Abstract
Background
Sirtuin 1 (SIRT1) is a class III histone deacetylase that may play a critical role in several biological functions, including lifespan, stress, and inflammation. Our main objective was to evaluate SIRT1 activity in peripheral blood mononuclear cells (PBMCs) in patients with osteoporosis and to analyze the relationship between the SIRT 1 activity and markers of inflammation and bone remodelling.
Material/Methods
We performed a prospective monocentric study of patients with osteoporosis and measured the nuclear and cytoplasmic activities of SIRT1 in PBMCs. Levels of proinflammatory cytokines were assessed in culture supernatants of PBMCs isolated from the osteoporosis patients. The level of serum C-terminal cross-linking telopeptide of type I collagen (CTX), a marker of bone resorption, was measured in the serum of osteoporosis patients.
Results
Sixteen women with osteoporosis were included. A statistically significant correlation between the cytoplasmic and nuclear SIRT 1 activities was found in PBMCs of patients with osteoporosis. Although non-significant, we observed a negative trend between nuclear SIRT 1 activity and the rate of serum CTX and a positive trend between IL-6 and CTX levels in patients with osteoporosis.
Conclusions
This study shows that the cytoplasmic and nuclear SIRT 1 activities are measurable in circulating PBMCs of patients with osteoporosis and that these 2 activities are correlated. The potential role of inflammation in bone resorption in patients with osteoporosis was also studied.
MeSH Keywords: Sirtuin 1, SIRT1, Osteoporosis, PBMCs
Background
Osteoporosis, which affects nearly 1 out of 2 women after 50 years of age, is a cause of morbidity and mortality in proven cases of fracture [1]. The sirtuin proteins (Silent Information Regulator proteins) belong to the class III histone deacetylases (HDAC) [2,3]. HDACs, of which 18 have been identified, are classified into 4 groups and are involved in epigenetic regulation, especially the deacetylation of histones [4,5]. These processes of acetylation and deacetylation are regulated by 2 enzyme systems, histone acetyl transferases (HATs) and HDACs, which include 7 sirtuins, especially sirtuin 1 (SIRT1) [6]. In mice, in the mesenchymal stem cells, SIRT1 activation promotes osteoblast differentiation via action on the p53 protein [7]. SIRT1 is regulated by a natural activator, resveratrol, which is a polyphenol abundant in red wine [8,9]. A study by Shakibaei demonstrated the role of resveratrol in inhibiting osteoclastogenesis via inhibition of RANK ligand [10]. Altogether studies suggested that SIRT1 dysregulation might play a role in the development of osteoporosis [3,5]. The presence of SIRT1 activity in animal mesenchymal cells has been highlighted, but so far no study has measured SIRT1 activity in human peripheral blood cells of osteoporotic patients [8]. We hypothesized that SIRT1 activity is measurable in PBMCs of patients with osteoporosis. The objective of our study was to evaluate the activity of SIRT 1 in PBMCs of osteoporotic patients and to analyze the relationship between SIRT1 activity and markers of inflammation IL-6, TNF alpha, and IL-8 and a marker of bone resorption, the serum C-terminal cross-linking telopeptide of type I collagen (CTX).
Material and Methods
Patients
A prospective monocentric study was conducted to measure the activity of SIRT1 in PBMCs of 16 osteoporotic patients. Sixteen control subjects were also enrolled. The criteria for inclusion of osteoporotic patients were the presence of a vertebral osteoporosis, defined by low bone mineral density (T score less than −2 SD) and the presence of at least 1 vertebral fracture without significant associated comorbidity. Exclusion criteria were the presence of significant comorbidity (e.g., diabetes and dyslipidemia) or treatment with corticosteroid or immunosuppressive therapy. The protocol was previously approved by the local Ethics Committee (CPP EST-II). All the patients and controls gave written informed consent.
PBMC isolation from whole blood
PBMCs were isolated by Ficoll gradient centrifugation. Blood from a patient was diluted with equal amounts of PBS, overlaid on Ficoll medium (Eurobio), and centrifuged at 900 × g for 30 min at 25°C. The PBMC band was removed and washed twice with PBS. Cell count was determined by Malassez cytometer (Poly Labo), and cells were resuspended in serum-free RPMI 1640.
Isolation of nuclear and cytoplasmic extracts from PBMCs
After isolation, PBMCs were harvested and washed with wash buffer (10 mM HEPES (pH 7.6), 10 mM KCl, 2 mM MgCl2, 1 mM EDTA). Cell pellets were then incubated on ice with cytoplasmic isolation buffer (10 mM HEPES (pH 7.6), 10 mM KCl, 2 mM MgCl2, 1 mM EDTA, and 0.02% Nonidet P-40). Cytoplasmic extracts were collected by centrifugation, and the nuclear pellets were washed twice in wash buffer, spun, and incubated for 15 min on ice with nuclear isolation buffer (20 mM HEPES (pH 7.6), 420 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, and 25% glycerol). Supernatants containing nuclear extracts were collected by centrifugation and stored at −80°C. Protease inhibitors (1 mM DTT, 1 mM PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin, and 1 μg/ml pepstatin) were added to all solutions. Protein concentration in nuclear and cytoplasmic extracts was determined by the Bradford method using a Bio-Photometer (Eppendorf).
Measurement of SIRT1 activity in PBMC extracts
SIRT1 activity was evaluated from cytoplasmic and nuclear compartments using a fluorometric assay at the 15-min point (SIRT1 fluorometric kit, BML-AK-555, Enzo Life Sciences, Villeurbanne, France) [11].
Measurement of proinflammatory cytokines and serum CTX
The levels of TNF alpha, IL-6, and IL-8 were quantified in PBMC culture supernatants at 48 h using ELISA assays according to the manufacturer’s recommendations (Quantikine Kits, R&D Systems, Minneapolis, MN). The biological marker of bone turnover sCTX-I was measured in the patient’s serum.
Statistical analysis
The correlation coefficient R2 was used to assess correlations between different data. This coefficient has been tested by a z-test at alpha=5% risk. Comparisons of continuous variables were made between groups using the t-test. The significance level used was p<0.05. Spearman’s Rank correlation coefficient was used to identify and test the strength of a relationship between 2 sets of data.
Results
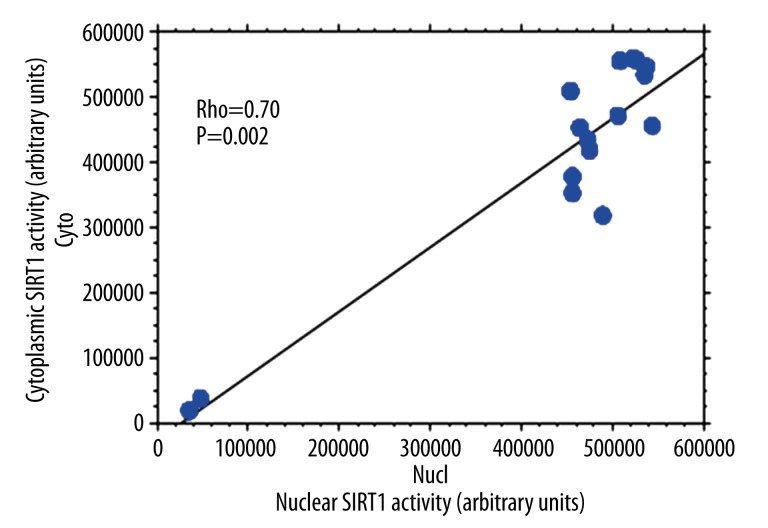
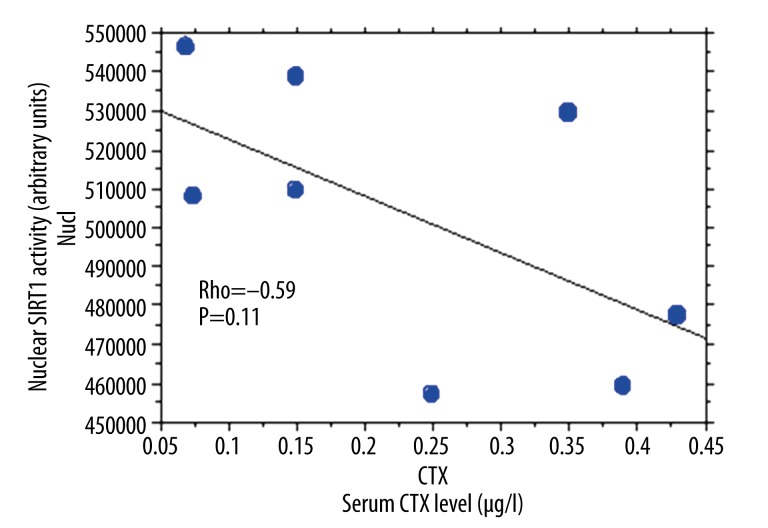
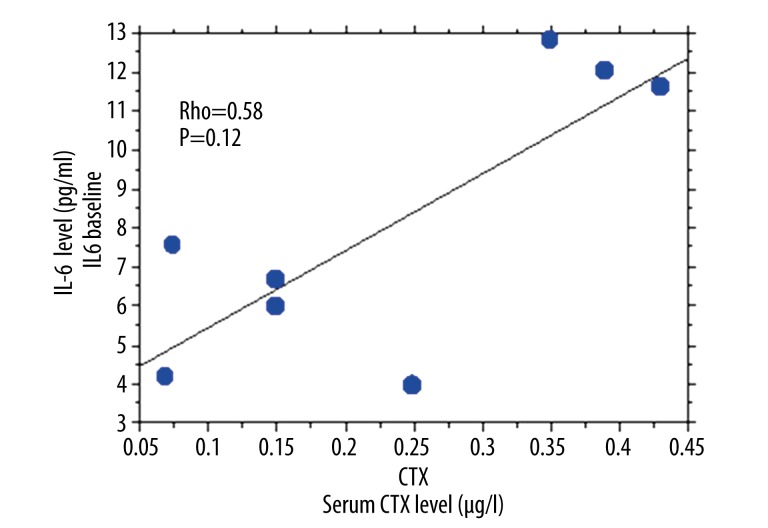
Sixteen osteoporotic women were included, with a mean age 68±9 years and an average of 2 vertebral fractures. We found a statistically significant correlation between the cytoplasmic and nuclear activity of SIRT1 in PBMCs of osteoporotic patients (R2=0.866) (Figure 1). By Spearman rank correlation analysis, cytoplasmic SIRT1 activity correlated positively with nuclear SIRT1 activity in PBMCs of osteoporotic patients (rho=0.70, P=0.002) (Figure 1). Nuclear and cytoplasmic SIRT1 activities were not significantly different in PBMCs of osteoporotic patients and healthy controls (p=0.16 and p=0.20, respectively). Erythrocyte sedimentation rate (ESR) was significantly higher in osteoporotic patients compared to controls (p=0.006). We then measured the levels of proinflammatory cytokines TNF alpha, IL-6, and IL-8 in the culture supernatants of PBMCs isolated from patients with osteoporosis and controls (Table 1). We did not find any statistically significant difference for TNF alpha (p=0.16), IL-6 (p=0.22), and IL-8 (p=0.09) levels in the culture supernatants of PBMCs isolated from osteoporotic patients compared to controls (Table 1). No significant correlation between the cytoplasmic and nuclear activity of SIRT 1 and the levels of the proinflammatory cytokines TNF alpha (rho=0.30, p=0.27), IL-6 (rho=0.05, p=0.82), and IL-8 (rho=0.40, p=0.12) was observed using Spearman rank correlation. Although not reaching significance, a negative trend was observed between the nuclear activity of SIRT1 and the levels of serum CTX using Spearman rank correlation (rho=−0.59, p=0.11) (Figure 2). Similarly, using Spearman rank correlation, a non-significant positive trend was observed between the level of serum CTX and IL-6 levels (rho=0.58, p=0.12) (Figure 3).
Figure 1.
Positive correlation between nuclear and cytoplasmic SIRT1 activity in PBMCs isolated from patients with osteoporosis (rho=0.70; p=0.002).
Table 1.
Values of biological parameters in patients with osteoporosis and controls.
| SIRT 1 nuclear activity | SIRT 1 cytoplasmic activity | CRP mg/l | ESR mm/h | TNFα pg/ml | IL 6 pg/ml | IL 8 pg/ml | |
|---|---|---|---|---|---|---|---|
| Osteoporosis patients (mean ±SD) (n=16) | 441,818±158,525 | 409,403±162,651 | 4.3±4.3 | 23.7±14.1 | 22.0±17.0 | 7.2±5.2 | 1,140.4±1,398.2 |
| Controls (mean ±SD) (n=16) | 377,564±196,262 | 354,613±200,762 | 2.8±1.6 | 9±5.5 | 30.5±27.1 | 8.7±4.9 | 564.8±720.4 |
| p | 0.16 | 0.20 | 0.11 | 0.006 | 0.16 | 0.22 | 0.09 |
Figure 2.
PBMC nuclear SIRT1 activity and serum CTX levels in patients with osteoporosis (rho=−0.59; p=0.11).
Figure 3.
IL-6 levels in PBMC culture supernatants and serum CTX levels in patients with osteoporosis (rho=0.58; p=0.12).
Discussion
Our study assessed the detection of SIRT1 activity in PBMCs of patients with osteoporosis. We observed a statistically significant relationship between nuclear and cytoplasmic activity of SIRT1 in PBMCs of osteoporotic patients. Our results indicate that SIRT1 also has cytoplasmic activity in human PBMCs, although the primarily nuclear activity of SIRT1 was shown previously in the literature [4]. Additionally, nucleo-cytoplasmic shuttling of SIRT1 may participate in the differentiation and in the modulation of cell death [12]. We did not find any significant association between the cytoplasmic and nuclear activities of SIRT1 and the levels of inflammation markers (CRP and ESR), and between the rate of bone resorption marker (CTX) and inflammation markers. We did not observe a statistical correlation between nuclear and cytoplasmic activities of SIRT1 and the levels of proinflammatory cytokines in the culture supernatants of PBMCs isolated from osteoporotic patients. Our results can be explained by the fact that in addition to SIRT1, several other HDACs, including HDAC1 and HDAC2, regulate gene expression of proinflammatory cytokines such as TNF alpha [13]. Although not reaching significance, a negative trend was observed between the nuclear activity of SIRT1 and the levels of serum CTX, a bone resorption marker, using Spearman rank correlation (rho=−0.59, p=0.11) (Figure 2). Similarly, using Spearman rank correlation, a non-significant positive trend was observed between the level of serum CTX and IL-6 levels (rho=0.58, p=0.12) (Figure 3). He et al. observed that resveratrol, a SIRT1 activator, inhibits osteoclastogenesis [14]. Further studies are needed to define the exact molecular mechanisms linking SIRT1 activity and bone homeostasis [15] and to support the potential role of inflammation in bone resorption.
Conclusions
We show here that the cytoplasmic and nuclear activities of SIRT1 are measurable in human blood PBMCs isolated from patients with osteoporosis, and that SIRT1 activity is correlated in these 2 cellular compartments. Our results also indicate a potential role of SIRT1 activity in the regulation of bone resorption. Further studies are needed to determine the exact role of SIRT1 and the molecular mechanisms involved in bone remodeling, with the goal to develop new therapeutic approaches to cure osteoporosis.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Source of support: Departmental sources
References
- 1.Briot K, Cortet B, Thomas T, et al. 2012 update of French guidelines for the pharmacological treatment of postmenopausal osteoporosis. Joint Bone Spine. 2012;79:304–13. doi: 10.1016/j.jbspin.2012.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Pang M, Hernandez M, Fernandez I, et al. Sirtuin activating compounds inhibit Rac1 activation, actin ring formation and osteoclastogenesis. ASBMR; 2010 october 18; Poster Session III, MO0258. [Google Scholar]
- 3.Cohen-Kfir E, Artsi H, Levin A, et al. Sirt1 is a regulator of bone mass and a repressor of Sost encoding for sclerostin, a bone formation inhibitor. Endocrinology. 2011;152:4514–24. doi: 10.1210/en.2011-1128. [DOI] [PubMed] [Google Scholar]
- 4.Herbein G, Wendling D. Histone deacetylases in viral infections. Clin Epigenet. 2010;1:13–24. doi: 10.1007/s13148-010-0003-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holroyd C, Harvey N, Dennison E. Epigenetic influences in the developmental origins of osteoporosis. Osteoporosis Int. 2012;23:401–10. doi: 10.1007/s00198-011-1671-5. [DOI] [PubMed] [Google Scholar]
- 6.Grabiec AM, Tak PP, Reedquist KA. Targeting histone deacetylase activity in rheumatoid arthritis and asthma as prototypes of inflammatory diseases: should we keep our HATs on? Arthritis Res Ther. 2008;10:226. doi: 10.1186/ar2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Backesjo CM, Li Y, Lindgren U, et al. Activation of Sirt1 decreases adipocyte formation during osteoblast differentiation of mesenchymal stem cells. J Bone Miner Res. 2006;21:993–1002. doi: 10.1359/jbmr.060415. [DOI] [PubMed] [Google Scholar]
- 8.Tseng PC, Hou SM, Chen RJ, et al. Resveratrol promotes osteogenesis of human mesenchymal stem cells by upregulating RUNX2 gene expression via the SIRT1/FOXO3A axis. J Bone Miner Res. 2011;26:2552–63. doi: 10.1002/jbmr.460. [DOI] [PubMed] [Google Scholar]
- 9.Renaud S, De Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–26. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 10.Shakibaei M, Buhrmann C, Mobasheri A. Resveratrol-mediated SIRT1 interactions with p300 modulate receptor activator of NF-κB Ligang (RANKL) activation of NF-κB signaling and inhibit osteoclastogenesis in bone-derived cells. J Biol Chem. 2011;286:11492–505. doi: 10.1074/jbc.M110.198713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wendling D, Abbas W, Godfrin-Valnet M, et al. Resveratrol, a sirtuin 1 activator, increases IL-6 production by peripheral blood mononuclear cells of patients with knee osteoarthritis. Clin Epigenetics. 2013;5:10. doi: 10.1186/1868-7083-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanno M, Sakamoto J, Miura T, et al. Nucleocytoplamsic shuttling of the NAD+-dependent histone deacetylase SIRT1. J Biol Chem. 2007;282:6823–32. doi: 10.1074/jbc.M609554200. [DOI] [PubMed] [Google Scholar]
- 13.Toussirot E, Abbas W, Khan KA, et al. Imbalance between HAT and HDAC activities in the PBMCs of patients with ankylosing spondylitis or rheumatoid arthritis and influence of HDAC inhibitors on TNF alpha production. PLoS One. 2013;8:e70939. doi: 10.1371/journal.pone.0070939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X, Andersson G, Lindgren U. Resveratrol prevents RANKL-induced osteoclast differentiation of murine osteoclast progenitor RAW 264.7 cells through inhibition of ROS production. Biochem Biophys Res Commun. 2010;401:356–62. doi: 10.1016/j.bbrc.2010.09.053. [DOI] [PubMed] [Google Scholar]
- 15.Braun T, Schett G. Pathways for bone loss in inflammatory disease. Curr Osteoporosis Rep. 2012;10:101–8. doi: 10.1007/s11914-012-0104-5. [DOI] [PubMed] [Google Scholar]