Abstract
Introduction
Postoperative atrial fibrillation after isolated coronary revascularization has been associated with increased morbidity and mortality. Aim of present investigation was to evaluate incidence of postoperative atrial fibrillation and its prognostic role in patients undergoing isolated coronary artery by-pass and disclose possible differences between off-pump and cardiopulmonary assisted revascularization.
Methods
Prospective cohort study of 229 patients undergoing isolated coronary artery by-pass at a tertiary heart surgery Centre. Off-pump treated patients were significantly older (70.5 vs 64.9 years, p<0.001). No other baseline differences were found. Patients who developed postoperative atrial fibrillation were followed up for an average period of 2 years.
Results
Post-operative occurred in 56/229 (24.1% after cardiopulmonary and 24.6% after off-pump coronary artery by-pass). Left atrium diameter was the only independent predictive factor (odds ratio =1.15, 95% confidence interval 1.02-1.30, p<0.001). All patients with postoperative atrial fibrillation were treated and discharged in sinus rhythm, in 6/56 recurred, only in one persisted. One patient died during follow up. No stroke was recorded.
Conclusions
After isolated surgical revascularization, atrial fibrillation occurred in 24% without differences related to operative technique. Recurrence of atrial fibrillation occurred in 6/56 patients (10.7%) however only in 1 persisted. Early and late mortality did not show relation with post-operative atrial fibrillation probably due to immediate treatment with recovery of sinus rhythm before discharge.
Keywords: atrial fibrillation, cardiac revascularization, stroke, mortality
Introduction
Atrial fibrillation occurs in 20-30% after surgical cardiac revascularization [1]. Several studies suggest that postoperative atrial fibrillation (POAF) is associated with an increased duration of hospitalization, early and long term morbidity and mortality [2, 3]. Off-pump coronary artery by-pass (CABG) grafting (OP-CABG) has been hypothesized to decrease incidence of POAF [4, 5]; however contrasting results has been reported [6,7]. Aim of present prospective investigation was to evaluate the incidence of POAF in patients undergoing isolated surgical revascularization in a tertiary heart surgery centre. Patients with POAF were followed for an average period of 2 years to assess the recurrence rate of the arrhythmia and its prognostic role on early and late risk of stroke and mortality. Finally, the role of cardiopulmonary by-pass (CPB) surgical revascularization (CPB-CABG) and OP-CABG on POAF was evaluated.
Methods
Study population. Among 822 patients who underwent heart surgery between Jan 1 2009 and Dec 31 2009 in a tertiary heart surgery Centre, 229 patients in sinus rhythm on hospital admission (179 males, 50 females) underwent isolated CABG (138 - OP-CABG, 91 - CPB-CABG). Patients with atrial fibrillation, hyperthyroidism or scheduled for Maze procedure were excluded from the study. In patients undergoing isolated CABG, bipolar Maze procedure was usually planned for subjects with persistent or frequent episodes of paroxysmal atrial fibrillation. Finally, patients with more than mild valvular disease and creatinine clearace < 30 ml/min were excluded.
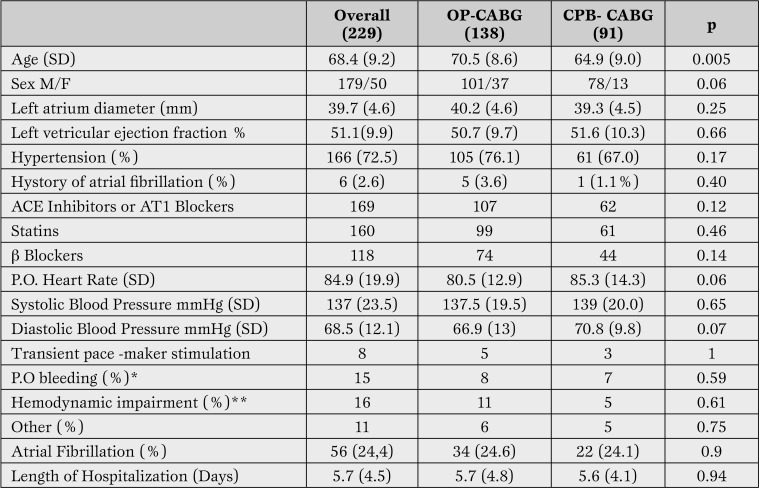
Echocardiographic evaluation was performed within 48 hours before surgery using a Sequoia Acuson Instrument (Siemens Medical Solution, Mount View, CA, USA). Echocardiography was performed according to the guidelines of the American Society of Echocardiography [8]. Clinical and echocardiographic characteristics of patients are reported in Table 1.
Table 1.
Clinical and echocardiographic characteristics of patients included in the study mean (standard deviation).
*Bleeding requiring transfusion at least 2 units of packed red blood cells or surgical revision.
**Hemodynamic deterioration with the need to infuse amines.
OP = off pump; CPB = cardipulonary-by pass; CABG = coronary artery by-pass; SD= standard deviation.
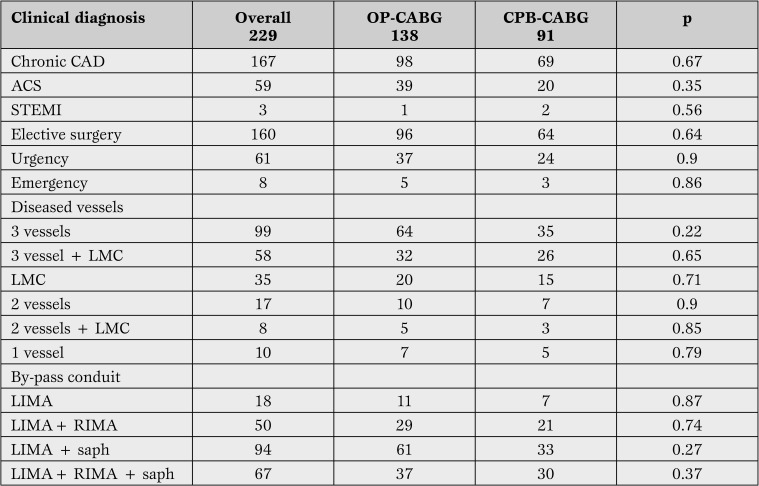
In Table 2 clinical diagnosis, indications for surgery (elective, urgency/emergency), the number of diseased vessels and graft performed in the groups under investigation are reported.
Table 2.
Clinical diagnosis, number of diseased vessels and grafts performed.
OP = off pump; CABG = coronary artery by-pass; CAD = coronary artery disease; ACS = acute coronary syndrome; STEMI = elevated ST acute myocardial infarction ; LMC = left main coronary; LIMA = left internal mammary artery; RIMA = right internal mammary artery; saph = saphene vein.
Thirty clinical and echocardiographic variables were considered to evaluate a relationship with occurrence of POAF.
After surgery all patients were continuously monitored electrocardiography (ECG), blood pressure, non-invasive oxygen saturation for at least the first 48 hours. ECG monitoring, both at bed and by telemetry, was maintained until discharge. Transient electric stimulation through epicardic wires was used for severe bradycardia or atrio-ventricular (AV) block until restoration of heart rhythm. All symptomatic arrhythmic episodes or asymptomatic atrial fibrillation lasting more than 15 minutes at ECG monitoring were considered as POAF and included in the analysis. Patients who did not recover sinus rhythm (SR) within 30 minutes were usually treated with intravenous amiodarone (300 mg in 1 hour followed by 900 mg/24 h e.v. continuous infusion) to control heart rate. Electrical cardioversion was considered when sinus rhythm was not restored within 24 hours after the beginning of pharmacological treatment. Amiodarone was continued for 3 months after discharge. Perioperative complications including bleeding needing transfusion of at least 2 units of packed red blood cells and/or surgical revision, severe hypotension requiring amines (norepinephrine, epinephrine, dobutamine or dopamine), and new onset AV block or severe bradycardia requiring electrical stimulation were recorded. Postoperative pericardial inflammation was diagnosed in the presence of pericardial rubs and/or ECG or echocardiogram signs of pericardial involvement. In the end, duration of hospitalization was examined. All patients was discharged to rehabilitation facilities. The study was approved by the ethic committee of our Institution and all participants gave their informed consent.
Follow-Up. Only patients with POAF were followed-up and entered the study. Follow-up visit were scheduled after 3, 12 and 24 months. Holter monitoring was performed every 3 months during the first year and thereafter every 6 months. Follow-up was closed on December 31 2012. No patient was lost at follow-up. Primary end point of the study was the evaluation of recurrence of atrial fibrillation and related hospitalization; secondary end points were all cause hospitalization and mortality. Finally we evaluated the role of surgical technique (CPB-CABG vs OP-CABG) on POAF.
Statistical Analysis. Data were described as mean and standard deviation (SD) for continuous variables and as number and percent for categorical variables. Preoperative and operative patient characteristics were compared according to the occurrence of postoperative AF by means of the Student t test or Fisher exact test for continuous and categorical variables, respectively, or finally by ANOVA. Multivariate logistic regression analysis was used to evaluate independent risk factors for atrial fibrillation.
Results
In-Hospital Outcomes. Overall incidence of POAF during hospitalization was 24.4% (56 of 229 patients), 38 males and 18 females. 1/229 patients died after surgery. Patients who developed AF after surgery were older than ones in stable sinus rhythm (70.5 vs 64.9 years, p=0.005). POAF was not related to clinical indication to surgery (elective vs urgency/emergency), number of diseased vessels or graft performed (Table 3).
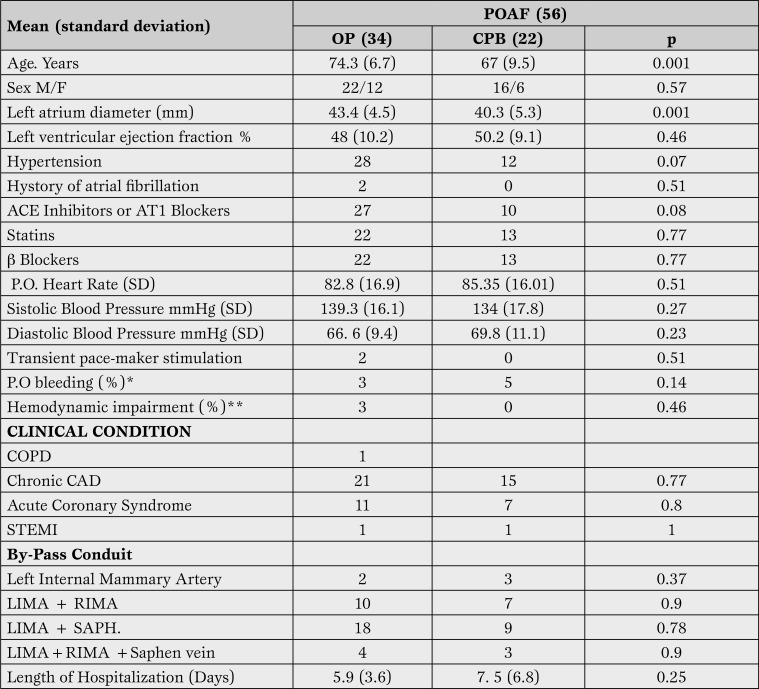
Table 3.
Comparison of clinical, echocardiographic characteristics and conduits used for grafting between patients with and without POAF according to surgical technique.
*Bleeding requiring transfusion at least 2 units of packed red blood cells or surgical revision.
** Hemodynamic deterioration with the need to infuse amines.
POAF = postoperative atrial fibrillation; OP = off pump; CPB = cardipulonary-by pas; COPD = chronic obstructive pulmonary disease; CAD = coronary artery disease; ACS = acute coronary syndrome; STEMI = elevated ST acute myocardial infarction ; LMC= left main coronary; LIMA = left internal mammary artery, RIMA = right internal mammary artery, saph = saphen vein; SD = stabdard deviation.
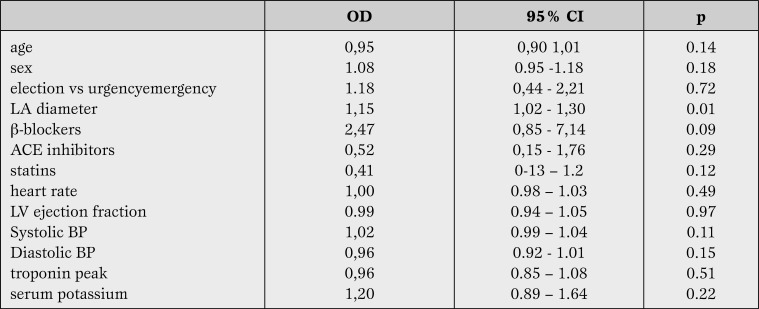
Postoperative troponin release did not differ between two groups. In patients with POAF left ventricular ejection fraction was not significantly lower than in sinus rhythm group (49% vs 51%). Two out of six patients with paroxysmal atrial fibrillation before surgery developed POAF. The use of beta-blockers, angiotensin converting enzyme (ACE) inhibitors/angiotensin A1 receptor (AT1) blockers and statins did not influence the prevalence of postoperative AF. Patients with AF did not show a different prevalence of intra-aortic balloon pump use or treatment with vasopressors or inotropic drugs after surgery. Transient electric stimulation after surgery was needed in 2 patients with POAF and in 3 who did not develop arrhythmias. No permanent pacing was required. Multivariate analysis revealed that only antero-posterior left atrium diameter was associated with an increased risk of POAF (odds ratio = 1.15; 95% confidence interval (CI) [1.02, 1.30], p<0.001) (Table 4).
Table 4.
Risk factors for POAF. Logistic regression analysis.
OD = odds ratio; CI = confidence interval; LA = left atrium; ACE = angiotensin-converting-enzyme; LV = left ventricle; BP = blood pressure.
The frequency of POAF was not statistically different between patients undergoing OP-CABG and those undergoing cardiopulmonary by-pass (24.6% for OP-CABG vs 24.1% for CPB-CABG.) Table 3 reports the relative number of elective in comparison to urgency/emergency procedures, and the number of grafts conduits employed in the two groups. Patients with POAF undergoing OP-CABG were on average 7 years older than CBP-CABG (74.3 vs 67 years, p<0.001). There were no other significant differences between the two groups. All patients with atrial fibrillation were successfully treated and discharged in sinus rhythm. Length of hospitalization was on average 2 days longer in patients with POAF after CPB-CABG.
Follow-up results. The 56 patients with postoperative atrial fibrillation were followed-up for a median of 685 days. 6 male patients, had recurrence of atrial fibrillation (10.7%). Age was not significant different in those patients (average age 72.5 vs 71.3 years). Among those patients, three underwent OP-CABG, and the others CPB-CABG. Preoperative left ventricular ejection fraction was not different in patients with AF in comparison to patients without recurrence (48% vs 49%), while mean left atrium anterior-posterior diameter was respectively 42 mm and 40 mm. On average, the AF recurrences occurred within 60 days after discharge. Amiodarone treatment was successful in 3 patients, electric cardioversion in one case. In one patient sinus rhythm recovered spontaneously. Ultimately, in the last patient sinus rhythm could not be restored. At the end of follow up only one patient died not for cardiovascular cause (lung cancer).
Discussion
Incidence of atrial arrhythmias after cardiac surgery has been reported to range from 10 to 65% [9,10]. According to a large multi-centre study, POAF after CABG occurs in near 30% [11]. Several factors, including type of surgical procedure, patient demographics, criteria used for diagnosis and methods of ECG monitoring, may account for the wide range of POAF incidence reported in literature. Several mechanisms are involved in the pathogenesis of POAF. Dispersion in atrial refractoriness induces multiple local re-entry wavelets; therefore, atrial fibrillation may be induced by several factors. Among these: trauma from surgical dissection and manipulation, myocardial ischemic damage, an exaggerated local inflammatory response with or without pericarditis, an elevation in atrial pressure from post operative ventricular stunning a chemical stimulation due to postoperative support with catecholamine and other inotropic agents, a reflex sympathetic activation from volume loss, anemia or pain, parasympathetic activation, fever from atelectasis or infection, hypoglycaemia, metabolic and electrolyte imbalance, fluid overload, prolonged post operative electrical stimulation.
Cardiopulmonary by-pass related hemodynamic changes may induce intraoperative atrial ischemia that has been hypothesized to play a role in the development of POAF.
Evidence supporting an association between AF after CABG surgery and late mortality is conflicting. Few data of patients with POAF after hospital discharge are available. Almassi et al. [3] reported at 6 months after surgery a significantly higher mortality in AF patients compared with patients without AF (9.4% vs 4.2%). Villareal et al. [2] showed in 6475 patients undergoing first isolated CABG that POAF was associated with at increased risk of death (odds ratio =1.5; 95% CI [1.3, 1.8]).
In this study, cumulative survival rate at 1 and 4 years was 87% and 74% in POAF patients versus 94% and 87% for non-AF population. In more than 8500 isolated CABG patients a significantly increased risk of death was observed among those who developed postoperative AF compared with those who did not (odds ratio =1.2; 95% CI, 1.1 to 1.3) [11]. Patients affected by postoperative AF had an increased 1-year mortality (4.6% versus 2.0%), and AF was confirmed to independently predict late mortality (hazard ratio, 1.7; 95% CI [1.2, 2.5]) [10]. Results from present investigation do not support an association of POAF with an increase of late mortality in patients undergoing surgical revascularization. Only one patient died at 2 years follow-up and not for cardiovascular cause. Restoration of sinus rhythm in all patients during hospitalization and low recurrence rate may have significantly decreased the risk of mortality, in particular due to stroke or complications of oral anticoagulant treatment.
Decreased risk of ischemic damage in beating heart CABG has been suggested to reduce incidence of POAF. Initial favourable results [6, 7] has not been confirmed by other authors [4, 12]. Siebert et al [5] during the postoperative intensive care unit stay reported a 9.8% rate of POAF in patients after CPB-CABG, 10.2% after OP-CABG, and 21% after CABG combined with valve replacement. A recent meta-analysis suggested a decreased incidence of AF in OP-CABG although overall mortality was not affected [13].
A not significant difference in the incidence of POAF between the two techniques was reported by several other studies [14, 15]. Two randomized, controlled trials and one large scale concurrent cohort study addressed the issue of beating-heart CABG. Ascione et al [7] found a significantly lower rate of postoperative AF in the OP-CABG group (11.0%) than in the CBP-pump CABG group (45.0%) in 200 patients who had been randomized to undergo CABG either with or without CPB. A significant difference in postoperative AF favouring the OP-CABG group (21.2%) compared to the on-pump CABG group (6.3%) was reported by Hernandez et al [16]. In contrast, in 281 patients randomized to CABG with or without CPB, Van Dijk et al [9] reported no difference in the rate of postoperative AF.
In patients undergoing emergency revascularization for acute coronary syndromes off-pump surgery vs CPB surgery was performed in patients with more severe clinical conditions: OP-CABG patients were more frequently in cardiogenic shock, had an impaired renal function, a log EUROSCORE >20 or a left ventricular ejection fraction <30% [17]. Overall survival and event rate, however, were similar at 5 five year follow-up. Postoperative AF occurred in 30.2% patients undergoing CPB-CABG vs 29.3% in OP-CABG surgery while the incidence of stroke was two-fold in the former group (6.7 vs 2.5%, p <0.035). Noteworthy the incidence of AF was two folds in patients with cardiogenic shock undergoing CPB versus OP surgery (62.5 vs 39.8%), with a significant increase in the number of stroke (33.3 vs 9.6%, p<0.009). Recently a TnI serum concentration >0.901 ng/ml at ICU admission has been identified as cut-off value for prediction of AF in patients undergoing elective CABG [18]. Patients with serum TnI > of 0.901 ng/ml showed an 11.5 times increased risk for the onset of AF after elective CABG. In present investigation no significant relation was found between TnI serum concentration after surgery and the risk of AF (odds ratio =0,96-95% CI [0,85, 1,08], p=0.17).
In our experience, the incidence of POAF resulted not significantly different in patients undergoing OP-CABG in comparison to CPB-CABG. The two groups were comparable for severity of coronary disease, number of grafts performed and clinical presentation (urgent versus elective surgery). However, mean age of patients undergoing OP-CABG was on average 7 years older in comparison to patients treated with CPB-CABG.
Limitations
The present study was limited by its observational nature, by a relative short follow-up period (2 years), and by the low number of patients investigated. In addition, patients were not randomized to either treatment. Otherwise the short time of enrolment (1 year), the similar characteristics of the two groups, with the cited exception of age, and the limited number of operators decreased the risk of non homogeneity of the population under investigation. The low number of recurrences occurred, similarly to other studies, may be related to potential bias due to the reliance on self-reporting for follow-up cardiac rhythm data. Although scheduled Holter monitoring in our investigation did not reveal paroxysmal episodes of atrial fibrillation, only continuous monitoring systems may provide definitive data. Similarly, the use of questionnaires and clinical examination during follow-up may not accurately identify paroxysmal episodes of AF and may potentially have underestimated the incidence of the arrhythmia recurrences.
Conclusion
Despite the reported limitations, our study does not support the hypothesis that postoperative AF is associated with an increased late mortality, rate of stroke or rehospitalization. Restoration of sinus rhythm before hospital discharge may have significantly limited the negative prognostic effects of post operative atrial fibrillation. At present, the guidelines of the American College of Chest Physician state that OP-CABG cannot be recommended to decrease post operative AF because of conflicting results resulted from randomized controlled trials or large-scale concurrent cohort studies [20].
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Rostagno C, Blanzola C, Pinelli F, Rossi A, Carone E, Stefàno PL. Atrial fibrillation after isolated coronary surgery. Incidence, long term effects and relation with operative technique. Heart, Lung and Vessels. 2014; 6(3): 171-179
References
- Hravnak M, Hoffman L A, Saul M I, Zullo T G, Whitman G R. Resource utilization related to atrial fibrillation after coronary artery bypass grafting. Am J Crit Care. 2002;11:228–238. [PMC free article] [PubMed] [Google Scholar]
- Villareal RP, Hariharan R, Liu B C, Kar B, Lee V V, Elayda M. et al. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. doi: 10.1016/j.jacc.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Almassi G H, Schowalter T, Nicolosi A C, Aggarwal A, Moritz T E, Henderson W G. et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501–511. doi: 10.1097/00000658-199710000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffolo E, de Andrade J C S, Branco J N, Teles C A, Aguiar L F, Gomes W J. Coronary artery bypass grafting without cardiopulmonary bypass. Ann Thorac SurAg. 1996;61:63–66. doi: 10.1016/0003-4975(95)00840-3. [DOI] [PubMed] [Google Scholar]
- Siebert J, Anisimowicz L, Lango R, Rogowski J, Pawlaczyk R, Brzezinski M. et al. Atrial fibrillation after coronary artery bypass grafting: does the type of procedure influence the early postoperative incidence? Eur J Cardiothorac Surg. 2001;19:455–459. doi: 10.1016/s1010-7940(01)00621-2. [DOI] [PubMed] [Google Scholar]
- Stamou S C, Dangas G, Hill P C, Pfister A J, Dullum M K, Boyce S W. et al. Atrial fibrillation after beating heart surgery. Am J Cardiol. 2000;86:64–67. doi: 10.1016/s0002-9149(00)00829-8. [DOI] [PubMed] [Google Scholar]
- Ascione R, Caputo M, Calori G, Lloyd C T, Underwood M J, Angelici G D. Predictors of atrial fibrillation after conventional and beating heart coronary surgery: a prospective, randomized study. Circulation. 2000;102:1530–1535. doi: 10.1161/01.cir.102.13.1530. [DOI] [PubMed] [Google Scholar]
- Douglas P S, Khandheria B, Stainback R F. ACCF/ASE/ACEP/ASNC/SCAI/SCC/SCMR 2007 appropriateness criteria for transthoracic and transesophageal echocardiography: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American Society of Echocardiography, American College of Emergency Physicians, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and the Society for Cardiovascular Magnetic Resonance. Endorsed by the American College of Chest Physicians and the Society of Critical Care Medicine. J Am Soc Echocardiogr. 2007;20:787–805. doi: 10.1016/j.echo.2007.06.011. [DOI] [PubMed] [Google Scholar]
- van Dijk D, Nierich A P, Jansen E W, Nathoe H M, Suyker W J, Diephuis J C. et al. Octopus Study Group. Early outcome after off-pump versus on-pump coronary bypass surgery: results from a randomized study. Circulation. 2001;104:1761–1766. doi: 10.1161/hc4001.097036. [DOI] [PubMed] [Google Scholar]
- Mathew J P, Parks R, Savino J S, Friedman A S, Koch C, Mangano D T. et al. Atrial fibrillation following coronary artery bypass graft surgery: predictors, outcomes, and resource utilization: Multicenter Study of Perioperative Ischemia Research Group. JAMA. 1996;276:300–306. [PubMed] [Google Scholar]
- Mathew J P, Fontes M L, Tudor I C, Ramsay J, Duke P, Mazer C D. et al. A multicenter risk index for atrial fibrillation after cardiac surgery. JAMA. 2004;291:1720–1729. doi: 10.1001/jama.291.14.1720. [DOI] [PubMed] [Google Scholar]
- Cohn W E, Sirois C A, Johnson R G. Atrial fibrillation after minimally invasive coronary artery bypass grafting: a retrospective, matched study. J Thorac Cardiovasc Surg. 1999;117:298–301. doi: 10.1016/S0022-5223(99)70426-5. [DOI] [PubMed] [Google Scholar]
- Moller C H, Penninga L, Wetterslev J, Steinbruchel D A. Off-pump versus on pump coronary artery bypass grafting for ischaemic heart disease. Cochrane Database Syst Rev. 2012;3 doi: 10.1002/14651858.CD007224.pub2. [CD007224] [DOI] [PubMed] [Google Scholar]
- Abreu J E, Reilly J, Salzano R P, Khachane V B, Jekel J F, Clyne C A. Comparison of frequencies of atrial fibrillation after coronary artery bypass. Am J Cardiol. 1999;83:775–776. doi: 10.1016/s0002-9149(98)00988-6. [DOI] [PubMed] [Google Scholar]
- Banach M, Goch A, Misztal M, Rysz J, Zaslonka J, Goch J H. et al. Relation between postoperative mortality and atrial fibrillation before surgical revascularization: 3-year follow-up. Thorac Cardiovasc Surg. 2008;56:20–23. doi: 10.1055/s-2007-989249. [DOI] [PubMed] [Google Scholar]
- Hernandez F, Cohn W E, Baribeau Y R, Tryzelaar J F, Charlesworth D C, Clough R A. et al. In-hospital outcomes of off-pump versus on-pump coronary artery bypassprocedures: a multicenter experience. Ann Thorac Surg. 2004;78:1528–1534. doi: 10.1016/s0003-4975(01)03202-7. [DOI] [PubMed] [Google Scholar]
- Rastan A J, Eckenstein J I, Hentschel B, Funkat A K, Gummert J F, Doll N. et al. Emergency coronary artery bypass graft surgery for acute coronary syndrome: beating heart versus conventional cardioplegic cardiac arrest strategies. Circulation. 2006;114:477–485. doi: 10.1161/CIRCULATIONAHA.105.001545. [Suppl I] [DOI] [PubMed] [Google Scholar]
- Leal J C, Petruccic O, Godoya M F, Brailea M L. Perioperative serum troponin I levels are associated with higher risk for atrial fibrillation in patients undergoing coronary artery bypass graft surgery. Intern CardioVascular and Thorac Surg. 2012;14:22–25. doi: 10.1093/icvts/ivr019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney E M, Thompson T D, Veledar E, Williams J, Weintraub W S. Cost-effectiveness of targeting patients undergoing cardiac surgery for therapy with intravenous amiodarone to prevent atrial fibrillation. J Am Coll Cardiol. 2002;40:737–745. doi: 10.1016/s0735-1097(02)02003-x. [DOI] [PubMed] [Google Scholar]
- Creswell L L, Alexander J C, Ferguson B, Lisbon A, Fleisher L A. Intra operative interventions: American College of Chest Physicians Guidelines for the Prevention and Management of Postoperative Atrial Fibrillation After Cardiac Surgery. Chest. 2005;128:28–35. doi: 10.1378/chest.128.2_suppl.28s. [Suppl II] [DOI] [PubMed] [Google Scholar]