Abstract
Approximately 3-8 million people in the United States suffer from interstitial cystitis/bladder pain syndrome (IC/BPS), a debilitating condition characterized by increased urgency and frequency of urination, as well as nocturia and general pelvic pain, especially upon bladder filling or voiding. Despite years of research, the cause of IC/BPS remains elusive and treatment strategies are unable to provide complete relief to patients. In order to study nervous system contributions to the condition, many animal models have been developed to mimic the pain and symptoms associated with IC/BPS. One such murine model is urinary bladder distension (UBD). In this model, compressed air of a specific pressure is delivered to the bladder of a lightly anesthetized animal over a set period of time. Throughout the procedure, wires in the superior oblique abdominal muscles record electrical activity from the muscle. This activity is known as the visceromotor response (VMR) and is a reliable and reproducible measure of nociception. Here, we describe the steps necessary to perform this technique in mice including surgical manipulations, physiological recording, and data analysis. With the use of this model, the coordination between primary sensory neurons, spinal cord secondary afferents, and higher central nervous system areas involved in bladder pain can be unraveled. This basic science knowledge can then be clinically translated to treat patients suffering from IC/BPS.
Keywords: Medicine, Issue 86, Bladder pain, electromyogram (EMG), interstitial cystitis/bladder pain syndrome (IC/BPS), urinary bladder distension (UBD), visceromotor response (VMR)
Introduction
Chronic pain is officially defined as pain that persists for approximately three months, or for longer than the normal tissue healing time1. This type of pain is one of the main reasons that people are driven to seek medical attention2 and can cost up to $635 billion dollars annually3. Current chronic pain coping strategies are considered archaic; after decades of medical advancements, NSAIDs (nonsteroidal anti-inflammatory drugs) and opioids are still the primary treatments prescribed by physicians. However these treatments target all different types of pain in the same way, providing analgesic effects all over the body as opposed to specifically focusing on the cause of that exact pain incident. In order to better help those suffering from chronic pain, research attention should be shifted towards the etiology and possible pain-specific treatments associated with the most common causes of chronic pain. The goal of this manuscript is to describe a model used to better understand a condition known as Interstitial Cystitis/Painful Bladder Syndrome (IC/BPS).
IC/BPS is a debilitating condition that affects millions of people, primarily women over the age of 40 4. The exact causes of IC/BPS are not known, however prevalence has been linked to genetics5, specific diets, and high stress levels6. Symptoms of IC/BPS include but are not limited to: Increased urgency to urinate, increased frequency of urination, aching, piercing, or burning pain during bladder filling and voiding, and nocturia7. Patients experiencing these conditions have higher stress levels and are more anxious8. This increased stress results in increased pain, which augments the original stress levels. Studies have shown that depression and anxiety levels decrease following treatments that relieve urinary pain, thus effectively breaking this positive-feedback cycle9. One of the challenges in discovering new treatments for IC/BPS has been the paucity of a basic science understanding of bladder pain.
In order to study nervous system contributions to the condition, many animal models have been developed to mimic the pain and symptoms associated with IC/BPS. Traditionally, cystitis has been recreated in animals with the introduction of various chemicals such as mustard oil, acetone, lipopolysaccharide, hydrochloric acid, and cyclophosphamide into the bladder. However no foreign agents are present in the sterile urine of IC/BPS patients, thus questioning the validity of these models. Another murine model of urological pain is urinary bladder distension (UBD)10. In this model, compressed air of a specific pressure is delivered to the bladder of an awake or lightly anesthetized animal over a set period of time. Throughout the procedure, wires in the superior oblique abdominal muscles record electrical activity from the muscle (with an electromyogram (EMG)). This activity is known as the visceromotor response (VMR) and is reliable, reproducible measure of nociception10. Similar hollow organ distention (e.g. bladder, rectum) in healthy human volunteers produces feelings of discomfort and significant increases in reported pain11,12, traits that are often used to diagnose IC/BPS. Thus, in conjunction with electrical, pharmacological, and optogenetic methods of stimulation or inhibition, UBD is a useful and valid model for understanding both the peripheral and central nervous system components of bladder nociception and pain.
Here, we describe the procedure for UBD as is currently used in our laboratory within female members of the common mouse strain, C57Bl/6J. Due to difficulties in the catheterization process of males, this procedure is primarily performed in female mice. This protocol has been adapted from that developed by Ness et al.10 and previously published from our lab13. The following protocol describes four primary components of this model in anesthetized animals: (1) bladder catheter and recording electrode surgery (2) partial anesthesia induction (3) bladder distention and VMR recording, and (4) data analysis of raw VMR/EMG traces. Subtle differences in the UBD-VMR procedure due to choice of organism, anesthesia depth and induction, and body temperature are discussed below.
Protocol
The following protocol has been approved by the Institutional Animal Care and Use Committee at Duquesne University and is consistent with guidelines from the National Institutes of Health.
1. Surgical Implantation of Wire Electrodes and Urinary Bladder Catheter Using Sterile Techniques (total time 5-10 min)
- The following items should be prepared prior to starting surgery:
- Actively monitor and adjust body temperature to 37.5±0.5 °C using temperature monitoring system with a battery-operated heating pad and animal body temperature probe (Materials List) Connect automatic system to a low-wattage heat lamp for additional control of body temperature.
- Equipment needed for isoflurane anesthesia includes compressed air (e.g. 100% O2), isoflurane vaporizer, isoflurane, isoflurane induction chamber (for initial knockdown), nose cone, and exhaust system.
- Assemble the proper surgical instruments, IV catheter, and electrodes (Materials List)
Induce anesthetic state in induction chamber at 2-3% isoflurane. Remove mouse from chamber when righting reflex is lost (0-1 min).
Place mouse in nose cone with 2-2.5% isoflurane. Pinch toe to check for full anesthesia. There should be no response from the animal. Start timer.
Place 1 drop of ophthalmic ointment on each eye of mouse.
Turn mouse over so dorsal side is facing down.
Insert catheter through urethra and into bladder.
- Dip catheter in surgical lube to lubricate catheter.
- Gently hold urethral opening with pointed forceps perpendicular to animal’s body. Holding catheter with the 2nd set of forceps, gently insert catheter end into urethra at an angle perpendicular to animal’s body.
- When catheter has gone into urethra ~1-2 mm, gently adjust catheter so that it is now parallel to the body aiming toward the head. This movement is necessary to avoid the pubic bone.
- Gently push catheter toward the mouse’s body. The catheter should slide into the body cavity smoothly and without resistance. Do not force catheter. If catheter does not enter smoothly, return to the perpendicular catheter position and start again.
- With catheter fully inserted in the bladder, gently push down on the mouse’s abdomen to expel bladder contents. Remove all urine from catheter by pushing a small piece of paper towel into catheter opening. When paper towel is soaked with urine, replace. Remove urine continuously throughout the remaining protocol including during bladder distention experiments (see Protocol 3 below.)
- Insert two silver wires into left abdominal muscles and one silver wire into chest (as a ground.)
- Use alcohol or iodine to disinfect area lateral to the right of catheter (animal’s left side of body.)
- Expose left abdominal muscle.
- Hold skin near left abdominal muscle with forceps. Make a small 1-2 cm incision with medium surgical scissors.
- Insert tip of scissors into incision and gently expand incision to 2 cm.
- Use forceps to move overlying fascia to expose superior oblique abdominal muscle. Be careful not to cut/rip any of the major blood vessels that are located in this region.
- Bend three silver wires in half. Use sharp 21 G needle to take a small bite of the muscle. Then, push needle through muscle. When inserting in muscle be careful not to puncture any internal organs or induce unnecessary damage to the muscle.
- Stick a free end of one silver wire into the opening of the 21 G needle. Gently pull needle back through the muscle to draw the silver wire through the muscle bite. When needle is free from muscle, gently pull silver wire through muscle until the loop in the wire is flush with the muscle bite.
- Repeat steps 1.7.3-1.7.4 for second muscle wire. This wire should be placed ~0.5 cm from the first muscle wire. It is important that the two wires do not touch after insertion.
- Insert the third silver wire (ground) in the chest inferior to the heart.
- Use sharp 21 G needle to take a small bite of skin (it is not necessary to make an incision or shave the skin) and push needle through the skin.
- Insert silver wire as described above and pull through skin.
Place a small amount of 37.5 °C sterile mineral oil over the exposed muscles to keep them moist and pull skin over as much exposed muscle as possible.
Inject 0.5 ml saline subcutaneously to help maintain fluid levels during subsequent steps.
Gently roll animal onto side so that body temperature can be easily maintained.
2. Partial Anesthesia Step-down Procedure (total time ~75 min)
The following anesthesia protocol has been approved by the Institutional Animal Care and Use Committee at Duquesne University and is consistent with guidelines from the National Institutes of Health.
Immediately after Section I (above) is complete, lower isoflurane to 1.5%. Hold at this level for 15 min.
- Lower isoflurane such that the animal is partially anesthetized.
- Lower isoflurane by 0.125% every 15 min until animal exhibits a flexion response to toe pinch but does not vocalize or otherwise move.
- Typically, a level of 0.8-1.0% isoflurane is optimal, however levels can vary due to differences in anesthesia setups. Vocalization and/or ambulation are extremely rare events when anesthesia is at the proper level.
3. Electromyogram (EMG) Recording During Bladder Distension with Graded Pressures (15-75 mmHg) (total time – variable depending on experiment)
- Setup the following items for EMG recording and bladder distention:
- Setup an amplifier, a digitizer, and recording software. In this setup, the digitizer has one input for the amplifier and two inputs for the pressure regulator (pressure and stimulus marker inputs, see below section 3.1.2). Exact hardware and software setup is customizable (see Materials List for specific instructions).
- Setup a system to deliver specific bursts of air pressure to animal. This system is referred to as the “timed pressure regulator” in the following protocol. It allows for automated pressure delivery including digitization of air pressure, control of trial length, inter-trial interval automation, control of trial number, and stimulus onset digital signal (see Anderson et al.14 for schematic of a timed pressure regulator).
Connect all lead wires from mouse (2 abdominal wires + 1 ground wire) to the amplifier and the digitizer and begin recording EMG signal in digitizer software. Note what time “Write” is selected in digitizer software.
Remove paper towel from bladder catheter. Connect catheter to air tubing (from timed pressure regulator.)
- Deliver a single 20 sec trial at 60 mmHg distention pressure.
- Set the flow regulator to 60 mmHg (check pressure using an analog sphygmomanometer.)
- Set the timed pressure regulator to deliver a 20 sec prepressure interval (this will be the 2V stimulus signal recorded in digitizer software) followed by a 20 sec pressure distention pulse.
- Start the trial (prepressure interval + pressure distention trial). When the actual pressure trial begins, double check the pressure using the 3 sphygmomanometers connected to the system.
- During the pressure distention trial, a strong EMG signal (>0.5 V) should be observed during and possibly after the 20 sec distention. The animal may exhibit abdominal movement but the animal should not vocalize or otherwise move. Although rare, if abnormal movements or vocalizations are observed, increase the isoflurane level.
If the 1st 60 mmHg distention produces an appropriate EMG signal, repeat step 3.4 two more times with an intertrial interval of 1-2 min.
- Upon receiving strong signals from the three 60 mmHg trials, perform experimental distentions.
- Many studies use graded distensions starting at low pressure (10-15 mmHg) and working up to noxious pressures (75-80 mmHg) in 10-15 mmHg increments. See representative results below for example traces at different pressures.
- Typical trials include a 20 sec prepressure interval, a 20 sec pressure distention trial, and a 1-2 min intertrial interval.
- Perform three distentions at each pressure and then average across the three trials (to obtain a single visceromotor response for each pressure). In an alternative method, perform five distensions at each pressure, discard the high and low trials, and then average the remaining three responses.
Once experimental distensions are complete, stop recording, save the experiment file, and sacrifice animal using approved methods.
4. Analysis of Raw EMG Traces
Analysis of visceromotor responses (i.e. EMG recordings) are easily done using on-board digitizer software or through a third-party program (Materials List) The following instructions are general instructions that could be used to analyze this data using multiple programs or using manual analysis.
- To export EMG, pressure, and stimulus signal data from digitizer software do the following:
- Open experimental file.
- Click “File” “Export as.”
- Change the file type to “spreadsheet text (.txt),” name the file and click “Save.”
- In the pop-up window, change the output sample rate to “1000,” change the start time to the moment data collection began and change the end time to “MaxTime().” Select the box marking “time shift output so first line is a 0.0 sec,” and click “OK.”
- The data will now be exported as a text file that can be opened in a variety of programs. The file should contain four columns (Timer, VMR (EMG), Pressure, Stimulus).
- For each experimental animal tested, perform the following mathematical operations.
- Rectify EMG signal for the entire data set by calculating the absolute value of each time point.
- Determine the average EMG response during the background section of the experiment.
- Subtract this average background EMG response from every rectified data point in the experiment to obtain the background corrected data set.
- Determine the area under the curve (AUC) for each 20 sec prepressure interval in the background corrected data set.
- Determine the AUC for each 20 sec pressure distension trial in the background corrected data set.
- Divide each pressure distension trial AUC (obtained in step 4.3.5) by the lowest prepressure interval AUC from the whole experiment (obtained in step 4.3.4.). This is the normalized and background corrected AUC for each complete pressure distention trial.
For each experimental animal, average multiple normalized trials from a single pressure to obtain the normalized VMR in Volts*sec (Vsec).
Perform the appropriate statistics on the data depending on the experimental design.
Representative Results
An illustration of the overall UBD-VMR setup can be seen in Figure 1A. Increasing pressure steps induce an increase in the raw VMR (i.e. EMG) (Figure 1B.) The raw VMR, pressure, and stimulus marker signal that should be observed during recording using digitizer software can be seen in Figure 2. The top channel seen in Figure 2 is the EMG trace (in V). The background signal of the EMG trace should be steady and low amplitude (<0.2 V). If background is high or contains sporadic large spikes (>0.5 V) the wires and amplifier settings may need to be adjusted. In some circumstances wrapping wires (from mouse to amplifier and/or amplifier to digitizer) in foil can reduce some noise. The middle channel in Figure 2 shows the pressure signal as quantified by the timed pressure regulator in mmHg. Note that the signal onset is a curve. This occurs because full distention of the bladder is not instantaneous. Once the pressure signal reaches the maximum value for that trial (e.g. 75 mmHg in Figure 2), the pressure should remain at that maximum value. On occasion, air can leak out of the urethra around the catheter. This happens most often at high pressures (e.g. >60 mmHg), can be observed by a change in the amplitude of the pressure signal, and can be reduced by adding a small amount of surgical lubricant around the urethral opening. The final channel (bottom of Figure 2) shows the stimulus marker channel. The stimulus marker channel shows two separate signals. At the start of the prepressure interval there is a momentary 2 V signal marker. This signal can be used to automate the data analysis process for a given experiment. Specifically, one can program a data analysis system to scan through a data file looking for stimulus markers that rise above 1.5 V. This allows the data analysis system to quickly find each complete trial. The second component of the stimulus channel is a 1 V signal that occurs during the entire pressure distention trial. Again, the presence of this second signal can be used for easy data analysis.
Representative data from a single experimental animal can be seen in Figure 3 and Table 1. The animal was distended three times at each of the following pressure: 15, 30, 45, 60, and 75 mmHg (Figure 3A; Table 1.) The three distentions are averaged and represented as a single data point in the graph. Notice that the normalized signal increases gradually with increasing bladder distention pressures. Following this initial distension set, this animal received two additional sets of graded bladder distention. As seen in Figure 3B, the second and third sets of distentions produced similar VMRs (at each pressure.) These three sets of distentions occurred over a 2.5 hr period demonstrating the stability of the recording setup and the reproducibility of the UBD stimulus and VMR response in a single animal. Importantly, the long-term stability of this prep only occurs when the isoflurane anesthesia is gradually stepped-down over the course of 1 hr. A shorter anesthesia procedure can lead to a progressive loss in the UBD VMR signal with each set of distentions (unpublished observations, Sadler and Kolber.) Another critical factor in the stability of the UBD VMR system is the maintenance of a stable body temperature. As seen in Figure 4, the UBD VMR (in response to 60 mmHg distention) is significantly lower at 33.5 °C body temperature compared to 37.5 °C.
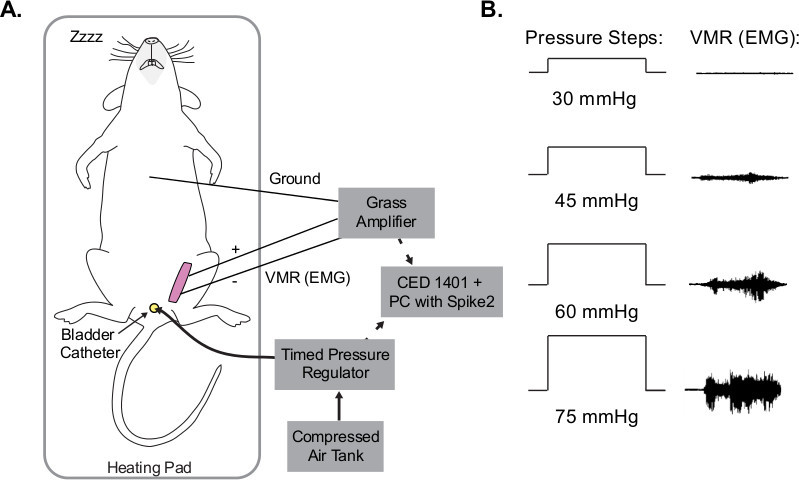 Figure 1. Visceromotor responses (VMR) from UBD. (A) Schematic of UBD setup. Compressed air is delivered into bladder via urethral catheter. During distensions, electrodes in abdominal muscle record EMG. Throughout the entire procedure, temperature is maintained using a battery-operated heating pad and overhead lamp. (B) Example EMG traces during UBD. As pressure increases, electrical output from abdominal muscles increases congruently.
Figure 1. Visceromotor responses (VMR) from UBD. (A) Schematic of UBD setup. Compressed air is delivered into bladder via urethral catheter. During distensions, electrodes in abdominal muscle record EMG. Throughout the entire procedure, temperature is maintained using a battery-operated heating pad and overhead lamp. (B) Example EMG traces during UBD. As pressure increases, electrical output from abdominal muscles increases congruently.
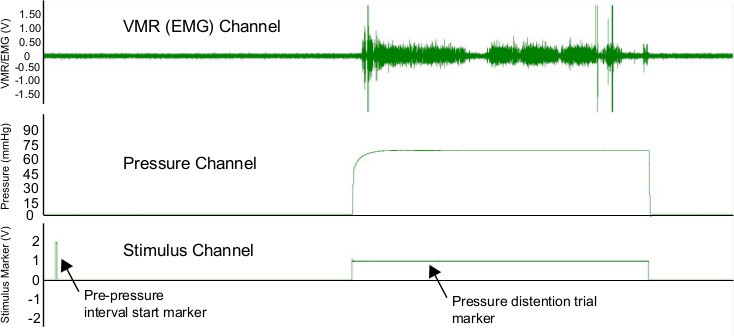 Figure 2. Screen shot showing data during UBD trial. Three channels are shown. The top channel shows the raw EMG trace (in V.) The middle channel shows the pressure (in mmHg) that is being delivered to the bladder. The bottom trace shows the stimulus marker channel that indicates the start of the prepressure interval and the entire pressure distention trial. Image is for a 40 sec complete trial (20 sec prepressure interval plus 20 sec pressure distention trial.)
Figure 2. Screen shot showing data during UBD trial. Three channels are shown. The top channel shows the raw EMG trace (in V.) The middle channel shows the pressure (in mmHg) that is being delivered to the bladder. The bottom trace shows the stimulus marker channel that indicates the start of the prepressure interval and the entire pressure distention trial. Image is for a 40 sec complete trial (20 sec prepressure interval plus 20 sec pressure distention trial.)
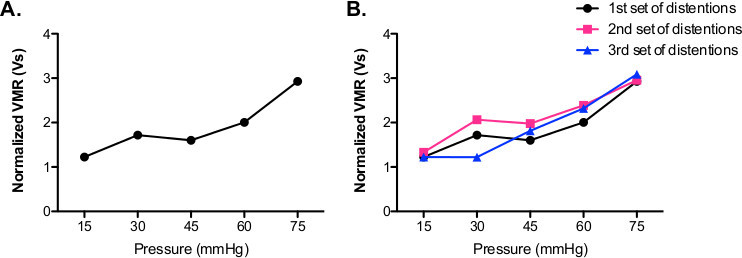 Figure 3. Representative data showing graded VMR. (A) The normalized VMR increases gradually with increasing pressure (15-75 mmHg) delivered to the bladder of the mouse. (B) Subsequent sets of bladder distentions (15-75 mmHg = 1 set) show similar VMRs compared to first set of distentions.
Figure 3. Representative data showing graded VMR. (A) The normalized VMR increases gradually with increasing pressure (15-75 mmHg) delivered to the bladder of the mouse. (B) Subsequent sets of bladder distentions (15-75 mmHg = 1 set) show similar VMRs compared to first set of distentions.
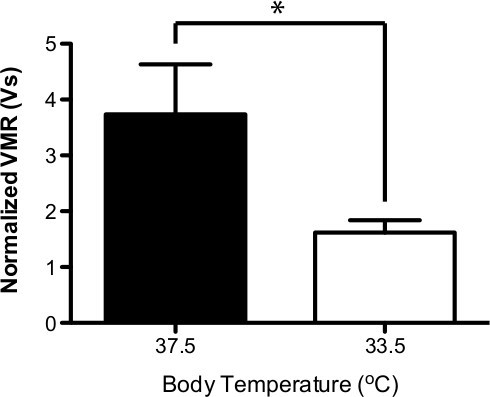 Figure 4. Body temperature decreases UBD VMR. A reduction in body temperature from 37.5 °C to 33.5 °C causes a significant decrease in the UBD VMR (n=6; paired t-test * P<0.05.)
Figure 4. Body temperature decreases UBD VMR. A reduction in body temperature from 37.5 °C to 33.5 °C causes a significant decrease in the UBD VMR (n=6; paired t-test * P<0.05.)
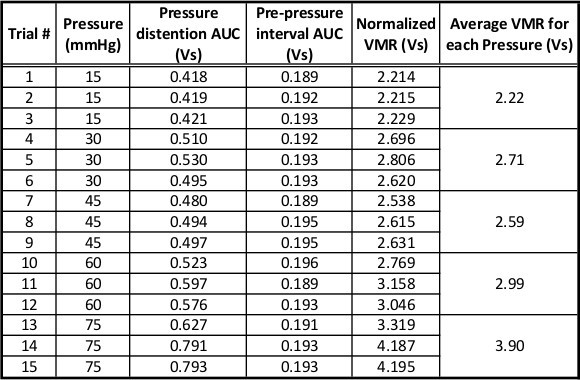 Table 1. Representative data for a full set of distentions. Table shows data including pressure distention AUC, prepressure interval AUC, background corrected pressure distention AUC, and normalized pressure distention AUC. For each pressure, the animal received three distention trials. The average of the normalized pressure distention AUC at each pressure is plotted in Figure 3A.
Table 1. Representative data for a full set of distentions. Table shows data including pressure distention AUC, prepressure interval AUC, background corrected pressure distention AUC, and normalized pressure distention AUC. For each pressure, the animal received three distention trials. The average of the normalized pressure distention AUC at each pressure is plotted in Figure 3A.
Discussion
In humans, interstitial cystitis/bladder pain syndrome (IC/BPS) represents a significant clinical problem because patients have debilitating pain that is often unresponsive to regular pain treatment15. One of the major challenges in understanding and ultimately treating chronic bladder pain is to understand the normal neural processes that are involved in the response to noxious bladder distention. In order to overcome this challenge, an animal model of bladder pain is necessary. This model should be reproducible, stable, and easily measured. Fortunately, Ness and colleagues10 developed a system that can be used to investigate the physiological responses to noxious bladder distention.
System setup is straightforward and can be done using primarily off-the-shelf hardware and software. The major custom piece of equipment involved in the UBD VMR system is the timed pressure regulator. This machine provides an analog-to-digital conversion of air pressure and sends stimulus onset markers to the digitizer hardware. The use of the timed pressure regulator greatly simplifies data analysis by automatically syncing the pressure stimulus with the recorded EMG (i.e. VMR) This is important because the VMR does not necessarily start precisely when the bladder is first distended and the VMR is often maintained for a short period (<5 sec) following the end of the distention. Nonetheless, with careful synchronization, one could easily perform the UBD VMR procedure with an on/off regulator to control distention on/off and a timer that is in sync with the recorded EMG signal.
A major advantage of the UBD VMR system for interrogating the nervous system role in bladder pain is that responses to graded bladder distention pressures fall along a standard curve with increasing pressures inducing greater VMRs. This allows the researcher to easily determine if an experimental manipulation causes an increase or decrease in the VMR at both innocuous and noxious bladder distention pressures. In addition, the signal from a single animal is very stable across time so that multiple rounds of graded distention can be done (Figure 3B.) This allows researchers to obtain a baseline UBD VMR and then manipulate the animal (e.g. deliver a drug) for within-subject matching13. Researchers have used the UBD-VMR system (in mice and rats) to record or manipulate spinal cord dorsal horn neurons16 and other neurons in the central nervous system (e.g. rostral ventral medulla17 or amygdala13,16.) These studies and others continue to add to our basic scientific understanding of the role of various components of the nervous system in the processing of bladder sensory input and ultimately the subjective pain felt from this nociceptive input. While the UBD VMR system includes many experimental advantages there are some limitations to the model. First, while visceral bladder distention evokes subjective feelings of “pain” in humans it is impossible to know if abdominal EMG recordings in mice are truly representative of pain. Nonetheless, UBD VMR responses are inhibited by common analgesics suggesting that the VMR is a pain-like response. Second, in the present UBD-VMR system, the animals are anesthetized during recording. Although a comparison of live versus anesthetized UBD VMR has validated the use of anesthesia10, there are possible unknown effects of isoflurane on pain-like responses. Third, the described protocol is not a survival surgery so multiple recordings of individual mice over time are not possible. Nonetheless, use of the UBD-VMR model as well as other visceral pain systems will allow for a more complete understanding of visceral pain and will lead to new therapeutic avenues for patients suffering from IC/BPS and other visceral pain conditions.
Disclosures
The authors have nothing to disclose.
Acknowledgments
We would like to acknowledge Drs. Robert Gereau, Henry Lai, and Lara Crock for their helpful assistance in setting up this system. We would also like to acknowledge funding sources for this work (BJK – International Association for the Study of Pain Early Career Research Grant by the Scan|Design Foundation by INGER & JENS BRUUN and the Duquesne University Hunkele Dreaded Disease Fund).
References
- Merskey H, Bogduk N. Classification of Chronic Pain. IASP Press; 1994. [Google Scholar]
- Gureje O, Korff MV, Simon GE, Gater R. Peristent pain and well-being. A World Health Organization study in primary care. J. Am. Med. Assoc. 1998;280:147–151. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- Gaskin DJ, Richard P. The economic costs of pain in the United States. J. Pain. 2012;13:715–754. doi: 10.1016/j.jpain.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Berry SH, et al. Prevalence of Symptoms of Bladder Pain Syndrome/Interstitial Cystitis Among Adult Females in the United States. J. Urol. 2011;186:540–544. doi: 10.1016/j.juro.2011.03.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunitsky E, et al. Bladder Pain Syndrome/Interstitial Cystitis in twin sisters. J. Urol. 2012;187:148–152. doi: 10.1016/j.juro.2011.09.051. [DOI] [PubMed] [Google Scholar]
- Tettamanti G, et al. Influence of smoking, coffee, and tea consumption on Bladder Pain Syndrome in female twins. Urology. 2011;77:1313–1317. doi: 10.1016/j.urology.2010.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren JW, et al. In: Harrison's Principles of Internal Medicine. Longo DL, et al., editors. New York: McGraw-Hill; 2012. [Google Scholar]
- Baldoni F, Ercolani M, Baldaro B, Trombini G. Stressful events and psychological symptoms in patients with functional urinary disorders. Perceptual Motor Skills. 1995;80:605–606. doi: 10.2466/pms.1995.80.2.605. [DOI] [PubMed] [Google Scholar]
- Tannenbaum C, Gray M, Hoffstetter S, Cardozo L. Comorbidities associated with bladder dysfunction. Int. J. Clin. Practice. 2013;67:105–113. doi: 10.1111/ijcp.12085. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Elhefni H. Reliable visceromotor responses are evoked by noxious bladder distention in mice. J. Urol. 2004;171:1704–1708. doi: 10.1097/01.ju.0000116430.67100.8f. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Richter HE, Varner RE, Fillingim RB. A psychophysical study of discomfort produced by repeated filling of the urinary bladder. Pain. 1998;76:61–69. doi: 10.1016/s0304-3959(98)00023-2. [DOI] [PubMed] [Google Scholar]
- Ness TJ, Gebhart GF. Visceral pain: a review of experimental studies. Pain. 1990;41:167–234. doi: 10.1016/0304-3959(90)90021-5. [DOI] [PubMed] [Google Scholar]
- Crock LW, et al. Central amygdala metabotropic glutamate receptor 5 in the modulation of visceral pain. J. Neurosci. 2012;32:14217–14226. doi: 10.1523/JNEUROSCI.1473-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RH, Ness TJ, Gebhart GF. A distension control device useful for quantitative studies of hollow organ sensation. Physiol. Behav. 1987;41:635–638. doi: 10.1016/0031-9384(87)90322-2. [DOI] [PubMed] [Google Scholar]
- Dimitrakov J, et al. Pharmacologic Management of Painful Bladder Syndrome/Interstitial Cystitis: A Systematic Review. Arch. Intern. Med. 2007;167:1922–1929. doi: 10.1001/archinte.167.18.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin C, Van Meerveld BGreenwood-, Foreman RD. Spinal neuronal responses to urinary bladder stimulation in rats with corticosterone or aldosterone onto the amygdala. J. Neurophysiol. 2003;90:2180–2189. doi: 10.1152/jn.00298.2003. [DOI] [PubMed] [Google Scholar]
- Randich A, Mebane H, DeBerry JJ, Ness TJ. Rostral ventral medulla modulation of the visceromotor reflex evoked by urinary bladder distension in female rats. J. Pain. 2008;9:920–926. doi: 10.1016/j.jpain.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]


