Abstract
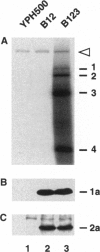
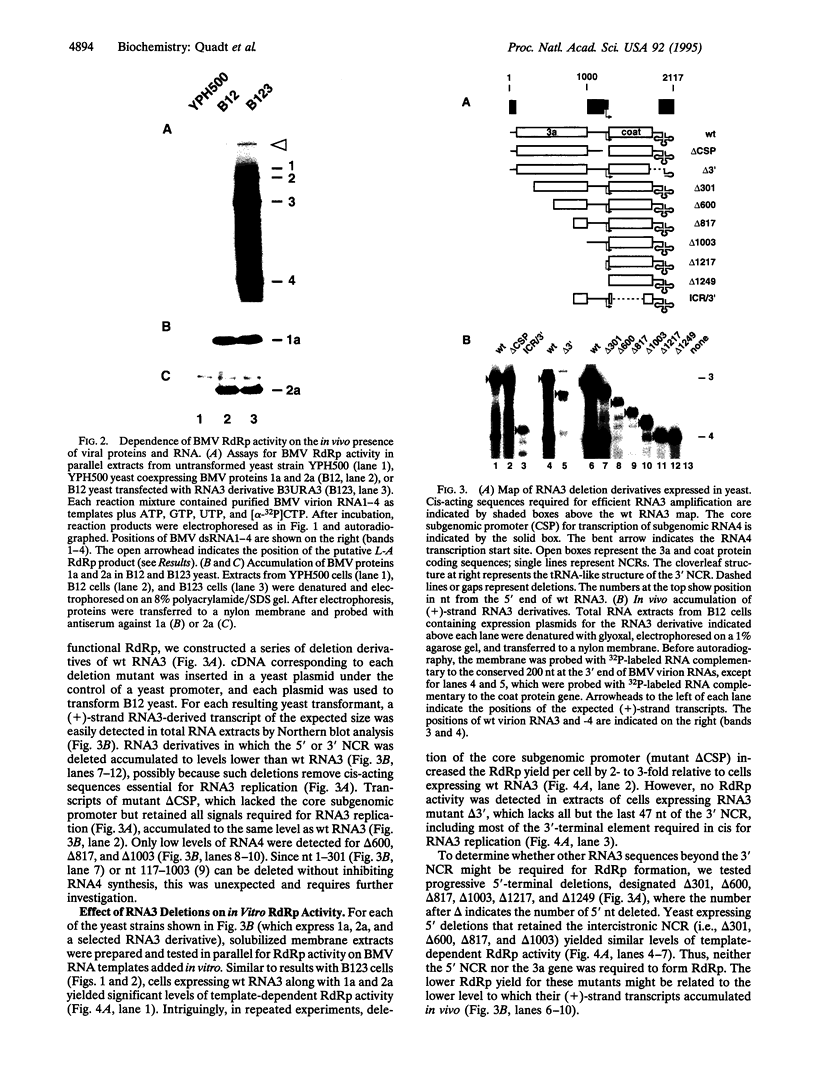
In this report we show that yeast expressing brome mosaic virus (BMV) replication proteins 1a and 2a and replicating a BMV RNA3 derivative can be extracted to yield a template-dependent BMV RNA-dependent RNA polymerase (RdRp) able to synthesize (-)-strand RNA from BMV (+)-strand RNA templates added in vitro. This virus-specific yeast-derived RdRp mirrored the template selectivity and other characteristics of RdRp from BMV-infected plants. Equivalent extracts from yeast expressing 1a and 2a but lacking RNA3 contained normal amounts of 1a and 2a but had no RdRp activity on BMV RNAs added in vitro. To determine which RNA3 sequences were required in vivo to yield RdRp activity, we tested deletions throughout RNA3, including the 5',3', and intercistronic noncoding regions, which contain the cis-acting elements required for RNA3 replication in vivo. RdRp activity was obtained only from cells expressing 1a, 2a, and RNA3 derivatives retaining both 3' and intercistronic noncoding sequences. Strong correlation between extracted RdRp activity and BMV (-)-strand RNA accumulation in vivo was found for all RNA3 derivatives tested. Thus, extractable in vitro RdRp activity paralleled formation of a complex capable of viral RNA synthesis in vivo. The results suggest that assembly of active RdRp requires not only viral proteins but also viral RNA, either to directly contribute some nontemplate function or to recruit essential host factors into the RdRp complex and that sequences at both the 3'-terminal initiation site and distant internal sites of RNA3 templates may participate in RdRp assembly and initiation of (-)-strand synthesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrera I., Schuppli D., Sogo J. M., Weber H. Different mechanisms of recognition of bacteriophage Q beta plus and minus strand RNAs by Q beta replicase. J Mol Biol. 1993 Jul 20;232(2):512–521. doi: 10.1006/jmbi.1993.1407. [DOI] [PubMed] [Google Scholar]
- De Jong W., Ahlquist P. A hybrid plant RNA virus made by transferring the noncapsid movement protein from a rod-shaped to an icosahedral virus is competent for systemic infection. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6808–6812. doi: 10.1073/pnas.89.15.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban R., Fujimura T., Wickner R. B. Internal and terminal cis-acting sites are necessary for in vitro replication of the L-A double-stranded RNA virus of yeast. EMBO J. 1989 Mar;8(3):947–954. doi: 10.1002/j.1460-2075.1989.tb03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Ahlquist P. Characterization and engineering of sequences controlling in vivo synthesis of brome mosaic virus subgenomic RNA. J Virol. 1988 Jul;62(7):2411–2420. doi: 10.1128/jvi.62.7.2411-2420.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Ahlquist P. Intercistronic as well as terminal sequences are required for efficient amplification of brome mosaic virus RNA3. J Virol. 1987 May;61(5):1457–1465. doi: 10.1128/jvi.61.5.1457-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R., Janda M., Ahlquist P. Bacterial gene inserted in an engineered RNA virus: efficient expression in monocotyledonous plant cells. Science. 1986 Mar 14;231(4743):1294–1297. doi: 10.1126/science.231.4743.1294. [DOI] [PubMed] [Google Scholar]
- Fujimura T., Esteban R., Wickner R. B. In vitro L-A double-stranded RNA synthesis in virus-like particles from Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1986 Jun;83(12):4433–4437. doi: 10.1073/pnas.83.12.4433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenni A. L., Joshi S., Chapeville F. tRNA-like structures in the genomes of RNA viruses. Prog Nucleic Acid Res Mol Biol. 1982;27:85–104. doi: 10.1016/s0079-6603(08)60598-x. [DOI] [PubMed] [Google Scholar]
- Hagen M., Chung T. D., Butcher J. A., Krystal M. Recombinant influenza virus polymerase: requirement of both 5' and 3' viral ends for endonuclease activity. J Virol. 1994 Mar;68(3):1509–1515. doi: 10.1128/jvi.68.3.1509-1515.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy S. F., German T. L., Loesch-Fries L. S., Hall T. C. Highly active template-specific RNA-dependent RNA polymerase from barley leaves infected with brome mosaic virus. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4956–4960. doi: 10.1073/pnas.76.10.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Kroner P., Ahlquist P., Meshi T. Biological activities of hybrid RNAs generated by 3'-end exchanges between tobacco mosaic and brome mosaic viruses. J Virol. 1991 Jul;65(7):3451–3459. doi: 10.1128/jvi.65.7.3451-3459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janda M., Ahlquist P. RNA-dependent replication, transcription, and persistence of brome mosaic virus RNA replicons in S. cerevisiae. Cell. 1993 Mar 26;72(6):961–970. doi: 10.1016/0092-8674(93)90584-d. [DOI] [PubMed] [Google Scholar]
- Kao C. C., Ahlquist P. Identification of the domains required for direct interaction of the helicase-like and polymerase-like RNA replication proteins of brome mosaic virus. J Virol. 1992 Dec;66(12):7293–7302. doi: 10.1128/jvi.66.12.7293-7302.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. C., Quadt R., Hershberger R. P., Ahlquist P. Brome mosaic virus RNA replication proteins 1a and 2a from a complex in vitro. J Virol. 1992 Nov;66(11):6322–6329. doi: 10.1128/jvi.66.11.6322-6329.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroner P. A., Young B. M., Ahlquist P. Analysis of the role of brome mosaic virus 1a protein domains in RNA replication, using linker insertion mutagenesis. J Virol. 1990 Dec;64(12):6110–6120. doi: 10.1128/jvi.64.12.6110-6120.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh L. E., Dreher T. W., Hall T. C. Mutational analysis of the core and modulator sequences of the BMV RNA3 subgenomic promoter. Nucleic Acids Res. 1988 Feb 11;16(3):981–995. doi: 10.1093/nar/16.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mise K., Allison R. F., Janda M., Ahlquist P. Bromovirus movement protein genes play a crucial role in host specificity. J Virol. 1993 May;67(5):2815–2823. doi: 10.1128/jvi.67.5.2815-2823.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacha R. F., Ahlquist P. Use of bromovirus RNA3 hybrids to study template specificity in viral RNA amplification. J Virol. 1991 Jul;65(7):3693–3703. doi: 10.1128/jvi.65.7.3693-3703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogue G. P., Hall T. C. The requirement for a 5' stem-loop structure in brome mosaic virus replication supports a new model for viral positive-strand RNA initiation. J Virol. 1992 Feb;66(2):674–684. doi: 10.1128/jvi.66.2.674-684.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadt R., Jaspars E. M. Purification and characterization of brome mosaic virus RNA-dependent RNA polymerase. Virology. 1990 Sep;178(1):189–194. doi: 10.1016/0042-6822(90)90393-6. [DOI] [PubMed] [Google Scholar]
- Quadt R., Kao C. C., Browning K. S., Hershberger R. P., Ahlquist P. Characterization of a host protein associated with brome mosaic virus RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1498–1502. doi: 10.1073/pnas.90.4.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadt R., Verbeek H. J., Jaspars E. M. Involvement of a nonstructural protein in the RNA synthesis of brome mosaic virus. Virology. 1988 Jul;165(1):256–261. doi: 10.1016/0042-6822(88)90679-4. [DOI] [PubMed] [Google Scholar]
- Smirnyagina E., Hsu Y. H., Chua N., Ahlquist P. Second-site mutations in the brome mosaic virus RNA3 intercistronic region partially suppress a defect in coat protein mRNA transcription. Virology. 1994 Feb;198(2):427–436. doi: 10.1006/viro.1994.1054. [DOI] [PubMed] [Google Scholar]
- Zhang X., Liao C. L., Lai M. M. Coronavirus leader RNA regulates and initiates subgenomic mRNA transcription both in trans and in cis. J Virol. 1994 Aug;68(8):4738–4746. doi: 10.1128/jvi.68.8.4738-4746.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]