Abstract
Background
The aim of this study was to compare the effects of the levonorgestrel-releasing intrauterine system (LNG-IUS) with conventional medical treatment in reducing heavy menstrual bleeding.
Material/Methods
Relevant studies were identified by a search of MEDLINE, EMBASE, the Cochrane Central Register of Controlled Trials, and clinical trials registries (from inception to April 2014). Randomized controlled trials comparing the LNG-IUS with conventional medical treatment (mefenamic acid, tranexamic acid, norethindrone, medroxyprogesterone acetate injection, or combined oral contraceptive pills) in patients with menorrhagia were included.
Results
Eight randomized controlled trials that included 1170 women (LNG-IUS, n=562; conventional medical treatment, n=608) met inclusion criteria. The LNG-IUS was superior to conventional medical treatment in reducing menstrual blood loss (as measured by the alkaline hematin method or estimated by pictorial bleeding assessment chart scores). More women were satisfied with the LNG-IUS than with the use of conventional medical treatment (odds ratio [OR] 5.19, 95% confidence interval [CI] 2.73–9.86). Compared with conventional medical treatment, the LNG-IUS was associated with a lower rate of discontinuation (14.6% vs. 28.9%, OR 0.39, 95% CI 0.20–0.74) and fewer treatment failures (9.2% vs. 31.0%, OR 0.18, 95% CI 0.10–0.34). Furthermore, quality of life assessment favored LNG-IUS over conventional medical treatment, although use of various measurements limited our ability to pool the data for more powerful evidence. Serious adverse events were statistically comparable between treatments.
Conclusions
The LNG-IUS was the more effective first choice for management of menorrhagia compared with conventional medical treatment. Long-term, randomized trials are required to further investigate patient-based outcomes and evaluate the cost-effectiveness of the LNG-IUS and other medical treatments.
MeSH Keywords: Menorrhagia, Meta-Analysis, Quality of Life
Background
Heavy menstrual bleeding, or menorrhagia, is defined as excessive menstrual blood loss that occurs alone or in combination with other symptoms and has a negative impact on a woman’s physical, social, emotional, and material quality of life [1]. Approximately 30% of women are negatively affected by menorrhagia during their reproductive years [2,3], resulting in increased health care costs [4]. Previous Cochrane reviews [5–7] found that tranexamic acid and danazol were more effective than non-steroidal anti-inflammatory drugs (NSAIDs), combined oral contraceptives, or oral progestogens; treatment with danazol caused more adverse events than other treatments, which may affect its acceptability and its long-term use. However, these conclusions were based on a small number of trials, all of which were underpowered, limiting the recommendations for clinical use.
The levonorgestrel-releasing intrauterine system (LNG-IUS) is a reversible method of contraception that has a known efficacy in the treatment of menorrhagia [8,9]. Several studies have assessed the clinical effectiveness and cost-effectiveness of LNG-IUS and surgical interventions (hysterectomy, first- and second-generation endometrial ablation), [10–12] but a clearly superior approach to management of menorrhagia remains elusive. While surgical approaches may be effective, they are associated with perioperative and long-term complication risks [13,14]. Moreover, 80% of women treated for menorrhagia have no uterine abnormality [5] and over a third of women undergoing hysterectomies for menorrhagia have normal uteri [15]. In addition, many women with menorrhagia desire to preserve their potential for childbearing. Therefore, surgical options should be reserved for women who have pelvic pathology and for those who fail medical treatment.1
The LNG-IUS is an inexpensive and minimally invasive procedure that could be an alternative to oral drug treatment as a first-line agent [16]. In 2007, guidelines from the UK National Institute for Health and Clinical Excellence recommended the LNG-IUS as a first-line treatment for menorrhagia on the basis of limited evidence [1]. Recently, several randomized trials have shown the superiority of LNG-IUS over conventional medical treatments in reducing menstrual blood loss in women with menorrhagia [17–20]. The ECLIPSE study, a more recent multicenter randomized trial, added evidence that the LNG-IUS was more effective than conventional medical treatment in reducing the effect of menorrhagia on quality of life [21]. However, a comprehensive summary of the pertinent evidence has not been published to date. Therefore, we performed a systematic review and meta-analysis to compare the effectiveness of LNG-IUS with that of conventional medical treatment in women with menorrhagia.
Material and Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement was followed for the performance and reporting of this systematic review [22].
A literature search of MEDLINE (1948–April 2014), EMBASE (1980–April 2014), and the Cochrane Central Register of Controlled Trials (CENTRAL) (up to April 2014) was performed in week 2 of April 2014. The following Medical Subject Headings (MeSH) terms and text words were combined and used: (levonorgestrel-releasing intrauterine system OR levonorgestrel intrauterine system OR levonorgestrel-IUS OR LNG-IUS OR progestogen releasing intrauterine systems OR Mirena); (norethisterone OR tranexamic acid OR mefenamic acid OR danazol OR oral contraceptives OR progestin OR medroxyprogesterone OR medical treatment OR medical therapy); (menorrhagia OR menometrorrhagia OR dysfunctional uterine bleeding OR heavy menstrual bleeding OR excessive uterine bleeding). A search of ongoing or recently completed trials was performed with the United Kingdom (www.controlled-trials.com) and United States Clinical Trials registries (www.clinicaltrials.gov). Relevant papers were also identified from the bibliographies of papers obtained through the search. All searches were performed by 2 independent researchers (Q.Y.W. and J.H.) without geographic or language restrictions.
Two reviewers (Q.Y.W. and J.H.) identified and screened the search findings for potentially eligible randomized controlled trials (RCTs) that compared the LNG-IUS with conventional medical treatment in patients with menorrhagia. For inclusion in the meta-analysis, a study had to fulfill the following criteria: an RCT article published in a peer-reviewed journal, with the report describing at least 1 of the primary outcomes mentioned below. When studies were reported by the same institution and/or authors, either the better quality study or the more recent publication was included. The following reports were excluded: abstracts, letters, editorials, expert opinions, case reports, reviews without original data, and studies lacking a control group. Any disagreements about inclusion were resolved by consensus or arbitration by a third reviewer (J.Q.).
Independently and in duplicate, the same 2 reviewers (Q.Y.W. and J.H.) extracted the data (first author, year of publication, country where the study was performed, sample size, study population characteristics, medical treatment option and dosage, follow-up, and outcomes of interest) into a standardized collection form and entered the data into the RevMan program (version 5.1.0, 2011; The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark). Some data in this meta-analysis may differ slightly from those included in the original studies because we standardized outcome definitions for data analysis.
Primary outcome measures were menstrual blood loss and rate of satisfaction. Although menstrual blood loss can be directly measured using the alkaline hematin method, indirect determination of menstrual bleeding using a validated pictorial bleeding assessment chart (PBAC) is a more practical approach and is commonly used in clinical trials [23]. A previous study demonstrated a correlation (correlation coefficient=0.847) between the objective measurement of blood loss (alkaline hematin method) and self-assessed PBAC scores, with a score of ≥100 to have a sensitivity of 86% and a specificity of 89% to diagnose a blood loss of ≥80 ml, which is the definition of menorrhagia [24]. In our study, data of direct measurement of menstrual blood loss and indirect evaluation using PBAC were extracted from the included trials to provide the most complete information on menstrual blood loss.
Because reduction in blood loss does not always correlate with satisfaction with treatment, rate of satisfaction with treatment was used as another primary outcome measure. As previously reported [11], “very satisfied” or “satisfied” were considered to be a positive response, and “very dissatisfied” or “dissatisfied” a negative response. “Not sure” or “uncertain” were conservatively judged to be a negative rating of treatment. For studies not reporting level of satisfaction, we used surrogate outcomes (major problem resolved/menstrual symptoms successfully treated/willing to continue with the treatment).
Secondary outcome measures included rate of discontinuation, treatment failure, serious adverse events, and quality of life. Although not consistently defined across studies, treatment failure included menstrual blood loss ≥80 ml or a PBAC score ≥100, unacceptable bleeding profile, persistent or recurrent heavy bleeding, major change in allocated treatment (one medical treatment to another, LNGIUS to medical treatment, or medical treatment to LNGIUS), removal of LNG-IUS, and discontinuation of medical treatment. Serious adverse events were defined as those resulting in death, disability, or hospitalization. To assess quality of life, the Medical Outcomes Study 36-Item Short-Form Health Survey (SF36) and the EuroQol Group 5-Dimension Self-Report (EQ-5D) questionnaires were utilized [25,26].
Studies were critically evaluated for design and risk of bias, according to criteria in the Cochrane Handbook for Systematic Reviews of Interventions [27]. Each RCT was assessed according to 7 criteria: (1) sequence generation; (2) allocation concealment; (3) blinding of participants, personnel, and outcome assessors; (4) incomplete outcome data; (5) selective outcome reporting; (6) other sources of bias; and (7) intention-to-treat analysis. In addition, trials were rated for levels of evidence according to the Oxford Centre for Evidence-Based Medicine in the UK [28].
Data analyses were performed using RevMan software version 5.1.0. All studies presented their data on menstrual blood loss using the alkaline hematin method as median (range) except for a study by Shabaan et al. [20], which presented it as mean ± standard deviation. Thus, these data were not used because of the relatively small number of studies available. To obtain a summary estimate of menstrual blood loss, we pooled PBAC scores and report the weighted mean difference (WMD). For dichotomous variables, odds ratios (ORs) were calculated with 95% confidence intervals (CIs). We estimated the degree of heterogeneity among studies using the Cochrane Q statistic (P <.10 was considered to be statistically significant heterogeneity) and the I2 statistic (I2 >50% was considered to be statistically significant heterogeneity) [29]. Initially, a fixed-effects model was used to synthesize all data. However, if there was evidence of heterogeneity among the included studies, random-effects analysis according to DerSimonian and Laird was used [30]. An estimation of potential publication bias was executed by funnel plot, in which the SE of log (OR) of each study was plotted against its log (OR). Asymmetry in such funnel plots, usually caused by small trials reporting greater effects on average than large trials, suggests a possible publication bias [31]. We used the GRADE methodology to assess the quality of evidence for each outcome across studies.
Results
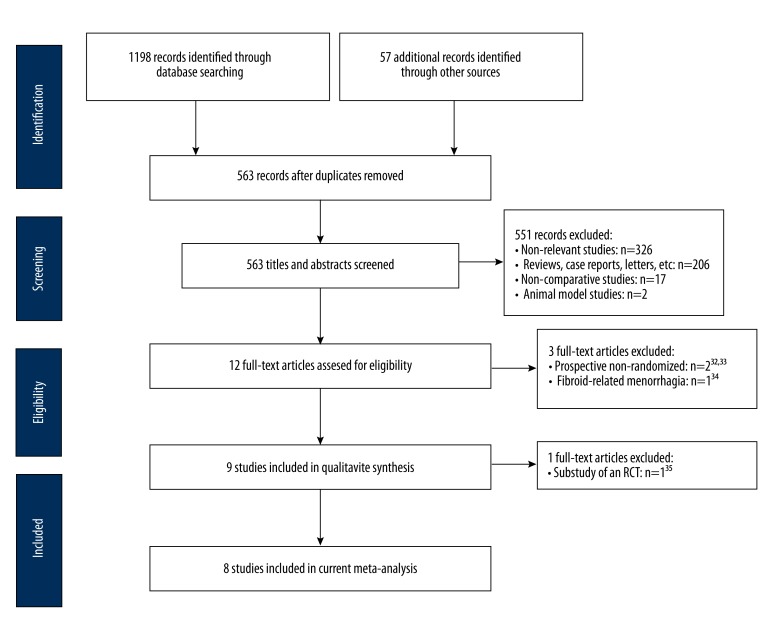
A flow diagram of our literature search is shown in Figure 1. The electronic database search resulted in 1198 citations: 481 from MEDLINE, 658 from EMBASE, and 59 from Cochrane Library. The searches of ClinicalTrials.gov and the Current Controlled Trials registry yielded 50 additional records. In addition, searching reference lists for all relevant papers, recent editorials, and related review articles yielded 7 more records. We identified 563 citations after excluding duplicates. Of these citations, we excluded 551 after screening the titles and abstracts. After further screening of the 12 full-text articles, 2 studies [32,33] were excluded because they were prospective, non-randomized studies. A study focusing on fibroid-related menorrhagia was excluded [34]. The remaining 9 studies were included in qualitative analysis, with 1 duplicate report of an RCT excluded [35], leaving 8 studies [17–21,36–38] in the final meta-analysis.
Figure 1.
Flow diagram of included studies. RCT, randomized controlled trials.
Table 1 outlines the characteristics of the 8 trials included in our meta-analysis. Of these studies, 3 were conducted in the UK [21,36,38], and the remainder were performed in Finland [37], USA [19], Canada [18], Turkey [17], and Egypt [20]. In total, there were 562 patients in the LNG-IUS group and 608 patients in the conventional medical treatment group. There were some differences in study design between trials. Medical treatments differed between studies: 2 studies used mefenamic acid [37,38], 2 studies used medroxyprogesterone [17,19], 2 studies used combined oral contraceptive pills [18,20], and 1 study used norethisterone [36]. In the largest multicenter randomized trial, conventional treatment included mefenamic acid, tranexamic acid, norethindrone, medroxyprogesterone acetate injection, combined oral contraceptive pills, and combinations of these methods [21]. Duration of follow-up varied from 3 months to 2 years. One study compared the LNG-IUS to both depot and oral medroxyprogesterone acetate, with 44 patients in each arm [17].
Table 1.
Characteristics of included randomized controlled trials.
| Author | Year | Country | Centers | No. of patients | Inclusion/exclusion criteria | Type of usual medical treatments | Duration, months | Lost to follow up | ||
|---|---|---|---|---|---|---|---|---|---|---|
| LNG-IUS | Medical therapy | LNG-IUS | Medical therapy | |||||||
| Irvine et al. [36] | 1998 | UK | Single | 22 | 22 |
Inclusion: parous women aged 18–45 y in good general health with a regular menstrual cycle, a normal pelvic examination with a sound measurement of the uterus of <10 cm, negative cervical cytology and a measured MBL ≥80 ml Exclusion: women had been treated with steroid hormones or anticoagulants during the previous three months, or had used injectable hormones for contraception during the previous 12 months |
Norethisterone | 3 | 0 | 0 |
| Reid et al. [38] | 2005 | UK | Single | 25 | 26 |
Inclusion: women aged 18–47 y in good general health with regular, ovulatory, menstrual cycles of 21–35 days and objective, idiopathic menorrhagia (MBL ≥80 ml) Exclusion: women had undiagnosed abnormal bleeding, were anovulatory, had submucous fibroids or fibroids with a total volume of >5 cm3, a uterine sound of >10 cm, abnormal cervical cytology, untreated hypertension, abnormal thyroid or liver function tests, asthma, had been treated for menorrhagia or used hormonal contraceptives within the previous four months |
Mefenamic acid | 6(6 cycles)† | 0 | 1 |
| Endrikat et al. [18] | 2009 | Canada | Nine | 20 | 19 |
Inclusion: healthy women aged 30 at entry, with a diagnosis of idiopathic menorrhagia (PBAC score ≥100 for 2 consecutive cycles) and with a normal or only slightly enlarged uterus Exclusion: contraindications for LNG-IUS and combined oral contraceptive, metabolic and endocrine diseases, diagnostically unclassified genital bleeding, a history of liver or vascular diseases, concomitant use of medications that could influence the study objectives, intramural or subserous fibroids of mean diameter ≥ 4cm or submucous fibroids, adenomyosis or endometrial abnormalities or perimenopausal |
Combined oral contraceptive pill | 12 | 3 | 7 |
| Kaunitz et al. [19] | 2010 | USA | Fifty-five | 82 | 83 |
Inclusion: parous women aged ≥18 y with idiopathic heavy menstrual bleeding (MBL ≥80 ml) desiring intrauterine contraception and willing to use barrier contraception if required Exclusion: changes in menstrual regularity, hot flushes, sleeping disorders, or changes in mood within the 3 months preceding the study; breastfeeding; congenital or acquired uterine abnormality, including fibroids if they distorted the uterine cavity or cervical canal; history of organic causes of abnormal uterine; use of LNG-IUS or a copper intrauterine device during the 30 days before the study; history of vascular or coagulation disorders; concomitant use of medication or presence of an underlying disease/condition known to affect the metabolism or pharmacokinetics of the study medication; and a body mass index ≥35 kg/m2 |
Oral medroxyprogesterone acetate | 6 (6 cycles)† | 2 | 1 |
| Shabaan et al. [20] | 2011 | Egypt | Single | 56 | 56 |
Inclusion: had self-described heavy menstrual bleeding, requested contraception, aged 20–50 y, had a regular cycle, and were living in a nearby area to make follow-up reasonably possible Exclusion: pregnancy, history of ectopic pregnancy, puerperal sepsis, pelvic inflammatory disease, or evidence of defective coagulation; fibroid of any size; history or evidence of malignancy or hyperplasia in the endometrial biopsy, incidental adnexal abnormality on ultrasound, contraindications to combined oral contraceptive pill, previous endometrial ablation or resection, uninvestigated postcoital bleeding and untreated abnormal cervical cytology |
Combined oral contraceptive pill | 12 | 8 | 9 |
| Gupta et al. [21] | 2013 | UK | Multiple | 285 | 286 |
Inclusion: women aged 25–50 y who presented to their primary care physicians with menorrhagia involving at least three consecutive menstrual cycles were eligible to participate Exclusion: women intended to become pregnant over the next 5 years, were taking hormone replacement therapy or tamoxifen, had intermenstrual bleeding or postcoital bleeding or findings suggestive of fibroids or other disorders, or had contraindications to or a preference for either the LNG-IUS or usual medical treatments; women with heavy, irregular bleeding were ineligible unless the results of endometrial biopsy were reported to be normal |
Mefenamic acid; tranexamic acid; norethindrone; medroxyprogesterone acetate injection; combined oral contraceptive pill | 24 | 13 | 16 |
| Küçük et a. [l17] | 2008 | Turkey | Single | 44 | 44/44‡ | Inclusion: perimenopausal patients (age ≥40 y) with heavy menstrual bleeding (MBL ≥80 ml) Exclusion: organic pathology; only irregular bleeding | Depot medroxyprogesterone acetate/oral medroxyprogesterone acetate | 6 | 0 | 0/0‡ |
| Lähtee-nmäki et al. [37] | 1998 | Finland | Three | 28 | 28 |
Inclusion: women who had spontaneous cycles and who were scheduled to undergo hysterectomy for treatment of excessive uterine bleeding with or without dysmenorrhagia Exclusion: had one fibroid ≥3 cm in diameter or more than three uterine fibroids, a history or current clinical evidence or suspicion of malignancy or active liver disease, adnexal tumours or cysts, or pelvic inflammatory disease within the previous 12 months |
Mefenamic acid | 6 | 0 | 0 |
LNG-IUS – levonorgestrel-releasing intrauterine system; MBL – menstrual blood loss; PBAC – pictorial bleeding assessment chart.
6 cycles of treatment with either LNG-IUS or medical therapy;
depot/oral medroxyprogesterone acetate.
Women with idiopathic menorrhagia (menstrual blood loss ≥80 ml) were included in these trials; however, there were differing enrollment criteria. One study enrolled women who were scheduled to undergo hysterectomy as the final treatment [37]. One study included only female smokers [17]. All but 1 study [36] reported the exclusion of women who had fibroids or other disorders.
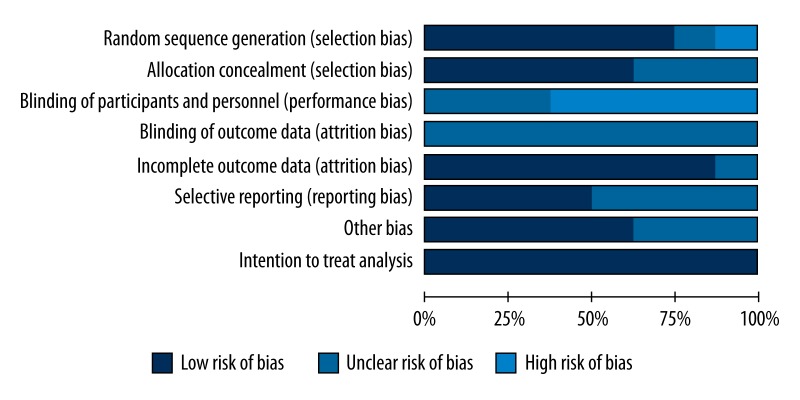
Figure 2 shows the methodological quality of the included trials. Six [19–21, 36–38] of the included trials had an adequate generation for randomization. Allocation concealment was unclear in 3 [17–19] of the 8 trials. None of the trials blinded the participants to treatment group, and none mentioned whether outcome assessors were blinded. One study did not provide the reasons for patients who were lost to follow-up; therefore, the risk of incomplete outcome data was unclear [20]. The risk of selective outcome reporting was unknown in 4 studies because of insufficient information [17,18,20,38]. Five studies [19–21,36,37] appeared to be free of other sources of bias, leaving the remaining studies unclear. Finally, all of the included studies reported intention-to-treat analysis, allowing us to use the results from intention-to-treat analysis in the meta-analysis.
Figure 2.
Quality assessment of included randomized controlled trials using the Cochrane Handbook for Systematic Reviews and Interventions.
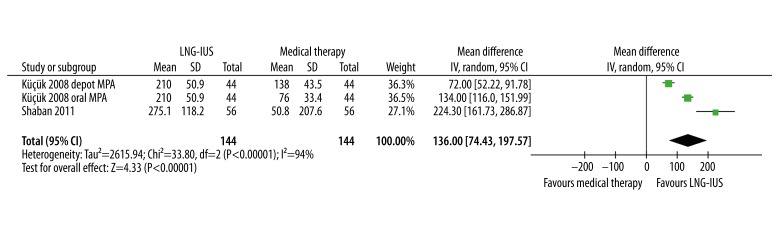
Six [17–20,36,38] of 8 studies provided measured menstrual blood loss or estimated PBAC scores (Table 2). Five studies [18–20,36,38] reported the mean percentage change from baseline in menstrual blood loss, which ranged from 70.8% to 94% in the LNG-IUS group and from 21.5% to 87% in the usual-treatment group. Five studies demonstrated the LNG-IUS to be superior to conventional medical treatment [17–20 38], while 1 study found no statistically significant difference between the LNG-IUS and norethisterone [36]. We were unable to pool data on menstrual blood loss measured by the alkaline hematin method because only 1 study reported the mean and standard deviation [20]; on the other hand, pooling data on PBAC scores showed significantly higher reductions of menstrual blood loss in the LNG-IUS group compared to the conventional treatment group (WMD 136.00, 95% CI 74.43–197.57, P<.001), although substantial heterogeneity across studies was observed (Q statistic=33.80, P<.001, I2=94%) (Figure 3).
Table 2.
Summary of menstrual blood loss outcomes.
| Study | Time | Alkaline haematin method† | P value | PBAC score† | P value | Percentage change from baseline | Superior | |||
|---|---|---|---|---|---|---|---|---|---|---|
| LNG-IUS | Medical therapy | LNG-IUS | Medical therapy | LNG-IUS | Medical therapy | |||||
| Irvine et al. [36] | Baseline | 105 (82–780) | 120 (82–336) | 0.56 | – | – | 94% | 87% | Equal‡ | |
| 3 months | 6 (0–284) | 20 (4–137) | — | – | ||||||
| Reid et al. [38] | Baseline | 122 (81–375) | 121 (85–389) | <0.001 | 240 (91–545) | 233 (77–469) | <0.001 | 79% | 23% | LNG-IUS |
| Cycle 6 | 5 (0–45) | 100 (46–168) | 25 (0–402) | 159 (50–307) | ||||||
| Endrikat et al. [18] | Baseline | – | – | – | 228 | 290 | 0.002 | 83% | 68% | LNG-IUS |
| 12 months | – | – | 13 | 72 | ||||||
| Kaunitz et al. [19] | Baseline | 148.0 (68.3–431.4) | 154.2 (63.4–456.0) | <0.001 | – | – | 70.8% | 21.5% | LNG-IUS | |
| Cycle 6 | 7.1 (0–1435.6) | 121.5 (0–437.7) | – | – | ||||||
| Shabaan et al. [20] | Baseline | 300.0±150.1 | 274.3±142.6 | <0.001 | 306.7±131.8 | 323.8±97.3 | <0.001 | 87.4% | 35.0% | LNG-IUS |
| 12 months | 44.4±34.9 | 118.2±75.0 | 31.6±35.1 | 273.0±238.4 | ||||||
| Küçük et al. [17] | Baseline | – | – | – | 287±57 | 284±50 | <0.05 | – | – | LNG-IUS§ |
| 6 months | – | – | 77±41 | 146±21 | ||||||
| Baseline | – | – | – | 287±57 | 230±36 | <0.05 | – | – | LNG-IUS¶ | |
| 6 months | – | – | 77±41 | 154±30 | ||||||
LNG-IUS – levonorgestrel-releasing intrauterine system; PBAC – pictorial bleeding assessment chart.
Data are expressed as median, median (range) or mean ± standard deviation;
LNG-IUS versus norethisterone;
LNG-IUS versus depot medroxyprogesterone acetate;
LNG-IUS versus oral medroxyprogesterone acetate.
Figure 3.
Pooled analysis of reduction of pictorial bleeding assessment chart (PBAC) scores. CI, confidence interval; IV, Inverse Variance method; LNG-IUS, levonorgestrel-releasing intrauterine system; MPA, medroxyprogesterone acetate; SD, standard deviation.
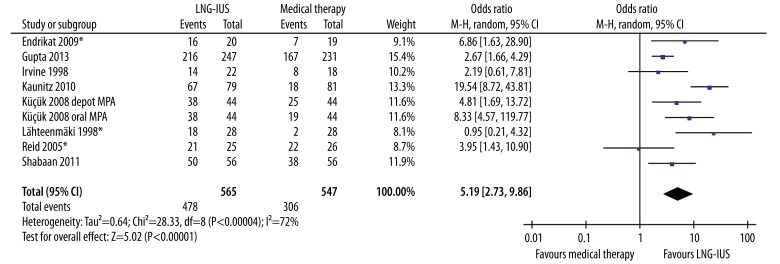
More women were satisfied with the LNG-IUS compared to conventional medical treatment (OR 5.19, 95% CI 2.73–9.86, P<.001), although there was significant heterogeneity between study estimates (Q statistic=28.33, P<.001, I2=72%) (Figure 4). Further sensitivity analyses demonstrated that the heterogeneity was the effect of all 8 varied outcomes, not the effect of any single study.
Figure 4.
Comparison of the levonorgestrel-releasing intrauterine system with medical therapy regarding rate of satisfaction. Surrogates were used for 3 studies (asterisk) not reporting level of satisfaction (Endrikat et al. 2009, Lähteenmäki et al. 1998, Reid et al. 2005). CI, confidence interval; LNG-IUS, levonorgestrel-releasing intrauterine system; M-H, Mantel-Haenszel method; MPA, medroxyprogesterone acetate.
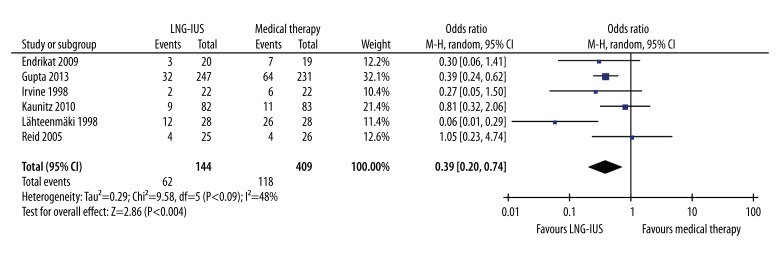
Six studies reported the rate of discontinuation [18,19,21,36–38]. A lower proportion of women in the LNG-IUS group discontinued the primary treatment as compared with the medical treatment group (14.6% vs. 28.9%, OR 0.39, 95% CI 0.20–0.74, P=.004) (Figure 5). Slight heterogeneity between studies was observed in this result (Q statistic=9.58, P=0.09, I2=48%). Sensitivity analyses showed that 1 independent study by Lähteenmäki et al. [37], which reported that 26 of 28 (93%) patients discontinued the treatment of mefenamic acid at 12 months, was the main origin of this heterogeneity. The heterogeneity was effectively decreased by excluding this study (Q statistic=3.67, P=0.45, I2=0%). However, results from pooled estimates of rate of discontinuation remained similar after excluding this study (OR 0.45, 95% CI 0.31–0.66, P<.001). The reasons for discontinuation were not available for further analysis.
Figure 5.
Pooled analysis of rate of discontinuation across studies. CI, confidence interval; LNG-IUS, levonorgestrel-releasing intrauterine system; M-H, Mantel-Haenszel method.
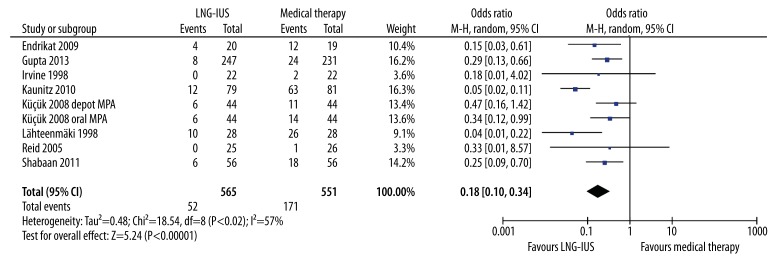
With respect to treatment failures, women receiving the LNG-IUS had a lower incidence of treatment failures than those receiving conventional medical treatment (OR 0.18, 95% CI 0.10–0.34, P<.001) (Figure 6). There was some evidence of heterogeneity between studies in this result (Q statistic=18.54, P=0.02, I2=57%). Sensitivity analyses were then performed by examining the influence on the overall effect of removing individual data from the analysis. The study by Kaunitz et al. [19], which reported significantly more successful treatment with the LNG-IUS (67/79, 84.8%) than medroxyprogesterone acetate (18/81, 22.2%), was the origin of the heterogeneity in this outcome. After exclusion of this study, the heterogeneity was effectively reduced (Q statistic=6.85, P=0.44, I2=0%). However, the incidence of treatment failure was still significantly lower for the LNG-IUS (OR 0.25, 95% CI 0.16–0.39, P<.001), compared with conventional medical treatment, after the exclusion of the study [19].
Figure 6.
Pooled analysis of treatment failures across studies. CI, confidence interval; LNG-IUS, levonorgestrel-releasing intrauterine system; M-H, Mantel-Haenszel method; MPA, medroxyprogesterone acetate.
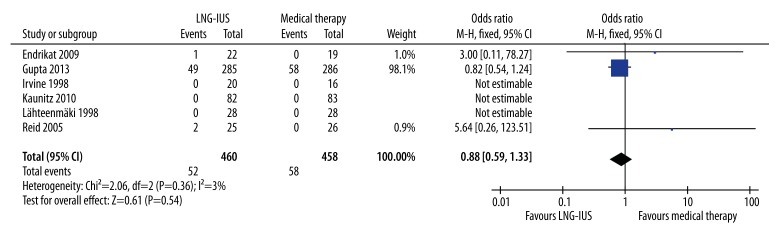
Serious adverse events were reported in 6 studies [18,19,21,36–38]. Three studies were not estimable, as no serious adverse events for either treatment were reported [19,36,37]. Pooled data from the remaining 3 studies [18,21,38] showed no statistically significant difference in serious adverse events between the LNG-IUS and medical treatment groups (OR 0.88, 95% CI 0.59–1.33, P=.54), with no obvious heterogeneity observed (Q statistic=2.06, P=0.36, I2=3%) (Figure 7).
Figure 7.
Pooled analysis of serious adverse events. CI, confidence interval; LNG-IUS, levonorgestrel-releasing intrauterine system; M-H, Mantel-Haenszel method.
Only 3 studies reported quality of life as an outcome measure [20,21,37]. This outcome was assessed in different ways; therefore, pooling the data for meta-analysis was not possible. Lähteenmäki et al. [37] assessed quality of life as general wellbeing, work performance, physical activity, sex life, and leisure time activity by the visual analogue scale. There was no improvement in quality of life scores in the medical treatment group (mefenamic acid) but there was significant improvement in patients with the LNG-IUS in all aspects. In a trial comparing the LNG-IUS with combined oral contraceptive pills, health-related quality of life and lost work days were analyzed via the HRQoL-4 questionnaire [20]. The authors found a significant reduction in the number of lost work days in the LNG-IUS group (P<.001), although overall health, physically ill days, and mentally ill days were comparable between groups. In the most recent randomized trial, 3 instruments were used to assess quality of life: the SF-36 and EQ-5D questionnaires, and the EQ-5D visual analogue scale [21]. The investigators demonstrated a significant improvement from baseline on the SF36 in both groups, with better scores in the LNGIUS group in 7 of the 8 domains (physical functioning, physical role, emotional role, social functioning, energy and vitality, pain, and perception of general health). No significant differences were observed between groups for the EQ5D questionnaire and EQ-5D visual analogue scale (P=.38 and P=.12, respectively).
Assessment of a funnel plot of rate of satisfaction suggests there was no publication bias. We used the GRADE approach to evaluate the quality of evidence. We found the quality of evidence for PBAC scores, rate of satisfaction, and serious adverse events to be moderate. We found the quality of evidence for treatment failure and rate of discontinuation to be moderate (Table 3).
Table 3.
Summary of findings table for the LNG-IUS compared with conventional medical treatment in patients with menorrhagia.
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk Medical therapy | Corresponding risk LNG-IUS | |||||
| PBAC scores | The mean PBAC score in the intervention group was 136 higher (74.43 to 197.57 higher) | 288 (3 studies) | ⊕⊕⊖⊖ low†,‡ | |||
| Rate of satisfaction | 559 per 1000 | 868 per 1000 (776 to 926) | OR 5.19 (2.73 to 9.86) | 1112 (9 studies) | ⊕⊕⊖⊖ low†,§ | |
| Treatment failures | 310 per 1000 | 75 per 1000 (43 to 133) | OR 0.18 (0.1 to 0.34) | 1116 (9 studies) | ⊕⊕⊕⊖ moderate† | |
| Rate of discontinuation | 289 per 1000 | 137 per 1000 (75 to 231) | OR 0.39 (0.2 to 0.74) | 833 (6 studies) | ⊕⊕⊕⊖ moderate† | |
| Serious adverse events | 127 per 1000 | 113 per 1000 (79 to 162) | OR 0.88 (0.59 to 1.33) | 918 (6 studies) | ⊕⊕⊖⊖ low†,¶ | |
| Quality of life | See comment | See comment | Not estimable | 646 (3 studies) | See comment | Three studies reported quality of life by different measurements |
CI – confidence interval; LNG-IUS – levonorgestrel-releasing intrauterine system; OR – odds ratio; PBAC – pictorial bleeding assessment chart. GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI);
some trials did not have adequate allocation concealment, blinding not clear, or the risk of selective outcome reporting unknown;
there was substantial heterogeneity across studies;
when studies did not report rate of satisfaction, we used surrogate outcomes (major problem resolved/menstrual symptoms successfully treated/willing to continue with the treatment);
small sample size, wide confidence intervals, or both reported.
Discussion
Based on the available evidence, the LNG-IUS significantly reduced menstrual blood loss from baseline and was more effective than conventional medical treatment. Women who received the LNG-IUS were more satisfied than those receiving conventional medical therapy. In an analysis of secondary outcomes, the LNG-IUS was associated with a lower rate of discontinuation and a lower incidence of treatment failures. Serious adverse events did not appear to significantly differ between treatment groups. In addition, quality of life indicators were superior for women with the LNG-IUS compared with those receiving conventional medical treatment, although the data were not pooled for meta-analysis because these indicators were inconsistent between studies.
Our review provides evidence that LNG-IUS is the more effective first choice for the treatment of menorrhagia, compared with medical treatment. Although the medical treatment approaches differed in each study reviewed, these agents are representative of current clinical practice and the majority of women with menorrhagia were treated solely with these agents in primary care [1]. In addition, meta-analyses of RCTs suggest that these agents are similar in their efficacy to reduce menstrual blood loss [5–7]. Therefore, our meta-analysis is a valid comparison of the LNG-IUS with conventional medical treatment.
The LNG-IUS has been approved in 120 countries worldwide for contraception and in 115 countries for the management of menorrhagia [16]. We found the LNG-IUS to be superior to conventional medical therapy in reducing menstrual blood loss, although this result was from pooled data on PBAC scores. As there is a good correlation between PBAC scores and menstrual blood volume, PBAC scores may substitute for the alkaline hematin method, which is not applicable to routine clinical use. In addition, more women were satisfied after using the LNG-IUS than medical treatment, suggesting that more women had their major problems resolved with the LNG-IUS. Moreover, the incidence of treatment failures was lower in the LNG-IUS group, which contributes to the strong evidence that the LNG-IUS is more effective than conventional medical therapies.
Although losses of 80 ml or more are traditionally considered the criterion for menorrhagia [1], which was used in trials included in this review and many other treatment trials, only about half of women seeking treatment meet this criterion [39] and there is heterogeneity among studies with regards to blood loss. Clinical guidelines now recommend the use of patient-based outcome measures (e.g., measures of quality of life) rather than menstrual blood loss because they capture the effect of heavy bleeding on women’s psychological and physical well-being [40]. SF36 and EQ-5D questionnaires have been shown to be practical, reliable, and valid instruments for measuring health status and medical outcomes [25,26]. Although women receiving the LNG-IUS had superior quality of life in 3 of 8 trials, we were unable to pool this data due to differing quality measurements among these studies, limiting the power of the evidence. Upcoming trials should access the impact of menorrhagia on women’s quality of life and use robust and clinically-accepted instruments.
It has been previously reported that a high proportion of women who were originally prescribed LNG-IUS had terminated its use at 2 years (28%) [11], and this rate increased after 4–5 years (50%) [41]. The most common reasons for discontinuation were lack of effectiveness and adverse events [32]. However, another randomized trial showed that depression may have also have been partly responsible [42]. It appears that success or failure of treatment with the LNG-IUS is multifactorial and difficult to predict on a case-by-case basis. Nonetheless, the discontinuation rate of the LNG-IUS was much lower than that of conventional medical treatment, according to our review. This is in agreement with a recent multicenter study also demonstrating a lower discontinuation rate at 12 months in the LNG-IUS group (13.3%) [32]. This may reflect greater symptom relief with the LNG-IUS, although another possible explanation is that the cessation of medications does not require consultation with a physician.
When comparing the efficacy of the LNG-IUS with that of conventional medical therapy, safety should also be carefully evaluated. There was heterogeneity in the description of adverse events in the studies. In the 2 studies [17,36] that analyzed occurrence of adverse events using statistical methods, there was no significant difference between the 2 groups. Also, most of the adverse events were reported to be mild-to-moderate in intensity in both groups. In the 6 trials reporting serious adverse events, there were also no significant differences between groups. Of note, no uterine perforations were reported with the LNG-IUS in these trials. Thus, both the LNG-IUS and medical treatment have favorable safety profiles and are well tolerated. With regards to long-term use of the LNG-IUS, hormone-related adverse events may occur if a second LNG-IUS is implemented after 5 years, due to increased serum LNG levels. One prospective, multicenter, non-comparative study examined the bleeding pattern and safety of consecutive use of multiple LNG-IUS devices, and found hormone-related adverse events to be uncommon; no discontinuations could be attributed to hormone-related adverse events [43]. Therefore, women contemplating LNG-IUS replacement after 5 years should be informed of the remote possibility of hormone-related adverse events, which wound not be likely to result in discontinuation of the LNG-IUS.
The incidence of amenorrhea was reported in 2 trials [20,36]. More women were amenorrheic after 3 months of treatment with the LNG-IUS compared to norethisterone (32% vs. 0%) [36]. In another study, 7 women (12.5%) in the LNG-IUS group had amenorrhea within 12 months of follow-up; there was no amenorrhea in the combined oral conceptive pills group [20]. Previous studies demonstrated that approximately 30% of women were amenorrheic after using the LNG-IUS for 5 years [43]; a higher proportion (up to 60%) was seen after using a second LNG-IUS [44]. Thus, women who do not wish to experience amenorrhea may not be the best candidates for use of the LNG-IUS as a first choice.
The LNG-IUS has consistently been shown to be cost-effective for the treatment of menorrhagia in multiple clinical settings [45,46]. A recent cost-effectiveness analysis by the National Health Service in the UK compared hysterectomy, endometrial ablation (both first and second generation), and the LNG-IUS for the treatment of menorrhagia and concluded that hysterectomy would be the preferred first intervention for treating menorrhagia [12]. Although hysterectomy is more expensive than endometrial ablation or long-term LNG-IUS use (10 years), it produced more quality-adjusted life-years (QALYs) in comparison. However, due to its invasiveness, hysterectomy should not be considered a first-line treatment. Another study demonstrated that initial use of the LNG-IUS was less costly and more effective compared with combined oral conception or progestogens for the treatment of dysfunctional uterine bleeding [47]. However, there are only limited data available on long-term follow-up. Other medical therapies with drugs such as tranexamic acid, a safe and cost-effective treatment for menorrhagia, have not been compared with the LNG-IUS.
This is the first systematic review and meta-analysis comparing the LNG-IUS with conventional medical treatment for menorrhagia. We used optimal methods, complying with guidelines on reporting of systematic reviews and meta-analyses. An extensive literature search was conducted, with no language restrictions, minimizing the risk of missing information and publication bias. Furthermore, this meta-analysis was conducted at an appropriate time, because enough data have accumulated to make an adequate analysis of the LNG-IUS, which is being used more frequently to replace conventional medical therapy worldwide. Our results did not change considerably after sensitivity analysis, indicating our analysis to be stable. However, there are several limitations to our study that should be acknowledged. First, the range of medications administered to patients in this review complicates any efforts to compare the LNG-IUS with individual agents. Second, the duration of follow-up varied from 3 months to 2 years, limiting our ability to assess long-term outcomes. Finally, there was notable statistical heterogeneity across studies, as reflected in our outcome measures, although the results were stable as indicated by sensitivity analysis.
Conclusions
Our findings concur with NICE guidelines for the use of LNG-IUS as a first-line treatment for menorrhagia [1]. Patient satisfaction rates were higher with the use of the LNG-IUS compared with conventional medical treatment. Use of the LNG-IUS is associated with a lower rate of discontinuation and fewer treatment failures compared with medical treatment. Quality of life analysis favored the LNG-IUS over medical therapy, although our study may be too underpowered to draw strong conclusions because of the varied outcome measures and the relatively small number of studies available. Serious adverse events were statistically comparable between groups. The LNG-IUS should be considered as first-line treatment in patients with menorrhagia if pharmaceutical treatment is indicated, whether or not contraception is needed. Long-term, randomized trials are required to further investigate patient-based outcomes and evaluate the cost-effectiveness of the LNG-IUS and other medical treatments.
Footnotes
Disclosure
No author has any potential conflict of interest.
Source of support: Self financing
References
- 1.National Institute for Health and Clinical Excellence (NICE) Heavy menstrual bleeding. 2007. [Cited 15 Jan 2014]. Available from URL: http://www.nice.org.uk/guidance/CG44.
- 2.Barnard K, Frayne SM, Skinner KM, Sullivan LM. Health status among women with menstrual symptoms. J Womens Health (Larchmt) 2003;12:911–19. doi: 10.1089/154099903770948140. [DOI] [PubMed] [Google Scholar]
- 3.Shapley M, Jordan K, Croft PR. Increased vaginal bleeding and psychological distress: a longitudinal study of their relationship in the community. BJOG. 2003;110:548–54. [PubMed] [Google Scholar]
- 4.Jensen JT, Lefebvre P, Laliberté F, et al. Cost burden and treatment patterns associated with management of heavy menstrual bleeding. J Womens Health (Larchmt) 2012;21:539–47. doi: 10.1089/jwh.2011.3147. [DOI] [PubMed] [Google Scholar]
- 5.Beaumont H, Augood C, Duckitt K, Lethaby A. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2007;3:CD001017. doi: 10.1002/14651858.CD001017.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lethaby A, Irvine G, Cameron I. Cyclical progestogens for heavy menstrual bleeding. Cochrane Database Syst Rev. 2008;1:CD001016. doi: 10.1002/14651858.CD001016.pub2. [DOI] [PubMed] [Google Scholar]
- 7.Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;1:CD000400. doi: 10.1002/14651858.CD000400.pub3. [DOI] [PubMed] [Google Scholar]
- 8.Kaunitz AM, Inki P. The levonorgestrel-releasing intrauterine system in heavy menstrual bleeding: a benefit-risk review. Drugs. 2012;72:193–215. doi: 10.2165/11598960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 9.Lethaby AE, Cooke I, Rees M. Progesterone or progestogen-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2005;4:CD002126. doi: 10.1002/14651858.CD002126.pub2. [DOI] [PubMed] [Google Scholar]
- 10.Kaunitz AM, Meredith S, Inki P, et al. Levonorgestrel-releasing intrauterine system and endometrial ablation in heavy menstrual bleeding: a systematic review and meta-analysis. Obstet Gynecol. 2009;113:1104–16. doi: 10.1097/AOG.0b013e3181a1d3ce. [DOI] [PubMed] [Google Scholar]
- 11.Middleton LJ, Champaneria R, Daniels JP, et al. Hysterectomy, endometrial destruction, and levonorgestrel releasing intrauterine system (Mirena) for heavy menstrual bleeding: systematic review and meta-analysis of data from individual patients. BMJ. 2010;341:c3929. doi: 10.1136/bmj.c3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roberts TE, Tsourapas A, Middleton LJ, et al. Hysterectomy, endometrial ablation, and levonorgestrel releasing intrauterine system (Mirena) for treatment of heavy menstrual bleeding: cost effectiveness analysis. BMJ. 2011;342:d2202. doi: 10.1136/bmj.d2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;3:CD003677. doi: 10.1002/14651858.CD003677.pub4. [DOI] [PubMed] [Google Scholar]
- 14.McCausland AM, McCausland VM. Long-term complications of endometrial ablation: cause, diagnosis, treatment, and prevention. J Minim Invasive Gynecol. 2007;14:399–406. doi: 10.1016/j.jmig.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Clarke A, Black N, Rowe P, et al. Indications for and outcome of total abdominal hysterectomy for benign disease: a prospective cohort study. Br J Obstet Gynaecol. 1995;102:611–20. doi: 10.1111/j.1471-0528.1995.tb11398.x. [DOI] [PubMed] [Google Scholar]
- 16.Nelson AL. Levonorgestrel intrauterine system: a first-line medical treatment for heavy menstrual bleeding. Womens Health (Lond Engl) 2010;6:347–56. doi: 10.2217/whe.10.16. [DOI] [PubMed] [Google Scholar]
- 17.Küçük T, Ertan K. Continuous oral or intramuscular medroxyprogesterone acetate versus the levonorgestrel releasing intrauterine system in the treatment of perimenopausal menorrhagia: a randomized, prospective, controlled clinical trial in female smokers. Clin Exp Obstet Gynecol. 2008;35:57–60. [PubMed] [Google Scholar]
- 18.Endrikat J, Shapiro H, Lukkari-Lax E, et al. A Canadian, multicentre study comparing the efficacy of a levonorgestrel-releasing intrauterine system to an oral contraceptive in women with idiopathic menorrhagia. J Obstet Gynaecol Can. 2009;31:340–47. doi: 10.1016/S1701-2163(16)34151-2. [DOI] [PubMed] [Google Scholar]
- 19.Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system or medroxyprogesterone for heavy menstrual bleeding: a randomized controlled trial. Obstet Gynecol. 2010;116:625–32. doi: 10.1097/AOG.0b013e3181ec622b. [DOI] [PubMed] [Google Scholar]
- 20.Shaaban MM, Zakherah MS, El-Nashar SA, Sayed GH. Levonorgestrel-releasing intrauterine system compared to low dose combined oral contraceptive pills for idiopathic menorrhagia: a randomized clinical trial. Contraception. 2011;83:48–54. doi: 10.1016/j.contraception.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia. N Engl J Med. 2013;368:128–37. doi: 10.1056/NEJMoa1204724. [DOI] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, et al. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janssen CA, Scholten PC, Heintz AP. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol. 1995;85:977–82. doi: 10.1016/0029-7844(95)00062-V. [DOI] [PubMed] [Google Scholar]
- 24.Higham JM, O’Brien PM, Shaw RW. Assessment of menstrual blood loss using a pictorial chart. Br J Obstet Gynaecol. 1990;97:734–39. doi: 10.1111/j.1471-0528.1990.tb16249.x. [DOI] [PubMed] [Google Scholar]
- 25.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 26.EuroQol Group. EuroQol – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions, version 5.1.0. United Kindom: The Cochrane Collaboration; 2008. [Google Scholar]
- 28.Phillips B, Ball C, Sackett D, et al. Levels of evidence. Oxford Centre for Evidence-based Medicine; [Cited 25 January 2014]. Available from URL: http://www.cebm.net/index.aspx?o=1025. [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 31.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–5. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee BS, Ling X, Asif S, et al. Levonorgestrel-releasing intrauterine system versus conventional medical therapy for heavy menstrual bleeding in the Asia-Pacific region. Int J Gynaecol Obstet. 2013;121:24–30. doi: 10.1016/j.ijgo.2012.10.028. [DOI] [PubMed] [Google Scholar]
- 33.Milsom I, Andersson K, Andersch B, Rybo G. A comparison of flurbiprofen, tranexamic acid, and a levonorgestrel-releasing intrauterine contraceptive device in the treatment of idiopathic menorrhagia. Am J Obstet Gynecol. 1991;164:879–83. doi: 10.1016/s0002-9378(11)90533-x. [DOI] [PubMed] [Google Scholar]
- 34.Sayed GH, Zakherah MS, El-Nashar SA, Shaaban MM. A randomized clinical trial of a levonorgestrel-releasing intrauterine system and a low-dose combined oral contraceptive for fibroid-related menorrhagia. Int J Gynaecol Obstet. 2011;112:126–30. doi: 10.1016/j.ijgo.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Kaunitz AM, Bissonnette F, Monteiro I, et al. Levonorgestrel-releasing intrauterine system for heavy menstrual bleeding improves hemoglobin and ferritin levels. Contraception. 2012;86:452–57. doi: 10.1016/j.contraception.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Irvine GA, Campbell-Brown MB, Lumsden MA, et al. Randomised comparative trial of the levonorgestrel intrauterine system and norethisterone for treatment of idiopathic menorrhagia. Br J Obstet Gynaecol. 1998;105:592–98. doi: 10.1111/j.1471-0528.1998.tb10172.x. [DOI] [PubMed] [Google Scholar]
- 37.Lähteenmäki P, Haukkamaa M, Puolakka J, et al. Open randomised study of use of levonorgestrel releasing intrauterine system as alternative to hysterectomy. BMJ. 1998;316:1122–26. doi: 10.1136/bmj.316.7138.1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid PC, Virtanen-Kari S. Randomised comparative trial of the levonorgestrel intrauterine system and mefenamic acid for the treatment of idiopathic menorrhagia: a multiple analysis using total menstrual fluid loss, menstrual blood loss and pictorial blood loss assessment charts. BJOG. 2005;112:1121–25. doi: 10.1111/j.1471-0528.2005.00642.x. [DOI] [PubMed] [Google Scholar]
- 39.Higham JM, Shaw RW. Clinical associations with objective menstrual blood volume. Eur J Obstet Gynecol Reprod Biol. 1999;82:73–76. doi: 10.1016/s0301-2115(98)00224-3. [DOI] [PubMed] [Google Scholar]
- 40.Committee on Practice Bulletins – Gynecology. Practice bulletin no. 128: diagnosis of abnormal uterine bleeding in reproductive-aged women. Obstet Gynecol. 2012;120:197–206. doi: 10.1097/AOG.0b013e318262e320. [DOI] [PubMed] [Google Scholar]
- 41.Nagrani R, Bowen-Simpkins P, Barrington JW. Can the levonorgestrel intrauterine system replace surgical treatment for the management of menorrhagia? BJOG. 2002;109:345–47. doi: 10.1111/j.1471-0528.2002.01274.x. [DOI] [PubMed] [Google Scholar]
- 42.Elovainio M, Teperi J, Aalto AM, et al. Depressive symptoms as predictors of discontinuation of treatment of menorrhagia by levonorgestrel-releasing intrauterine system. Int J Behav Med. 2007;14:70–75. doi: 10.1007/BF03004171. [DOI] [PubMed] [Google Scholar]
- 43.Gemzell-Danielsson K, Inki P, Boubli L, et al. Bleeding pattern and safety of consecutive use of the levonorgestrel-releasing intrauterine system (LNG-IUS) – a multicentre prospective study. Hum Reprod. 2010;25:354–59. doi: 10.1093/humrep/dep426. [DOI] [PubMed] [Google Scholar]
- 44.Rönnerdag M, Odlind V. Health effects of long-term use of the intrauterine levonorgestrel-releasing system. A follow-up study over 12 years of continuous use. Acta Obstet Gynecol Scand. 1999;78:716–21. [PubMed] [Google Scholar]
- 46.Hurskainen R, Teperi J, Rissanen P, et al. Quality of life and cost-effectiveness of levonorgestrel-releasing intrauterine system versus hysterectomy for treatment of menorrhagia: a randomised trial. Lancet. 2001;357:273–77. doi: 10.1016/S0140-6736(00)03615-1. [DOI] [PubMed] [Google Scholar]
- 47.Lete I, Cristóbal I, Febrer L, et al. Economic evaluation of the levonorgestrel-releasing intrauterine system for the treatment of dysfunctional uterine bleeding in Spain. Eur J Obstet Gynecol Reprod Biol. 2011;154:71–80. doi: 10.1016/j.ejogrb.2010.08.019. [DOI] [PubMed] [Google Scholar]