Abstract
Introduction
Diabetes mellitus is associated with cardiovascular disease. Anti-diabetic therapy has a limited capability (if any) of changing the incidence of either death or major cardiovascular disease, and cardiovascular safety concerns have been raised. We aimed at identifying episodes of acute myocardial infarction associated to a relatively new class of drugs, dipeptidyl peptidase-4 inhibitors.
Methods
Retrospective study: from 954 admissions (15 month period) in the coronary care unit, we selected 200 admissions corresponding to 196 patients with myocardial infarction and diabetes. 35 of these patients were receiving therapy with dipeptidyl peptidase-4 inhibitors (the vast majority, in association to metformin). We evaluated the peak plasma cardiac troponin I as the main study parameter.
Results
Patients on dipeptidyl peptidase-4 inhibitors therapy had a mean peak cardiac troponin plasma level of 50.2±121.3 ng/ml (n=35), the corresponding value for insulin being 39.2±108.4 ng/ml (n=56), for metformin the value was 45.8±97.3 ng/ml (n=93) and for sulfonylureas, 42.4±77.7 ng/ml (n=52). None of these values differed significantly from the corresponding control group of patients not taking each class of drug. The linear regression study also yielded a negative result relating therapy with dipeptidyl peptidase-4 inhibitors and peak troponin values. Acute myocardial infarctions associated to dipeptidyl peptidase-4 inhibitors varied widely in the clinical characteristics of the patients.
Conclusions
We found no evidence that peak plasma troponin I was different between patient with acute myocardial infarction and use of dipeptidyl peptidase-4 inhibitors when compared to cases not under such therapy.
Keywords: acute myocardial infarction, diabetes mellitus, dipeptidyl peptidase-4 inhibitors, metformin, troponin.
Introduction
Diabetes mellitus is a highly prevalent disease that acts as a cardiovascular risk factor [1]. The presence of diabetes mellitus is associated with an increase in the mortality rate of patients, both with or without a previous myocardial infarction [2]. Diabetes mellitus has been shown to be associated to an increased incidence of coronary artery disease and stroke [3]. Clinical trials have shown anti-diabetic therapies to have very limited, if any, capability to change the incidence of either death or major cardiovascular disease, such as myocardial infarction or stroke [4]. Issues of cardiovascular safety associated to anti-diabetic therapy have been put forward [5].
Dipeptidyl peptidase-4 inhibitors (DPP-4 inhibitors) are a relatively new class of anti-diabetic drugs that have been shown to decrease glycated hemoglobin, either if used alone or in association to other drugs such as metformin.
In patients with myocardial infarction, the presence of diabetes mellitus is relatively common. In the present investigation, we aimed to characterize episodes of acute myocardial infarction associated to the use of DPP-4 inhibitors, as well as to other anti-diabetic drugs. For that purpose, data from the admissions that took place during 15 months in an acute coronary care unit were retrospectively evaluated. Peak plasma cardiac troponin I was the major parameter under study, since plasma troponin provides an estimate of the importance of the myocardial injury in myocardial infarction [6].
Methods
The present study was retrospective. From all patients admitted to an intensive coronary care unit from January 2011 to March 2012, patients with both acute myocardial infarction and diabetes mellitus were identified. A patient was considered to have diabetes mellitus if anti-diabetic therapy was being taken, if the diagnosis had been previously established on the basis of current recommendations [7] or if glycated hemoglobin greater than 6.5% [7] was present at admission.
Acute myocardial infarction was diagnosed following the recommendations in use [8]. Patients with in-hospital acute myocardial infarction were excluded. Patients who were initially admitted to another hospital, and who were later transferred into our institution were only included if the peak value for plasma troponin I could be clearly identified.
Data on previous anti-diabetic drug use was searched in the electronic file(s) corresponding to each patient. Peak plasma cardiac troponin I levels was also searched in the corresponding electronic files. Additional data were obtained for each patient on the following parameters: presence of ST segment elevation in the electrocardiogram; previous history of myocardial infarction; previous coronary revascularization, either percutaneous or surgical; primary coronary angioplasty in the current episode; plasma creatinine at admission.
Peak cardiac troponin levels in patients under anti-diabetic therapy with DPP-4 inhibitors were compared to the corresponding values for patients under no such therapy. The same comparison was carried out regarding insulin, metformin and sulfonylureas.
Troponin I was measured using the ARCHITECT STAT system, of Abbott Diagnostics (Abbott Park, Illinois, USA). The 99th percentile of troponin I in a normal population with this assay was established at 0.012 ng/ml.
The present protocol was approved by the ethics committee of our institution.
Statistical Methods. Data are presented as arithmetic means and standard deviations. Pairs of means were compared using Mann Whitney U test. Linear regression analysis was carried out, taking peak plasma troponin I as dependent variable, and age, sex, plasma creatinine at admission, presence of ST segment elevation and use of DPP-4 inhibitors, use of metformin, use of insulin and use of a sulfonylurea as independent variables.
For all comparisons a two-sided significance level of 0.05 was considered statistically significant. Data analysis was performed using the SPSS 20 software program, from IBM.
Results
A total number of 954 patients were admitted in the period under study. From those, 200 admissions, corresponding to 196 patients (2 patients were admitted twice and 1 patient for three different times), were selected as meeting the inclusion criteria. 127 patients were of the male sex and 69 were female. The mean age was 67.7±10.6 years. ST segment elevation myocardial infarction was present in 62 patients. Primary coronary angioplasty was carried out in 44 patients.
The mean peak plasma cardiac troponin I values for the 200 admissions was 48.5±94.9 ng/ml.
DPP-4 inhibitors (either vildagliptin or sitagliptin) were being taken on admission by 35 patients, insulin by 56 patients, metformin by 93 patients, and sulfonylureas by 52 patients. A small number of patients were taking other types of antidiabetic dugs. 31 patients were taking no antidiabetic therapy at admission. Nineteen patients were taking oral antidiabetic drugs, but it was impossible to establish which drugs were in use (either the patients did not recall the names of the drugs in use or the record was incomplete).
As for the most commonly found specific anti-diabetic therapies the following mean values for peak plasma troponin I (in ng/ml) were seen: no anti-diabetic therapy, 68.6±93.1 (n=31); insulin alone 22.8±70.7 (n=37), metformin alone 48.9±98.5 (n= 31), sulfonylurea alone 106.9±166.5 (n=7), sulfonylurea plus metformin 27.9±38.2 (n=17); DPP-4 inhibitors plus metformin 32.7±50.8 (n=14).
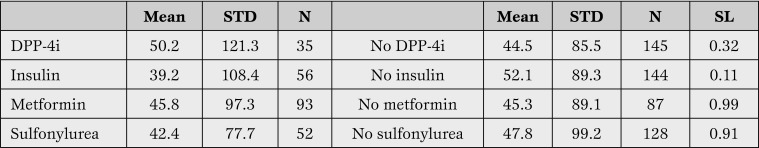
The mean value for peak plasma troponin for patients either taking or not taking DPP-4 inhibitors, insulin, metformin or sulfonylureas are shown in Table 1.
Table 1.
Arithmetic mean and standard deviation (STD) for peak troponin I plasma values for patients with diabetes mellitus and acute myocardial infarction.
N = number; SL = significance level (Mann-Whitney U test); DPP-4i = dipeptidyl peptidase-4 inhibitors.
Patients under DPP-4 inhibitors therapy had a mean peak cardiac troponin plasma level of 50.2±121.3 ng/ml (n=35), the corresponding value for insulin being 39.2±108.4 ng/ml (n=56), for metformin the value being 45.8±97.3 ng/ml (n=93) and for sulfonylureas, 42.4±77.7 ng/ml (n=52). None of these values was significantly different from the corresponding group of patients not taking each class of drug (Table 1). As Table 2 shows, almost all (32/35) patients under DPP-4 inhibitors were simultaneously using metformin, and many were using other anti-diabetic drugs.
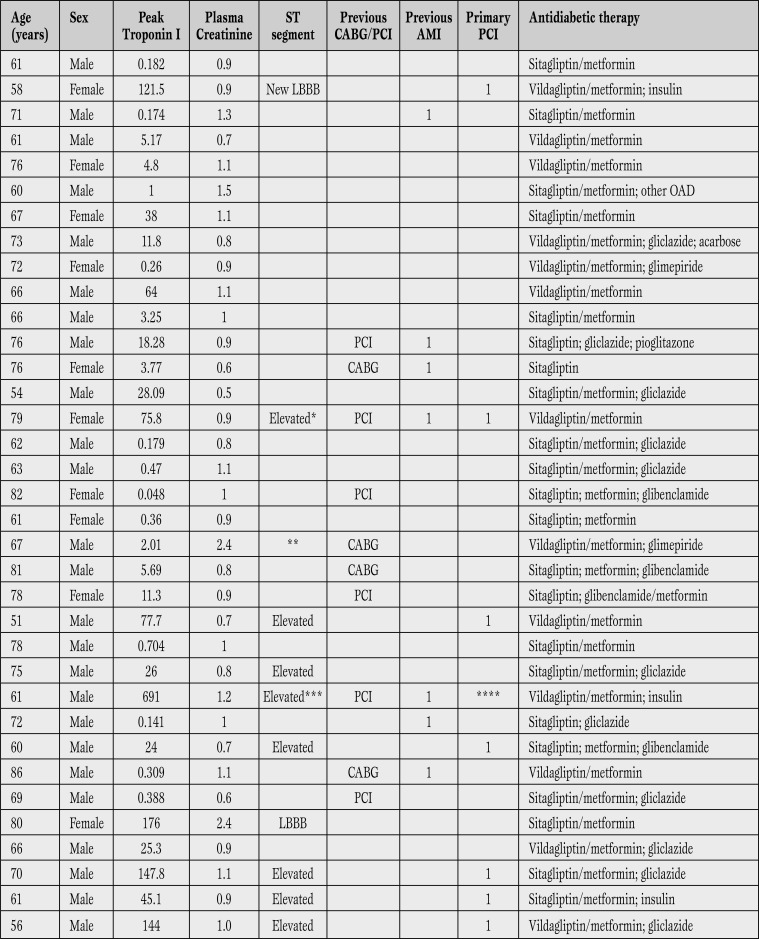
Table 2.
Clinical characteristics of 35 patients with diabetes mellitus and acute myocardial infarction associated to the use of DDP-4 inhibitors. Plasma creatinine (mg/dL); troponin I (ng/mL).
*Acute intra-stent thrombosis. **Severe aortic stenosis. ***Deceased. ****Thrombolysis.
LBBB = left bundle branch block; PCI = coronary percutaneous intervention; CABG = coronary artery bypass graft surgery; AMI = acute myocardial infarction; OAD = oral anti-diabetic drug.
Linear regression analysis, taking peak plasma troponin I as dependent variable, and age, sex, plasma creatinine at admission, ST segment elevation and use of DPP-4 inhibitors as independent variables, yielded an overall significant result (ANOVA with F 5.1, significance level <0.01), however only the presence of ST segment elevation reached a significance level < 0.05 (the presence of DPP-4 inhibitors had a significance level of 0.35).
Table 2 shows some clinical characteristics of patients with acute myocardial infarction admitted while currently taking DPP-4 inhibitors. Eight cases of elevated ST-segment infarction, including one case of intra-stent thrombosis, and a case with new left bundle branch block were seen. One patient died. Peak plasma levels for cardiac troponin I varied in a relatively wide range, from minor elevations under 1 ng/ml, to values over 100 ng/ml (Table 2).
Discussion
In the present study we describe a group of 35 diabetic patients with acute myocardial infarction under current DPP-4 inhibitors therapy. The vast majority of the patients were also taking metformin. Myocardial infarctions associated to the use of DPP-4 inhibitors have been shown to be very variable in terms of peak plasma cardiac troponin levels, the major parameter evaluated in the present study. Mean peak plasma troponin in myocardial infarctions associated to the use of DPP-4 inhibitors, however, was not significantly different from the corresponding value in patients under other forms of anti-diabetic therapy, the same happening to myocardial infarctions associated to the use of insulin, metformin or sulfonylureas.
The treatment of type 2 diabetes mellitus has been associated to modest results in what concerns mortality and major cardiovascular disease, such as myocardial infarction and stroke. The clinical trials published in recent years, the Action to Control Cardiovascular Risk in Diabetes Study (ACCORD), the Action in Diabetes and Vascular Disease: Preterax and Diamicron MR Controlled Evaluation (ADVANCE) and the Veterans Affairs Diabetes Study (VADT), all failed to show results of interest associated to intensive therapy, in what concerns cardiovascular disease. ACCORD has even shown an increased mortality rate associated to intensive anti-diabetic therapy. A meta-analysis (also including data from the United Kingdom Prospective Diabetes [UKPDS] Study), however, has shown that “intensive glucose control reduced the risk for some cardiovascular disease outcomes (such as nonfatal myocardial infarction), but did not reduce the risk for cardiovascular death or all-cause mortality, and increased the risk for severe hypoglycemia” [9], findings essentially corroborated by a similar study [10].
The UKPDS 80 study did show favorable long-term effects of intensive therapy (“legacy effect”), however the cohort under study in UKPDS 80 was only a fraction of the original UKPDS group of patients.
It has been speculated that “increases in levels of insulin, not glucose, may be etiologic in cardio-vascular disease risk” [11], and it has also been stated that “it can be argued that lowering HbA1c is not, in and by itself, a meaningful outcome” [12].
It is in this setting that new classes of anti-diabetic drugs have been created, among which the DPP-4 inhibitors have attracted a considerable degree of interest. Promising laboratory data concerning DPP-4 inhibition have been published, including improved endothelial function [13] and reduction of experimental infarct size in the rat [14].
This group of drugs, which includes, among others, sitagliptin, vildagliptin and saxagliptin, are believed to act by inhibiting the enzyme dipeptidyl peptidase 4, which in turn degrades incretins such as GLP-1, hormones released postprandially, thereby increasing insulin and decreasing glucagon. DPP-4 inhibitors have been shown to decrease glycated hemoglobin with a neutral effect on body weight and a low risk for hypoglycemia [15]. According to Jose and Inzucchi, DPP-4 substrates are extensive, and “DPP-4 is not specific for GLP-1 and therefore has the potential to mediate a wide range of pleiotropic effects [16].”
Two major clinical trials on DPP-4 inhibitors have been published. Saxagliptin was compared to placebo in the Saxagliptin assessment of vascular outcomes recorded in patients with diabetes mellitus (SAVOR- TIMI 53) clinical trial [17]. After a median follow-up of 2.1 years, the study concluded that “DPP-4 inhibition with saxagliptin did not increase or decrease the rate of ischemic events, though the rate of hospitalization for heart failure was increased” [17].
In the Examination of cardiovascular outcomes with Alogliptin versus standard of care (EXAMINE) clinical trial, a total of 5380 patients with acute coronary syndrome were randomized to take alogliptin or placebo and followed for a median of 18 months [18]. The authors concluded that the rates of major adverse cardiovascular events were not increased with alogliptin as compared with placebo [18].
Meta-analyses and a pooled analysis [19,20,21] failed to show an unfavorable cardiovascular profile for these drugs, however in the comparator arm there were not only data obtained with placebo but also with active comparators, thereby limiting the evaluation of DPP-4 inhibitors.
In the present study, the vast majority of the patients with myocardial infarction associated to the use of DPP-4 inhibitors were also taking metformin. Linear regression analysis failed to indicate any significant influence on peak troponin I levels associated to the presence of DPP-4 inhibitors. From a theoretical standpoint, the presence of metformin could mask any possible effect of DPP-4 inhibitors on the patho-physiology of acute myocardial infarction. Peak plasma troponin I values in the 31 patients treated with metformin alone was 48.9±98.5 ng/ml, a value which is very similar to the value for the 35 patients under DPP-4 therapy, 50.2±121.3 ng/ml. It is clearly difficult to evaluate, using the present data, any possible effects of DPP-4 inhibitors, by themselves, on myocardial infarction.
We can reasonably suggest, however, that DPP-4 inhibitors therapy doesn’t seem to be associated to any increased importance of acute myocardial infarction in patients under metformin therapy, in what concerns peak plasma troponin I levels. In any case, both vildagliptin/metformin and sitagliptin/metformin have become extremely popular fixed drug associations, at least in Portugal, where they represented the second and third absolute top-selling drugs in the period January/March 2012 [22].
Study limitations - The present study has significant limitations: it is a retrospective study; indication bias is probably present, in the sense that patients for whom doctors chose different types of anti-diabetic therapy were probably different from each other; for a considerable number of patients, it is known that they were under oral anti-diabetic therapy but the exact nature of that therapy is unknown; the duration of anti-diabetic drug usage is also unknown; the small dimension of the sample limits the strength of conclusions; finally there are many factors influencing troponin levels in patients with myocardial infarction, reperfusion therapy being one of them [23].
Conclusion
In conclusion, we have described a group of 35 diabetic patients with acute myocardial infarction under current anti-diabetic therapy including a DPP-4 inhibitor. The vast majority of the patients were also taking metformin.
We found no evidence that peak plasma troponin I was different between patient with acute myocardial infarction and use of DPP-4i, when compared to cases not under such therapy.
Footnotes
Source of Support Nil.
Disclosures None declared.
Cite as: Nunes JPL, Rodrigues JD, Melão F. Acute myocardial infarction associated to DPP-4 inhibitors. Heart, Lung and Vessels. 2014; 6(3): 180-186
References
- Stamler J, Vaccaro O, Neaton J D, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- Haffner S M, Lehto S, Ronnemaa T, Ronnemaa K, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. New Engl J Med. 1998;339:229–234. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- Sarwar N, Gao P, Seshasai S R, Gobin R, Kaptoge S, Di Angelantonio E. et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda V P, Rodrigues M J, Goncalves F R, Nunes J P L. Effects of hypoglycemic agents on mortality and major cardiovascular outcomes in patients with type 2 diabetes mellitus: a narrative review. Rev Port Cardiol. 2009;28:1099–1119. [PubMed] [Google Scholar]
- Nissen S E, Wolski K. Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- Steen H, Giannitsis E, Futterer S, Merten C, Juenger C, Katus H A. Cardiac troponin T at 96 hours after acute myocardial infarction correlates with infarct size and cardiac function. J Am Coll Cardiol. 2006;48:2192–2194. doi: 10.1016/j.jacc.2006.06.002. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35:S11–63. doi: 10.2337/dc12-s011. [(Suppl. 1)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thygesen K, Alpert J S, White H D. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- Kelly T N, Bazzano L A, Fonseca V A, Thethi T K, Reynolds K, He J. Systematic review: glucose control and cardiovascular disease in type 2 diabetes. Ann Intern Med. 2009;151:394–403. doi: 10.7326/0003-4819-151-6-200909150-00137. [DOI] [PubMed] [Google Scholar]
- Turnbull F M, Abraira C, Anderson R J, Byington R P, Chalmers J P, Duckworth W C. et al. Intensive glucose control and macrovascular outcomes in type 2 diabetes. Diabetologia. 2009;52:2288–2298. doi: 10.1007/s00125-009-1470-0. [DOI] [PubMed] [Google Scholar]
- Goodarzi M O, Psaty B M. Glucose lowering to control macrovascular disease in type 2 diabetes: treating the wrong surrogate end point? JAMA. 2008;300:2051–2053. doi: 10.1001/jama.2008.510. [DOI] [PubMed] [Google Scholar]
- Zannad F, Stough W G, Pocock S J, Sleight P, Cushman W C, Cleland J G. et al. Diabetes clinical trials: helped or hindered by the current shift in regulatory requirements? Eur Heart J. 2012;33:1049–1057. doi: 10.1093/eurheartj/ehr437. [DOI] [PubMed] [Google Scholar]
- Matsubara J, Sugiyama S, Akiyama E, Iwashita S, Kurokawa H, Ohba K. et al. Dipeptidyl peptidase-4 inhibitor, sitagliptin, improves endothelial dysfunction in association with its anti-inflammatory effects in patients with coronary artery disease and uncontrolled diabetes. Circ J. 2013;77:1337–1344. doi: 10.1253/circj.cj-12-1168. [DOI] [PubMed] [Google Scholar]
- Hausenloy D J, Whittington H J, Wynne A M, Begum S S, Theodorou L, Riksen N. et al. Dipeptidyl peptidase-4 inhibitors and GLP-1 reduce myocardial infarct size in a glucose-dependent manner. Cardiovasc Diabetol. 2013;12:154–154. doi: 10.1186/1475-2840-12-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karagiannis T, Paschos P, Paletas K, Matthews D R, Tsapas A. Dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus in the clinical setting: systematic review and meta-analysis. BMJ. 2012;344:1369–1369. doi: 10.1136/bmj.e1369. [DOI] [PubMed] [Google Scholar]
- Jose T, Inzucchi S E. Cardiovascular effects of the DPP-4 inhibitors. Diab Vasc Dis Res. 2012;9:109–116. doi: 10.1177/1479164111436236. [DOI] [PubMed] [Google Scholar]
- Scirica B M, Bhatt D L, Braunwald E, Steg P G, Davidson J, Hirshberg B. et al. Saxagliptin and Cardiovascular Outcomes in Patients with Type 2 Diabetes Mellitus. N Engl J Med. 2013;369:1317–1326. doi: 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- White W B, Cannon C P, Heller S R, Nissen S E, Bergenstal R M, Bakris G L. et al. Alogliptin after Acute Coronary Syndrome in Patients with Type 2 Diabetes. N Engl J Med. 2013;369:1327–1335. doi: 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- Monami M, Dicembrini I, Martelli D, Mannucci E. Safety of dipeptidyl peptidase-4 inhibitors: a meta-analysis of randomized clinical trials. Curr Med Res Opin. 2011;27:57–64. doi: 10.1185/03007995.2011.602964. [(Suppl. 3)] [DOI] [PubMed] [Google Scholar]
- Patil H R, Al Badarin F J, Al Shami H A, Bhatti S K, Lavie C J, Bell D S. et al. Meta-analysis of effect of dipeptidyl peptidase-4 inhibitors on cardiovascular risk in type 2 diabetes mellitus. Am J Cardiol. 2012;110:826–833. doi: 10.1016/j.amjcard.2012.04.061. [DOI] [PubMed] [Google Scholar]
- White W B, Pratley R, Fleck P, Munsaka M, Hisada M, Wilson C. et al. Cardiovascular safety of the dipetidyl peptidase-4 inhibitor alogliptin in type 2 diabetes mellitus. Diabetes Obes Metab. 2013;15:668–673. doi: 10.1111/dom.12093. [DOI] [PubMed] [Google Scholar]
- Analise do Mercado de Medicamentos em Ambulatorio Março 2012. Lisbon, Portugal: Instituto da Farmacia e Medicamento. INFARMED; 2012.
- Katus H A, Remppis A, Scheffold T, Diederich K W, Kuebler W. Intracellular compartmentation of cardiac troponin T and its release kinetics in patients with reperfused and nonreperfused myocardial infarction. Am J Cardiol. 1991;67:1360–1367. doi: 10.1016/0002-9149(91)90466-x. [DOI] [PubMed] [Google Scholar]