Abstract
Background
70 years ago, it was put forward that the diseased liver was not a favorable soil for metastatic tumor cells. In addition, a few studies have demonstrated that rare occurrence of colorectal liver metastases among patients with fatty liver, cirrhosis or chronic hepatitis B and C virus infection. We performed a meta-analysis to verify the association between the incidences of colorectal liver metastases with chronically diseased livers.
Methods
Relevant studies were identified by a search of electronic database PubMed, Cochrane Library, OVID, Web of Science and CNKI (up to February 24, 2014). Pooled odds ratio (OR) with 95% confidence interval (CI) was calculated using random- or fixed-effect models when appropriate. Meta-analysis and publication bias (Bgger's test) was evaluated with STATA 12.0.
Results
A total of 10,349 colorectal cancer patients from 10 studies were included. The meta-analysis result showed there was a significant difference in the incidences of colorectal liver metastases between patients with normal and chronically diseased livers (OR = 0.32; 95% CI 95%: 0.26–0.38, P = 0.000 fixed-effects model). The result of Begg's test (Pr>|z| = 0.089; P>0.05) revealed no publication bias.
Conclusions
The results of this meta-analysis demonstrated that patients with chronically diseased livers had significantly lower incidences of colorectal liver metastases than those with normal livers.
Introduction
Colorectal cancer (CRC) is a high morbidity malignancy tumor over the world, which is predicted will account for 8% of new cancer cases in the United States in 2014 [1]. Tumor recurrence and distant metastasis are the main causes of death in CRCs [2]. The liver is the most common site for metastatic colorectal cancer. It was reported that approximately 40% of advanced colorectal cancer patients developed liver metastases [3], [4]. In 1942, Lisa et al [5] reported that the cirrhotic liver was not a favorable soil for metastatic tumor cells. They found that, in 782 autopsy cases with malignant tumors, there were only 6 cases with metastatic cancer in cirrhotic liver. A theory for this phenomenon was that an inappropriate environment for the transplanted tumor cells was formed by the diseased liver; this meant the “soil” was not favorable for the “seed” to grow [5].
During the past twenty years, several studies have discovered a low incidence of colorectal liver metastases in patients with liver diseases, including fatty liver [6], cirrhosis [7] and chronic hepatitis B and C virus infection [8]. However, the precise reason of the above findings is still unclear. Since only few articles reported this issue, we collected all relevant articles and carried out a meta-analysis to compare the incidences of colorectal liver metastases in normal and chronically diseased livers.
Methods
Literature collection
We collected the potentially relevant studies through a search in electronic database PubMed, Cochrane Library, OVID, Web of Science and Chinese National Knowledge Infrastructure (CNKI). Chronically diseased liver included cirrhosis, fatty liver and hepatitis virus infection. The keywords used for the search including “colorectal cancer”, “diseased liver”, “hepatitis”, “fatty liver”, “cirrhosis” and “liver metastasis”. All non-English articles were translated in English and then analyzed. The latest search was updated on February 24, 2014.
Inclusion and exclusion criteria
Inclusion criteria: (1) studies evaluating the association between colorectal liver metastases and chronically diseased livers; (2) only patients with advanced colorectal cancer were included in the study, because early-staged colorectal cancers such as a lesion confined to the mucosa or submucosa rarely metastasized to the liver; (3) all patients were closely followed up; (4) containing useful figures including numbers of the two groups (diseased liver group and normal liver group) and numbers of liver metastases in each group. Exclusion criteria: (1) animal studies, pharmaceutical researches, case reports; (2) reports without usable data; (3) duplicate publications.
Date extraction
Two investigators (CB and LK) extracted data from eligible studies independently, according to the inclusion and exclusion criteria above. For disagreements, a consensus was reached by a third investigator (ZS). The following information was collected from each study: first author, publication date, country of origin, ethnicity, type of liver diseases, number of each group (diseased liver group and normal liver group), and number of liver metastases in each group.
Statistical methods
Meta-analysis was performed by using Stata 12.0 software (Stata Corporation, College Station, TX, USA). To determine the statistical heterogeneity of the studies, Chi-square-based Q test and I2 statistics were used. For the Q test, A P value less than 0.05 indicated significant heterogeneity; for the I2 statistics, an I2 value greater than 50% was considered severe heterogeneity. The potential publication bias was assessed using a “funnel plot” and the Begg's test. The fixed-effects model was adopted in the initial calculation of odds ratio with corresponding 95% CIs. If there was a significant statistical heterogeneity among the studies, the random-effects model was applied for the analysis. By comparing the incidences of colorectal liver metastases in normal and chronically diseased livers, we tried to explore the impact of liver diseases on colorectal liver metastases.
Results
Studies characteristics
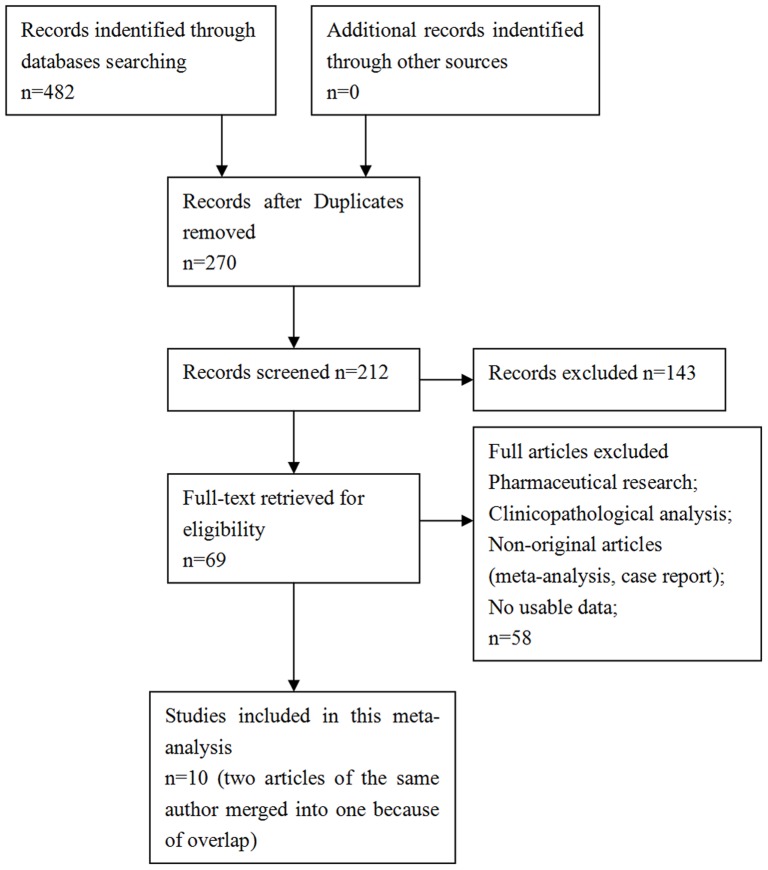
A total of 10 retrospective studies published between 1992 and 2013 were identified as eligible according to the selection criteria Figure 1 . Five studies evaluated patients from China, four evaluated patients from Japan and one evaluated patients from Italy [6]–[16]. These studies included a total of 10,349 patients. The overall incidence of colorectal liver metastases in diseased liver groups was 8.92% (138/1547), and in normal liver groups it was 21.445% (1887/8802). All the diagnoses of colorectal cancer relied on pathology, all the judgements of synchronous or metachronous liver metastases were confirmed by biopsy or diagnostic imaging (CT, MRI or PET-CT). All the diagnoses of hepatitis infection were based on serological tests of hepatitis virus, including HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc. The patients who had one or more items positive in the hepatitis tests were considered as the chronic hepatitis virus infection group. The diagnoses of liver cirrhosis had no unified standard. Some were according to the assessment of a combination of laboratory parameters (serum bilirubin, serum albumin, prothrombin time international normalized ratio) and clinical parameters (encephalopathy and ascites). Some were based on histological diagnosis or ultrasound/CT scan. The diagnoses of fatty liver were based on ultrasound/CT scan. The main characteristics were summarized in Table 1 .
Figure 1. Flowchart presenting the steps of literature search and selection.
Table 1. Main characteristics of the eligible studies.
| First author | Year | country | Ethnicity | types of liver diseases | Total number | Diseased liver | Diseased liver with liver metastases | Non-diseased liver | Non-diseased liver with livermetastases |
| Uetsuji | 1992 | Japan | Asian | Cirrhosis | 250 | 46 | 0 | 204 | 40 |
| Hayashi | 1997 | Japan | Asian | Fatty liver | 839 | 121 | 2 | 718 | 115 |
| Utsunomiya | 1999 | Japan | Asian | Hepatitis infection | 438 | 37 | 3 | 401 | 85 |
| Song | 2001 | China | Asian | Hepatitis infection | 512 | 74 | 10 | 438 | 119 |
| Iasome | 2005 | Italy | Caucasian | Hepatitis infection/cirrhosis/fatty liver | 834 | 291 | 28 | 543 | 174 |
| Qian | 2010 | China | Asian | Hepatitis infection/cirrhosis | 2352 | 140 | 11 | 2212 | 517 |
| Qiu | 2011 | China | Asian | Hepatitis infection | 1298 | 332 | 47 | 966 | 272 |
| Wang | 2012 | China | Asian | Hepatitis infection | 354 | 70 | 2 | 284 | 48 |
| Zeng | 2013 | China | Asian | Hepatitis infection | 2868 | 373 | 33 | 2495 | 465 |
| Murono | 2013 | Japan | Asian | Fatty liver | 604 | 63 | 2 | 541 | 52 |
Meta-analysis Results
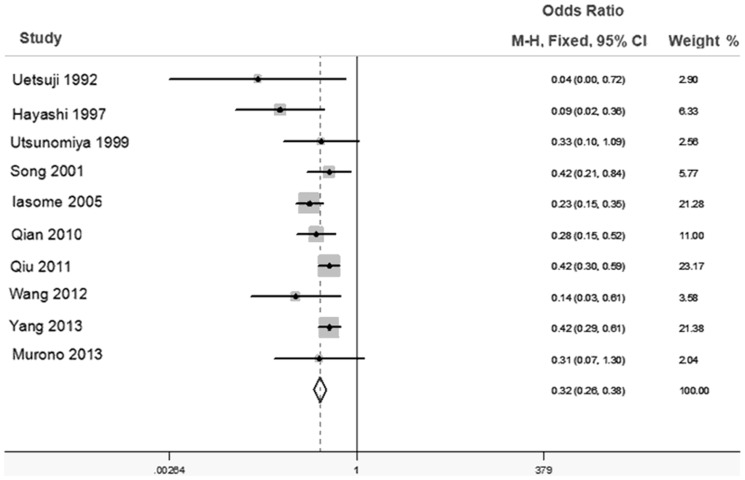
As Figure 2 showed there was a moderate heterogeneity among the trials (I2 = 37.4% P>0.05), and then the fixed-effects model was adopted. The odds ratio, expressed as diseased liver group versus normal liver group, was 0.32 (95% CI: 0.26–0.38, P = 0.000 fixed-effects model). Through comparing the incidences of colorectal liver metastases between the diseased liver group and normal liver group, we found there was a significant difference in the incidences of colorectal liver metastases between the two groups. This result demonstrated that patients with chronically diseased livers had significantly lower incidences of colorectal liver metastases than those with normal livers.
Figure 2. Forest plot for the association between incidences of colorectal liver metastases with chronically diseased liver.
Forest plot of OR was assessed for the differences of incidence of colorectal liver metastases between the diseased liver group and normal liver group (OR = 0.32, 95%CI = 0.26–0.38 fixed effects model).
Publication bias
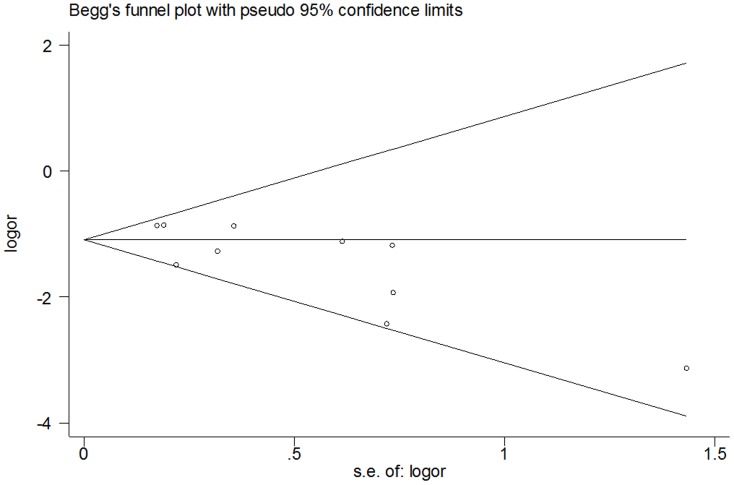
Figure 3 showed the Begg's funnel plot with pseudo 95% CIs. No significant publication bias was observed. Bias was assessed statistically using the Begg's test; still, the results of Begg's test revealed no publication bias (Pr>|z| = 0.089; P>0.05).
Figure 3. Funnel plot analysis of potential publication bias.
Discussion
This meta-analysis showed that colorectal cancer patients with chronically diseased livers had significantly lower incidences of colorectal liver metastases than those with normal livers. The reason for this phenomenon is still unknown. Studies have shown that clinicopathological characteristics of primary colorectal cancers were not altered by viral hepatitis [8], [13], suggesting that the different incidences of colorectal liver metastases between the diseased liver group and normal liver group were not caused by variation of pathological factors in primary cancers.
Karube [17] found the pyrimidine nucleoside phosphorylase (PyNPase) activity and the microvessel density in the metastatic liver lesion in fatty liver rats were significantly lower than non-fatty liver rats. They suggested that the decreased activity of PyNPase and neovascularization in the metastatic lesion were closely related to fewer liver metastases. In addition, evidences showed that disorders of fat metabolism, such as increased omega-3 fatty acids and medium-chain triglycerides in the liver cells inhibited tumor cell proliferation and angiogenesis, which could prevent the growth of tumor cells [18].
Cirrhosis, as defined by the World Health Organization (WHO), was “a diffuse process characterized by fibrosis and conversion of normal liver architecture into structurally abnormal nodules” [19]. In cirrhotic livers, Kupffer cells were activated and released constitutive amount of proinflammatory factors, such as tumor necrosis factor-α (TNF-α) and interleukin −l (IL-l). Song et al [20] investigated the influence of activated Kupffer cells from cirrhotic rat livers on hepatic colonization and FasR-mediated apoptosis of colon cancer cells. They found Kupffer cells in cirrhotic livers sensitized metastatic colon cancer cells to FasR mediated apoptosis by up-regulating their expression of Fas receptors, which thus prepared the malignancies to be eliminated by tumour-infiltrating lymphocytes. Hence, activation of Kupffer cells during hepatic cirrhosis on one hand resulted in tissue damage and fibrogenesis in livers, but on the other hand inhibited the hepatic matastasis formation of colon cancers.
Seitz [21] reported that high metalloproteinase inhibitor contents and especially altered lectins or lectin binding sites in cirrhosis of the liver might help to explain the rare event “metastasis in cirrhosis”. Pathophysiological pathway of cirrhosis underwent through the process of extracellular matrix remodeling leading to new collagen formation and deposition [22]. Matrix metalloproteinases (MMPs) and their tissue inhibitors (TIMPs) played important roles in the process of matrix degrading and remodeling. In the process of fibrosis, the overall MMP activity decreased, due to increased expression of TIMPs and other anti-proteases expressed by hepatic stellate cells and hepatocytes. Therefore, increased expression of TIMPs may have inhibitory role in the process of colonization and formation of colorectal metastasis in chronically injured liver. In addition, cirrhosis was associated with increased intrahepatic resistance to portal flow. Mittal et al [23] demonstrated that there was a significant fall in peak venous velocity (PVV) with the increasing severity of the grade of cirrhosis. Moreover, a reversed flow in the portal venous system was observed in cirrhotic patients. These hemodynamic events were responsible for the progressive fall in the portal venous velocity with an increasing severity of the portal hypertension. The disruption of portal blood flow with venovenous shunting may prevent tumour cells reaching the liver.
It has been reported that hepatitis virus infection resulted in a high state of the immune response in livers [24]. Cytotoxic T lymphocytes (CTL) and Kupffer cells were main components of the immune response during HBV infection. HBV replication activated the specific lytic pathways of cell injury by CTL and Kupffer cells [25], which effectively killed metastatic cancer cells just after lodging in the sinusoids [26], [27]. This coincides with the result reported by Song et al [13] that HBV infection with viral replication, which was determined by the presence of HBeAg and HBV DNA in serum, reduced the incidence of colorectal liver metastases in CRCs; whereas, occurrence of liver metastases in patients with nonreplicative HBV infection was close to those without HBV infection. Moreover, HBV replication promoted immune cells to secrete tumor necrosis factor a (TNF-a) [28], which also killed metastatic cancer cells. In addition, Wang et al [29] found that HBV replication resulted in elevated protein levels of Polo-like kinase1 (Plk1) and down-regulation of SUZ12 (suppressor of zeste 12). Plk1 was found overexpressed in a variety of human tumors and its expression was associated with cellular proliferation and prognosis of tumor patients. Deregulation of Plk1 activity contributed to genetic instability, which in turn leaded to oncogenic transformation [30]. SUZ12 could combine EZH2 (enhancer of zeste homolog 2) and EED (embryonic ectoderm development), and formed polycomb repressive complex (PRC2). PRC2 participated in epigenetic silence of several tumor suppressor genes by catalyzing the trimethylation of histone H3 at lysine 27, which served as a docking site for DNA methyltransferases and histone deacetylases [31]. Recent studies implied that PRC2 and its subunits (SUZ12 and EZHZ) were often deregulated in various cancer types and their overexpressions were closely associated with carcinogenesis [32], [33].
Conclusion
The phenomenon of the rare occurrence of metastatic colorectal cancers in chronically diseased livers has been observed for more than 70 years, but few related articles had been reported. This meta-analysis collected all related reports so far, and verified that patients with diseased livers have significantly lower incidences of colorectal liver metastases than those with normal livers. Nevertheless, it is still necessary to conduct larger size and better design studies to confirm our results. Moreover, of the ten included studies, nine studies evaluated patients from Asia. Because of this, our finding may just represent cancer patients from Asia. In addition, we suggest particular attention should be given to the precise mechanism of this phenomenon, so as to provide a research strategy on basic research and clinical prevention of colorectal liver metastases.
Supporting Information
PRISMA Checklist.
(PDF)
Acknowledgments
We thank all our colleagues working in the Department of Colorectal Surgery, First Affiliated Hospital of Guangxi Medical University, Nanning, Guangxi, People's Republic of China.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
This article is supported by the National Natural Science Foundation of China (grants No. 81160235). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Ma J, Zou Z, Jemal A (2014) Cancer statistics, 2014. CA Cancer J Clin 64: 9–29. [DOI] [PubMed] [Google Scholar]
- 2. Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, et al. (2010) Colorectal cancer. Lancet 375: 1030–1047. [DOI] [PubMed] [Google Scholar]
- 3. August DA, Ottow RT, Sugarbaker TS (1984) Clinical perspectives on human colon cancer metastases. Cancer Metast Rev 3: 303–324. [DOI] [PubMed] [Google Scholar]
- 4. Asbun HJ, Tsao JI, Hughes KS (1994) Resection of hepatic metastases from colorectal carcinoma. Cancer Treat Res 69: 33–41. [DOI] [PubMed] [Google Scholar]
- 5. Lieber MM (1957) The rare occurrence of metastatic carcinoma in the cirrhotic liver. Am J Med Sci 233 2: 145–52. [DOI] [PubMed] [Google Scholar]
- 6. Hayashi S, Masuda H, Shigematsu M (1996) Liver metastasis rare in colorectal cancer patients with fatty liver. Hepatogastroenterology 44: 1069–1075. [PubMed] [Google Scholar]
- 7. Iascone C, Ruperto M, Barillari P (2005) Occurrence of synchronous colorectal cancer metastasis in the cirrhotic or fatty liver. Minerva Chir 60: 185–90. [PubMed] [Google Scholar]
- 8. Utsunomiya T, Saitsu H, Saku M, Yoshida K, Matsumata T, et al. (1999) Rare occurrence of colorectal cancer metastasis in livers infected with hepatitis B or C virus. Am J Surg 177: 279–281. [DOI] [PubMed] [Google Scholar]
- 9. Zeng Y, He XW, He XS, Wu XJ, Ma JP, et al. (2013) Chronic Hepatic Viral Infection Could be a Protective Factor for Colorectal Cancer Liver Metastases: Analysis in a Single Institute. Hepatogastroenterology 60: 37–41. [DOI] [PubMed] [Google Scholar]
- 10. Wang FS, Shao ZG, Zhang JL, Liu YF (2012) Colorectal liver metastases rarely occur in patients with chronic hepatitis virus infection. Hepatogastroenterology 59: 1390–1392. [DOI] [PubMed] [Google Scholar]
- 11. Qiu HB, Zhang LY, Zeng ZL, Wang ZQ, Luo HY, et al. (2011) HBV infection decreases risk of liver metastasis in patients with colorectal cancer: A cohort study. World J Gastroenterol 17: 804–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Qian HG, Zhang J, Leng JH, Zhou GQ, Wu JH, et al. (2010) Association of hepatitis B virus infection and cirrhosis with liver metastasis in colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi 13: 202–204. [PubMed] [Google Scholar]
- 13. Song E, Chen J, Ou Q, Su F (2001) Rare occurrence of metastatic colorectal cancers in livers with replicative hepatitis B infection. Am J Surg 181: 529–33. [DOI] [PubMed] [Google Scholar]
- 14. Iascone C, Ruperto M, Barillari P (2005) Colorectal carcinoma metastasis in livers infected with hepatitis B or C virus. Minerva Chir 60: 77–81. [PubMed] [Google Scholar]
- 15. Murono K, Kitayama J, Tsuno NH, Nozawa H, Kawai K, et al. (2013) Hepatic steatosis is associated with lower incidence of liver metastasis from colorectal cancer. Int J Colorectal Dis 28: 1065–1072. [DOI] [PubMed] [Google Scholar]
- 16. Uetsuji S, Yamamura M, Yamamichi K, Okuda Y, Takada H, et al. (1992) Absence of colorectal cancer metastasis to the cirrhotic liver. Am J Surg 164: 176–7. [DOI] [PubMed] [Google Scholar]
- 17. Karube H, Masuda H, Hayashi S, Ishii Y, Nemoto N (2000) Fatty liver suppressed the angiogenesis in liver metastatic lesions. Hepatogastroenterology 47: 1541–1545. [PubMed] [Google Scholar]
- 18. Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, et al. (2008) Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer 8: 122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anthony PP, Ishak KG, Nayak NC, Poulsen HE, Scheuer PJ, et al. (1977) The morphology of cirrhosis: definition, nomenclature, and classification. Bull World Health Organ 55: 521–40. [PMC free article] [PubMed] [Google Scholar]
- 20. Song E, Chen J, Ouyang N, Wang M, Exton MS, et al. (2001) Kupffer cells of cirrhotic rat livers sensitize colon cancer cells to Fas-mediated apoptosis. Br J Cancer 84: 1265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Seitz G (1989) Why are metastases in cirrhotic livers so rare? Ultraschall Med 10 3: 123–126. [DOI] [PubMed] [Google Scholar]
- 22. Consolo M, Amoroso A, Spandidos DA, Mazzarino MC (2009) Matrix metalloproteinases and their inhibitors as markers of inflammation and fibrosis in chronic liver disease (Review). Int J Mol Med 24: 143–152. [DOI] [PubMed] [Google Scholar]
- 23. Mittal P1, Gupta R, Mittal G, Kalia V (2011) Association between portal vein color Doppler findings and the severity of disease in cirrhotic patients with portal hypertension. Iran J Radiol 8: 211–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Villeneuve JP (2005) The natural history of chronic hepatitis B virus infection. J Clin Virol 34: S139–142. [DOI] [PubMed] [Google Scholar]
- 25. Tordjmann T, Soulie A, Guettier C, Schmidt M, Berthou C, et al. (1998) Perforin and granzyme B lytic protein expression during chronic viral and autoimmune hepatitis. Liver 18: 391–397. [DOI] [PubMed] [Google Scholar]
- 26. Winwood PJ, Arthur MJ (1993) Kupffer cells: their activation and role in animal models of liver injury and human liver disease. Semin Liver Dis 13: 50–9. [DOI] [PubMed] [Google Scholar]
- 27. Ando K, Hiroishi K, Kaneko T, Moriyama T, Muto Y, et al. (1997) Perforin, Fas/Fas ligand, and TNF-alpha pathways as specific and bystander killing mechanisms of hepatitis C virus-specific human CTL. J Immunol 158: 5283–91. [PubMed] [Google Scholar]
- 28. Lara-Pezzi E, Majano PL, Gómez-Gonzalo M, García-Monzón C, Moreno-Otero R, et al. (1998) The hepatitis B virus X protein up-regulates tumor necrosis factor a gene expression in hepatocytes. Hepatology 28: 1013–1021. [DOI] [PubMed] [Google Scholar]
- 29. Wang WH, Studach LL, Andrisani OM (2011) Proteins ZNF198 and SUZ12 are down-regulated in hepatitis B virus (HBV) X protein-mediated hepatocyte transformation and in HBV replication. Hepatology 53: 1137–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Eckerdt F, Yuan J, Strebhardt K (2005) Polo-like kinases and oncogenesis. Oncogene 24: 267–276. [DOI] [PubMed] [Google Scholar]
- 31. Deb G, Singh AK, Gupta S (2014) EZH2: Not EZHY (Easy) to Deal. Mol Cancer Res 12: 639–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yu H, Simons DL, Segall I, Carcamo-Cavazos V, Schwartz EJ, et al. (2012) PRC2/EED-EZH2 complex is up-regulated in breast cancer lymph node metastasis compared to primary tumor and correlates with tumor proliferation in situ. PLoS One 7: e51239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Coe BP, Thu KL, Aviel-Ronen S, Vucic EA, Gazdar AF, et al. (2013) Genomic deregulation of the E2F/Rb pathway leads to activation of the oncogene EZH2 in small cell lung cancer. PLoS One 8: e71670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(PDF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.