Abstract
Serotonin (5-hydroxytryptamine or 5-HT) is an important neurotransmitter regulating a wide range of physiological and pathological functions via activation of heterogeneously expressed 5-HT receptors. The 5-HT7 receptor is one of the most recently described members of the 5-HT receptor family. Functionally, 5-HT7 receptor is associated with a number of physiological and pathological responses, including serotonin-induced phase shifting of the circadian rhythm, control of memory as well as locomotor and exploratory activity. A large body of evidence indicates involvement of the 5-HT7 receptor in anxiety and depression, and recent studies suggest that 5-HT7 receptor can be highly relevant for the treatment of major depressive disorders. The 5-HT7 receptor is coupled to the stimulatory Gs-protein, and receptor stimulation results in activation of adenylyl cyclase (AC) leading to a rise of cAMP concentration. In addition, this receptor is coupled to the G12-protein to activate small GTPases of the Rho family. This review focuses on molecular mechanisms responsible for the 5-HT7 receptor-mediated signaling. We provide detailed overview of signaling cascades controlled and regulated by the 5-HT7 receptor and discuss the functional impact of 5-HT7 receptor for the regulation of different cellular and subcellular processes.
Keywords: serotonergic signaling, G-protein coupled receptors, serotonin 5-HT7 receptor, heterotrimeric G-protein, oligomerization, palmitoylation
General principles of G-protein coupled receptor signaling
G-protein coupled receptors (GPCRs) represent the largest and most diverse superfamily of transmembrane receptors divided into five different families: rhodopsin, secretin, glutamate, adhesion and frizzled receptors (Bjarnadóttir et al., 2006). Initial studies with first discovered GPCRs, bovine rhodopsin and β2 adrenergic receptor, arouse great interest in the field of GPCRs, whose structures and functions became a subject of extensive research (Nathans and Hogness, 1983; Dixon et al., 1986). All these receptors function as signal-transducers by translating extracellular stimuli into intracellular responses resulting in multiple physiological as well as pathophysiological responses (Thompson et al., 2008). All known GPCRs consist of an extracellular amino-terminus, seven membrane-spanning α-helices (for which reason they are often referred to as 7 transmembrane receptors), and an intracellular carboxyl-terminus. Hence GPCR activity is induced by many different ligands, the mechanism of sensing ligands and transducing signals are highly variable (reviewed in Kristiansen, 2004). According to the “allosteric ternary complex model”, GPCRs exist in equilibrium between an inactive and active state (Christopoulos and Kenakin, 2002), explaining the agonist-independent, constitutive activity of some receptors (Seifert and Wenzel-Seifert, 2002).
Heterotrimeric G-proteins
Heterotrimeric G-proteins are the main downstream effectors of GPCRs acting as molecular switches by turning on intracellular downstream signaling cascades. They consist of three subunits, α, β and γ and are divided into four subgroups according to the structural and functional similarities of the Gα subunit. The members of the stimulatory Gαs family stimulate adenylyl cyclases (ACs), whereas inhibitory Gαi proteins inhibit ACs. The Gαq class of G-proteins couples to phospholipase Cβ (PLCβ), while Gα12 family members activate Rho guanine-nucleotide exchange factors (Rho GEFs; Kristiansen, 2004). To date at least 16 different genes encoding Gα subunits, 5 genes encoding Gβ subunits and 12 different genes encoding Gγ subunits have been discovered. Although not all subunits do interact with each other, the diversity of heterotrimeric G-proteins is still enormous, and this represents an additional level of complexity by the regulation of multiple signaling pathways (Cabrera-Vera et al., 2003).
Heterotrimeric G-proteins become activated by GPCRs via complex conformational changes, which are also facilitated by Gβγ dimers (Ford et al., 1998). Upon discovery of the heterotrimeric G-proteins, they were thought to conduct signals exclusively via Gα-subunits. Later on, Gβγ dimer has also been shown to directly modulate downstream effectors. First identified downstream target of Gβγ dimer was G-protein coupled inward rectifier potassium (GIRK) channel (Logothetis et al., 1987). Nowadays, a list of downstream effectors regulated by Gβγ dimers is permanently extending (Woehler and Ponimaskin, 2009).
In parallel with this classical G-protein mediated GPCR signaling, non-classical (G-protein independent) signaling became obvious during the last decade. This type of signaling will be also discussed below.
G-protein independent signaling
Beside the canonical GPCR signaling pathways via heterotrimeric G-proteins, GPCRs can participate in non-canonical, G-protein independent signaling. Main players of the G-protein independent signaling are arrestins - a small family of cytosolic adaptor proteins consisting of four members (Krupnick and Benovic, 1998). In contrast to arrestin 1 and arrestin 4 (X arrestin), which are primary involved in adaption processes of opsins in rods or cones, arrestin 2 and 3 (β-arrestin 1 and 2) are ubiquitously expressed and can interact with different GPCRs (Lefkowitz and Shenoy, 2005). Shortly after receptor stimulation, the C-terminal tail of a GPCR often becomes substrate for the phosphorylation by G-protein coupled receptor kinases (GRKs; Gehret and Hinkle, 2010). Phosphorylated receptors display a high affinity for β-arrestin 1 and 2, which hinder interactions between receptor and heterotrimeric G-protein resulting in desensitization and damping of G-protein dependent signaling (Perry et al., 2002). However, differently than thought at the beginning, arrestins not only switch-off the GPCR-signaling, but can also lead to the activation of alternative signaling pathways. Thus, β-arrestins serve as a signaling hub, linking activated GPCRs to multiple (G-protein independent) signaling pathways such as receptor trafficking as well as in extending GPCR mediated signaling to non-receptor tyrosine kinases (nRTKs) like proto-oncogene c-Src (c-Src) and mitogen-activated protein kinases (MAPK) signaling pathways.
5-HT7 receptor: physiological functions and distribution in the brain
The 5-HT7 receptor is one of the most recently discovered members of the serotonin receptor family, which was cloned in 1993 independently by researchers in three laboratories (Bard et al., 1993; Lovenberg et al., 1993; Ruat et al., 1993). The 5-HT7 receptor gene is located on human chromosome 10q23.3-q24.3 with an open reading frame containing 1335 base pairs and encoding a protein of 445 amino acids (Bard et al., 1993). The 5-HT7 receptor is broadly expressed in the central nervous system including spinal cord (Dogrul and Seyrek, 2006), thalamus, hypothalamus, hippocampus, prefrontal cortex, and the amygdala where it is expressed in both neurons and glial cells (Hedlund and Sutcliffe, 2004; Thomas and Hagan, 2004; Russo et al., 2005). Significant density of 5-HT7 receptor was observed in raphe nuclei area. In contrast, receptor expression level detected in putamen and cerebellum was relatively low (Horisawa et al., 2013). The 5-HT7 receptor is also expressed in the suprachiasmatic nucleus, and one of the first functions proposed for the 5-HT7 receptor was the regulation of sleep/wake cycles (Lovenberg et al., 1993). Functional analysis demonstrated association of the 5-HT7 receptor with central processes such as learning and memory, including specific aspects of hippocampus-dependent information processing (Hedlund and Sutcliffe, 2004; Ballaz et al., 2007; Eriksson et al., 2008; Gasbarri et al., 2008; Hedlund, 2009). Moreover, 5-HT7 receptor can be implicated in several neurological diseases (Hedlund and Sutcliffe, 2004; Thomas and Hagan, 2004). It has been shown that pharmacological blockade or knock-down of the 5-HT7 receptor induces antidepressant-like behavior in animal models (Guscott et al., 2005; Hedlund et al., 2005; Wesołowska et al., 2007). In addition, certain antidepressants may act directly on the 5-HT7 receptor (Mullins et al., 1999), suggesting this receptor as a novel target by the treatment of depression (Hedlund, 2009; Mnie-Filali et al., 2009). Analysis of mRNA expression level revealed that the amount of 5-HT7 gene transcripts in the dorsolateral prefrontal cortex of schizophrenic patients was increased, demonstrating that 5-HT7 receptor can also be associated with schizophrenia (East et al., 2002; Pouzet et al., 2002; Ikeda et al., 2006).
So far, three splice variants of the 5-HT7 receptor have been identified in human, including 5-HT7(a), 5-HT7(b), 5-HT7(d), three in mouse - 5-HT7(a), 5-HT7(b), 5-HT7(d), and four in rat - 5-HT7(a), 5-HT7(b), 5-HT7(c), 5-HT7(e) (Heidmann et al., 1997; Liu et al., 2001). These splice variants differ only in their short carboxyl-terminal amino acid sequence. Receptor isoforms have altered patterns of tissue distribution, while no difference in their pharmacological properties and coupling to ACs was observed (Heidmann et al., 1997, 1998; Krobert et al., 2001). The human 5-HT7(d) receptor represents an exception, because this isoform possesses a differential pattern of receptor internalization which can affect receptor-mediated signaling (Guthrie et al., 2005). In this regard, 5-HT7(d) receptor was constitutively internalized in the absence of agonist suggesting that its carboxyl-terminal tail, which is the longest among known human 5-HT7 receptor isoforms, may contain a motif that interacts with cellular transport machinery that is distinct from 5-HT7(a) and 5-HT7(b) receptors.
Gαs signaling mediated by the 5-HT7 receptor
The canonical signaling pathway of the 5-HT7 receptor is activation of Gs-protein which in turn can activate different AC isoforms (Shen et al., 1993). ACs show a unique tissue distribution as well as regulatory properties (Krupinski et al., 1989; Bakalyar and Reed, 1990; Premont et al., 1996). In vitro, all known AC isoforms are sensitive to the Gs activation (Cooper et al., 1995; Taussig and Gilman, 1995; Sunahara et al., 1996). In contrast, it has been demonstrated that Ca2+/calmodulin-stimulated neural-specific isoforms AC1 and AC8 are insensitive to Gs in vivo (Impey et al., 1994; Wayman et al., 1994; Nielsen et al., 1996), and that 5-HT7(a) receptor isoform can stimulate AC1 and AC8 by increasing intracellular Ca2+ concentration (Baker et al., 1998). The coupling between 5-HT7 receptor and Gs-protein results in increased AC activity leading to production of cAMP, which in turn activates protein kinase A (PKA) thereby inducing phosphorylation of different target proteins (Figure 1). This results in activation of multiple downstream signaling cascades, including Ras-dependent and Rap1-independent activation of the neuroprotective extracellular signal-regulated kinases (ERK) and Akt (protein kinase B) pathways (Errico et al., 2001; Johnson-Farley et al., 2005). Noteworthy, 5-HT7 receptor-mediated activation of Akt requires increases both in [cAMP] and intracellular [Ca2+], while activation of ERK is inhibited by Ca2+ (Figure 1). However, neither an influx of extracellular Ca2+ nor release of intracellular Ca2+ stores was required for 5-HT7 receptor-mediated activation of ERK in cultured primary hippocampal neurons (Lin et al., 2003). The authors of this study also demonstrated that increase in cAMP concentration causes activation of ERK in neurons via a pathway independent of PKA and Raf-1 (Li et al., 1991; Kyriakis et al., 1992). It is widely accepted, that intracellular pathways regulating ERK1/2 and Akt signaling are involved in actin filament reorganization. On the other hand, studies with LM2 cells, which are able to invade into the lung tissue in vivo, revealed no significant inhibition in cell motility after Ras-ERK pathway blockade, while PI3K pathways was critically involved in regulation of motility of LM2 cells (Choi and Helfman, 2014). It has been also shown that activation of PI3K activity alone is sufficient to remodel actin filaments and to increase cell migration through the activation of Akt in chicken embryo fibroblast (Qian et al., 2004). Thus, 5-HT7 receptor-mediated activation of Gs-protein can be involved in the activation of effector molecules regulating the cellular motility and cytoskeleton formation.
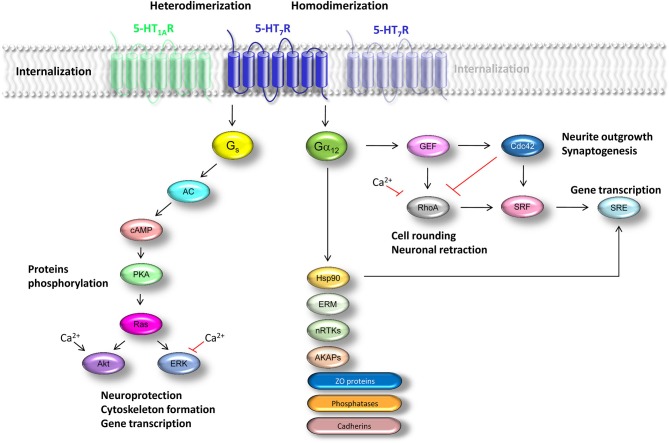
Figure 1.
Schematic representation of signaling pathways regulated by the 5-HT7 receptor. Effects mediated by Gs-proteins are in the left section. Summary of the G12-mediated signaling processes is shown in the right section. Abbreviations: GIRK—G-protein coupled inward rectifier potassium channel; AC—adenylyl cyclase; cAMP—cyclic adenosine monophosphate; PKA—protein kinase A; ERK—extracellular signal-regulated kinases; Akt—protein kinase B, Hsp90—heat shot shock protein 90; ERM—proteins of the ezrin-radixin-moesin family; GEF—guanine-nucleotide exchange factor (represented by the leukemia-associated RhoGEF LARG and p115Rho); nRTKs—non-receptor tyrosine kinases; AKAPs—A-kinase anchoring proteins; ZO—zona occludens proteins; SRF—serum response factor; SRE—serum response element.
Gα12 signaling mediated by the 5-HT7 receptor
In our previous studies we have demonstrated that 5-HT7 receptor is coupled not only to the Gs-protein, but can also activate G12-protein (Figure 1; Kvachnina et al., 2005; Kobe et al., 2012).
The G12-proteins have been shown to activate multiple signaling pathways, and their prominent downstream effectors are members of the Rho family of small GTPases (Rho, Rac, and Cdc42). The G12-protein can modulate the activity of Rho GTPases by activation of guanine-nucleotide exchange factor (GEF) p115Rho which was the first identified downstream effector of Gα12 proteins (Hart et al., 1998; Kozasa et al., 1998). Later on, plethora of additional downstream targets of G12-proteins has been discovered. In addition to other RhoGEFs, such as leukemia-associated RhoGEF (LARG) and RhoGEF homologs in Caenorhabditis elegans, regulator of G-protein signaling (RGS) family members, proteins of the ezrin-radixin-moesin (ERM) family, nRTKs, protein phosphatases, A-kinase anchoring proteins (AKAPs), zona occludens proteins and heat shot shock protein 90 (Hsp90) have been identified to directly interact with heterotrimeric G12-protein (Figure 1; Hiley et al., 2006; Kelly et al., 2007). The Gα12 subunit can also interact with C-terminal parts of cadherins leading to release of β-catenin into cytoplasm and nucleus, thus triggering gene transcription (Meigs et al., 2001).
In case of 5-HT7 receptor, it has been reported that receptor-mediated stimulation of G12-protein results in Rho-dependent activation of a transcription factor, serum response factor (SRF), which binds to the serum response element (SRE; Figure 1). Noteworthy, stimulation of 5-HT7 receptor led to the dose-dependent increase in SRE-driven gene expression even in the presence of a PKA-inhibitor or pertussis toxin (PTX), suggesting a receptor-mediated SRE activation in a PKA-independent manner (Kvachnina et al., 2005). Recent findings also elucidated Rho-independent mechanism of Gα12-mediated SRE activation via Hsp90 (Figure 1; Montgomery et al., 2014). Interaction between Gα12 and Hsp90 might also be critically involved in a selective transport of the G12-protein to the lipid rafts (Waheed and Jones, 2002).
Detailed analysis of 5-HT7 receptor-mediated signaling revealed that coupling of receptor to the heterotrimeric G12-protein selectively activates both RhoA and Cdc42 (Kvachnina et al., 2005), suggesting existence of cross-talk between Cdc42 and RhoA pathways. This might be mediated via convergent actions of these GTPases on the downstream effector myosin (Manser et al., 1994; Amano et al., 1996). Alternatively, Cdc42 and RhoA may function in a hierarchical cascade wherein Cdc42 downregulates RhoA activity (Figure 1; Li et al., 2002).
In neuroblastoma cells, agonist-dependent activation of recombinant 5-HT7 receptor induces pronounced filopodia formation via a Cdc42-mediated pathway paralleled by the RhoA-induced cell rounding (Kvachnina et al., 2005). Stimulation of the 5-HT7R/G12 signaling pathway in cultured hippocampal neurons promotes formation of dendritic spines and accelerates synaptogenesis, leading to enhanced spontaneous synaptic activity (Kobe et al., 2012). Morphogenic action of 5-HT7 receptor was further confirmed in experiments with striatal and cortical neuronal cultures (Speranza et al., 2013). In this study authors observed pronounced neurite outgrowth after specific activation of 5-HT7 receptor and demonstrated involvement of ERK and Cdk5 in this process, presuming both proteins to be downstream signaling molecules of Gα12 (Speranza et al., 2013).
Noteworthy that 5-HT7/G12 signaling in hippocampus undergoes strong developmental regulation. In organotypic hippocampal cultures from juvenile mice, 5-HT7R/G12 signaling potentiates formation of dendritic spines, increases the basal neuronal excitability and modulates synaptic plasticity. In contrast, in older neuronal preparations, stimulation of 5-HT7 receptor had no effect on neuronal morphology, synaptogenesis and synaptic plasticity (Kobe et al., 2012). Accordingly, the expression level of both 5-HT7 receptor and G12-protein in the hippocampus is progressively decreased during postnatal development (Kobe et al., 2012). Thus, 5-HT-induced activation of the 5-HT7R/G12 signaling pathways and the consequent reorganization of the dendritic morphology appear to be a part of the molecular cascade required for the growth of new synapses and the formation of initial neuronal networks, which then become the subject of activity-dependent structural and functional plasticity (Citri and Malenka, 2008; Ibata et al., 2008).
Homo- and heterodimerization of 5-HT7 receptors
G-protein-coupled receptors were initially assumed to exist and function as monomeric units that interact with corresponding G-proteins in 1:1 stoichiometry. Recent studies revealed the capability of GPCRs to form oligomers (Devi, 2001; Bulenger et al., 2005), and it is now widely accepted that homo- and heterodimerization can represent an additional mechanism regulating GPCR-mediated signaling.
Pharmacological analysis in combination with BRET experiments demonstrated that 5-HT7 receptor can form homooligomers in recombinant system (Teitler et al., 2010; Figure 1). Existence of 5-HT7 receptor homodimers has also been shown in primary cultures of rat cortical astrocytes (Smith et al., 2011). Homooligomerization of 5-HT7 receptor at the single-cell level has been further confirmed using two different FRET assays (Renner et al., 2012).
By combined application of biochemical and biophysical approaches we have recently demonstrated that 5-HT7 receptors can form heterodimers with 5-HT1A receptors both in vitro and in vivo (Renner et al., 2012; Figure 1). From the functional point of view, heterodimerization decreases Gi-protein coupling of 5-HT1A receptor and attenuates receptor-mediated activation of G-protein-gated potassium (GIRK) channels, without substantial changes in the coupling of 5-HT7 receptor to the Gs-protein. Moreover, heterodimerization significantly facilitated internalization of 5-HT1A receptor, while internalization kinetics of 5-HT7 receptor was decelerated upon heterodimerization (Renner et al., 2012).
Palmitoylation of the 5-HT7 receptor
Many signaling molecules involved in GPCR-mediated signaling are modified by post-translational modifications (Escribá et al., 2007), such as phosphorylation, ubiquitination, glycosylation, palmitoylation and others. The experiments with mutations of two predicted N-glycosylation sites in 5-HT7(a) receptor (N5Q and N66Q) revealed, that 5-HT7(a) receptor glycosylation neither influence the binding of 5-CT agonist to the receptor, nor the potency or efficacy with respect to activation of second messenger cascades, although a decrease in receptor density is apparent for the non-glycosylated receptor (Gellynck et al., 2012). To date, no data about the phosphorylation or ubiquitination of 5-HT7 receptor are available.
Covalent attachment of long chain saturated fatty acids (i.e., palmitate) to cysteine residue(s) within the protein via a labile thioester linkage (S-palmitoylation) represents a widespread post-translational modification of GPCRs since approximately 80% of all known receptors contain the potentially palmitoylable cysteine residue(s) downstream of their seventh transmembrane domain (Escribá et al., 2007). GPCR palmitoylation is involved in the modulation of different receptor functions from coupling to G-proteins and regulation of endocytosis to receptor phosphorylation and desensitization. Also the serotonin receptors represent potential substrates for palmitoylation, and palmitoylation was experimentally demonstrated for 5-HT1A, 5-HT1B, 5-HT4 and 5-HT7 receptors (reviewed in Gorinski and Ponimaskin, 2013).
The mouse 5-HT7 receptor has been shown to undergo dynamic palmitoylation in an agonist-dependent manner after expression in Sf.9 insect cells. Mutation analysis demonstrated that cysteines located in the C-terminal receptor domain at positions 404, 438 and 441 represent the main potential palmitoylation sites (Figure 2). Although these cysteine residues were responsible for the attachment of more than 90% of the receptor-bound palmitate, palmitoylation of 5-HT7 receptor was still not restricted to its C-terminus, pointing to the existence of additional acylation site(s) within the receptor.
Figure 2.
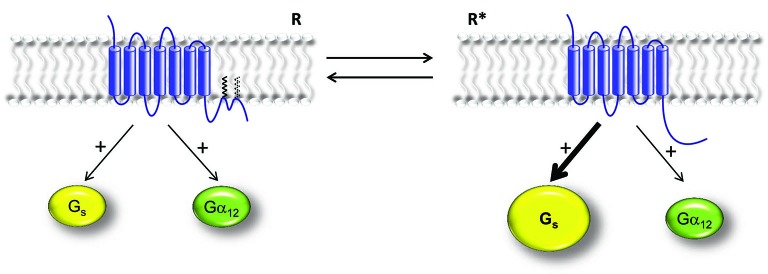
Hypothetical model for the regulation of 5-HT7 receptor activity by dynamic palmitoylation. This model suggests existence of two different receptor populations: Palmitoylated receptors with two additional intracellular C-terminal loops (left) and non-palmitoylated receptors with no intracellular C-terminal loops (right). These populations exist in dynamic equilibrium regulated by basal or agonist-promoted palmitate turnover. Depalmitoylation results in significant increase of the receptor’s capacity to convert from the inactive (R) to the active (R*) form in the absence of an agonists. Palmitoylated receptor shows activation of both Gαs- and Gα12-proteins (left). Non-palmitoylated receptor possesses increased agonist-independent, constitutive activity (R*) towards Gαs-mediated signaling, while basal receptor-mediated activation of Gα12-protein is unaltered (right).
Functional analysis of palmitoylation-deficient mutants revealed that agonist-induced activation of Gs- and G12-proteins was unaffected. However, mutation of the Cys404 either alone or in combination with Cys438/Cys441 significantly increased the agonist-independent, Gs-mediated constitutive 5-HT7 receptor activity, while the activation of G12-protein was not affected (Figure 2; Kvachnina et al., 2009). Generally, these data suggest that palmitoylation of 5-HT7 receptor might be directly involved in the isomerization of the receptor from the inactive to the active form in the absence of agonists. This transformation can be realized by dictating the conformation of receptor’s flexible cytoplasmic loops which might be involved either in the receptor/Gs-protein recognition or in Gs-protein binding and/or receptor-mediated Gs-protein activation (Figure 2). In combination with the previous findings on the functional role of 5-HT4 receptor palmitoylation (Ponimaskin et al., 2002, 2005), this observation suggests that palmitoylation can represent a general feature regulating constitutive receptor activity. Moreover, in case of 5-HT7 receptor (which is coupled to both, Gs- and G12-proteins) dynamic palmitoylation can represent a molecular mechanism responsible for selective Gs- or G12-mediated signaling.
Pharmacological properties of 5-HT7 receptor
During the last decade, several selective agonists and antagonists for 5-HT7 receptors have been developed and applied to investigate its pharmacology. Pharmacological analysis revealed that application of risperidone, 9-OH-risperidone, methiothepin, bromocryptine, lisuride, and metergoline resulted in irreversible inhibition of the recombinant 5-HT7 receptor expressed in HEK-293 cells (Smith et al., 2006; Knight et al., 2009). In contrast, action of other potent 5-HT7 receptor antagonists, including clozapine, mesulergine, penfluridol, amperozide and cinanserin is reversible and can be washed out (Knight et al., 2009). In other study receptor-inactivating properties of risperidone, 9-OH-risperidone, bromocriptine, methiothepin, metergoline, and lisuride have been demonstrated. Noteworthy that methiothepin and bromocriptine maximally inhibited forskolin-stimulated adenylate cyclase, whereas the other drugs produced partial inhibition, indicating the drugs are inducing slightly different inactive conformations of the 5-HT7 receptor (Toohey et al., 2009). Nowadays, the highly specific 5-HT7 receptor antagonist SB-269970 (pKi = 8.9 nM) is a mostly used receptor antagonist for in vitro and in vivo studies (Kobe et al., 2012; Renner et al., 2012; Tokarski et al., 2012; Vasefi et al., 2013; Guseva et al., 2014; Monti and Jantos, 2014). For the pharmacological activation of the receptor, a high-affinity receptor agonist 5-CT (IC50 = 0.83 nM, EC50 13 nM) is widely used in a numerous in vitro and in vivo studies (Guscott et al., 2003; Kobe et al., 2012; Vasefi et al., 2013). However, 5-CT is known to activate 5-HT1A, 5-HT1B, and 5-HT1D receptors. Therefore, analysis of 5-HT7 receptor functions by 5-CT requires parallel application of 5-HT1A/1B/1D receptor antagonists. Recently, various novel selective agonists such as AS-19, LP-44, LP-12, LP-211 and E-55888 were developed in addition to 5-CT (reviewed in Di Pilato et al., 2014). Amongst them two novel agonists, LP-211 and LP-378, have been investigated in regard to exploratory motivation, anxiety-related profiles, and spontaneous circadian rhythm (Adriani et al., 2012). The authors have shown that three- to four-fold dosage of LP-378 was necessary to induce the same effect as LP-211. The latest studies, both in vitro and in vivo, indicated LP-211 (Ki = 379 nM) as a more specific 5-HT7 receptor agonist with great potential for future investigations (Speranza et al., 2013; Monti and Jantos, 2014).
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The work was supported by Deutsche Forschungsgemeinschaft through the grant PO732 and Cluster of Excellence REBIRTH. Authors thank ERA-NET Neuron EU program for support of the project TargetECM.
References
- Adriani W., Travaglini D., Lacivita E., Saso L., Leopoldo M., Laviola G. (2012). Modulatory effects of two novel agonists for serotonin receptor 7 on emotion, motivation and circadian rhythm profiles in mice. Neuropharmacology 62, 833–842 10.1016/j.neuropharm.2011.09.012 [DOI] [PubMed] [Google Scholar]
- Amano M., Ito M., Kimura K., Fukata Y., Chihara K., Nakano T., et al. (1996). Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase). J. Biol. Chem. 271, 20246–20249 10.1074/jbc.271.34.20246 [DOI] [PubMed] [Google Scholar]
- Bakalyar H. A., Reed R. R. (1990). Identification of a specialized adenylyl cyclase that may mediate odorant detection. Science 250, 1403–1406 10.1126/science.2255909 [DOI] [PubMed] [Google Scholar]
- Baker L. P., Nielsen M. D., Impey S., Metcalf M. A., Poser S. W., Chan G., et al. (1998). Stimulation of type 1 and type 8 Ca2+/calmodulin-sensitive adenylyl cyclases by the Gs-coupled 5-hydroxytryptamine subtype 5-HT7A receptor. J. Biol. Chem. 273, 17469–17476 10.1074/jbc.273.28.17469 [DOI] [PubMed] [Google Scholar]
- Ballaz S. J., Akil H., Watson S. J. (2007). The 5-HT7 receptor: role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience 149, 192–202 10.1016/j.neuroscience.2007.07.043 [DOI] [PubMed] [Google Scholar]
- Bard J. A., Zgombick J., Adham N., Vaysse P., Branchek T. A., Weinshank R. L. (1993). Cloning of a novel human serotonin receptor (5-HT7) positively linked to adenylate cyclase. J. Biol. Chem. 268, 23422–23426 [PubMed] [Google Scholar]
- Bjarnadóttir T. K., Gloriam D. E., Hellstrand S. H., Kristiansson H., Fredriksson R., Schiöth H. B. (2006). Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 88, 263–273 10.1016/j.ygeno.2006.04.001 [DOI] [PubMed] [Google Scholar]
- Bulenger S., Marullo S., Bouvier M. (2005). Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol. Sci. 26, 131–137 10.1016/j.tips.2005.01.004 [DOI] [PubMed] [Google Scholar]
- Cabrera-Vera T. M., Vanhauwe J., Thomas T. O., Medkova M., Preininger A., Mazzoni M. R., et al. (2003). Insights into G protein structure, function and regulation. Endocr. Rev. 24, 765–781 10.1210/er.2000-0026 [DOI] [PubMed] [Google Scholar]
- Choi C., Helfman D. M. (2014). The Ras-ERK pathway modulates cytoskeleton organization, cell motility and lung metastasis signature genes in MDA-MB-231 LM2. Oncogene 33, 3668–3676 10.1038/onc.2013.341 [DOI] [PubMed] [Google Scholar]
- Christopoulos A., Kenakin T. (2002). G protein-coupled receptor allosterism and complexing. Pharmacol. Rev. 54, 323–374 10.1124/pr.54.2.323 [DOI] [PubMed] [Google Scholar]
- Citri A., Malenka R. C. (2008). Synaptic plasticity: multiple forms, functions and mechanisms. Neuropsychopharmacology 33, 18–41 10.1038/sj.npp.1301559 [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Mons N., Karpen J. W. (1995). Adenylyl cyclases and the interaction between calcium and cAMP signalling. Nature 374, 421–424 10.1038/374421a0 [DOI] [PubMed] [Google Scholar]
- Devi L. A. (2001). Heterodimerization of G-protein-coupled receptors: pharmacology, signaling and trafficking. Trends Pharmacol. Sci. 22, 532–537 10.1016/s0165-6147(00)01799-5 [DOI] [PubMed] [Google Scholar]
- Di Pilato P., Niso M., Adriani W., Romano E., Travaglini D., Berardi F., et al. (2014). Selective agonists for serotonin 7 (5-HT7) receptor and their applications in preclinical models: an overview. Rev. Neurosci. 25, 401–415 10.1515/revneuro-2014-0009 [DOI] [PubMed] [Google Scholar]
- Dixon R. A., Kobilka B. K., Strader D. J., Benovic J. L., Dohlman H. G., Frielle T., et al. (1986). Cloning of the gene and cDNA for mammalian beta-adrenergic receptor and homology with rhodopsin. Nature 321, 75–79 10.1038/321075a0 [DOI] [PubMed] [Google Scholar]
- Dogrul A., Seyrek M. (2006). Systemic morphine produce antinociception mediated by spinal 5-HT7, but not 5-HT1A and 5-HT2 receptors in the spinal cord. Br. J. Pharmacol. 149, 498–505 10.1038/sj.bjp.0706854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- East S. Z., Burnet P. W., Kerwin R. W., Harrison P. J. (2002). An RT-PCR study of 5-HT(6) and 5-HT(7) receptor mRNAs in the hippocampal formation and prefrontal cortex in schizophrenia. Schizophr. Res. 57, 15–26 10.1016/s0920-9964(01)00323-1 [DOI] [PubMed] [Google Scholar]
- Eriksson T. M., Golkar A., Ekström J. C., Svenningsson P., Ogren S. O. (2008). 5-HT7 receptor stimulation by 8-OH-DPAT counteracts the impairing effect of 5-HT(1A) receptor stimulation on contextual learning in mice. Eur. J. Pharmacol. 596, 107–110 10.1016/j.ejphar.2008.08.026 [DOI] [PubMed] [Google Scholar]
- Errico M., Crozier R. A., Plummer M. R., Cowen D. S. (2001). 5-HT(7) receptors activate the mitogen activated protein kinase extracellular signal related kinase in cultured rat hippocampal neurons. Neuroscience 102, 361–367 10.1016/s0306-4522(00)00460-7 [DOI] [PubMed] [Google Scholar]
- Escribá P. V., Wedegaertner P. B., Goñi F. M., Vögler O. (2007). Lipid-protein interactions in GPCR-associated signaling. Biochim. Biophys. Acta 1768, 836–852 10.1016/j.bbamem.2006.09.001 [DOI] [PubMed] [Google Scholar]
- Ford C. E., Skiba N. P., Bae H., Daaka Y., Reuveny E., Shekter L. R., et al. (1998). Molecular basis for interactions of G protein betagamma subunits with effectors. Science 280, 1271–1274 10.1126/science.280.5367.1271 [DOI] [PubMed] [Google Scholar]
- Gasbarri A., Cifariello A., Pompili A., Meneses A. (2008). Effect of 5-HT(7) antagonist SB-269970 in the modulation of working and reference memory in the rat. Behav. Brain Res. 195, 164–170 10.1016/j.bbr.2007.12.020 [DOI] [PubMed] [Google Scholar]
- Gehret A. U., Hinkle P. M. (2010). Importance of regions outside the cytoplasmic tail of G-protein-coupled receptors for phosphorylation and dephosphorylation. Biochem. J. 428, 235–245 10.1042/bj20100139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellynck E., Andressen K. W., Lintermans B., Haegeman G., Levy F. O., Vanhoenacker P., et al. (2012). Biochemical and pharmacological study of N-linked glycosylation of the human serotonin 5-HT7a receptor. FEBS J. 279, 1994–2003 10.1111/j.1742-4658.2012.08581.x [DOI] [PubMed] [Google Scholar]
- Gorinski N., Ponimaskin E. (2013). Palmitoylation of serotonin receptors. Biochem. Soc. Trans. 41, 89–94 10.1042/BST20120235 [DOI] [PubMed] [Google Scholar]
- Guscott M., Bristow L. J., Hadingham K., Rosahl T. W., Beer M. S., Stanton J. A., et al. (2005). Genetic knockout and pharmacological blockade studies of the 5-HT7 receptor suggest therapeutic potential in depression. Neuropharmacology 48, 492–502 10.1016/j.neuropharm.2004.11.015 [DOI] [PubMed] [Google Scholar]
- Guscott M. R., Egan E., Cook G. P., Stanton J. A., Beer M. S., Rosahl T. W., et al. (2003). The hypothermic effect of 5-CT in mice is mediated through the 5-HT7 receptor. Neuropharmacology 44, 1031–1037 10.1016/s0028-3908(03)00117-5 [DOI] [PubMed] [Google Scholar]
- Guseva D., Holst K., Kaune B., Meier M., Keubler L., Glage S., et al. (2014). Serotonin 5-HT7 receptor is critically involved in acute and chronic inflammation of the gastrointestinal tract. Inflamm. Bowel Dis. 20, 1516–1529 10.1097/MIB.0000000000000150 [DOI] [PubMed] [Google Scholar]
- Guthrie C. R., Murray A. T., Franklin A. A., Hamblin M. W. (2005). Differential agonist-mediated internalization of the human 5-hydroxytryptamine 7 receptor isoforms. J. Pharmacol. Exp. Ther. 313, 1003–1010 10.1124/jpet.104.081919 [DOI] [PubMed] [Google Scholar]
- Hart M. J., Jiang X., Kozasa T., Roscoe W., Singer W. D., Gilman A. G., et al. (1998). Direct stimulation of the guanine nucleotide exchange activity of p115 RhoGEF by Galpha13. Science 280, 2112–2114 10.1126/science.280.5372.2112 [DOI] [PubMed] [Google Scholar]
- Hedlund P. B. (2009). The 5-HT7 receptor and disorders of the nervous system: an overview. Psychopharmacology (Berl) 206, 345–354 10.1007/s00213-009-1626-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund P. B., Huitron-Resendiz S., Henriksen S. J., Sutcliffe J. G. (2005). 5-HT7 receptor inhibition and inactivation induce antidepressantlike behavior and sleep pattern. Biol. Psychiatry 58, 831–837 10.1016/j.biopsych.2005.05.012 [DOI] [PubMed] [Google Scholar]
- Hedlund P. B., Sutcliffe J. G. (2004). Functional, molecular and pharmacological advances in 5-HT7 receptor research. Trends Pharmacol. Sci. 25, 481–486 10.1016/j.tips.2004.07.002 [DOI] [PubMed] [Google Scholar]
- Heidmann D. E., Metcalf M. A., Kohen R., Hamblin M. W. (1997). Four 5-hydroxytryptamine7 (5-HT7) receptor isoforms in human and rat produced by alternative splicing: species differences due to altered intron-exon organization. J. Neurochem. 68, 1372–1381 10.1046/j.1471-4159.1997.68041372.x [DOI] [PubMed] [Google Scholar]
- Heidmann D. E., Szot P., Kohen R., Hamblin M. W. (1998). Function and distribution of three rat 5-hydroxytryptamine7 (5-HT7) receptor isoforms produced by alternative splicing. Neuropharmacology 37, 1621–1632 10.1016/s0028-3908(98)00070-7 [DOI] [PubMed] [Google Scholar]
- Hiley E., McMullan R., Nurrish S. J. (2006). The Galpha12-RGS RhoGEF-RhoA signalling pathway regulates neurotransmitter release in C. elegans. EMBO J. 25, 5884–5895 10.1038/sj.emboj.7601458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horisawa T., Ishiyama T., Ono M., Ishibashi T., Taiji M. (2013). Binding of lurasidone, a novel antipsychotic, to rat 5-HT7 receptor: analysis by [3H]SB-269970 autoradiography. Prog. Neuropsychopharmacol. Biol. Psychiatry 40, 132–137 10.1016/j.pnpbp.2012.08.005 [DOI] [PubMed] [Google Scholar]
- Ibata K., Sun Q., Turrigiano G. G. (2008). Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57, 819–826 10.1016/j.neuron.2008.02.031 [DOI] [PubMed] [Google Scholar]
- Ikeda M., Iwata N., Kitajima T., Suzuki T., Yamanouchi Y., Kinoshita Y., et al. (2006). Positive association of the serotonin 5-HT7 receptor gene with schizophrenia in a Japanese population. Neuropsychopharmacology 31, 866–871 10.1038/sj.npp.1300901 [DOI] [PubMed] [Google Scholar]
- Impey S., Wayman G., Wu Z., Storm D. R. (1994). Type I adenylyl cyclase functions as a coincidence detector for control of cyclic AMP response element-mediated transcription: synergistic regulation of transcription by Ca2+ and isoproterenol. Mol. Cell. Biol. 14, 8272–8281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Farley N. N., Kertesy S. B., Dubyak G. R., Cowen D. S. (2005). Enhanced activation of Akt and extracellular-regulated kinase pathways by simultaneous occupancy of Gq-coupled 5-HT2A receptors and Gs-coupled 5-HT7A receptors in PC12 cells. J. Neurochem. 92, 72–82 10.1111/j.1471-4159.2004.02832.x [DOI] [PubMed] [Google Scholar]
- Kelly P., Casey P. J., Meigs T. E. (2007). Biologic functions of the G12 subfamily of heterotrimeric g proteins: growth, migration and metastasis. Biochemistry 46, 6677–6687 10.1021/bi700235f [DOI] [PubMed] [Google Scholar]
- Knight J. A., Smith C., Toohey N., Klein M. T., Teitler M. (2009). Pharmacological analysis of the novel, rapid and potent inactivation of the human 5-Hydroxytryptamine7 receptor by risperidone, 9-OH-Risperidone and other inactivating antagonists. Mol. Pharmacol. 75, 374–380 10.1124/mol.108.052084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobe F., Guseva D., Jensen T. P., Wirth A., Renner U., Hess D., et al. (2012). 5-HT7R/G12 signaling regulates neuronal morphology and function in an age-dependent manner. J. Neurosci. 32, 2915–2930 10.1523/JNEUROSCI.2765-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozasa T., Jiang X., Hart M. J., Sternweis P. M., Singer W. D., Gilman A. G., et al. (1998). p115 RhoGEF, a GTPase activating protein for Galpha12 and Galpha13. Science 280, 2109–2111 10.1126/science.280.5372.2109 [DOI] [PubMed] [Google Scholar]
- Kristiansen K. (2004). Molecular mechanisms of ligand binding, signaling and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol. Ther. 103, 21–80 10.1016/j.pharmthera.2004.05.002 [DOI] [PubMed] [Google Scholar]
- Krobert K. A., Bach T., Syversveen T., Kvingedal A. M., Levy F. O. (2001). The cloned human 5-HT7 receptor splice variants: a comparative characterization of their pharmacology, function and distribution. Naunyn Schmiedebergs Arch. Pharmacol. 363, 620–632 10.1007/s002100000369 [DOI] [PubMed] [Google Scholar]
- Krupnick J. G., Benovic J. L. (1998). The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmacol. Toxicol. 38, 289–319 10.1146/annurev.pharmtox.38.1.289 [DOI] [PubMed] [Google Scholar]
- Krupinski J., Coussen F., Bakalyar H. A., Tang W. J., Feinstein P. G., Orth K., et al. (1989). Adenylyl cyclase amino acid sequence: possible channel- or transporter-like structure. Science 244, 1558–1564 10.1126/science.2472670 [DOI] [PubMed] [Google Scholar]
- Kvachnina E., Dumuis A., Wlodarczyk J., Renner U., Cochet M., Richter D. W., et al. (2009). Constitutive Gs-mediated, but not G12-mediated, activity of the 5-hydroxytryptamine 5-HT7(a) receptor is modulated by the palmitoylation of its C-terminal domain. Biochim. Biophys. Acta 1793, 1646–1655 10.1016/j.bbamcr.2009.08.008 [DOI] [PubMed] [Google Scholar]
- Kvachnina E., Liu G., Dityatev A., Renner U., Dumuis A., Richter D. W., et al. (2005). 5-HT7 receptor is coupled to G alpha subunits of heterotrimeric G12-protein to regulate gene transcription and neuronal morphology. J. Neurosci. 25, 7821–7830 10.1523/jneurosci.1790-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., et al. (1992). Raf-1 activates MAP kinase-kinase. Nature 358, 417–421 10.1038/358417a0 [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Shenoy S. K. (2005). Transduction of receptor signals by beta-arrestins. Science 308, 512–517 10.1126/science.1109237 [DOI] [PubMed] [Google Scholar]
- Li Z., Aizenman C. D., Cline H. T. (2002). Regulation of rho GTPases by crosstalk and neuronal activity in vivo. Neuron 33, 741–750 10.1016/s0896-6273(02)00621-9 [DOI] [PubMed] [Google Scholar]
- Li P., Wood K., Mamon H., Haser W., Roberts T. (1991). Raf-1: a kinase currently without a cause but not lacking in effects. Cell 64, 479–482 10.1016/0092-8674(91)90228-q [DOI] [PubMed] [Google Scholar]
- Lin S. L., Johnson-Farley N. N., Lubinsky D. R., Cowen D. S. (2003). Coupling of neuronal 5-HT7 receptors to activation of extracellular-regulated kinase through a protein kinase A-independent pathway that can utilize Epac. J. Neurochem. 87, 1076–1085 10.1046/j.1471-4159.2003.02076.x [DOI] [PubMed] [Google Scholar]
- Liu H., Irving H. R., Coupar I. M. (2001). Expression patterns of 5-HT7 receptor isoforms in the rat digestive tract. Life Sci. 69, 2467–2475 10.1016/s0024-3205(01)01318-2 [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. (1987). The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature 325, 321–326 10.1038/325321a0 [DOI] [PubMed] [Google Scholar]
- Lovenberg T. W., Baron B. M., de Lecea L., Miller J. D., Prosser R. A., Rea M. A., et al. (1993). A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11, 449–458 10.1016/0896-6273(93)90149-l [DOI] [PubMed] [Google Scholar]
- Manser E., Leung T., Salihuddin H., Zhao Z. S., Lim L. (1994). A brain serine/threonine protein kinase activated by Cdc42 and Rac1. Nature 367, 40–46 10.1038/367040a0 [DOI] [PubMed] [Google Scholar]
- Meigs T. E., Fields T. A., McKee D. D., Casey P. J. (2001). Interaction of Galpha 12 and Galpha 13 with the cytoplasmic domain of cadherin provides a mechanism for beta -catenin release. Proc. Natl. Acad. Sci. U S A 98, 519–524 10.1073/pnas.021350998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnie-Filali O., Lambas-Señas L., Scarna H., Haddjeri N. (2009). Therapeutic potential of 5-HT7 receptors in mood disorders. Curr. Drug Targets 10, 1109–1117 10.2174/138945009789735129 [DOI] [PubMed] [Google Scholar]
- Montgomery E. R., Temple B. R., Peters K. A., Tolbert C. E., Booker B. K., Martin J. W., et al. (2014). Gα12 structural determinants of Hsp90 interaction are necessary for serum response element-mediated transcriptional activation. Mol. Pharmacol. 85, 586–597 10.1124/mol.113.088443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti J. M., Jantos H. (2014). The role of serotonin 5-HT7 receptor in regulating sleep and wakefulness. Rev. Neurosci. 25, 429–437 10.1515/revneuro-2014-0016 [DOI] [PubMed] [Google Scholar]
- Mullins U. L., Gianutsos G., Eison A. S. (1999). Effects of antidepressants on 5-HT7 receptor regulation in the rat hypothalamus. Neuropsychopharmacology 21, 352–367 10.1016/s0893-133x(99)00041-x [DOI] [PubMed] [Google Scholar]
- Nathans J., Hogness D. S. (1983). Isolation, sequence analysis and intron-exon arrangement of the gene encoding bovine rhodopsin. Cell 34, 807–814 10.1016/0092-8674(83)90537-8 [DOI] [PubMed] [Google Scholar]
- Nielsen M. D., Chan G. C., Poser S. W., Storm D. R. (1996). Differential regulation of type I and type VIII Ca2+-stimulated adenylyl cyclases by Gi-coupled receptors in vivo. J. Biol. Chem. 271, 33308–33316 10.1074/jbc.271.52.33308 [DOI] [PubMed] [Google Scholar]
- Perry S. J., Baillie G. S., Kohout T. A., McPhee I., Magiera M. M., Ang K. L., et al. (2002). Targeting of cyclic AMP degradation to beta 2-adrenergic receptors by beta-arrestins. Science 298, 834–836 10.1126/science.1074683 [DOI] [PubMed] [Google Scholar]
- Ponimaskin E., Dumuis A., Gaven F., Barthet G., Heine M., Glebov K., et al. (2005). Palmitoylation of the 5-hydroxytryptamine4a receptor regulates receptor phosphorylation, desensitization and beta-arrestin-mediated endocytosis. Mol. Pharmacol. 67, 1434–1443 10.1124/mol.104.008748 [DOI] [PubMed] [Google Scholar]
- Ponimaskin E. G., Profirovic J., Vaiskunaite R., Richter D. W., Voyno-Yasenetskaya T. A. (2002). 5-Hydroxytryptamine 4(a) receptor is coupled to the Galpha subunit of heterotrimeric G13 protein. J. Biol. Chem. 277, 20812–20819 10.1074/jbc.m112216200 [DOI] [PubMed] [Google Scholar]
- Pouzet B., Didriksen M., Arnt J. (2002). Effects of the 5-HT(7) receptor antagonist SB-258741 in animal models for schizophrenia. Pharmacol. Biochem. Behav. 71, 655–665 10.1016/s0091-3057(01)00744-4 [DOI] [PubMed] [Google Scholar]
- Premont R. T., Matsuoka I., Mattei M. G., Pouille Y., Defer N., Hanoune J. (1996). Identification and characterization of a widely expressed form of adenylyl cyclase. J. Biol. Chem. 271, 13900–13907 10.1074/jbc.271.23.13900 [DOI] [PubMed] [Google Scholar]
- Qian Y., Corum L., Meng Q., Blenis J., Zheng J. Z., Shi X., et al. (2004). PI3K induced actin filament remodeling through Akt and p70S6K1: implication of essential role in cell migration. Am. J. Physiol. Cell Physiol. 286, C153–C163 10.1152/ajpcell.00142.2003 [DOI] [PubMed] [Google Scholar]
- Renner U., Zeug A., Woehler A., Niebert M., Dityatev A., Dityateva G., et al. (2012). Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 125, 2486–2499 10.1242/jcs.101337 [DOI] [PubMed] [Google Scholar]
- Ruat M., Traiffort E., Leurs R., Tardivel-Lacombe J., Diaz J., Arrang J. M., et al. (1993). Molecular cloning, characterization and localization of a high-affinity serotonin receptor (5-HT7) activating cAMP formation. Proc. Natl. Acad. Sci. U S A 90, 8547–8551 10.1073/pnas.90.18.8547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A., Pellitteri R., Monaco S., Romeo R., Stanzani S. (2005). “In vitro” postnatal expression of 5-HT7 receptors in the rat hypothalamus: an immunohistochemical analysis. Brain Res. Dev. Brain Res. 154, 211–216 10.1016/j.devbrainres.2004.11.002 [DOI] [PubMed] [Google Scholar]
- Seifert R., Wenzel-Seifert K. (2002). Constitutive activity of G-protein-coupled receptors: cause of disease and common property of wild-type receptors. Naunyn Schmiedebergs Arch. Pharmacol. 366, 381–416 10.1007/s00210-002-0588-0 [DOI] [PubMed] [Google Scholar]
- Shen Y., Monsma F. J., Jr., Metcalf M. A., Jose P. A., Hamblin M. W., Sibley D. R. (1993). Molecular cloning and expression of a 5-hydroxytryptamine7 serotonin receptor subtype. J. Biol. Chem. 268, 18200–18204 [PubMed] [Google Scholar]
- Smith C., Rahman T., Toohey N., Mazurkiewicz J., Herrick-Davis K., Teitler M. (2006). Risperidone irreversibly binds to and inactivates the h5-HT7 serotonin receptor. Mol. Pharmacol. 70, 1264–1270 10.1124/mol.106.024612 [DOI] [PubMed] [Google Scholar]
- Smith C., Toohey N., Knight J. A., Klein M. T., Teitler M. (2011). Risperidone-induced inactivation and clozapine-induced reactivation of rat cortical astrocyte 5-hydroxytryptamine7 receptors: evidence for in situ G protein-coupled receptor homodimer protomer cross-talk. Mol. Pharmacol. 79, 318–325 10.1124/mol.110.069278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speranza L., Chambery A., Di Domenico M., Crispino M., Severino V., Volpicelli F., et al. (2013). The serotonin receptor 7 promotes neurite outgrowth via ERK and Cdk5 signaling pathways. Neuropharmacology 67, 155–167 10.1016/j.neuropharm.2012.10.026 [DOI] [PubMed] [Google Scholar]
- Sunahara R. K., Dessauer C. W., Gilman A. G. (1996). Complexity and diversity of mammalian adenylyl cyclases. Annu. Rev. Pharmacol. Toxicol. 36, 461–480 10.1146/annurev.pharmtox.36.1.461 [DOI] [PubMed] [Google Scholar]
- Taussig R., Gilman A. G. (1995). Mammalian membrane-bound adenylyl cyclases. J. Biol. Chem. 270, 1–4 10.1074/jbc.270.1.1 [DOI] [PubMed] [Google Scholar]
- Teitler M., Toohey N., Knight J. A., Klein M. T., Smith C. (2010). Clozapine and other competitive antagonists reactivate risperidone-inactivated h5-HT7 receptors: radioligand binding and functional evidence for GPCR homodimer protomer interactions. Psychopharmacology (Berl) 212, 687–697 10.1007/s00213-010-2001-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. R., Hagan J. J. (2004). 5-HT7 receptors. Curr. Drug Targets CNS Neurol. Disord. 3, 81–90 10.2174/1568007043482633 [DOI] [PubMed] [Google Scholar]
- Thompson M. D., Cole D. E., Jose P. A. (2008). Pharmacogenomics of G protein-coupled receptor signaling: insights from health and disease. Methods Mol. Biol. 448, 77–107 10.1007/978-1-59745-205-2_6 [DOI] [PubMed] [Google Scholar]
- Tokarski K., Zelek-Molik A., Duszyńska B., Satała G., Bobula B., Kusek M., et al. (2012). Acute and repeated treatment with the 5-HT7 receptor antagonist SB 269970 induces functional desensitization of 5-HT7 receptors in rat hippocampus. Pharmacol. Rep. 64, 256–265 10.1016/s1734-1140(12)70763-6 [DOI] [PubMed] [Google Scholar]
- Toohey N., Klein M. T., Knight J., Smith C., Teitler M. (2009). Human 5-HT7 receptor-induced inactivation of forskolin-stimulated adenylate cyclase by risperidone, 9-OH-risperidone and other “inactivating antagonists”. Mol. Pharmacol. 76, 552–559 10.1124/mol.109.056283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasefi M. S., Yang K., Li J., Kruk J. S., Heikkila J. J., Jackson M. F., et al. (2013). Acute 5-HT7 receptor activation increases NMDA-evoked currents and differentially alters NMDA receptor subunit phosphorylation and trafficking in hippocampal neurons. Mol. Brain 6:24 10.1186/1756-6606-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waheed A. A., Jones T. L. (2002). Hsp90 interactions and acylation target the G protein Galpha 12 but not Galpha 13 to lipid rafts. J. Biol. Chem. 277, 32409–32412 10.1074/jbc.c200383200 [DOI] [PubMed] [Google Scholar]
- Wayman G. A., Impey S., Wu Z., Kindsvogel W., Prichard L., Storm D. R. (1994). Synergistic activation of the type I adenylyl cyclase by Ca2+ and Gs-coupled receptors in vivo. J. Biol. Chem. 269, 25400–25405 [PubMed] [Google Scholar]
- Wesołowska A., Tatarczyńska E., Nikiforuk A., Chojnacka-Wójcik E. (2007). Enhancement of the anti-immobility action of antidepressants by a selective 5-HT7 receptor antagonist in the forced swimming test in mice. Eur. J. Pharmacol. 555, 43–47 10.1016/j.ejphar.2006.10.001 [DOI] [PubMed] [Google Scholar]
- Woehler A., Ponimaskin E. G. (2009). G protein–mediated signaling: same receptor, multiple effectors. Curr. Mol. Pharmacol. 2, 237–248 10.2174/1874467210902030237 [DOI] [PubMed] [Google Scholar]