depend upon the primary hemodynamic pathology.
Control of contributing factors, general ICU care and reversible causes of ARHS
Infection prevention, treatment of sepsis in accordance with the surviving sepsis campaign bundles, normoxia, normocapnia, thromboprophylaxis, peptic ulcer prophylaxis, correction of acid-base imbalance and electrolytes and glycemic control are mandatory and applied to most ICU patients [12]. Pulmonary vasodilators and/or inodilators (in acutely decompensated PAH), thrombolysis (in massive PE), revascularization (in RV infarction) and sequential AV pacing and/or cardioversion (in significant dysrrhythmias), could potentially correct the abnormal RV physiology [44].
Optimization of intravascular fluid status
Fluid loading in ARHS remains controversial. RV ejection fraction is dependent on RV pre-load, in the abscence of PAH and it is likely that RV output will be inadequate in hypovolemia. The RV can increase the stroke work through an increase in RV free wall stretch (via the Frank-Starling mechanism) [29]. Therefore, optimization of pre-load may improve RV ejection fraction.
The role of CVP as a guide to fluid therapy remains controversial. A systematic review of 24 studies demonstrated a poor relationship between CVP and intravascular fluid status and the inability of CVP/delta-CVP to predict the hemodynamic response to a fluid challenge [32, 45].
Depending on where the patient is on the Frank-Starling curve, some may be adequately resuscitated with a CVP of 6-7 mm Hg, while others may still be intravascularly volume depleted at a CVP of 10 mm Hg [31]. In a recent meta-analysis, Marik et al. showed that there is paucity of data to support the widespread practice of using CVP to assess intravascular fluid status and guide fluid therapy [46]. More reliable hemodynamic assessment tools, such as PAC, pulse contour analysis, TTE and/or TEE when available, may be utilized to guide fluid therapy in ARHS [32].
Mercat et al. showed that in critically ill medical patients with circulatory failure (defined by CI 2), due to massive PE, fluid loading with 500 ml of colloid increased the cardiac index significantly and improved hemodynamic status [47]. If the hemodynamic response to initial fluid challenge is poor, in the context of ARHS, further volume loading may cause RV overdistension, increased ventricular interdependence, decreased LV filling and RV ischemia, leading to worsening shock [44]. In RV volume overload, acute kidney injury due to venous congestion (cardiorenal syndrome), continuous veno-venous hemofiltration (CVVH) facilitates greater clinical improvement compared with aggressive diuretic therapy, in heart failure patients, who are diuretic resistant [48].
The role of vasopressors in ARHS
In order to preserve adequate right coronary blood flow, systemic pressure should be maintained above the PA pressures. It has been shown that in patients with sepsis, PAH and RV dysfunction, norepinephrine increases systemic pressure through alpha-1 receptor agonism and may improve the RV oxygen supply/demand ratio, but this potentially beneficial effect on RV ejection fraction may be offset by a concomitant increase in PVR and RV afterload, at high doses (>0.5 mcg/kg/min) [43, 49]. Besides, norepinephrine, through beta-1 receptor agonism could potentially improve RV-PA coupling and CO [22]. Low dose vasopressin (0.033-0.067 U/min) mediates pulmonary arterial vasodilation and may be useful in vasodilatory shock and pulmonary vascular dysfunction, especially in norepinephrine resistant patients [43, 49, 50].
Inotropes and inodilators in ARHS
Dobutamine (beta-1 receptor agonist) can be used as the first-line inotropic agent in ARHS due to RV contractile dysfunction. Low dose dobutamine (2-5 mcg/kg/min) increases CI, SV and decreases PVR and SVR [12, 43, 51]. At higher doses (>10 mcg/kg/min) dobutamine causes tachycardia, increased oxygen consumption, increased PVR and leads to systemic hypotension and addition of a vasopressor might be required [12, 51]. High quality evidence suggests that dopamine is associated with increased tachyarrhythmias and is not recommended in cardiogenic shock [45]. It has been demonstrated that in patients with septic shock and ARHS, who are unresponsive to fluid loading, dopamine or dobutamine, epinephrine improves RV contractility in spite of a rise in mean PAP by 11% (p<0.05) [52]. Selective phosphodiresterase (PDE) III inhibitors (enoximone, milrinone, amrinone), augment myocardial contractility and cause systemic and pulmonary vasodilaton, by increasing cyclic adenosine monophosphate (cAMP) and thus reducing PA pressures and improving RV function in patients with ARHS due to pressure overloaded RV [43]. Systemic hypotension should be expected and addition of a vasopressor might be needed. It has been demonstrated that levosimendan, a calcium sensitizer with pulmonary vasodilator properties (inodilator), improves RV performance in ARHS secondary to sepsis-induced ARDS and in experimental ARHS restores RV-PA coupling better than dobutamine [53]. In ARHS, levosimendan has been shown to reduce the increased RV afterload and ventricular interdependence, improve RV contractility and RV diastolic function, without significant increase in oxygen consumption, mediated by opening of sarcolemmal and mitochondrial potassium-adenosine triphosphate channels [54, 55]. Although levosimendan is indicated for the treatment of acute heart failure (class of recommendation IIa, level of evidence B), it is has not yet been approved in all countries [54, 55].
Pulmonary vasodilation in ARHS
The goals of pulmonary vasodilation in ARHS are:
1) decrease PVR and impedance;
2) increase RV stroke volume and output;
3) avoid systemic hypotension and maintain coronary perfusion;
4) avoid hypoxemia from ventilation-perfusion mismatch.
It is recommended that inhaled nitric oxide (NO), which increases intra-cellular cyclic guanosine monophosphate (cGMP), should be considered as short term therapy to improve PaO2/FiO2 ratio and CO, in ventilated patients with ARHS secondary to ARDS [43]. It has also been suggested that NO may be effective in stabilizing patients with ARHS due to massive PE until more definitive treatment is available [29, 56].
Prostanoid formulations (epoprostenol, iloprost) are potent pulmonary and systemic vasodilators with anti-thrombotic and anti-proliferative actions. They reduce PVR and improve RV function and they have been used in ARHS due to RV pressure overload [57].
It has been shown that use of intravenous epoprostenol in mechanically ventilated patients with ARDS reduces PVR and improves RV performance [58].
Sildenafil, a PDE 5 inhibitor, increases downstream cGMP signaling and potentiates the beneficial effects of NO. It reduces PVR and increases CO and myocardial perfusion [29]. Karakitsos et al. showed that mechanically ventilated patients with ARHS from PAH, who were dependent on dobutamine, were treated with oral sildenafil and in many cases they were successfully weaned from inotropic and ventilatory support [59].
Mechanical ventilation strategies in ARHS
Optimal MV ventilation management in ARHS consists of: avoidance of hypoxemia, hypercapnia, high levels of PEEP (>10 cmH2O) and both high and low extremes of lung volumes and use lung protective ventilation strategies if possible [43, 60,61,62].
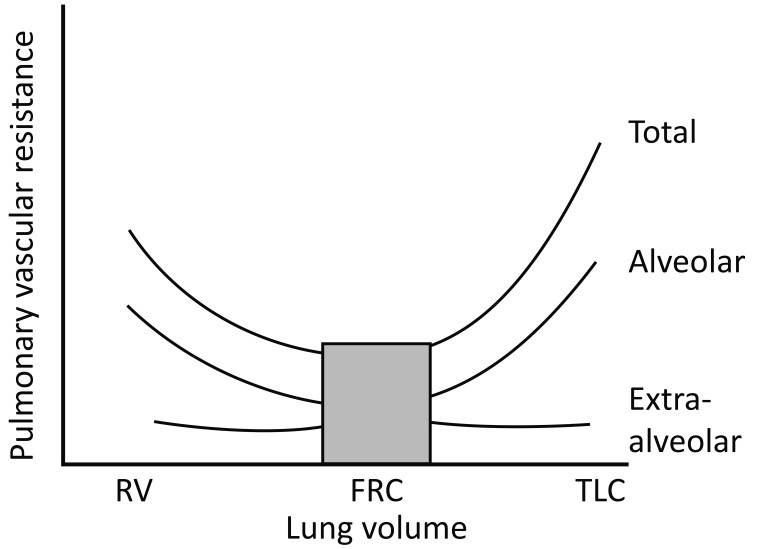
The RV afterload is governed by PVR which is directly affected by changes in lung volume [61]. Increased PVR occurs at both low and high lung volumes. At low volumes this is due to the elastic recoil forces of the lung parenchyma leading to extra-alveolar vessel collapse and terminal airway collapse leading to alveolar hypoxia and hypoxic pulmonary vasoconstriction (HPV) and at high lung volumes due to collapse of the alveolar vessels via stretch of the alveolar wall. When PVR is plotted against lung volume, a typical U-shaped curve occurs with the lowest PVR occurring at functional residual capacity (FRC) (Figure 2) [62].
Figure 2.
Effect of changing lung volume on pulmonary vascular resistance (PVR) (62). (Adopted from: Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child 1999; 80: 475-480). Permission to reproduce granted under BMJ Publishing Group Ltd’s general terms.
RV = residual volume; FRC = functional residual capacity; TLC = total lung capacity.
Schmitt et al. assessed the impact of PEEP on the RV outflow impedance using doppler data obtained by TEE, in mechanically ventilated ICU patients with ARDS. They demonstrated that high PEEP (13±4 cmH2O) was associated with increased RV afterload and worsening RV systolic dysfunction [60].
The significant decrease in the incidence of ARHS (from 61% to 25%) in ARDS since ARDSnet trial was published, reflects a change in MV practice and suggests that lung protective strategies (tidal volume: 6-8 ml/kg predicted body weight (PBW), low plateau pressures and PEEP) reduce the incidence of ARHS [63].
In patients with ARHS, during lung protective ventilation, permissive hypercapnia should be avoided as acute hypercapnia could lead to pulmonary vasoconstriction or exacerbate hypoxic pulmonary vasoconstriction and could potentially worsen RV dysfunction [64]. A prospective observational study, which evaluated the relative roles of acute permissive hypercapnia and PEEP variations on RV function, in severe ARDS patients, showed that increasing PEEP at constant Pplat induces acute hypercapnia that may impair RV function and decrease CI. It is therefore recommended that in cases of ARHS, lung protective ventilation should be gradually adapted to limit hypercapnia and RV overload [65].
In mechanically ventilated ICU patients with ARHS, refractory hypoxemia and/or hypercapnia and high PEEP requirements, extracorporeal membrane oxygenation (ECMO) could be used as a bridge to the recovery of respiratory function. Oxygenation and carbon dioxide clearance are provided by the extracorporeal circuit, minimizing pulmonary vasoconstriction due to hypoxemia and/or hypercapnia [66, 67]. In patients with ARHS due to severe ARDS, where lung protective ventilation may not be adequate in managing hypercapnic acidosis, extracorporeal carbon dioxide (ECCO2) removal devices are an option, as they are less invasive than ECMO and may play a role in instituting “ultra-protective” lung ventilation (tidal volume: 4 ml/kg PBW) [68].
It should be noted that the cardiac consequences of weaning from MV may be responsible for weaning failure in patients with ARHS. In these patients, an increase in weaning-induced RV afterload may occur due to marked increase in work of breathing, hypoxemia or high intrinsic PEEP, leading to further worsening RV enlargement during weaning. This may result in leftward shift of the interventricular septum, impeding LV diastolic filling and LV output (ventricular interdependence), causing pulmonary edema and failure to wean from MV [69].
Mechanical circulatory support
Low cardiac output syndrome caused by ARHS after cardiac surgery, particularly coronary artery bypass graft surgery and heart transplant, may be an indication for intra-aortic balloon pump (IABP) [70, 71]. It has been demonstrated that IABP improves hemodynamics and RV efficiency in acute ischemic RV failure [72]. However, a recent RCT failed to demonstrate any mortality benefit in patients with cardiogenic shock complicating acute myocardial infarction [73].
Veno-arterial (VA) ECMO has been used as a salvage therapy in cases of ARHS due to massive PE and refractory cardiogenic shock, after systemic thrombolysis. It can be used as a means of unloading the RV and supporting systemic circulation, in medically refractory RV failure with accompanying hypotension and end-organ failure and as a bridge to transplant [74, 75].
Right ventricular assist devices (RVADs) in ARHS may be used as a bridge to recovery or transplant, or as a definitive surgical treatment, in primary RV dysfunction. In patients who are successfully weaned from the RVAD, residual RV dysfunction is compatible with survival [76]. It has been suggested that RVADs should be avoided in patients with ARHS secondary to RV afterload resistance (with severely elevated PVR), as pumping blood into the PA could potentially cause worsening PAH and lung injury, whereas CO and CI remain low. In such cases VA ECMO might be more effective in off-loading the RV [77].
The use of mechanical cardiovascular support devices depends largely on local availability of specialized facilities, cardiopulmonary pathophysiology expertise and operator experience.
Conclusion
ARHS can occur in many critical illnesses and carries substantial morbidity and mortality. ARHS is difficult to diagnose in the critically ill as those patients have ongoing physiological derangement presenting the intensive care specialists with a diagnostic dilemma. Cardiac echo and right heart catheterization are invaluable diagnostic tools in the assessment of the RV at the bedside, which also provide a rapid risk stratification and could direct treatment strategies.
Characterizing, identifying and correcting reversible factors is of paramount importance. Minimizing RV afterload (pulmonary vasodilators, inodilators, RV “protective” MV strategies) and maximizing RV performance (preload, inotropy, mechanical circulatory support) are the major components of ARHS management. Need for mechanical circulatory support, merits referral to specialized treatment centres, if there is insufficient local expertise or capacity. There is lack of definitive data regarding the management of ARHS in ICU patients, without pre-existing cardiopulmonary disease.
Therefore, some recommendations may rely on lower level of evidence or expert opinion. Well-designed and adequately powered RCTs are required to estimate the prevalence of ARHS among critically patients receiving MV, improve the understanding of its mechanisms in the context of critical illness and evaluate the efficacy of therapy guided by invasive and non-invasive hemodynamic monitoring tools.
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Zochios V, Jones N. Acute right heart syndrome in the critically ill patient. Heart, Lung and Vessels. 2014; 6(3): 157-170
References
- Greyson C R. Pathophysiology of right ventricular failure. Crit Care Med. 2008;36:S57–S65. doi: 10.1097/01.CCM.0000296265.52518.70. [DOI] [PubMed] [Google Scholar]
- Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L. Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate. Am Heart J. 1997;134:479–487. doi: 10.1016/s0002-8703(97)70085-1. [DOI] [PubMed] [Google Scholar]
- Bleeker G B, Steendijk P, Holman E R, Yu C M, Breithardt O A, Kaandorp T A. et al. Assessing right ventricular function: the role of echocardiography and complementary technologies. Heart. 2006;92:19–26. doi: 10.1136/hrt.2005.082503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alaverdian A, Cohen R. The Right Ventricle in critical illness. The Open Critical Care Medicine Journal. 2010;3:38–42. [Google Scholar]
- Kevin L G, Barnard M. Right ventricular failure. Contin Educ Anaesth Crit Care Pain. 2007;7:89–94. [Google Scholar]
- Zochios V, Keeshan A. Pulmonary embolism in the mechanically-ventilated critically ill patient: is it different? JICS. 2013;14:36–44. [Google Scholar]
- Reis Miranda D, Gommers D, Lachman B. In: Gullo A, eds. Anaesthesia, Intensive Care and Emergency Medicine. 2006. Effect of mechanical ventilation on right ventricular afterload. pp. 361–366. [Trieste: Springer] [Google Scholar]
- Cecconi M, Johnston E, Rhodes A. What role does the right side of the heart play in circulation? Crit Care. 2006;10:6–6. doi: 10.1186/cc4832. [Suppl III] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddad F, Hunt S A, Rosenthal D N, Murphy D J. Right ventricular function in cardiovascular disease. I. Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. doi: 10.1161/CIRCULATIONAHA.107.653576. [DOI] [PubMed] [Google Scholar]
- Forrest P. Anaesthesia and right ventricular failure. Anaesth Intensive Care. 2009;37:370–385. doi: 10.1177/0310057X0903700314. [DOI] [PubMed] [Google Scholar]
- Giovambattista R D. Hyperthyroidism as a reversible cause of right ventricular overload and congestive heart failure. Cardiovasc Ultrasound. 2008;6:29–29. doi: 10.1186/1476-7120-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahm T, McCaslin C A, Wozniak T C, Ghumman W, Fadl Y Y, Obeidat O S. et al. Medical and surgical treatment of acute right ventricular failure. J Am Coll Cardiol. 2010;56:1435–1446. doi: 10.1016/j.jacc.2010.05.046. [DOI] [PubMed] [Google Scholar]
- Santambrogio L, Bianchi T, Fuardo M, Gazzoli F, Veronesi R, Braschi A. et al. Right ventricular failure after left ventricular assist device insertion: preoperative risk factors. Interact Cardiovasc Thorac Surg. 2006;5:379–382. doi: 10.1510/icvts.2006.128322. [DOI] [PubMed] [Google Scholar]
- Michael R, Pinsky M. Recent advances in the clinical application of heart-lung interactions. Curr Opin Crit Care. 2002;8:26–31. doi: 10.1097/00075198-200202000-00005. [DOI] [PubMed] [Google Scholar]
- Jardin F, Gueret P, Dubourg O, Farcot J C, Margairaz A, Bourdarias J P. Two-dimensional echocardiographic evaluation of right ventricular size and contractility in acute respiratory failure. Crit Care Med. 1985;13:952–956. doi: 10.1097/00003246-198511000-00035. [DOI] [PubMed] [Google Scholar]
- Vieillard-Baron A, Schmitt J M, Augarde R, Fellahi J L, Prin S, Page E. et al. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications and prognosis. Crit Care Med. 2001;29:1551–1555. doi: 10.1097/00003246-200108000-00009. [DOI] [PubMed] [Google Scholar]
- Snow R L, Davies P, Pontoppidan H, Zapol W M, Reid L. Pulmonary vascular remodeling in adult respiratory distress syndrome. Am Rev Respir Dis. 1982;126:887–892. doi: 10.1164/arrd.1982.126.5.887. [DOI] [PubMed] [Google Scholar]
- Cook D, Attia J, Weaver B, McDonald E, Meade M, Crowther M. Venous thromboembolic disease: an observational study in medical-surgical intensive care unit patients. J Crit Care. 2000;15:127–132. doi: 10.1053/jcrc.2000.19224. [DOI] [PubMed] [Google Scholar]
- Vieillard-Baron A, Page B, Augarde R, Prin S, Qanadli S, Beauchet A. et al. Acute cor pulmonale in massive pulmonary embolism: incidence, echocardiographic pattern, clinical implications and recovery rate. Intensive Care Med. 2001;27:1481–1486. doi: 10.1007/s001340101032. [DOI] [PubMed] [Google Scholar]
- Matthews J C, McLaughlin V. Acute right ventricular failure in the setting of acute pulmonary embolism or chronic pulmonary hypertension: a detailed review of the pathophysiology, diagnosis, and management. Curr Cardiol Rev. 2008;4:49–59. doi: 10.2174/157340308783565384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furian T, Aguiar C, Prado K, Ribeiro R V, Becker L, Martinelli N. et al. Ventricular dysfunction and dilation in severe sepsis and septic shock: relation to endothelial function and mortality. J Crit Care. 2012;27:319–319. doi: 10.1016/j.jcrc.2011.06.017. [e9-15.] [DOI] [PubMed] [Google Scholar]
- Chan C M, Klinger J R. The right ventricle in sepsis. Clin Chest Med. 2008;29:661–676. doi: 10.1016/j.ccm.2008.07.002. [DOI] [PubMed] [Google Scholar]
- Markel T A, Wairiuko G M, Lahm T, Crisostomo P R, Wang M, Herring C M. et al. The right heart and its distinct mechanisms of development, function, and failure. J Surg Res. 2007;146:304–313. doi: 10.1016/j.jss.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Methvin A B, Owens A T, Emmi A G, Allen M, Wiegers S E, Dries D L. et al. Ventilatory inefficiency reflects right ventricular dysfunction in systolic heart failure. Chest. 2011;139:617–625. doi: 10.1378/chest.10-0318. [DOI] [PubMed] [Google Scholar]
- Kevin L G, Barnard M. Right ventricular failure. Contin Educ Anaesth Crit Care Pain. 2007;7:89–94. [Google Scholar]
- Kucher N, Walpoth N, Wustmann K, Noveanu M, Gertsch M. QR in V1-an ECG sign associated with right ventricular strain and adverse clinical outcome in pulmonary embolism. Eur Heart J. 2003;24:1113–1119. doi: 10.1016/s0195-668x(03)00132-5. [DOI] [PubMed] [Google Scholar]
- Miller G A, Sutton G C. Acute massive pulmonary embolism. Clinical and haemodynamic findings in 23 patients studied by cardiac catheterization and pulmonary arteriography. Br Heart J. 1970;32:518–523. doi: 10.1136/hrt.32.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reems M M, Aumann M. Central venous pressure: principles, measurement, and interpretation. Compend Contin Educ Vet. 2012;34:1–1. [PubMed] [Google Scholar]
- Greyson C R. Right heart failure in the intensive care unit. Curr Opin Crit Care. 2012;18:424–431. doi: 10.1097/MCC.0b013e3283577070. [DOI] [PubMed] [Google Scholar]
- Matthay M A, Chatterjee K. Bedside catheterization of the pulmonary artery: risks compared with benefits. Ann Intern Med. 1988;109:826–826. doi: 10.7326/0003-4819-109-10-826. [DOI] [PubMed] [Google Scholar]
- Goldstein J A, Barzilai B, Rosamond T L, Eisenberg P R, Jaffe A S. Determinants of hemodynamic compromise with severe right ventricular infarction. Circulation. 1990;82:359–368. doi: 10.1161/01.cir.82.2.359. [DOI] [PubMed] [Google Scholar]
- Zochios V, Wilkinson V. Assessment of intravascular fluid status and fluid responsiveness during mechanical ventilation in surgical and intensive care patients. JICS. 2011;12:295–300. [Google Scholar]
- Wyler von Ballmoos M, Takala J, Roeck M, Porta F, Tueller D, Ganter C C. et al. Pulse-pressure variation and hemodynamic response in patients with elevated pulmonary artery pressure: a clinical study. Crit Care. 2010;14:111–111. doi: 10.1186/cc9060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daudel F, Tüller D, Krähenbühl S, Jakob S M, Takala J. Pulse pressure variation and volume responsiveness during acutely increased pulmonary artery pressure: an experimental study. Crit Care. 2010;14:R122–R122. doi: 10.1186/cc9080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard F, Richards G, Biais M, Lopes M, Auler J O. Using pulse pressure variation or stroke volume variation to diagnose right ventricular failure? Crit Care. 2010;14:451–451. doi: 10.1186/cc9303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudski L G, Lai W W, Afilalo J, Hua L, Handschumacher M D, Chandrasekaran K. et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr. 2010;23:685–713. doi: 10.1016/j.echo.2010.05.010. [Quiz 786-8] [DOI] [PubMed] [Google Scholar]
- Jardin F, Dubourg O, Guéret P, Delorme G, Bourdarias J P. Quantitative two-dimensional echocardiography in massive pulmonary embolism: Emphasis on ventricular interdependence and leftward septal displacement. J Am Coll Cardiol. 1987;10:1201–1206. doi: 10.1016/s0735-1097(87)80119-5. [DOI] [PubMed] [Google Scholar]
- Hüttemann E. Transoesophageal echocardiography in critical care. Minerva Anestesiol. 2006;72:891–913. [PubMed] [Google Scholar]
- Kang D K, Thilo C, Schoepf U J, Barraza J M Jr, Nance J W Jr, Bastarrika G. et al. CT signs of right ventricular dysfunction: prognostic role in acute pulmonary embolism. JACC Cardiovasc Imaging. 2011;4:841–849. doi: 10.1016/j.jcmg.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Mitoff P R, Beauchesne L, Dick A J, Chow B J, Beanlands R S, Haddad H. et al. Imaging the failing right ventricle. Curr Opin Cardiol. 2012;27:148–153. doi: 10.1097/HCO.0b013e32834fec4e. [DOI] [PubMed] [Google Scholar]
- Lim W, Cook D J, Griffith L E, Crowther M A, Devereaux P J. Elevated cardiac troponin levels in critically ill patients: prevalence, incidence, and outcomes. Am J Crit Care. 2006;15:280–288. [PubMed] [Google Scholar]
- Cepkova M, Kapur V, Ren X, Quinn T, Zhuo H, Foster E. et al. Clinical significance of elevated B-type natriuretic peptide in patients with acute lung injury with or without right ventricular dilatation: an observational cohort study. Ann Intensive Care. 2011;1:18–18. doi: 10.1186/2110-5820-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price L C, Wort S J, Finney S J, Marino P S, Brett S J. Pulmonary vascular and right ventricular dysfunction in adult critical care. Crit Care. 2010;14:169–169. doi: 10.1186/cc9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza G, Goldhaber S Z. The acutely decompensated right ventricle: pathways for diagnosis and management. Chest. 2005;128:1836–1852. doi: 10.1378/chest.128.3.1836. [DOI] [PubMed] [Google Scholar]
- Marik P E, Baram M, Vahid B. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Chest. 2008;134:172–178. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- Marik P E, Cavallazzi R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit Care Med. 2013;41:1774–1781. doi: 10.1097/CCM.0b013e31828a25fd. [DOI] [PubMed] [Google Scholar]
- Mercat A, Diehl J L, Meyer G, Teboul J L, Sors H. Hemodynamic effects of fluid loading in acute massive pulmonary embolism. Crit Care Med. 1999;27:540–544. doi: 10.1097/00003246-199903000-00032. [DOI] [PubMed] [Google Scholar]
- Giglioli C, Landi D, Cecchi E, Chiostri M, Gensini G F, Valente S. et al. Effects of ULTRAfiltration vs. DIureticS on clinical, biohumoral and haemodynamic variables in patients with deCOmpensated heart failure: the ULTRADISCO study. Eur J Heart Fail. 2011;13:337–346. doi: 10.1093/eurjhf/hfq207. [DOI] [PubMed] [Google Scholar]
- Kerbaul F, Rondelet B, Motte S, Fesler P, Hubloue I, Ewalenko P. et al. Effects of norepinephrine and dobutamine on pressure load-induced right ventricular failure. Crit Care Med. 2004;32:1035–1040. doi: 10.1097/01.ccm.0000120052.77953.07. [DOI] [PubMed] [Google Scholar]
- Luckner G, Mayr V D, Jochberger S, Wenzel V, Ulmer H, Hasibeder W R. et al. Comparison of two dose regimens of arginine vasopressin in advanced vasodilatory shock. Crit Care Med. 2007;35:2280–2285. doi: 10.1097/01.ccm.0000281853.50661.23. [DOI] [PubMed] [Google Scholar]
- Haddad F, Doyle R, Murphy D J, Hunt S A. Right ventricular function in cardiovascular disease, part II: pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- Le Tulzo Y, Seguin P, Gacouin A, Camus C, Suprin E, Jouannic I. et al. Effects of epinephrine on right ventricular function in patients with severe septic shock and right ventricular failure: a preliminary descriptive study. Intensive Care Med. 1997;23:664–670. doi: 10.1007/s001340050391. [DOI] [PubMed] [Google Scholar]
- Kerbaul F, Rondelet B, Demester J P, Fesler P, Huez S, Naeije R. et al. Effects of levosimendan versus dobutamine on pressure load-induced right ventricular failure. Crit Care Med. 2006;34:2814–2819. doi: 10.1097/01.CCM.0000242157.19347.50. [DOI] [PubMed] [Google Scholar]
- Morelli A, Teboul J L, Maggiore S M, Vieillard-Baron A, Rocco M, Conti G. Effects of levosimendan on right ventricular afterload in patients with acute respiratory distress syndrome: a pilot study. Crit Care Med. 2006;34:2287–2293. doi: 10.1097/01.CCM.0000230244.17174.4F. [DOI] [PubMed] [Google Scholar]
- Pathak A, Lebrin M, Vaccaro A, Senard J M, Despas F. Pharmacology of levosimendan: inotropic, vasodilatory and cardioprotective effects. J Clin Pharm Ther. 2013;38:341–349. doi: 10.1111/jcpt.12067. [DOI] [PubMed] [Google Scholar]
- Summerfield D T, Desai H, Levitov A, Grooms D A, Marik P E. Inhaled nitric oxide as salvage therapy in massive pulmonary embolism: a case series. Respir Care. 2012;57:444–448. doi: 10.4187/respcare.01373. [DOI] [PubMed] [Google Scholar]
- Clapp L H, Finney P, Turcato S, Tran S, Rubin L J, Tinker A. Differential effects of stable prostacyclin analogs on smooth muscle proliferation and cyclic AMP generation in human pulmonary artery. Am J Respir Cell Mol Biol. 2002;26:194–201. doi: 10.1165/ajrcmb.26.2.4695. [DOI] [PubMed] [Google Scholar]
- Radermacher P, Santak B, Wust H J, Tarnow J, Falke K J. Prostacyclin and right ventricular function in patients with pulmonary hypertension associated with ARDS. Intensive Care MED. 1990;16:227–232. doi: 10.1007/BF01705156. [DOI] [PubMed] [Google Scholar]
- Karakitsos D, Papanikolaou J, Karabinis A, Alalawi R, Jumper C, Alexopoulos D. et al. Acute effect of sildenafil on central hemodynamics in mechanically ventilated patients with WHO group III pulmonary hypertension and right ventricular failure necessitating administration of dobutamine. J Cardiol. 2013;167:848–854. doi: 10.1016/j.ijcard.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Schmitt J M, Vieillard-Baron A, Augarde R, Prin S, Page B, Jardin F. Positive end-expiratory pressure titration in acute respiratory distress syndrome patients: impact on right ventricular outflow impedance evaluated by pulmonary artery Doppler flow velocity measurements. Crit Care Med. 2001;29:1154–1158. doi: 10.1097/00003246-200106000-00012. [DOI] [PubMed] [Google Scholar]
- Hakim T S, Michel R P, Chang H K. Effect of lung inflation on pulmonary vascular resistance by venous and arterial occlusion. J Appl Physiol. 1982;53:1110–1115. doi: 10.1152/jappl.1982.53.5.1110. [DOI] [PubMed] [Google Scholar]
- Shekerdemian L, Bohn D. Cardiovascular effects of mechanical ventilation. Arch Dis Child. 1999;80:475–480. doi: 10.1136/adc.80.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower R G, Matthay M A, Morris A, Schoenfeld D, Thompson B T, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Balanos G M, Talbot N P, Dorrington K L, Robbins P A. Human pulmonary vascular response to 4 h of hypercapnia and hypocapnia measured using Doppler echocardiography. J Appl Physiol. 2003;94:1543–1551. doi: 10.1152/japplphysiol.00890.2002. [DOI] [PubMed] [Google Scholar]
- Mekontso Dessap A, Charron C, Devaquet J, Aboab J, Jardin F, Brochard L. et al. Impact of acute hypercapnia and augmented positive end-expiratory pressure on right ventricle function in severe acute respiratory distress syndrome. Intensive Care Med. 2009;35:1850–1858. doi: 10.1007/s00134-009-1569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattinoni L, Carlesso E, Langer T. Clinical review: Extracorporeal membrane oxygenation. Crit Care. 2011;15:243–243. doi: 10.1186/cc10490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratta C, Lavezzo B, Ballaris M A, Panio A, Crucitti M, Andruetto P. et al. Extracorporeal membrane oxygenation rescue therapy in a case of portopulmonary hypertension during liver transplantation: a case report. Transplant Proc. 2013;45:2774–2775. doi: 10.1016/j.transproceed.2013.07.001. [DOI] [PubMed] [Google Scholar]
- Tiruvoipati R, Botha J A, Pilcher D, Bailey M. Carbon dioxide clearance in critical care. Anaesth Intensive Care. 2013;41:157–162. doi: 10.1177/0310057X1304100129. [DOI] [PubMed] [Google Scholar]
- Teboul J L, Monnet X, Richard C. Weaning failure of cardiac origin: recent advances. Crit Care. 2010;14:211–211. doi: 10.1186/cc8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeken U, Feindt P, Litmathe J, Kurt M, Gams E. Intraaortic balloon pumping in patients with right ventricular insufficiency after cardiac surgery: parameters to predict failure of IABP Support. Thorac Cardiovasc Surg. 2009;57:324–328. doi: 10.1055/s-0029-1185766. [DOI] [PubMed] [Google Scholar]
- Arafa O E, Geiran O R, Andersen K, Fosse E, Simonsen S, Svennevig J L. Intraaortic balloon pumping for predominantly right ventricular failure after heart transplantation. Ann Thorac Surg. 2000;70:1587–1593. doi: 10.1016/s0003-4975(00)01864-6. [DOI] [PubMed] [Google Scholar]
- Nordhaug D, Steensrud T, Muller S, Husnes K V, Myrmel T. Intraaortic balloon pumping improves hemodynamics and right ventricular efficiency in acute ischemic right ventricular failure. Ann Thorac Surg. 2004;78:1426–1432. doi: 10.1016/j.athoracsur.2003.12.077. [DOI] [PubMed] [Google Scholar]
- Thiele H, Zeymer U, Neumann F J, Ferenc M, Olbrich H G, Hausleiter J. et al. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–1296. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- Belohlavek J, Rohn V, Jansa P, Tosovsky J, Kunstyr J, Semrad M. et al. Veno-arterial ECMO in severe acute right ventricular failure with pulmonary obstructive hemodynamic pattern. J Invasive Cardiol. 2010;22:365–369. [PubMed] [Google Scholar]
- Gregoric I D, Chandra D, Myers T J, Scheinin S A, Loyalka P, Kar B. Extracorporeal membrane oxygenation as a bridge to emergency heart-lung transplantation in a patient with idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2008;27:466–468. doi: 10.1016/j.healun.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Moazami N, Pasque M K, Moon M R, Herren R L, Bailey M S, Lawton J S. et al. Mechanical support for isolated right ventricular failure in patients after cardiotomy. J Heart Lung Transplant. 2004;23:1371–1375. doi: 10.1016/j.healun.2003.09.022. [DOI] [PubMed] [Google Scholar]
- Berman M, Tsui S, Vuylsteke A, Klein A, Jenkins D P. Lifethreatening right ventricular failure in pulmonary hypertension: RVAD or ECMO? J Heart Lung Transplant. 2008;27:1188–1189. doi: 10.1016/j.healun.2008.07.017. [DOI] [PubMed] [Google Scholar]