Abstract
Introduction
Oncologists treating patients with targeted therapies encounter adverse events (AEs) that pose management challenges, lead to dosing inconsistencies, and impact patient quality of life. Oncologists' practices and attitudes in the management of targeted therapy-related AEs in renal cell carcinoma (RCC) patients are poorly understood. We sought to identify unmet needs associated with AE management and understand oncologists' treatment optimization strategies.
Methods
A 24-item online survey was administered in August 2012 to 119 U.S. oncologists treating advanced RCC patients. The survey solicited responses regarding demographics, practice settings, AE management practice patterns and beliefs, treatment barriers, and patient education.
Results
Respondents indicated between 25-50% of patients require dose modification/discontinuation due to AEs. The greatest barrier to optimizing treatment for RCC is the unpredictability of patient responses to treatment (43%). Most respondents (78%) discuss AE management with patients, but only a minority proactively reaches out to patients (46%). Most practitioners (70%) refer patients to non-oncology specialists when faced with unfamiliar AEs, although finding interested physicians (43%) and time constraints (40%) were the most commonly cited barriers to consulting with other specialties.
Conclusion
Results suggest that many patients require dose modifications/discontinuation due to AEs, and that non-oncologists are a frequently utilized resource to manage these events. There is a need for predictive drug toxicity markers to establish counseling and prevention, along with opportunities for increased education on supportive care techniques to maintain quality of life and consistent dosing.
Keywords: advanced kidney cancer, targeted therapy-related side effects, side effect management practices, anti-cancer therapy, oncologist practice patterns
Introduction
Targeted therapies are emerging as a viable treatment option in the management of advanced renal cell carcinoma (RCC). Molecules critical to the growth and survival of cancer cells, such as the vascular endothelial growth factor (VEGF) or its receptor (VEGFR) and the mammalian target of rapamycin (mTOR), all implicated in the pathogenesis of RCC, are being increasingly exploited as primary drug targets. These agents can delay time to disease progression and have shown improved progression-free survival in phase II/III clinical trials,[1],[2] with many promising drugs in the pipeline. To date, the anti-VEGF monoclonal antibody bevacizumab (in combination with IFN-α), the VEGFR inhibitors sorafenib, sunitinib, pazopanib, and axitinib, and the mTOR inhibitors temsirolimus and everolimus have received regulatory approval in the treatment of advanced RCC.[3, 4]
Although the target specificity of these newer therapies circumvents some of the systemic adverse effects associated with conventional chemotherapy, accumulating clinical experience and the unique adverse events (AEs) being reported,[5] warrant attention. This is important to maintain dose intensity of anticancer regimens and enhance patient quality of life. Several authors have attempted to address the clinical and management aspects of treatment-related AEs, in cancer patients who are now living longer because of targeted therapies.[5-7] There is, however, a lack of evidence-based treatment strategies and consensus among healthcare providers regarding the management of AEs, which would require significant collaboration between centers and supportive care specialists. In addition, the rapid pace of development of these innovative oncologic therapies, and a paucity of supportive care specialists familiar with this niche therapeutic area, are all hindering the constitution of effective AE management strategies.
Understanding current clinical practices is important to assess potential knowledge gaps, which can permit the optimization of existing treatment strategies to improve patient care and the design of effective educational efforts. Only a few studies have explored the prevailing clinical scenario in the management of AEs, with epidermal growth factor receptor inhibitors (EGFRIs) having received most attention,[8-10] and none with therapies used in patients with renal cell carcinoma (RCC). This study reports the lts of a national survey of oncologists, conducted to identify unmet needs associated with the management of patients treated with targeted cancer therapies for advanced RCC, and gain a better understanding of oncologists' perceptions and comprehensive care strategies used with these treatments.
Materials and Methods
Survey Development
A structured questionnaire was developed, in collaboration with a panel of experts (M.E.L., M.E., A.C., P.C.), Pfizer Inc., and Sermo. The self-administered survey was field-tested and refined based on the feedback received with regards to readability, usability, clarity, and randomization of questions. The finalized survey consisted of a 24-item questionnaire with one and/or multiple options to choose from, as applicable. The first five supplementary questions (QS1-QS5) were related to the practice demographics and the rest (Q1-Q19) pertained to clinical practices; the complete questionnaire is available as supplementary material (Appendix 1). Pfizer Inc. provided the funding for survey development, administration, data collection, and analysis.
Survey Administration and Participant Characteristics
The online survey was administered to 119 participants between August 29th and 30th, 2012. The responders consisted of institution-based and community-based practicing oncologists and hematologists, involved in the primary management and/or active monitoring of RCC patients treated with targeted therapies. Responders were not provided any remuneration. The data was collected and captured in an excel database, and subsequently retrieved for statistical analysis.
Statistical Analysis
Descriptive statistics were used to analyze participants' responses. The Pearson chi-square test, Fisher's exact test, and independent samples T-test (2-tailed) with equal variance were used to compare institution-based and community-based providers. Statistical significance was considered at P < 0.05. All statistical analyses were performed using Stata/SE 12.0.
Results
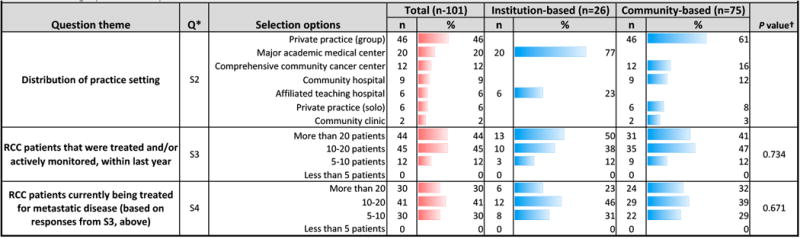
Response rate, clinical demographics, practice setting (Table 1)
Table 1. Demographics and experience of respondent sample.
|
Q: Question number
Statistical analysis was used to assess differences between institution- and community-based respondents; Pearson chi-square or Fisher's exact test (when anycell contained a value <5) was used to calculate P values. Statistical significance was considered at P <0.05
Of 119 responders, 101 institution-based (n=26) and community-based (n=75) physicians completed the survey. Institution-based respondents had more RCC patients [QS3], though community physicians managed more RCC patients for metastatic disease [QS4]. Institution-based physicians practiced at an academic medical center or affiliated teaching hospital. Community practice settings included private practices (group or solo) or community cancer centers, hospitals, or clinics [QS2]. Geographically, practice locations were distributed relatively equally [QS1].
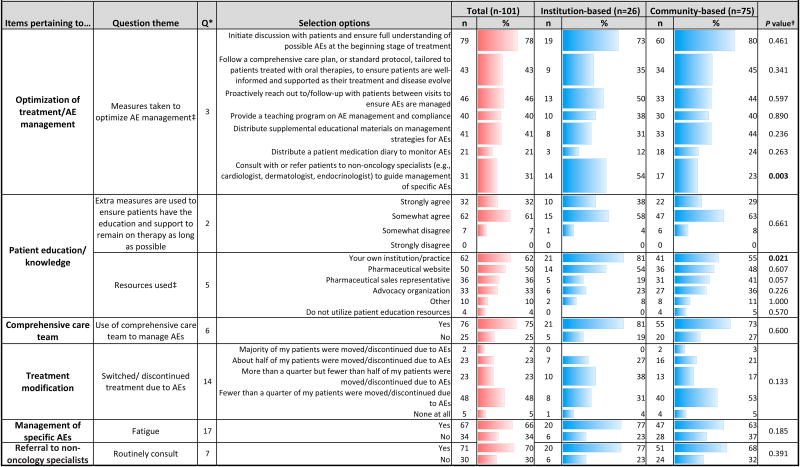
Management of adverse events: Practice patterns and opinions(Tables 2, 3)
Table 2. Management of adverse events: Practice patterns.
|
Q: Question number
Statistical analysis was used to assess differences between institution- and community-based respondents; Pearson chi-square or Fisher's exact test (when anycell contained a value <5) was used to calculate P values. Statistical significance was considered at P <0.05
Respondents were allowed to make multiple selections
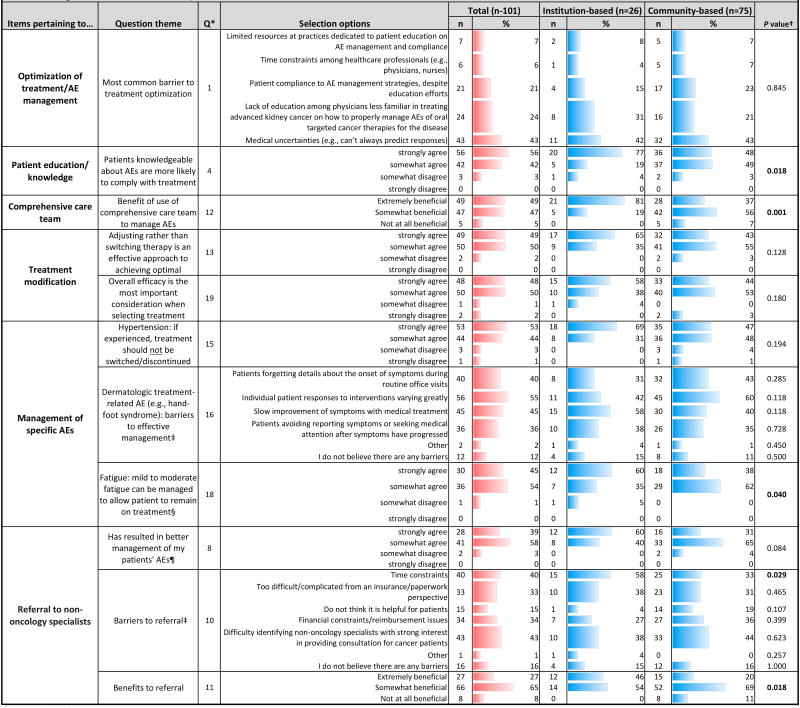
Table 3. Management of adverse events: Practice patterns.
|
Q: Question number
Statistical analysis was used to assess differences between institution- and community-based respondents; Pearson chi-square or Fisher's exact test (when anycell contained a value <5) was used to calculate P values. Statistical significance was considered at P <0.05
Respondents were allowed to make multiple selections
Includes only data from respondents who answered “yes” to Q17(n=67)
Includes only data from respondents who answered “yes” to Q7(n=71)
Most respondents initiate patient discussions at the start of treatment to ensure understanding of AEs [Q3]; institution-based doctors were more likely to strongly agree that well-informed patients comply with treatment (P=0.018) [Q4]. Yet only 43% of physicians followed a comprehensive care plan to provide patient support, and just 46% followed up to ensure AEs were managed [Q3]. The most commonly used resources for patient education in AE management included respondent's own institution, pharmaceutical websites, sales representatives, and advocacy organizations [Q5]. Institution-based physicians more often turned to their own institution (P=0.021), while community-based physicians tended to use information from sales representatives or advocacy organizations [Q5]. While 55% of respondents agreed that patients who are knowledgeable about AEs are more likely to comply with treatment [Q4], only 32% of physicians strongly agreed that their practice takes extra measures to educate/support patients [Q2]. For effective AE management, 75% utilized a comprehensive care team [Q6], and nearly all found this beneficial [Q12]. Most agreed that adjusting therapy dosing based on patient needs/safety, rather than switching therapy, can achieve optimal outcomes [Q13]. More than half of respondents stated that fewer than 25% of their patients changed/discontinued treatment due to AEs. Forty-six percent stated that AEs caused 25-50% of their patients to discontinue treatment [Q14].
Medical uncertainties were identified as the greatest treatment barrier [Q1]. Among other barriers, institution-based respondents most often cited lack of physician education, while community respondents more often mentioned patient compliance. Most respondents agreed that when selecting treatment, drug efficacy is the most important consideration [Q19].
Among specific treatment-related AEs, hypertension did not warrant treatment modification [Q15]. Sixty-six percent of practices routinely implement interventions for cancer-related fatigue [Q17]. The majority agreed that fatigue could be managed, allowing patients to remain on treatment [Q18]; institution-based physicians tended to strongly agree (P=0.040). Barriers to effective management of dermatologic AEs included varying intervention response, slow improvement, inconsistent patient histories, and non-reporting or treatment delay [Q16].
Referral to non-oncology specialists (Tables 2, 3)
Most oncologists consulted with non-oncology specialists for management of unfamiliar AEs [Q7]. A majority agreed that this resulted in better AE management [Q8]; institution-based respondents tended to strongly agree (P=0.084). Nearly a third of respondents found consultations extremely beneficial; institution-based physicians were more likely to hold this opinion (P=0.018) [Q11] and to refer patients to non-oncologists for specific AEs (P=0.003) [Q3]. Dermatologists were referred to most often, a trend driven by community physicians (P=0.005); institution-based respondents consulted cardiologists most frequently [Q9]. Still, respondents cited barriers to consulting specialists, including difficulty identifying those interested in consulting for cancer patients, time/financial constraints, and insurance/paperwork complexity [Q10]. Institution-based doctors more frequently selected the time constraint barrier (P=0.029).
Discussion
Our survey found significant differences among institution-based and community-based practices in relation to reference sources used for AE management, belief in the benefit of comprehensive care teams and non-oncology specialists to assist in managing treatment-related AEs, and referral patterns (to non-oncology specialists). Both practices took into account treatment efficacy, dose titration (vs. switching), and utilization of a comprehensive care team in patient management. At the time of the survey, axitinib, bevacizumab (with IFN-α), everolimus, pazopanib, sorafenib, sunitinib and temsirolimus had received regulatory approval.
Optimization of AE management
Our survey highlights the emphasis placed by a majority of practitioners (especially institution-based) on imparting AE-related education to patients, prior to initiation of therapy. Most believe that this practice allows patients to adhere to treatment and remain on therapy for as long as possible. The preference for utilization of institutional practice/resources for patient education, especially among institution-based physicians, may be a reflection of the robust set-up and academic environment of major medical centers. In addition, institution-based physicians have increased access to and familiarity with the extensive resources of academic institutions.
The aforementioned pre-emptive educational efforts appeared to wane after commencement of treatment. It is during this period that the treatment and disease evolve and patients actually experience AEs. We noted that a substantially lower number (<50%) of physicians implement AE management strategies (e.g. proactive interval follow-ups, consultations with non-oncology specialists, distribution of supplemental AE educational materials), ensure compliance (e.g. teaching programs), and monitor AEs (e.g. patient medication diaries), all of which are measures critical for good clinical outcomes. Other authors have provided a number of recommendations for the effective management of targeted therapy-related AEs. [5, 11, 12]
Nevertheless, it is noteworthy that almost all physicians considered that titration of the dose of lifesaving anticancer drugs (vis-à-vis switching) is often an effective approach to mitigating toxicities in patients. Whereas dose modification may represent a useful strategy in decreasing AEs, its effects on clinical outcome are concerning, in part because the severity of AEs such as HFSR and hypertension have been correlated with an improved response.[13, 14] Furthermore, nearly all respondents indicated that treatment should not be switched/discontinued for hypertension or fatigue, two key AEs commonly seen with these therapies, as strategies exist for their effective control. Intriguingly, nearly half of the participants indicated that they had switched or discontinued treatment due to AEs for 25%-50% of their patients. This probably reflects one instance of discordance between oncologists' beliefs and practices.
Most physicians utilized a comprehensive care team for AE management, yet only half found it extremely beneficial; of the latter, a vast majority were from institution-based practices (P = 0.018). This discrepancy could be explained by patient demographic differences between the two practice settings, physician time constraints, and the utility of such teams to the type of practice setting itself. For example, comprehensive care teams can reduce emergency room visits, hospital admissions/stays, fragmented care (multiple specialists), or duplication of services.
Time constraints appear to result in non-referral to outside specialists for AE management, specifically for institution-based physicians (P = 0.029), and to a lesser extent, seem to hinder treatment optimization for RCC patients, thus potentially leading to sub-optimal patient care. This may be partially explained by a higher patient volume, especially among institution-based physicians. The issue is further intensified by the fact that medical uncertainties were cited as the most common treatment optimization barrier, in addition to others such as the lack of education among physicians in managing these AEs and patient compliance to AE management strategies.
Consultation with non-oncology specialists
Previous studies have highlighted the importance of multidisciplinary teams to better manage targeted therapy-related AEs in cancer patients.[11, 15] Around 70% of our participants noted that their practice does routinely consult with non-oncology specialists for management of targeted therapy-related AEs [Q7], which is in contrast to 31% in a previous item [Q3]. This discrepancy may be attributed to the difference in wording between the two questions (specific versus unfamiliar AEs) and the multiple-selection format of Q3; respondents may have implicitly ranked their most frequent management measure rather than selecting all that were applicable, leading to an underestimation of the true prevalence of outside referral. Also, half of our respondents were from a private practice setting, which might account for the high referral rates due to less familiarity with non-oncologic AEs, as compared to their academic peers.
Dermatologists and cardiologists are the most frequently consulted specialists. However, not all agreed that referral to non-oncology specialists offered benefit for management of AEs suggesting that there is an urgent need to devise better evidence-based AE treatment strategies. The main barriers to consulting non-oncology specialists among institution-based and community-based physicians were noted to include difficulty finding physicians with an interest in consulting for cancer patients and time constraints, respectively.
Our findings highlight a need to increase oncologists' awareness of specialty centers, including supportive care specialists and programs. Furthermore, it highlights a need to train and increase non-oncology specialists adept in AE management in cancer patients. Lastly, dermatologic AEs such as rash[16] and hand-foot skin reaction (HSFR), besides being common with sunitinib, sorafenib, and axitinib (which has a higher risk of HSFR relative to pazopanib),[17-20] are correlates that may identify patients likely to benefit from treatment.[13] Thus, incorporation of dermatologists in the multidisciplinary management of RCC patients is crucial.
Besides the expertise of multidisciplinary specialists, oncologists should be well-equipped with their own management strategies for targeted therapy-related AEs that can cause disruption of treatment[12, 21] and negatively impact patient quality of life.[22, 23] Our survey identified a number of AE management strategies, while others have published recommendations highlighting AEs that can be best addressed by non-oncology specialists.[6, 7] Notwithstanding, the evidence for suggested management measures remains largely anecdotal.[6]
Limitations
Survey-based studies are subject to a number of limitations. First, because data is collected at a single time point, changes in practices and/or patient populations are not accounted for. Further, it is difficult to discern event chronology or recognize temporal associations. Second, surveys are retrospective and may introduce recall bias. Additionally, our responders may not have been exclusively administering targeted therapy for RCC management. Third, although efforts were made to include a random sample of responders, selection bias may have been inadvertently introduced, as reflected by the respondents' practice setting. A thorough analysis was performed for both institutional- and community-based groups; yet caution must be exercised in making conclusions regarding the former due to the smaller sample size. Fourth, because our responders were from the US, we cannot extrapolate our results to other countries. Lastly, although management strategies are driven primarily by physicians, inclusion of other clinical personnel (e.g. nurses, pharmacists) involved in patient care, and even patients or their families/caregivers may broaden our understanding. A follow-up research endeavor (reflecting a different time point) would be ideal, although survey administration can be expensive and time-consuming.
Conclusion
In summary, this survey fills an important gap by drawing attention to oncologists' current practices and beliefs in addressing targeted therapy-related AEs during the treatment of RCC, and their perceptions regarding the utility of outside referral to assist in AE management. Our study highlights the urgent need for a concerted inter-disciplinary approach and multi-center effort to address unpredictable and unfamiliar AEs from targeted therapies. Physician and patient education, and interval patient follow-ups are perhaps the most inexpensive interventions to yield improved outcomes. Although comprehensive care teams are relatively difficult to set-up, they are crucial in maintaining consistent dosing of anticancer therapy, AE management and patient quality of life. Development of evidence-based strategies and consensus guidelines for the comprehensive management of AEs from targeted therapies has yet to gain momentum, and further research in this arena will immensely benefit cancer patients and survivors.
Supplementary Material
Figure 1.
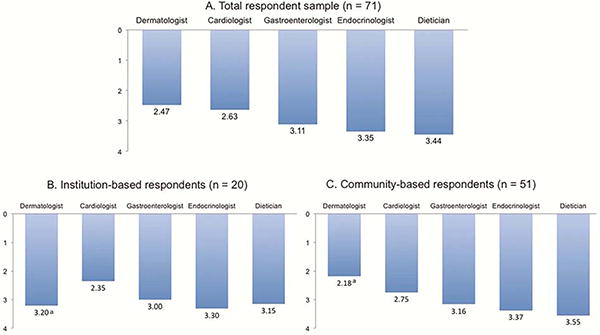
A, B, C Mean rank order of specific non-oncology specialists with whom respondents consulted with or referred patients to (1 = most often, 5 = least often), stratified by total respondent sample (n=71) (A), institution-based respondents (n=20) (B), and community-based respondents (n=51) (C)*
* Includes only data from respondents who answered “yes” to Q7: Do you routinely consult with or refer to non-oncology specialists, for management of unfamiliar AEs?
aMean values were significantly different between institution-based and community-based respondents (P < 0.01)
Clinical Practice Points.
Although the specificity of targeted therapies circumvent the systemic side effects associated with conventional chemotherapy, unique AEs are commonly reported with the use of these drugs, which necessitate oncologists' attention and careful management.
Our survey revealed that RCC patients require dose modifications/discontinuation due to targeted therapy-related AEs. Non-oncologists are frequently consulted to manage these AEs, although physician time constraints appear to be a barrier to referral. Most oncologists discuss AE management with patients, but only a minority proactively reach out to patients.
Institution-based physicians were more likely to utilize their own institution/practice for AE management information, whereas community-based physicians most often used sales representatives. Medical uncertainties were identified as the greatest barrier to treatment optimization.
Less than half of physicians in our sample implement AE management strategies, ensure patient compliance, and monitor AEs, measures that are critical for good clinical outcomes.
There is a need to increase oncologists' awareness of outside specialty centers and a need to train and increase the number of non-oncology specialists adept in the management of AEs in cancer patients.
Incorporation of dermatologists and cardiologists in the multidisciplinary management of RCC patients is crucial. Developing a concerted inter-disciplinary approach to address unpredictable/unfamiliar AEs from targeted therapies will allow patients to achieve a greater quality of life.
Acknowledgments
The authors thank Sermo and Young & Rubicom for their assistance with the materials. This study was funded by Pfizer Inc.
Funding sources: Memorial Sloan-Kettering Cancer Center, NY; Pfizer, Inc.
Abbreviations (alphabetical order)
- AE
Adverse events
- RCC
Renal Cell Carcinoma
Footnotes
Conflict of Interest Page: MEL has a consultant or advisory role with Advancell, AstraZeneca, Aveo, Bayer, BergPharma, Bristol-Myers Squibb, Galderma, Genentech, Genzyme, GlaxoSmithKline, Helsinn, Imclone, Lilly, LindiSkin, Merck, Novocure, Onyx, Pfizer, Roche, Sandoz, Sanofi Aventis and Wyeth. PC, AC, and ME each have a consultant role for Pfizer. JNR and VRB have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ljungberg B, et al. Guidelines on Renal Cell Carcinoma. [Accessed: September 23, 2013];European Association of Urology. 2013 Available from: http://www.uroweb.org/gls/pdf/10_Renal_Cell_Carcinoma_LRV2.pdf.
- 2.Motzer RJ, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27(22):3584–90. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Demetri GD, et al. Efficacy and safety of regorafenib for advanced gastrointestinal stromal tumours after failure of imatinib and sunitinib (GRID): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):295–302. doi: 10.1016/S0140-6736(12)61857-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santoni M, et al. Novel agents, combinations and sequences for the treatment of advanced renal cell carcinoma: when is the revolution coming? Curr Cancer Drug Targets. 2013;13(3):313–25. doi: 10.2174/1568009611313030009. [DOI] [PubMed] [Google Scholar]
- 5.Ravaud A. Treatment-associated adverse event management in the advanced renal cell carcinoma patient treated with targeted therapies. Oncologist. 2011;16 Suppl 2:32–44. doi: 10.1634/theoncologist.2011-S2-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eisen T, et al. Targeted therapies for renal cell carcinoma: review of adverse event management strategies. J Natl Cancer Inst. 2012;104(2):93–113. doi: 10.1093/jnci/djr511. [DOI] [PubMed] [Google Scholar]
- 7.Boers-Doets CB, et al. Oral adverse events associated with tyrosine kinase and mammalian target of rapamycin inhibitors in renal cell carcinoma: a structured literature review. Oncologist. 2012;17(1):135–44. doi: 10.1634/theoncologist.2011-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boone SL, et al. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: survey results. Oncology. 2007;72(3-4):152–9. doi: 10.1159/000112795. [DOI] [PubMed] [Google Scholar]
- 9.Hassel JC, et al. Treatment of epidermal growth factor receptor antagonist-induced skin rash: results of a survey among German oncologists. Onkologie. 2010;33(3):94–8. doi: 10.1159/000277656. [DOI] [PubMed] [Google Scholar]
- 10.Peuvrel L, et al. Survey on the management of skin toxicity associated with EGFR inhibitors amongst French Physicians. Journal of the European Academy of Dermatology and Venereology. 2013;27(4):419–429. doi: 10.1111/j.1468-3083.2011.04421.x. [DOI] [PubMed] [Google Scholar]
- 11.Livingstone E, et al. Interdisciplinary management of cutaneous adverse events of EGFR inhibitors and multityrosine kinase inhibitors in oncology. Dtsch Med Wochenschr. 2011;136(1-2):39–44. doi: 10.1055/s-0030-1269440. [DOI] [PubMed] [Google Scholar]
- 12.Négrier S, Ravaud A. Optimisation of sunitinib therapy in metastatic renal cell carcinoma: adverse-event management. European Journal of Cancer Supplements. 2007;5(7):12–19. [Google Scholar]
- 13.Poprach A, et al. Skin toxicity and efficacy of sunitinib and sorafenib in metastatic renal cell carcinoma: a national registry-based study. Ann Oncol. 2012;23(12):3137–43. doi: 10.1093/annonc/mds145. [DOI] [PubMed] [Google Scholar]
- 14.Rini BI, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103(9):763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Escudier B, et al. Multidisciplinary management of metastatic renal cell carcinoma in the era of targeted therapies. Cancer Treatment Reviews. 2012;38(2):127–132. doi: 10.1016/j.ctrv.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Balagula Y, et al. Clinical and histopathologic characteristics of rash in cancer patients treated with mammalian target of rapamycin inhibitors. Cancer. 2012;118(20):5078–83. doi: 10.1002/cncr.27505. [DOI] [PubMed] [Google Scholar]
- 17.Chu D, et al. Risk of hand-foot skin reaction with sorafenib: a systematic review and meta-analysis. Acta Oncol. 2008;47(2):176–86. doi: 10.1080/02841860701765675. [DOI] [PubMed] [Google Scholar]
- 18.Chu D, et al. Risk of hand-foot skin reaction with the multitargeted kinase inhibitor sunitinib in patients with renal cell and non-renal cell carcinoma: a meta-analysis. Clin Genitourin Cancer. 2009;7(1):11–9. doi: 10.3816/CGC.2009.n.002. [DOI] [PubMed] [Google Scholar]
- 19.Balagula Y, et al. The risk of hand foot skin reaction to pazopanib, a novel multikinase inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs. 2012;30(4):1773–81. doi: 10.1007/s10637-011-9652-2. [DOI] [PubMed] [Google Scholar]
- 20.Fischer A, et al. The risk of hand-foot skin reaction to axitinib, a novel VEGF inhibitor: a systematic review of literature and meta-analysis. Invest New Drugs. 2013;31(3):787–97. doi: 10.1007/s10637-013-9927-x. [DOI] [PubMed] [Google Scholar]
- 21.Robert C, et al. Dermatologic symptoms associated with the multikinase inhibitor sorafenib. J Am Acad Dermatol. 2009;60(2):299–305. doi: 10.1016/j.jaad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Nardone B, et al. The effect of hand-foot skin reaction associated with the multikinase inhibitors sorafenib and sunitinib on health-related quality of life. J Drugs Dermatol. 2012;11(11):e61–5. [PubMed] [Google Scholar]
- 23.Rosen AC, et al. Impact of dermatologic adverse events on quality of life in 283 cancer patients: a questionnaire study in a dermatology referral clinic. Am J Clin Dermatol. 2013;14(4):327–33. doi: 10.1007/s40257-013-0021-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


